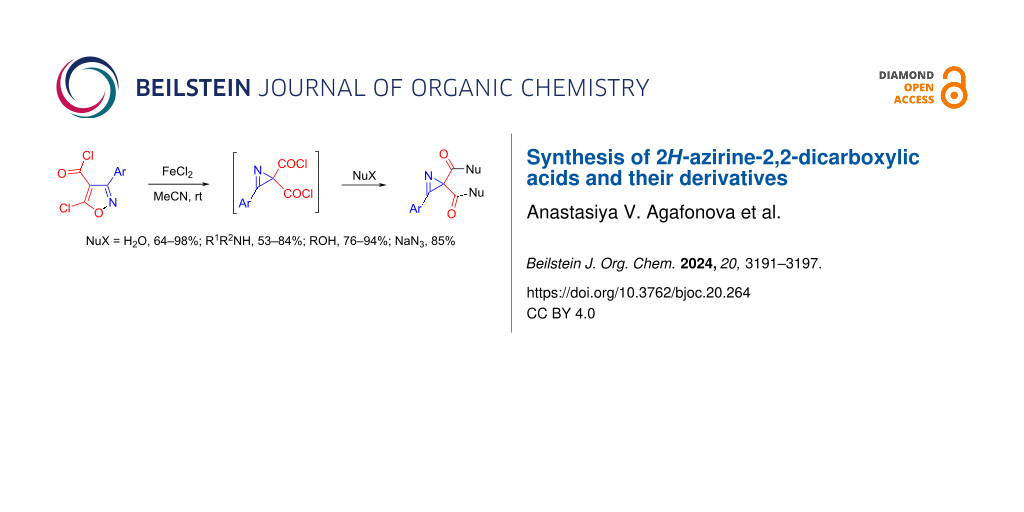
Abstract
Methods for the preparation of 3-aryl-2H-azirine-2,2-dicarboxylic acids and their amides, esters, and azides by FeCl2-catalyzed isomerization of 3-aryl-5-chloroisoxazole-4-carbonyl chlorides into 3-aryl-2H-azirine-2,2-dicarbonyl dichlorides followed by their reaction with nucleophiles are reported. Two approaches to the preparation of 3-aryl-5-chloroisoxazole-4-carbonyl chlorides have been developed.
Graphical Abstract
Introduction
The isomerization of isoxazoles, containing a heteroatomic substituent at C5, to 2H-azirines is a powerful method for the preparation of 2H-azirine-2-carboxylic acid derivatives [1]. In particular, the catalytic isomerization of 5-chloroisoxazoles allows the generation of azirine-2-carbonyl chlorides, which can be easily converted into a variety of azirine-2-carboxylic acid derivatives by reactions with nucleophilic reagents. Using this approach, numerous 2-(1H-pyrazol-1-ylcarbonyl)-2H-azirines, 1-(2H-azirine-2-carbonyl)benzotriazoles, 2H-azirine-2-carbonyl azides, anhydrides, amides, esters, and thioesters of azirine carboxylic acids, as well as azirine carboxylic acids themselves, have been prepared over the last decade (see [2] and references therein). Azirine-2-carboxylic acid derivatives are not only valuable synthetic building blocks [3-11] but also show useful biological activities [12-18]. Although many 2,2-bifunctionalized azirines have been synthesized [3-11], the synthesis of only one 2H-azirine-2,2-dicarboxylic acid derivative, dimethyl 3-phenyl-2H-azirine-2,2-dicarboxylate, has been reported to date. This compound was prepared by a Rh2(Piv)4-catalyzed isomerization of methyl 5-methoxy-3-phenylisoxazole-4-carboxylate [19]. The described linear synthesis, unfortunately, allows obtaining only one azirine-2,2-dicarboxylic acid derivative from a certain isoxazole precursor. Herein, we would like to report a method for the synthesis of 2H-azirine-2,2-dicarboxylic acids and their various derivatives from a single starting material, 3-substituted 2H-azirine-2,2-dicarbonyl dichloride 2, via the reaction with nucleophiles (Scheme 1). Two approaches to the preparation of diacyl chlorides 2 without using noble metals have also been developed.
Scheme 1: Approaches to 2H-azirine-2,2-dicarboxylic acid derivatives.
Scheme 1: Approaches to 2H-azirine-2,2-dicarboxylic acid derivatives.
Results and Discussion
5-Сhloroisoxazole-4-carbonyl chlorides 1, required for the preparation of 2H-azirine-2,2-dicarboxylic acids and their derivatives, were synthesized using two reaction sequences (Table 1). The first sequence involved the chloroformylation of isoxazolones 3 to 5-chloroisoxazole-4-carbaldehydes 4 by POCl3/DMF [20-23], followed by radical chlorination of 4 with SO2Cl2/AIBN [24]. The alternative route to acid chlorides 1 included oxidation of aldehydes 4 with Oxone to acids 5 and the conversion of the latter into acid chlorides with thionyl chloride.
Table 1: Synthesis of 5-chloroisoxazole-4-carbonyl chlorides.
|
|
|||||
| entry | 1, 3, 4, 5 | R | yield of 4 (%) | yield of 5 (%) | yield of 1 (%) |
| 1 | a | Ph | 53 | – | 77a (b) |
| 2 | b | 4-MeC6H4 | 20 | 98 | 25 (b)/84 (d) |
| 3 | c | 4-CF3C6H4 | 64 | – | 83a (b) |
| 4 | d | 3-MeOC6H4 | 35 | 97 | – (b)/84 (d) |
| 5 | e | 4-FC6H4 | 47 | – | 84a (b) |
| 6 | f | 4-ClC6H4 | 63 | – | 94a (b) |
| 7 | g | 4-BrC6H4 | 50 | 97 | – (b)/77 (d) |
| 8 | h | 2-BrC6H4 | 14 | 99 | – (b)/84(d) |
| 9 | i | 4-NO2C6H4 | 64 | 99 | 0 (b)/92 (d) |
| 10 | j | t-Bu | 69 | 92 | –/99 (d) |
aIsolation without chromatography.
The first reaction sequence was suitable for obtaining compounds 1a–c,e,f with substituents tolerant to radical reaction conditions. A significant advantage of the method is that chromatography was not required to isolate the products. At the same time, compound 4i proved to be inactive under the used chlorination conditions, compounds 4g,h underwent partial hydrodebromination in the aryl substituent in the same step, while compound 4j yielded a product with difficult to separate impurities. In these cases, as well as in reactions giving low aldehyde yields in the first step (4b,d), the second developed reaction sequence turned out to be more effective. In the second approach, the oxidation of aldehydes 4 with Oxone to acids 5 occurs with yields close to quantitative, and the conversion of the latter to the acid chlorides 1 with thionyl chloride proceeded with yields of 77–92%. This made it possible to synthesize the target isoxazoles 1b,d,g–j with fairly high yields.
Having in hand a set of isoxazoles 1a–i containing aryl substituents at the 3-position of the isoxazole ring, both with electron-donating and electron-withdrawing groups, and the tert-butyl-substituted isoxazole 1j, we proceeded to obtain dicarboxylic acids 6 (Scheme 2). The isomerization of isoxazoles 1 into diacyl chlorides 2 was achieved by applying the conditions for the isomerization of 3-aryl-5-chloroisoxazoles [25-27] using anhydrous FeCl2 as a catalyst and carrying out the reaction in acetonitrile at rt for 2 h. After TLC showed the disappearance of the starting isoxazoles 1, the reaction mixture was treated with water and acids 6a–i were isolated in 64–98% yield. Isoxazole 1j did not isomerize at room temperature, which is typical for highly sterically congested isoxazoles containing a 3-tert-butyl substituent [26]. The mechanism of such isomerizations of isoxazoles has been previously discussed using DFT calculations [25,26], which revealed the formation of an isoxazole–Fe complex, which facilitates the cleavage of the N–O bond and subsequent 1,3-cyclization, ultimately leading to the formation of 2H-azirine.
Scheme 2: Synthesis of 2H-azirine-2,2-dicarboxylic acids 6.
Scheme 2: Synthesis of 2H-azirine-2,2-dicarboxylic acids 6.
Therefore, the isomerization of isoxazole 1j was carried out at a higher temperature, 82 °C, but after hydrolysis of the reaction mixture, instead of the expected azirine dicarboxylic acid 6j, oxazole-4-carboxylic acid 9 was isolated. Apparently, azirine 2j underwent ring opening at higher temperature to nitrile ylide 7, which after cyclization and hydrolysis gave acid 9 (Scheme 3) (cf., e.g. [23]).
Scheme 3: Transformations of 3-(tert-butyl)-5-chloroisoxazole-4-carbonyl chloride (1j).
Scheme 3: Transformations of 3-(tert-butyl)-5-chloroisoxazole-4-carbonyl chloride (1j).
Next, given that the preparation of 2H-azirine-2-carboxamides from 2H-azirine-2-carbonyl chlorides is challenging [27], we proceeded to carefully optimize the conversion of 2H-azirine-2,2-dicarbonyl dichlorides 2 to 2H-azirine-2,2-dicarboxamides 10 using isoxazole 1a and benzylamine as starting materials (Table 2). It turned out that the previously found optimal reaction conditions for the preparation of amides from azirine-2-carbonyl chlorides [27] are not suitable for obtaining bis-amides from azirine-2,2-dicarbonyl dichlorides. In order to obtain a maximum yield, it is better in this case, to carry out the reaction with 2 equiv of the amine in the presence of 4 equiv of Cs2CO3 to trap hydrogen chloride. Additionally, the workup procedure, in which the product is isolated by filtration through celite after reaction with the amine, often allows one to obtain higher yields than an aqueous treatment of the reaction mixture.
Table 2: Optimization of amide preparation.
|
|
||||||
| Entry | FeCl2 (mol %) | time 1 (h) | additive (equiv) | BnNH2 (equiv) | solvent 2 | yield of 10a (%) |
| 1a | 20 | 2 | 2-MePy (2) | 2 | PhMe | 14 |
| 2 | 20 | 2 | 2-MePy (2) | 2 | PhMe | 39 |
| 3 | 20 | 2 | 2-MePy (2) | 2 | – | 38 |
| 4 | 20 | 2 | 2-MePy (2) | 3 | – | 30 |
| 5 | 20 | 2 | DMAP (2) | 3 | – | 9 |
| 6 | 20 | 2 |
ClC(O)OEt (1)
+ 2-TMSPy (1) |
6 | – | 19 |
| 7 | 20 | 2 | – | 4 | – | 14 |
| 8 | 20 | 2 | K2CO3 (4) | 2 | – | 45 |
| 9 | 20 | 2 | Cs2CO3 (4) | 2 | – | 72 |
| 10b | 20 | 2 | Cs2CO3 (4) | 2 | – | 73 |
| 11 | 5 | 4 | Cs2CO3 (4) | 2 | – | 10 |
aThe residue obtained from the isomerization of 1a → 2a was diluted with dry Et2O (50 mL), the precipitated FeCl2 was filtered off and after evaporation of Et2O, 2a was dissolved in anhydrous toluene. bFiltration through celite after reaction with the amine (without aqueous work-up).
A number of amides 10a–h were obtained from isoxazole 1a and primary and secondary amines according to the conditions described in entries 9 and 10 in Table 2, with yields of up to 78% (Scheme 4). An experiment with benzylamine and isoxazole 1a on a 1.5 mmol scale gave diamide 10a in 84% yield. The structure of compound 10h was confirmed by single-crystal X-ray diffraction analysis. The reaction of azetidine with diacyl chloride 2a gave a complex mixture of products, and O-methyl hydroxylamine did not react.
Scheme 4: Synthesis of amides 10. aFiltration through celite after reaction with amine (without aqueous workup). bWork up with H2O.
Scheme 4: Synthesis of amides 10. aFiltration through celite after reaction with amine (without aqueous worku...
Diacyl chloride 2a reacts with methanol and ethanol to give diesters 11a,b (Scheme 5). An experiment with isoxazole 1a and methanol on a 4 mmol scale gave dimethyl ester 11a in 99% yield. Unexpectedly, the reaction of branched alcohols with diacyl chloride 2a failed. For example, the reaction with benzyl alcohol resulted in the formation of an overly complex mixture of products. Adding bases to trap HCl did not improve the situation. Dibenzyl ester 11c was prepared using traditional activation of carboxylic acid 6a, although the yield was only 23%. A higher yield of the branched ester 11d (86%, as a mixture of diastereomers) was obtained by carbene insertion, generated by blue LED irradiation of methyl 2-diazo-2-phenylacetate, into the O–H bonds of diacid 6a (Scheme 5). Apparently, in this case, the reaction proceeds through a less sterically congested transition state.
Diacyl chloride 2a was also reacted with sodium azide as nucleophile at room temperature giving dicarbonyl azide 12 in 85% yield (Scheme 6).
Scheme 6: Synthesis of dicarbonyl azide 12.
Scheme 6: Synthesis of dicarbonyl azide 12.
Conclusion
Two reaction sequences for the synthesis of 3-aryl-5-chloroisoxazole-4-carbonyl chlorides have been developed. These compounds are convenient precursors for the preparation of 2H-azirine-2,2-dicarboxylic acids and their derivatives such as amides, esters and azides, via an Fe(II)-catalyzed room temperature isomerization to 3-aryl-2H-azirine-2,2-dicarbonyl dichlorides followed by their fast reaction at the same temperature with O- and N-nucleophiles. 3-Aryl-2H-azirine-2,2-dicarboxylic acids were prepared in 64–98% yield, whereas 3-(tert-butyl)-2H-azirine-2,2-dicarboxylic acid could not be obtained by this method because the isomerization of 3-(tert-butyl)-5-chloroisoxazole-4-carbonyl chloride did not occur at room temperature, but at elevated temperature (82 °C) the reaction proceeded via the formation of the nitrile ylide, which cyclized to 2-(tert-butyl)-5-chlorooxazole-4-carbonyl chloride. 3-Phenyl-2H-azirine-2,2-dicarboxamides were prepared using primary and secondary amines in 53–84% yield, and the reaction is scalable. Methyl and ethyl esters of 3-phenyl-2H-azirine-2,2-dicarboxylic acid were prepared in 76–99% yield from 3-phenyl-2H-azirine-2,2-dicarbonyl dichloride and methanol or ethanol, but the reaction of more branched alcohols failed. Such esters could be prepared from the dicarboxylic acids using traditional activation or OH-insertion reaction of carbenes formed by irradiation of the appropriate diazo compound.
Supporting Information
| Supporting Information File 1: Experimental procedures and characterization data of new compounds. | ||
| Format: PDF | Size: 7.1 MB | Download |
Acknowledgements
In commemoration of the 300th anniversary of St Petersburg State University’s founding. This research was carried out using resources of the Centre for Magnetic Resonance, the Research Centre for X-ray Diffraction Studies, the Centre for Chemical Analysis and Materials, and the Computer Centre of the Science Park of St. Petersburg State University.
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Galenko, E. E.; Khlebnikov, A. F.; Novikov, M. S. Chem. Heterocycl. Compd. 2016, 52, 637–650. doi:10.1007/s10593-016-1944-1
Return to citation in text: [1] -
Prokop'eva, I. N.; Tomashenko, O. A.; Matveeva, D. R.; Galenko, E. E.; Novikov, M. S.; Khlebnikov, A. F. Tetrahedron 2024, 167, 134255. doi:10.1016/j.tet.2024.134255
Return to citation in text: [1] -
Palacios, F.; de Retana, A. M. O.; de Marigorta, E. M.; de los Santos, J. M. Eur. J. Org. Chem. 2001, 2401–2414. doi:10.1002/1099-0690(200107)2001:13<2401::aid-ejoc2401>3.0.co;2-u
Return to citation in text: [1] [2] -
Palacios, F.; de Retana, A. M. O.; Martínez de Marigorta, E.; Manuel de los Santos, J. Org. Prep. Proced. Int. 2002, 34, 219–269. doi:10.1080/00304940209356770
Return to citation in text: [1] [2] -
Pinho e Melo, T. M. V. D.; Rocha-Gonsalves, A. M. d’A. Curr. Org. Synth. 2004, 1, 275–292. doi:10.2174/1570179043366729
Return to citation in text: [1] [2] -
Lemos, A. Molecules 2009, 14, 4098–4119. doi:10.3390/molecules14104098
Return to citation in text: [1] [2] -
Padwa, A. Adv. Heterocycl. Chem. 2010, 99, 1–31. doi:10.1016/s0065-2725(10)09901-0
Return to citation in text: [1] [2] -
Khlebnikov, A. F.; Novikov, M. S. Tetrahedron 2013, 69, 3363–3401. doi:10.1016/j.tet.2013.02.020
Return to citation in text: [1] [2] -
De, A.; Majee, A. J. Heterocycl. Chem. 2022, 59, 422–448. doi:10.1002/jhet.4415
Return to citation in text: [1] [2] -
Xu, F.; Zeng, F.-W.; Luo, W.-J.; Zhang, S.-Y.; Huo, J.-Q.; Li, Y.-P. Eur. J. Org. Chem. 2024, 27, e202301292. doi:10.1002/ejoc.202301292
Return to citation in text: [1] [2] -
Charushin, V. N.; Verbitskiy, E. V.; Chupakhin, O. N.; Vorobyeva, D. V.; Gribanov, P. S.; Osipov, S. N.; Ivanov, A. V.; Martynovskaya, S. V.; Sagitova, E. F.; Dyachenko, V. D.; Dyachenko, I. V.; Krivokolylsko, S. G.; Dotsenko, V. V.; Aksenov, A. V.; Aksenov, D. A.; Aksenov, N. A.; Larin, A. A.; Fershtat, L. L.; Muzalevskiy, V. M.; Nenajdenko, V. G.; Gulevskaya, A. V.; Pozharskii, A. F.; Filatova, E. A.; Belyaeva, K. V.; Trofimov, B. A.; Balova, I. A.; Danilkina, N. A.; Govdi, A. I.; Tikhomirov, A. S.; Shchekotikhin, A. E.; Novikov, M. S.; Rostovskii, N. V.; Khlebnikov, A. F.; Klimochkin, Y. N.; Leonova, M. V.; Tkachenko, I. M.; Mamedov, V. A. O.; Mamedova, V. L.; Zhukova, N. A.; Semenov, V. E.; Sinyashin, O. G.; Borshchev, O. V.; Luponosov, Y. N.; Ponomarenko, S. A.; Fisyuk, A. S.; Kostyuchenko, A. S.; Ilkin, V. G.; Beryozkina, T. V.; Bakulev, V. A.; Gazizov, A. S.; Zagidullin, A. A.; Karasik, A. A.; Kukushkin, M. E.; Beloglazkina, E. K.; Golantsov, N. E.; Festa, A. A.; Voskresenskii, L. G.; Moshkin, V. S.; Buev, E. M.; Sosnovskikh, V. Y.; Mironova, I. A.; Postnikov, P. S.; Zhdankin, V. V.; Yusubov, M. S. O.; Yaremenko, I. A.; Vil', V. A.; Krylov, I. B.; Terent'ev, A. O.; Gorbunova, Y. G.; Martynov, A. G.; Tsivadze, A. Y.; Stuzhin, P. A.; Ivanova, S. S.; Koifman, O. I.; Burov, O. N.; Kletskii, M. E.; Kurbatov, S. V.; Yarovaya, O. I.; Volcho, K. P.; Salakhutdinov, N. F.; Panova, M. A.; Burgart, Y. V.; Saloutin, V. I.; Sitdikova, A. R.; Shchegravina, E. S.; Fedorov, A. Y. Russ. Chem. Rev. 2024, 93, RCR5125. doi:10.59761/rcr5125
Return to citation in text: [1] [2] -
Sakharov, P. А.; Novikov, M. S.; Rostovskii, N. V. Chem. Heterocycl. Compd. 2021, 57, 512–521. doi:10.1007/s10593-021-02934-2
Return to citation in text: [1] -
Molinski, T. F.; Ireland, C. M. J. Org. Chem. 1988, 53, 2103–2105. doi:10.1021/jo00244a049
Return to citation in text: [1] -
Salomon, C. E.; Williams, D. H.; Faulkner, D. J. J. Nat. Prod. 1995, 58, 1463–1466. doi:10.1021/np50123a021
Return to citation in text: [1] -
Keffer, J. L.; Plaza, A.; Bewley, C. A. Org. Lett. 2009, 11, 1087–1090. doi:10.1021/ol802890b
Return to citation in text: [1] -
Skepper, C. K.; Dalisay, D. S.; Molinski, T. F. Org. Lett. 2008, 10, 5269–5271. doi:10.1021/ol802065d
Return to citation in text: [1] -
Skepper, C. K.; Dalisay, D. S.; Molinski, T. F. Bioorg. Med. Chem. Lett. 2010, 20, 2029–2032. doi:10.1016/j.bmcl.2010.01.068
Return to citation in text: [1] -
Rostovskii, N. V.; Koronatov, A. N.; Sakharov, P. A.; Agafonova, A. V.; Novikov, M. S.; Khlebnikov, A. F.; Rogacheva, E. V.; Kraeva, L. A. Org. Biomol. Chem. 2020, 18, 9448–9460. doi:10.1039/d0ob02023k
Return to citation in text: [1] -
Rostovskii, N. V.; Agafonova, A. V.; Smetanin, I. A.; Novikov, M. S.; Khlebnikov, A. F.; Ruvinskaya, J. O.; Starova, G. L. Synthesis 2017, 49, 4478–4488. doi:10.1055/s-0036-1590822
Return to citation in text: [1] -
Anderson, D. J. J. Org. Chem. 1986, 51, 945–947. doi:10.1021/jo00356a039
Return to citation in text: [1] -
Rajender, P. S.; Sridevi, G.; Reddy, K. K. Synth. Commun. 2012, 42, 2191–2200. doi:10.1080/00397911.2011.555051
Return to citation in text: [1] -
Beccalli, E. M.; Marchesini, A. J. Org. Chem. 1987, 52, 3426–3434. doi:10.1021/jo00391a048
Return to citation in text: [1] -
Serebryannikova, A. V.; Galenko, E. E.; Novikov, M. S.; Khlebnikov, A. F. J. Org. Chem. 2019, 84, 15567–15577. doi:10.1021/acs.joc.9b02536
Return to citation in text: [1] [2] -
Ryabova, O. B.; Makarov, V. A.; Alekseeva, L. M.; Shashkov, A. S.; Chernyshev, V. V.; Granik, V. G. Russ. Chem. Bull. 2005, 54, 1907–1914. doi:10.1007/s11172-006-0057-x
Return to citation in text: [1] -
Mikhailov, K. I.; Galenko, E. E.; Galenko, A. V.; Novikov, M. S.; Ivanov, A. Y.; Starova, G. L.; Khlebnikov, A. F. J. Org. Chem. 2018, 83, 3177–3187. doi:10.1021/acs.joc.8b00069
Return to citation in text: [1] [2] -
Galenko, E. E.; Bodunov, V. A.; Galenko, A. V.; Novikov, M. S.; Khlebnikov, A. F. J. Org. Chem. 2017, 82, 8568–8579. doi:10.1021/acs.joc.7b01351
Return to citation in text: [1] [2] [3] -
Agafonova, A. V.; Novikov, M. S.; Khlebnikov, A. F. Molecules 2023, 28, 275. doi:10.3390/molecules28010275
Return to citation in text: [1] [2] [3]
| 1. | Galenko, E. E.; Khlebnikov, A. F.; Novikov, M. S. Chem. Heterocycl. Compd. 2016, 52, 637–650. doi:10.1007/s10593-016-1944-1 |
| 3. | Palacios, F.; de Retana, A. M. O.; de Marigorta, E. M.; de los Santos, J. M. Eur. J. Org. Chem. 2001, 2401–2414. doi:10.1002/1099-0690(200107)2001:13<2401::aid-ejoc2401>3.0.co;2-u |
| 4. | Palacios, F.; de Retana, A. M. O.; Martínez de Marigorta, E.; Manuel de los Santos, J. Org. Prep. Proced. Int. 2002, 34, 219–269. doi:10.1080/00304940209356770 |
| 5. | Pinho e Melo, T. M. V. D.; Rocha-Gonsalves, A. M. d’A. Curr. Org. Synth. 2004, 1, 275–292. doi:10.2174/1570179043366729 |
| 6. | Lemos, A. Molecules 2009, 14, 4098–4119. doi:10.3390/molecules14104098 |
| 7. | Padwa, A. Adv. Heterocycl. Chem. 2010, 99, 1–31. doi:10.1016/s0065-2725(10)09901-0 |
| 8. | Khlebnikov, A. F.; Novikov, M. S. Tetrahedron 2013, 69, 3363–3401. doi:10.1016/j.tet.2013.02.020 |
| 9. | De, A.; Majee, A. J. Heterocycl. Chem. 2022, 59, 422–448. doi:10.1002/jhet.4415 |
| 10. | Xu, F.; Zeng, F.-W.; Luo, W.-J.; Zhang, S.-Y.; Huo, J.-Q.; Li, Y.-P. Eur. J. Org. Chem. 2024, 27, e202301292. doi:10.1002/ejoc.202301292 |
| 11. | Charushin, V. N.; Verbitskiy, E. V.; Chupakhin, O. N.; Vorobyeva, D. V.; Gribanov, P. S.; Osipov, S. N.; Ivanov, A. V.; Martynovskaya, S. V.; Sagitova, E. F.; Dyachenko, V. D.; Dyachenko, I. V.; Krivokolylsko, S. G.; Dotsenko, V. V.; Aksenov, A. V.; Aksenov, D. A.; Aksenov, N. A.; Larin, A. A.; Fershtat, L. L.; Muzalevskiy, V. M.; Nenajdenko, V. G.; Gulevskaya, A. V.; Pozharskii, A. F.; Filatova, E. A.; Belyaeva, K. V.; Trofimov, B. A.; Balova, I. A.; Danilkina, N. A.; Govdi, A. I.; Tikhomirov, A. S.; Shchekotikhin, A. E.; Novikov, M. S.; Rostovskii, N. V.; Khlebnikov, A. F.; Klimochkin, Y. N.; Leonova, M. V.; Tkachenko, I. M.; Mamedov, V. A. O.; Mamedova, V. L.; Zhukova, N. A.; Semenov, V. E.; Sinyashin, O. G.; Borshchev, O. V.; Luponosov, Y. N.; Ponomarenko, S. A.; Fisyuk, A. S.; Kostyuchenko, A. S.; Ilkin, V. G.; Beryozkina, T. V.; Bakulev, V. A.; Gazizov, A. S.; Zagidullin, A. A.; Karasik, A. A.; Kukushkin, M. E.; Beloglazkina, E. K.; Golantsov, N. E.; Festa, A. A.; Voskresenskii, L. G.; Moshkin, V. S.; Buev, E. M.; Sosnovskikh, V. Y.; Mironova, I. A.; Postnikov, P. S.; Zhdankin, V. V.; Yusubov, M. S. O.; Yaremenko, I. A.; Vil', V. A.; Krylov, I. B.; Terent'ev, A. O.; Gorbunova, Y. G.; Martynov, A. G.; Tsivadze, A. Y.; Stuzhin, P. A.; Ivanova, S. S.; Koifman, O. I.; Burov, O. N.; Kletskii, M. E.; Kurbatov, S. V.; Yarovaya, O. I.; Volcho, K. P.; Salakhutdinov, N. F.; Panova, M. A.; Burgart, Y. V.; Saloutin, V. I.; Sitdikova, A. R.; Shchegravina, E. S.; Fedorov, A. Y. Russ. Chem. Rev. 2024, 93, RCR5125. doi:10.59761/rcr5125 |
| 12. | Sakharov, P. А.; Novikov, M. S.; Rostovskii, N. V. Chem. Heterocycl. Compd. 2021, 57, 512–521. doi:10.1007/s10593-021-02934-2 |
| 13. | Molinski, T. F.; Ireland, C. M. J. Org. Chem. 1988, 53, 2103–2105. doi:10.1021/jo00244a049 |
| 14. | Salomon, C. E.; Williams, D. H.; Faulkner, D. J. J. Nat. Prod. 1995, 58, 1463–1466. doi:10.1021/np50123a021 |
| 15. | Keffer, J. L.; Plaza, A.; Bewley, C. A. Org. Lett. 2009, 11, 1087–1090. doi:10.1021/ol802890b |
| 16. | Skepper, C. K.; Dalisay, D. S.; Molinski, T. F. Org. Lett. 2008, 10, 5269–5271. doi:10.1021/ol802065d |
| 17. | Skepper, C. K.; Dalisay, D. S.; Molinski, T. F. Bioorg. Med. Chem. Lett. 2010, 20, 2029–2032. doi:10.1016/j.bmcl.2010.01.068 |
| 18. | Rostovskii, N. V.; Koronatov, A. N.; Sakharov, P. A.; Agafonova, A. V.; Novikov, M. S.; Khlebnikov, A. F.; Rogacheva, E. V.; Kraeva, L. A. Org. Biomol. Chem. 2020, 18, 9448–9460. doi:10.1039/d0ob02023k |
| 3. | Palacios, F.; de Retana, A. M. O.; de Marigorta, E. M.; de los Santos, J. M. Eur. J. Org. Chem. 2001, 2401–2414. doi:10.1002/1099-0690(200107)2001:13<2401::aid-ejoc2401>3.0.co;2-u |
| 4. | Palacios, F.; de Retana, A. M. O.; Martínez de Marigorta, E.; Manuel de los Santos, J. Org. Prep. Proced. Int. 2002, 34, 219–269. doi:10.1080/00304940209356770 |
| 5. | Pinho e Melo, T. M. V. D.; Rocha-Gonsalves, A. M. d’A. Curr. Org. Synth. 2004, 1, 275–292. doi:10.2174/1570179043366729 |
| 6. | Lemos, A. Molecules 2009, 14, 4098–4119. doi:10.3390/molecules14104098 |
| 7. | Padwa, A. Adv. Heterocycl. Chem. 2010, 99, 1–31. doi:10.1016/s0065-2725(10)09901-0 |
| 8. | Khlebnikov, A. F.; Novikov, M. S. Tetrahedron 2013, 69, 3363–3401. doi:10.1016/j.tet.2013.02.020 |
| 9. | De, A.; Majee, A. J. Heterocycl. Chem. 2022, 59, 422–448. doi:10.1002/jhet.4415 |
| 10. | Xu, F.; Zeng, F.-W.; Luo, W.-J.; Zhang, S.-Y.; Huo, J.-Q.; Li, Y.-P. Eur. J. Org. Chem. 2024, 27, e202301292. doi:10.1002/ejoc.202301292 |
| 11. | Charushin, V. N.; Verbitskiy, E. V.; Chupakhin, O. N.; Vorobyeva, D. V.; Gribanov, P. S.; Osipov, S. N.; Ivanov, A. V.; Martynovskaya, S. V.; Sagitova, E. F.; Dyachenko, V. D.; Dyachenko, I. V.; Krivokolylsko, S. G.; Dotsenko, V. V.; Aksenov, A. V.; Aksenov, D. A.; Aksenov, N. A.; Larin, A. A.; Fershtat, L. L.; Muzalevskiy, V. M.; Nenajdenko, V. G.; Gulevskaya, A. V.; Pozharskii, A. F.; Filatova, E. A.; Belyaeva, K. V.; Trofimov, B. A.; Balova, I. A.; Danilkina, N. A.; Govdi, A. I.; Tikhomirov, A. S.; Shchekotikhin, A. E.; Novikov, M. S.; Rostovskii, N. V.; Khlebnikov, A. F.; Klimochkin, Y. N.; Leonova, M. V.; Tkachenko, I. M.; Mamedov, V. A. O.; Mamedova, V. L.; Zhukova, N. A.; Semenov, V. E.; Sinyashin, O. G.; Borshchev, O. V.; Luponosov, Y. N.; Ponomarenko, S. A.; Fisyuk, A. S.; Kostyuchenko, A. S.; Ilkin, V. G.; Beryozkina, T. V.; Bakulev, V. A.; Gazizov, A. S.; Zagidullin, A. A.; Karasik, A. A.; Kukushkin, M. E.; Beloglazkina, E. K.; Golantsov, N. E.; Festa, A. A.; Voskresenskii, L. G.; Moshkin, V. S.; Buev, E. M.; Sosnovskikh, V. Y.; Mironova, I. A.; Postnikov, P. S.; Zhdankin, V. V.; Yusubov, M. S. O.; Yaremenko, I. A.; Vil', V. A.; Krylov, I. B.; Terent'ev, A. O.; Gorbunova, Y. G.; Martynov, A. G.; Tsivadze, A. Y.; Stuzhin, P. A.; Ivanova, S. S.; Koifman, O. I.; Burov, O. N.; Kletskii, M. E.; Kurbatov, S. V.; Yarovaya, O. I.; Volcho, K. P.; Salakhutdinov, N. F.; Panova, M. A.; Burgart, Y. V.; Saloutin, V. I.; Sitdikova, A. R.; Shchegravina, E. S.; Fedorov, A. Y. Russ. Chem. Rev. 2024, 93, RCR5125. doi:10.59761/rcr5125 |
| 27. | Agafonova, A. V.; Novikov, M. S.; Khlebnikov, A. F. Molecules 2023, 28, 275. doi:10.3390/molecules28010275 |
| 2. | Prokop'eva, I. N.; Tomashenko, O. A.; Matveeva, D. R.; Galenko, E. E.; Novikov, M. S.; Khlebnikov, A. F. Tetrahedron 2024, 167, 134255. doi:10.1016/j.tet.2024.134255 |
| 27. | Agafonova, A. V.; Novikov, M. S.; Khlebnikov, A. F. Molecules 2023, 28, 275. doi:10.3390/molecules28010275 |
| 25. | Mikhailov, K. I.; Galenko, E. E.; Galenko, A. V.; Novikov, M. S.; Ivanov, A. Y.; Starova, G. L.; Khlebnikov, A. F. J. Org. Chem. 2018, 83, 3177–3187. doi:10.1021/acs.joc.8b00069 |
| 26. | Galenko, E. E.; Bodunov, V. A.; Galenko, A. V.; Novikov, M. S.; Khlebnikov, A. F. J. Org. Chem. 2017, 82, 8568–8579. doi:10.1021/acs.joc.7b01351 |
| 27. | Agafonova, A. V.; Novikov, M. S.; Khlebnikov, A. F. Molecules 2023, 28, 275. doi:10.3390/molecules28010275 |
| 25. | Mikhailov, K. I.; Galenko, E. E.; Galenko, A. V.; Novikov, M. S.; Ivanov, A. Y.; Starova, G. L.; Khlebnikov, A. F. J. Org. Chem. 2018, 83, 3177–3187. doi:10.1021/acs.joc.8b00069 |
| 26. | Galenko, E. E.; Bodunov, V. A.; Galenko, A. V.; Novikov, M. S.; Khlebnikov, A. F. J. Org. Chem. 2017, 82, 8568–8579. doi:10.1021/acs.joc.7b01351 |
| 24. | Ryabova, O. B.; Makarov, V. A.; Alekseeva, L. M.; Shashkov, A. S.; Chernyshev, V. V.; Granik, V. G. Russ. Chem. Bull. 2005, 54, 1907–1914. doi:10.1007/s11172-006-0057-x |
| 23. | Serebryannikova, A. V.; Galenko, E. E.; Novikov, M. S.; Khlebnikov, A. F. J. Org. Chem. 2019, 84, 15567–15577. doi:10.1021/acs.joc.9b02536 |
| 20. | Anderson, D. J. J. Org. Chem. 1986, 51, 945–947. doi:10.1021/jo00356a039 |
| 21. | Rajender, P. S.; Sridevi, G.; Reddy, K. K. Synth. Commun. 2012, 42, 2191–2200. doi:10.1080/00397911.2011.555051 |
| 22. | Beccalli, E. M.; Marchesini, A. J. Org. Chem. 1987, 52, 3426–3434. doi:10.1021/jo00391a048 |
| 23. | Serebryannikova, A. V.; Galenko, E. E.; Novikov, M. S.; Khlebnikov, A. F. J. Org. Chem. 2019, 84, 15567–15577. doi:10.1021/acs.joc.9b02536 |
| 19. | Rostovskii, N. V.; Agafonova, A. V.; Smetanin, I. A.; Novikov, M. S.; Khlebnikov, A. F.; Ruvinskaya, J. O.; Starova, G. L. Synthesis 2017, 49, 4478–4488. doi:10.1055/s-0036-1590822 |
| 26. | Galenko, E. E.; Bodunov, V. A.; Galenko, A. V.; Novikov, M. S.; Khlebnikov, A. F. J. Org. Chem. 2017, 82, 8568–8579. doi:10.1021/acs.joc.7b01351 |
© 2024 Agafonova et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.