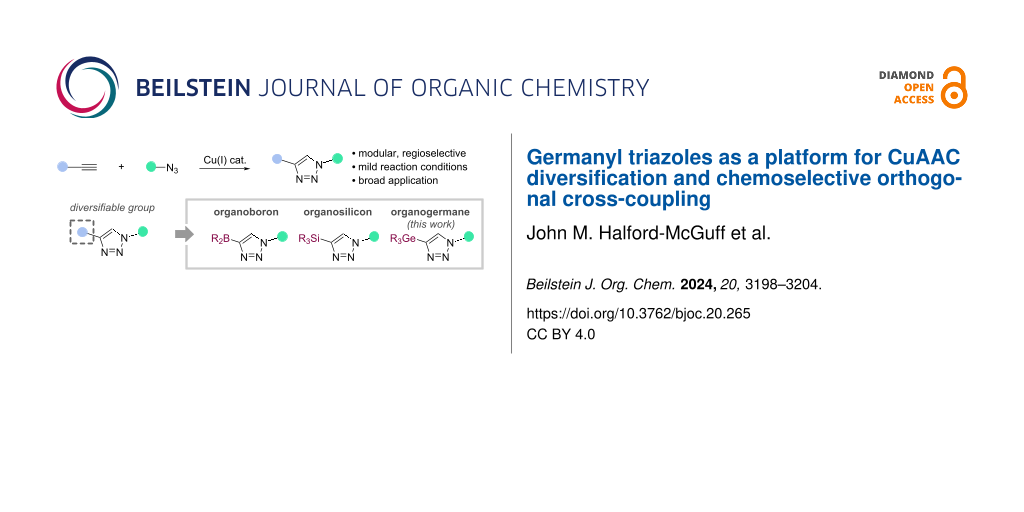
Abstract
We report the synthesis of germanyl triazoles formed via a copper-catalysed azide–alkyne cycloaddition (CuAAC) of germanyl alkynes. The reaction is often high yielding, functional group tolerant, and compatible with complex molecules. The installation of the Ge moiety enables further diversification of the triazole products, including chemoselective transition metal-catalysed cross-coupling reactions using bifunctional boryl/germyl species.
Graphical Abstract
Introduction
Since its inception, click chemistry has been established as a powerful approach for molecule synthesis. Strategies within click chemistry include several widely used reactions such as the (hetero-)Diels–Alder reaction [1,2], alkene hydrothiolation [3], and an array of amide-bond-forming chemistries [4]. However, by virtue of the access to alkyne and azide precursors and the formation of a single 1,4-disubstituted triazole product, the copper-catalysed azide–alkyne cycloaddition (CuAAC) remains the archetypal click reaction (Scheme 1) [5].
Scheme 1: The CuAAC reaction and installation of functional groups for product diversification.
Scheme 1: The CuAAC reaction and installation of functional groups for product diversification.
The reaction has shown applicability on small and large scale, as well as under flow conditions [6], and extensive scope across a range of benign solvent conditions [7-10]. In addition, the CuAAC reaction uses inexpensive Cu catalysts [11], is insensitive towards oxygen and water [12,13], and consistently delivers high yields and (where relevant) enantioselectivities [8-10,14-19]. As such, the reaction has been used extensively throughout drug discovery [20,21], chemical biology [22,23], and materials science [24-27]. Orthogonal alkyne reactivity can also be observed under certain systems [28-30]. The reaction typically uses a Cu(II) pre-catalyst, which is converted to a mechanistically-required Cu(I) species in situ through the addition of a reductant (e.g., sodium ascorbate, NaAsc) [31,32], or via Glaser–Hay alkyne homocoupling [33,34].
The mild and accessible nature of the CuAAC reaction has allowed the use of azide or alkyne components that bear functional groups for subsequent product diversification (Scheme 1). For example, protected alkynylboron reagents can be employed [35-37], such as N-methyliminodiacetic acid (MIDA)boronate esters [38], potassium trifluoroborates [39], and others [40-42]. Similarly, organosilicon reagents have proven useful in various Cu- and Pd-catalysed C–X-bond-forming strategies [43-51], including widespread use across several CuAAC methodologies [52-54].
Germanium-based functional groups have recently emerged as highly useful components for transition-metal-catalysed cross-couplings. Schoenebeck and co-workers have shown that Ge-based compounds are versatile reagents within chemoselective cross-coupling processes for the formation of a variety of C–C and C–X bonds [55-63]. Importantly, these transformations can take place in the presence of borylated functional groups, allowing orthogonal cross-coupling, whilst also offering excellent stability compared to boron-based reagents [57-67].
Based on their utility and stability, germanium units could therefore be useful within CuAAC reactions and offer potential as functional handles for downstream elaboration of CuAAC products. To date, the main use of germanyl alkynes in (3 + 2) cycloadditions has been limited to a small number of Huisgen (non-Cu-catalysed) reactions [68,69]. Zaitsev and co-workers reported the synthesis and CuAAC reactions of a dialkynyl germane to access 1,2-bis(triazolyl)tetraphenyldigermanes [70]. Here, we report the development of germanyl alkynes as CuAAC components, with exploration of their scope and downstream diversification.
Results and Discussion
We undertook an exploratory survey of CuAAC reaction conditions using benzyl azide and triethylgermanyl acetylene (see Supporting Information File 1). The most effective conditions were found to be based on the classical combination of CuSO4/NaAsc, with optimisation (see Supporting Information File 1) delivering the general conditions shown in Scheme 2. These afforded a clean conversion to the desired triazole products 1–21 without any observable degermylation or other side reactions that could be anticipated based on transmetalation to Cu [43].
Scheme 2: Scope of germanyl acetylene CuAAC. Alkyne (1.0 equiv), azide (1.1 equiv), CuSO4·5H2O (5.0 mol %), NaAsc (50 mol %), NEt3 (1.0 equiv), t-BuOH/H2O 1:1 (250 mM), N2, rt, 16 h. Isolated yields. aReaction performed with CsF (2.0 equiv) as an additive. bReaction performed at rt for 64 h.
Scheme 2: Scope of germanyl acetylene CuAAC. Alkyne (1.0 equiv), azide (1.1 equiv), CuSO4·5H2O (5.0 mol %), N...
The generality of the CuAAC process was explored using a range of azides (Scheme 2a), with variation of the germanyl alkyne motif (Scheme 2b), and with variation of both components (Scheme 2c). In general, the CuAAC process worked effectively, tolerating the functional groups for which the CuAAC is well-known – in all cases the remaining mass balance was accounted for by the germanyl acetylene, suggesting sluggish CuAAC reactivity compared to other alkynes, which typically require much shorter reaction times. Extending the reaction time provided a higher conversion to the product 14. Yields were observed to be greater for aryl azides (e.g., 4 vs 6). Heterocycles such as pyridine (1), pyrimidine (10), phenothiazine (11), and chromene (12) were tolerated. Benzylic azides were accommodated including those bearing nitro (2), iodo (3), and boronic ester groups (5, 21). Strained rings were effective including cubane (18) and bicyclopentane (20). While 18 and 20 were isolated in lower yield, no evidence of ring opening was observed and the starting material could be recovered in each case, consistent with observations by Lam and MacMillan [71,72]. Variation of the steric and electronic parameters of the germanyl acetylene was straightforward (14–17; Scheme 2b). Several limitations were observed (Scheme 2d): benzyl azides displaying an arylboronic acid and MIDA ester (22 and 23) gave no reaction, side reactions were observed with a dialkynyl germane (24), and the product derived from azide 25 was unstable to purification.
To further demonstrate the compatibility and utility of germanyl alkynes in CuAAC reactions, we applied the CuAAC process to more challenging substrates. Using fluorophore- and cholesterol-derived azides, coupling with the triethylgermanyl alkyne delivered the expected products 26 and 27, respectively, in good yield, enabling possible downstream diversification of these functional molecules of relevance to chemical biology (Scheme 3a).
Scheme 3: (a) Application of Ge-alkyne CuAAC to functional molecules. (b) Functionalisation of germylated triazoles. Isolated yields unless stated. (i) Pd(PPh3)4 (10 mol %), 2-bromothiazole (1.2 equiv), KCl (3.0 equiv), PhMe/EtOH 4:1, N2, 100 °C, 16 h. NMR yield in parentheses. (ii) Pd2(dba)3 (2.5 mol %), iodobenzene (1.5 equiv), AgBF4 (1.5 equiv), DMF, N2, 80 °C, 16 h. (iii) NBS (2.0 equiv), DMF, air, rt, 2 h. (iv) Pd(dtbpf)Cl2 (10 mol %), 2-acetylthiophen-3-ylboronic acid (1.2 equiv), K3PO4 (2.0 equiv), iPrOH/H2O 3:4, N2, 85 °C, 16 h. (v) Pd(dtbpf)Cl2 (2.0 mol %), 1-naphthylzinc bromide (1.2 equiv), THF, N2, 45 °C, 16 h. (vi) Cu(OAc)2·H2O (30 mol %), B(OH)3 (2.0 equiv), DBU (2.0 equiv), MeCN, air, 70 °C, 24 h. (vii) Cu(OAc)2·H2O (30 mol %), B(OH)3 (2.0 equiv), piperidine (2.0 equiv), MeCN, air, 70 °C, 24 h. See Supporting Information File 1 for full details.
Scheme 3: (a) Application of Ge-alkyne CuAAC to functional molecules. (b) Functionalisation of germylated tri...
The utility of the germanyl triazole products was then assessed by subsequent derivatisation of exemplar compounds 15 and 21 (Scheme 3b). Chemoselective Suzuki–Miyaura cross-coupling of the BPin moiety in 21 was straightforward, giving 28 in excellent yield [73]. Similarly, cross-coupling of the GeEt3 moiety in 15 under conditions developed by Schoenebeck and co-workers gave 29 [57]. Bromodegermanylation using NBS employing conditions from Schoenebeck gave bromotriazoles 30 and 31 in moderate to excellent yield [62]. These could then undergo Suzuki–Miyaura cross-coupling to give 32 or chemoselective Negishi coupling to give 33 [74]. Finally, BPin 21 could be oxidised to the phenol derivative 34 or cross-coupled with piperidine under Chan–Lam conditions to give the aniline derivative 35 in good yield [75].
Conclusion
In summary, we have developed a general method towards the synthesis of germanyl triazoles. These reagents are generally compatible but seem to be less reactive than other classes of alkyne. The germanyl alkyne CuAAC is applicable to functional group-rich molecules, opening opportunities for downstream diversification by chemoselective functionalisation strategies [76]. The germanyl group installed in the triazole products can be used as a reactive handle for further diversification including cross-coupling reactions.
Supporting Information
The research data supporting this publication can be accessed at https://doi.org/10.17630/53959471-068e-483e-bcd4-920e6761926b and CCDC 2355570 contains the supplementary crystallographic data for this study.
| Supporting Information File 1: Characterization data and copies of NMR spectra. | ||
| Format: PDF | Size: 6.6 MB | Download |
| Supporting Information File 2: Crystallographic information file (cif) for compound 13. | ||
| Format: CIF | Size: 1.6 MB | Download |
| Supporting Information File 3: Checkcif file for compound 13. | ||
| Format: PDF | Size: 140.0 KB | Download |
Funding
J.M.H.-M. thanks the EPSRC Centre for Doctoral Training EaSI-CAT for a Ph.D. studentship. T.M.R. thanks the EPSRC and the University of St Andrews for Ph.D. studentship. G.A.B., F.P., and A.J.B.W. thank the Leverhulme Trust (RPG-2020-380). A.J.B.W. thanks the Leverhulme Trust for a Research Fellowship (RF-2022-014) and the EPSRC Programme Grant ‘‘Boron: Beyond the Reagent’’ (EP/W007517/1) for support.
Data Availability Statement
Data generated and analyzed during this study is openly available at https://doi.org/10.17630/53959471-068e-483e-bcd4-920e6761926b.
References
-
Tasdelen, M. A. Polym. Chem. 2011, 2, 2133–2145. doi:10.1039/c1py00041a
Return to citation in text: [1] -
Eschenbrenner‐Lux, V.; Kumar, K.; Waldmann, H. Angew. Chem., Int. Ed. 2014, 53, 11146–11157. doi:10.1002/anie.201404094
Return to citation in text: [1] -
Hoyle, C. E.; Bowman, C. N. Angew. Chem., Int. Ed. 2010, 49, 1540–1573. doi:10.1002/anie.200903924
Return to citation in text: [1] -
Li, H.; Aneja, R.; Chaiken, I. Molecules 2013, 18, 9797–9817. doi:10.3390/molecules18089797
Return to citation in text: [1] -
Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004–2021. doi:10.1002/1521-3773(20010601)40:11<2004::aid-anie2004>3.0.co;2-5
Return to citation in text: [1] -
Hatit, M. Z. C.; Reichenbach, L. F.; Tobin, J. M.; Vilela, F.; Burley, G. A.; Watson, A. J. B. Nat. Commun. 2018, 9, 4021. doi:10.1038/s41467-018-06551-0
Return to citation in text: [1] -
Melo, A.; Monteiro, L.; Lima, R. M. F.; de Oliveira, D. M.; de Cerqueira, M. D.; El-Bachá, R. S. Oxid. Med. Cell. Longevity 2011, 467180. doi:10.1155/2011/467180
Return to citation in text: [1] -
Meldal, M.; Tornøe, C. W. Chem. Rev. 2008, 108, 2952–3015. doi:10.1021/cr0783479
Return to citation in text: [1] [2] -
Haldón, E.; Nicasio, M. C.; Pérez, P. J. Org. Biomol. Chem. 2015, 13, 9528–9550. doi:10.1039/c5ob01457c
Return to citation in text: [1] [2] -
García-Álvarez, J.; Díez, J.; Gimeno, J. Green Chem. 2010, 12, 2127–2130. doi:10.1039/c0gc00342e
Return to citation in text: [1] [2] -
Wang, K.; Bi, X.; Xing, S.; Liao, P.; Fang, Z.; Meng, X.; Zhang, Q.; Liu, Q.; Ji, Y. Green Chem. 2011, 13, 562–565. doi:10.1039/c0gc00848f
Return to citation in text: [1] -
Fu, F.; Martinez, A.; Wang, C.; Ciganda, R.; Yate, L.; Escobar, A.; Moya, S.; Fouquet, E.; Ruiz, J.; Astruc, D. Chem. Commun. 2017, 53, 5384–5387. doi:10.1039/c7cc02504a
Return to citation in text: [1] -
Nebra, N.; García-Álvarez, J. Molecules 2020, 25, 2015. doi:10.3390/molecules25092015
Return to citation in text: [1] -
Vala, D. P.; Vala, R. M.; Patel, H. M. ACS Omega 2022, 7, 36945–36987. doi:10.1021/acsomega.2c04883
Return to citation in text: [1] -
Cook, T. L.; Walker, J. A.; Mack, J. Green Chem. 2013, 15, 617–619. doi:10.1039/c3gc36720g
Return to citation in text: [1] -
Girard, C.; Önen, E.; Aufort, M.; Beauvière, S.; Samson, E.; Herscovici, J. Org. Lett. 2006, 8, 1689–1692. doi:10.1021/ol060283l
Return to citation in text: [1] -
Chtchigrovsky, M.; Primo, A.; Gonzalez, P.; Molvinger, K.; Robitzer, M.; Quignard, F.; Taran, F. Angew. Chem., Int. Ed. 2009, 48, 5916–5920. doi:10.1002/anie.200901309
Return to citation in text: [1] -
Zhu, R.-Y.; Chen, L.; Hu, X.-S.; Zhou, F.; Zhou, J. Chem. Sci. 2020, 11, 97–106. doi:10.1039/c9sc04938j
Return to citation in text: [1] -
Liu, E.-C.; Topczewski, J. J. J. Am. Chem. Soc. 2019, 141, 5135–5138. doi:10.1021/jacs.9b01091
Return to citation in text: [1] -
Lal, K.; Yadav, P.; Kumar, A.; Kumar, A.; Paul, A. K. Bioorg. Chem. 2018, 77, 236–244. doi:10.1016/j.bioorg.2018.01.016
Return to citation in text: [1] -
Rani, A.; Singh, G.; Singh, A.; Maqbool, U.; Kaur, G.; Singh, J. RSC Adv. 2020, 10, 5610–5635. doi:10.1039/c9ra09510a
Return to citation in text: [1] -
Wright, M. H.; Sieber, S. A. Nat. Prod. Rep. 2016, 33, 681–708. doi:10.1039/c6np00001k
Return to citation in text: [1] -
Sapienza, P. J.; Currie, M. M.; Lancaster, N. M.; Li, K.; Aubé, J.; Goldfarb, D.; Cloer, E. W.; Major, M. B.; Lee, A. L. ACS Chem. Biol. 2021, 16, 2766–2775. doi:10.1021/acschembio.1c00617
Return to citation in text: [1] -
Döhler, D.; Michael, P.; Binder, W. H. Acc. Chem. Res. 2017, 50, 2610–2620. doi:10.1021/acs.accounts.7b00371
Return to citation in text: [1] -
Meldal, M. Macromol. Rapid Commun. 2008, 29, 1016–1051. doi:10.1002/marc.200800159
Return to citation in text: [1] -
Pacini, A.; Nitti, A.; Vitale, M.; Pasini, D. Int. J. Mol. Sci. 2023, 24, 7620. doi:10.3390/ijms24087620
Return to citation in text: [1] -
Zaccaria, C. L.; Cedrati, V.; Nitti, A.; Chiesa, E.; Martinez de Ilarduya, A.; Garcia-Alvarez, M.; Meli, M.; Colombo, G.; Pasini, D. Polym. Chem. 2021, 12, 3784–3793. doi:10.1039/d1py00737h
Return to citation in text: [1] -
Hatit, M. Z. C.; Sadler, J. C.; McLean, L. A.; Whitehurst, B. C.; Seath, C. P.; Humphreys, L. D.; Young, R. J.; Watson, A. J. B.; Burley, G. A. Org. Lett. 2016, 18, 1694–1697. doi:10.1021/acs.orglett.6b00635
Return to citation in text: [1] -
Hatit, M. Z. C.; Seath, C. P.; Watson, A. J. B.; Burley, G. A. J. Org. Chem. 2017, 82, 5461–5468. doi:10.1021/acs.joc.7b00545
Return to citation in text: [1] -
Seath, C. P.; Burley, G. A.; Watson, A. J. B. Angew. Chem., Int. Ed. 2017, 56, 3314–3318. doi:10.1002/anie.201612288
Return to citation in text: [1] -
Rodionov, V. O.; Fokin, V. V.; Finn, M. G. Angew. Chem., Int. Ed. 2005, 44, 2210–2215. doi:10.1002/anie.200461496
Return to citation in text: [1] -
Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596–2599. doi:10.1002/1521-3773(20020715)41:14<2596::aid-anie2596>3.0.co;2-4
Return to citation in text: [1] -
Hein, J. E.; Fokin, V. V. Chem. Soc. Rev. 2010, 39, 1302–1315. doi:10.1039/b904091a
Return to citation in text: [1] -
Bunschoten, R. P.; Peschke, F.; Taladriz-Sender, A.; Alexander, E.; Andrews, M. J.; Kennedy, A. R.; Fazakerley, N. J.; Lloyd Jones, G. C.; Watson, A. J. B.; Burley, G. A. J. Am. Chem. Soc. 2024, 146, 13558–13570. doi:10.1021/jacs.4c03348
Return to citation in text: [1] -
Huang, J.; Macdonald, S. J. F.; Harrity, J. P. A. Chem. Commun. 2009, 436–438. doi:10.1039/b817052e
Return to citation in text: [1] -
Huang, J.; Macdonald, S. J. F.; Cooper, A. W. J.; Fisher, G.; Harrity, J. P. A. Tetrahedron Lett. 2009, 50, 5539–5541. doi:10.1016/j.tetlet.2009.07.085
Return to citation in text: [1] -
Dai, C.; Cheng, Y.; Cui, J.; Wang, B. Molecules 2010, 15, 5768–5781. doi:10.3390/molecules15085768
Return to citation in text: [1] -
Grob, J. E.; Nunez, J.; Dechantsreiter, M. A.; Hamann, L. G. J. Org. Chem. 2011, 76, 10241–10248. doi:10.1021/jo201973t
Return to citation in text: [1] -
Jung, S. h.; Choi, K.; Pae, A. N.; Lee, J. K.; Choo, H.; Keum, G.; Cho, Y. S.; Min, S.-J. Org. Biomol. Chem. 2014, 12, 9674–9682. doi:10.1039/c4ob01967a
Return to citation in text: [1] -
Zu, B.; Guo, Y.; He, C. J. Am. Chem. Soc. 2021, 143, 16302–16310. doi:10.1021/jacs.1c08482
Return to citation in text: [1] -
Van Belois, A.; Maar, R. R.; Workentin, M. S.; Gilroy, J. B. Inorg. Chem. 2019, 58, 834–843. doi:10.1021/acs.inorgchem.8b02966
Return to citation in text: [1] -
Li, J.; Tanaka, H.; Imagawa, T.; Tsushima, T.; Nakamoto, M.; Tan, J.; Yoshida, H. Chem. – Eur. J. 2024, 30, e202303403. doi:10.1002/chem.202303403
Return to citation in text: [1] -
Lam, P. Y. S.; Deudon, S.; Hauptman, E.; Clark, C. G. Tetrahedron Lett. 2001, 42, 2427–2429. doi:10.1016/s0040-4039(01)00203-9
Return to citation in text: [1] [2] -
Denmark, S. E.; Smith, R. C.; Chang, W.-T. T.; Muhuhi, J. M. J. Am. Chem. Soc. 2009, 131, 3104–3118. doi:10.1021/ja8091449
Return to citation in text: [1] -
Denmark, S. E.; Regens, C. S. Acc. Chem. Res. 2008, 41, 1486–1499. doi:10.1021/ar800037p
Return to citation in text: [1] -
Hirabayashi, K.; Mori, A.; Kawashima, J.; Suguro, M.; Nishihara, Y.; Hiyama, T. J. Org. Chem. 2000, 65, 5342–5349. doi:10.1021/jo000679p
Return to citation in text: [1] -
Nakao, Y.; Takeda, M.; Matsumoto, T.; Hiyama, T. Angew. Chem., Int. Ed. 2010, 49, 4447–4450. doi:10.1002/anie.201000816
Return to citation in text: [1] -
Hagiwara, E.; Gouda, K.-i.; Hatanaka, Y.; Hiyama, T. Tetrahedron Lett. 1997, 38, 439–442. doi:10.1016/s0040-4039(96)02320-9
Return to citation in text: [1] -
Hatanaka, Y.; Hiyama, T. J. Org. Chem. 1988, 53, 918–920. doi:10.1021/jo00239a056
Return to citation in text: [1] -
Denmark, S. E.; Wehrli, D. Org. Lett. 2000, 2, 565–568. doi:10.1021/ol005565e
Return to citation in text: [1] -
Denmark, S. E.; Choi, J. Y. J. Am. Chem. Soc. 1999, 121, 5821–5822. doi:10.1021/ja9908117
Return to citation in text: [1] -
Yamamoto, K.; Kanezashi, M.; Tsuru, T.; Ohshita, J. Polym. J. 2017, 49, 401–406. doi:10.1038/pj.2016.128
Return to citation in text: [1] -
Venkatesh, G. B.; Hari Prasad, S. Phosphorus, Sulfur Silicon Relat. Elem. 2015, 190, 335–341. doi:10.1080/10426507.2014.947405
Return to citation in text: [1] -
Li, L.; Shang, T.; Ma, X.; Guo, H.; Zhu, A.; Zhang, G. Synlett 2015, 26, 695–699. doi:10.1055/s-0034-1379970
Return to citation in text: [1] -
Fricke, C.; Schoenebeck, F. Acc. Chem. Res. 2020, 53, 2715–2725. doi:10.1021/acs.accounts.0c00527
Return to citation in text: [1] -
Rogova, T.; Ahrweiler, E.; Schoetz, M. D.; Schoenebeck, F. Angew. Chem., Int. Ed. 2024, 63, e202314709. doi:10.1002/anie.202314709
Return to citation in text: [1] -
Fricke, C.; Sherborne, G. J.; Funes‐Ardoiz, I.; Senol, E.; Guven, S.; Schoenebeck, F. Angew. Chem., Int. Ed. 2019, 58, 17788–17795. doi:10.1002/anie.201910060
Return to citation in text: [1] [2] [3] -
Dahiya, A.; Schoetz, M. D.; Schoenebeck, F. Angew. Chem., Int. Ed. 2023, 62, e202310380. doi:10.1002/anie.202310380
Return to citation in text: [1] [2] -
Dahiya, A.; Gevondian, A. G.; Schoenebeck, F. J. Am. Chem. Soc. 2023, 145, 7729–7735. doi:10.1021/jacs.3c01081
Return to citation in text: [1] [2] -
Dahiya, A.; Fricke, C.; Schoenebeck, F. J. Am. Chem. Soc. 2020, 142, 7754–7759. doi:10.1021/jacs.0c02860
Return to citation in text: [1] [2] -
Sherborne, G. J.; Gevondian, A. G.; Funes‐Ardoiz, I.; Dahiya, A.; Fricke, C.; Schoenebeck, F. Angew. Chem., Int. Ed. 2020, 59, 15543–15548. doi:10.1002/anie.202005066
Return to citation in text: [1] [2] -
Fricke, C.; Deckers, K.; Schoenebeck, F. Angew. Chem., Int. Ed. 2020, 59, 18717–18722. doi:10.1002/anie.202008372
Return to citation in text: [1] [2] [3] -
Kaithal, A.; Sasmal, H. S.; Dutta, S.; Schäfer, F.; Schlichter, L.; Glorius, F. J. Am. Chem. Soc. 2023, 145, 4109–4118. doi:10.1021/jacs.2c12062
Return to citation in text: [1] [2] -
Luo, Y.; Tian, T.; Nishihara, Y.; Lv, L.; Li, Z. Chem. Commun. 2021, 57, 9276–9279. doi:10.1039/d1cc03907e
Return to citation in text: [1] -
Xu, Q.-H.; Xiao, B. Org. Chem. Front. 2022, 9, 7016–7027. doi:10.1039/d2qo01467j
Return to citation in text: [1] -
Li, W.-F.; Xu, Q.-H.; Miao, Q.-Y.; Xiao, B. J. Org. Chem. 2024, 89, 16269–16281. doi:10.1021/acs.joc.3c02348
Return to citation in text: [1] -
Han, A.-C.; Xiao, L.-J.; Zhou, Q.-L. J. Am. Chem. Soc. 2024, 146, 5643–5649. doi:10.1021/jacs.3c14386
Return to citation in text: [1] -
Piterskaya, Y. L.; Khramchikhin, A. V.; Stadnichuk, M. D. Zh. Obshch. Khim. 1996, 66, 1188–1194.
Return to citation in text: [1] -
Demina, M. M.; Nguyen, T. L. H.; Shaglaeva, N. S.; Mareev, A. V.; Medvedeva, A. S. Russ. J. Org. Chem. 2012, 48, 1582–1584. doi:10.1134/s1070428012120196
Return to citation in text: [1] -
Zaitsev, K. V.; Veshchitsky, G. A.; Oprunenko, Y. F.; Kharcheva, A. V.; Moiseeva, A. A.; Gloriozov, I. P.; Lermontova, E. K. Chem. – Asian J. 2023, 18, e202300753. doi:10.1002/asia.202300753
Return to citation in text: [1] -
Smith, E.; Jones, K. D.; O’Brien, L.; Argent, S. P.; Salome, C.; Lefebvre, Q.; Valery, A.; Böcü, M.; Newton, G. N.; Lam, H. W. J. Am. Chem. Soc. 2023, 145, 16365–16373. doi:10.1021/jacs.3c03207
Return to citation in text: [1] -
Wiesenfeldt, M. P.; Rossi-Ashton, J. A.; Perry, I. B.; Diesel, J.; Garry, O. L.; Bartels, F.; Coote, S. C.; Ma, X.; Yeung, C. S.; Bennett, D. J.; MacMillan, D. W. C. Nature 2023, 618, 513–518. doi:10.1038/s41586-023-06021-8
Return to citation in text: [1] -
Pérez‐Perarnau, A.; Preciado, S.; Palmeri, C. M.; Moncunill‐Massaguer, C.; Iglesias‐Serret, D.; González‐Gironès, D. M.; Miguel, M.; Karasawa, S.; Sakamoto, S.; Cosialls, A. M.; Rubio‐Patiño, C.; Saura‐Esteller, J.; Ramón, R.; Caja, L.; Fabregat, I.; Pons, G.; Handa, H.; Albericio, F.; Gil, J.; Lavilla, R. Angew. Chem., Int. Ed. 2014, 53, 10150–10154. doi:10.1002/anie.201405758
Return to citation in text: [1] -
Wang, C.; Tobrman, T.; Xu, Z.; Negishi, E.-i. Org. Lett. 2009, 11, 4092–4095. doi:10.1021/ol901566e
Return to citation in text: [1] -
Vantourout, J. C.; Miras, H. N.; Isidro-Llobet, A.; Sproules, S.; Watson, A. J. B. J. Am. Chem. Soc. 2017, 139, 4769–4779. doi:10.1021/jacs.6b12800
Return to citation in text: [1] -
Peschke, F.; Taladriz-Sender, A.; Andrews, M. J.; Watson, A. J. B.; Burley, G. A. Angew. Chem., Int. Ed. 2023, 62, e202313063. doi:10.1002/anie.202313063
Return to citation in text: [1]
| 73. | Pérez‐Perarnau, A.; Preciado, S.; Palmeri, C. M.; Moncunill‐Massaguer, C.; Iglesias‐Serret, D.; González‐Gironès, D. M.; Miguel, M.; Karasawa, S.; Sakamoto, S.; Cosialls, A. M.; Rubio‐Patiño, C.; Saura‐Esteller, J.; Ramón, R.; Caja, L.; Fabregat, I.; Pons, G.; Handa, H.; Albericio, F.; Gil, J.; Lavilla, R. Angew. Chem., Int. Ed. 2014, 53, 10150–10154. doi:10.1002/anie.201405758 |
| 57. | Fricke, C.; Sherborne, G. J.; Funes‐Ardoiz, I.; Senol, E.; Guven, S.; Schoenebeck, F. Angew. Chem., Int. Ed. 2019, 58, 17788–17795. doi:10.1002/anie.201910060 |
| 62. | Fricke, C.; Deckers, K.; Schoenebeck, F. Angew. Chem., Int. Ed. 2020, 59, 18717–18722. doi:10.1002/anie.202008372 |
| 1. | Tasdelen, M. A. Polym. Chem. 2011, 2, 2133–2145. doi:10.1039/c1py00041a |
| 2. | Eschenbrenner‐Lux, V.; Kumar, K.; Waldmann, H. Angew. Chem., Int. Ed. 2014, 53, 11146–11157. doi:10.1002/anie.201404094 |
| 6. | Hatit, M. Z. C.; Reichenbach, L. F.; Tobin, J. M.; Vilela, F.; Burley, G. A.; Watson, A. J. B. Nat. Commun. 2018, 9, 4021. doi:10.1038/s41467-018-06551-0 |
| 33. | Hein, J. E.; Fokin, V. V. Chem. Soc. Rev. 2010, 39, 1302–1315. doi:10.1039/b904091a |
| 34. | Bunschoten, R. P.; Peschke, F.; Taladriz-Sender, A.; Alexander, E.; Andrews, M. J.; Kennedy, A. R.; Fazakerley, N. J.; Lloyd Jones, G. C.; Watson, A. J. B.; Burley, G. A. J. Am. Chem. Soc. 2024, 146, 13558–13570. doi:10.1021/jacs.4c03348 |
| 5. | Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004–2021. doi:10.1002/1521-3773(20010601)40:11<2004::aid-anie2004>3.0.co;2-5 |
| 35. | Huang, J.; Macdonald, S. J. F.; Harrity, J. P. A. Chem. Commun. 2009, 436–438. doi:10.1039/b817052e |
| 36. | Huang, J.; Macdonald, S. J. F.; Cooper, A. W. J.; Fisher, G.; Harrity, J. P. A. Tetrahedron Lett. 2009, 50, 5539–5541. doi:10.1016/j.tetlet.2009.07.085 |
| 37. | Dai, C.; Cheng, Y.; Cui, J.; Wang, B. Molecules 2010, 15, 5768–5781. doi:10.3390/molecules15085768 |
| 4. | Li, H.; Aneja, R.; Chaiken, I. Molecules 2013, 18, 9797–9817. doi:10.3390/molecules18089797 |
| 28. | Hatit, M. Z. C.; Sadler, J. C.; McLean, L. A.; Whitehurst, B. C.; Seath, C. P.; Humphreys, L. D.; Young, R. J.; Watson, A. J. B.; Burley, G. A. Org. Lett. 2016, 18, 1694–1697. doi:10.1021/acs.orglett.6b00635 |
| 29. | Hatit, M. Z. C.; Seath, C. P.; Watson, A. J. B.; Burley, G. A. J. Org. Chem. 2017, 82, 5461–5468. doi:10.1021/acs.joc.7b00545 |
| 30. | Seath, C. P.; Burley, G. A.; Watson, A. J. B. Angew. Chem., Int. Ed. 2017, 56, 3314–3318. doi:10.1002/anie.201612288 |
| 3. | Hoyle, C. E.; Bowman, C. N. Angew. Chem., Int. Ed. 2010, 49, 1540–1573. doi:10.1002/anie.200903924 |
| 31. | Rodionov, V. O.; Fokin, V. V.; Finn, M. G. Angew. Chem., Int. Ed. 2005, 44, 2210–2215. doi:10.1002/anie.200461496 |
| 32. | Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596–2599. doi:10.1002/1521-3773(20020715)41:14<2596::aid-anie2596>3.0.co;2-4 |
| 8. | Meldal, M.; Tornøe, C. W. Chem. Rev. 2008, 108, 2952–3015. doi:10.1021/cr0783479 |
| 9. | Haldón, E.; Nicasio, M. C.; Pérez, P. J. Org. Biomol. Chem. 2015, 13, 9528–9550. doi:10.1039/c5ob01457c |
| 10. | García-Álvarez, J.; Díez, J.; Gimeno, J. Green Chem. 2010, 12, 2127–2130. doi:10.1039/c0gc00342e |
| 14. | Vala, D. P.; Vala, R. M.; Patel, H. M. ACS Omega 2022, 7, 36945–36987. doi:10.1021/acsomega.2c04883 |
| 15. | Cook, T. L.; Walker, J. A.; Mack, J. Green Chem. 2013, 15, 617–619. doi:10.1039/c3gc36720g |
| 16. | Girard, C.; Önen, E.; Aufort, M.; Beauvière, S.; Samson, E.; Herscovici, J. Org. Lett. 2006, 8, 1689–1692. doi:10.1021/ol060283l |
| 17. | Chtchigrovsky, M.; Primo, A.; Gonzalez, P.; Molvinger, K.; Robitzer, M.; Quignard, F.; Taran, F. Angew. Chem., Int. Ed. 2009, 48, 5916–5920. doi:10.1002/anie.200901309 |
| 18. | Zhu, R.-Y.; Chen, L.; Hu, X.-S.; Zhou, F.; Zhou, J. Chem. Sci. 2020, 11, 97–106. doi:10.1039/c9sc04938j |
| 19. | Liu, E.-C.; Topczewski, J. J. J. Am. Chem. Soc. 2019, 141, 5135–5138. doi:10.1021/jacs.9b01091 |
| 22. | Wright, M. H.; Sieber, S. A. Nat. Prod. Rep. 2016, 33, 681–708. doi:10.1039/c6np00001k |
| 23. | Sapienza, P. J.; Currie, M. M.; Lancaster, N. M.; Li, K.; Aubé, J.; Goldfarb, D.; Cloer, E. W.; Major, M. B.; Lee, A. L. ACS Chem. Biol. 2021, 16, 2766–2775. doi:10.1021/acschembio.1c00617 |
| 76. | Peschke, F.; Taladriz-Sender, A.; Andrews, M. J.; Watson, A. J. B.; Burley, G. A. Angew. Chem., Int. Ed. 2023, 62, e202313063. doi:10.1002/anie.202313063 |
| 12. | Fu, F.; Martinez, A.; Wang, C.; Ciganda, R.; Yate, L.; Escobar, A.; Moya, S.; Fouquet, E.; Ruiz, J.; Astruc, D. Chem. Commun. 2017, 53, 5384–5387. doi:10.1039/c7cc02504a |
| 13. | Nebra, N.; García-Álvarez, J. Molecules 2020, 25, 2015. doi:10.3390/molecules25092015 |
| 24. | Döhler, D.; Michael, P.; Binder, W. H. Acc. Chem. Res. 2017, 50, 2610–2620. doi:10.1021/acs.accounts.7b00371 |
| 25. | Meldal, M. Macromol. Rapid Commun. 2008, 29, 1016–1051. doi:10.1002/marc.200800159 |
| 26. | Pacini, A.; Nitti, A.; Vitale, M.; Pasini, D. Int. J. Mol. Sci. 2023, 24, 7620. doi:10.3390/ijms24087620 |
| 27. | Zaccaria, C. L.; Cedrati, V.; Nitti, A.; Chiesa, E.; Martinez de Ilarduya, A.; Garcia-Alvarez, M.; Meli, M.; Colombo, G.; Pasini, D. Polym. Chem. 2021, 12, 3784–3793. doi:10.1039/d1py00737h |
| 11. | Wang, K.; Bi, X.; Xing, S.; Liao, P.; Fang, Z.; Meng, X.; Zhang, Q.; Liu, Q.; Ji, Y. Green Chem. 2011, 13, 562–565. doi:10.1039/c0gc00848f |
| 74. | Wang, C.; Tobrman, T.; Xu, Z.; Negishi, E.-i. Org. Lett. 2009, 11, 4092–4095. doi:10.1021/ol901566e |
| 7. | Melo, A.; Monteiro, L.; Lima, R. M. F.; de Oliveira, D. M.; de Cerqueira, M. D.; El-Bachá, R. S. Oxid. Med. Cell. Longevity 2011, 467180. doi:10.1155/2011/467180 |
| 8. | Meldal, M.; Tornøe, C. W. Chem. Rev. 2008, 108, 2952–3015. doi:10.1021/cr0783479 |
| 9. | Haldón, E.; Nicasio, M. C.; Pérez, P. J. Org. Biomol. Chem. 2015, 13, 9528–9550. doi:10.1039/c5ob01457c |
| 10. | García-Álvarez, J.; Díez, J.; Gimeno, J. Green Chem. 2010, 12, 2127–2130. doi:10.1039/c0gc00342e |
| 20. | Lal, K.; Yadav, P.; Kumar, A.; Kumar, A.; Paul, A. K. Bioorg. Chem. 2018, 77, 236–244. doi:10.1016/j.bioorg.2018.01.016 |
| 21. | Rani, A.; Singh, G.; Singh, A.; Maqbool, U.; Kaur, G.; Singh, J. RSC Adv. 2020, 10, 5610–5635. doi:10.1039/c9ra09510a |
| 75. | Vantourout, J. C.; Miras, H. N.; Isidro-Llobet, A.; Sproules, S.; Watson, A. J. B. J. Am. Chem. Soc. 2017, 139, 4769–4779. doi:10.1021/jacs.6b12800 |
| 40. | Zu, B.; Guo, Y.; He, C. J. Am. Chem. Soc. 2021, 143, 16302–16310. doi:10.1021/jacs.1c08482 |
| 41. | Van Belois, A.; Maar, R. R.; Workentin, M. S.; Gilroy, J. B. Inorg. Chem. 2019, 58, 834–843. doi:10.1021/acs.inorgchem.8b02966 |
| 42. | Li, J.; Tanaka, H.; Imagawa, T.; Tsushima, T.; Nakamoto, M.; Tan, J.; Yoshida, H. Chem. – Eur. J. 2024, 30, e202303403. doi:10.1002/chem.202303403 |
| 38. | Grob, J. E.; Nunez, J.; Dechantsreiter, M. A.; Hamann, L. G. J. Org. Chem. 2011, 76, 10241–10248. doi:10.1021/jo201973t |
| 39. | Jung, S. h.; Choi, K.; Pae, A. N.; Lee, J. K.; Choo, H.; Keum, G.; Cho, Y. S.; Min, S.-J. Org. Biomol. Chem. 2014, 12, 9674–9682. doi:10.1039/c4ob01967a |
| 43. | Lam, P. Y. S.; Deudon, S.; Hauptman, E.; Clark, C. G. Tetrahedron Lett. 2001, 42, 2427–2429. doi:10.1016/s0040-4039(01)00203-9 |
| 71. | Smith, E.; Jones, K. D.; O’Brien, L.; Argent, S. P.; Salome, C.; Lefebvre, Q.; Valery, A.; Böcü, M.; Newton, G. N.; Lam, H. W. J. Am. Chem. Soc. 2023, 145, 16365–16373. doi:10.1021/jacs.3c03207 |
| 72. | Wiesenfeldt, M. P.; Rossi-Ashton, J. A.; Perry, I. B.; Diesel, J.; Garry, O. L.; Bartels, F.; Coote, S. C.; Ma, X.; Yeung, C. S.; Bennett, D. J.; MacMillan, D. W. C. Nature 2023, 618, 513–518. doi:10.1038/s41586-023-06021-8 |
| 68. | Piterskaya, Y. L.; Khramchikhin, A. V.; Stadnichuk, M. D. Zh. Obshch. Khim. 1996, 66, 1188–1194. |
| 69. | Demina, M. M.; Nguyen, T. L. H.; Shaglaeva, N. S.; Mareev, A. V.; Medvedeva, A. S. Russ. J. Org. Chem. 2012, 48, 1582–1584. doi:10.1134/s1070428012120196 |
| 70. | Zaitsev, K. V.; Veshchitsky, G. A.; Oprunenko, Y. F.; Kharcheva, A. V.; Moiseeva, A. A.; Gloriozov, I. P.; Lermontova, E. K. Chem. – Asian J. 2023, 18, e202300753. doi:10.1002/asia.202300753 |
| 55. | Fricke, C.; Schoenebeck, F. Acc. Chem. Res. 2020, 53, 2715–2725. doi:10.1021/acs.accounts.0c00527 |
| 56. | Rogova, T.; Ahrweiler, E.; Schoetz, M. D.; Schoenebeck, F. Angew. Chem., Int. Ed. 2024, 63, e202314709. doi:10.1002/anie.202314709 |
| 57. | Fricke, C.; Sherborne, G. J.; Funes‐Ardoiz, I.; Senol, E.; Guven, S.; Schoenebeck, F. Angew. Chem., Int. Ed. 2019, 58, 17788–17795. doi:10.1002/anie.201910060 |
| 58. | Dahiya, A.; Schoetz, M. D.; Schoenebeck, F. Angew. Chem., Int. Ed. 2023, 62, e202310380. doi:10.1002/anie.202310380 |
| 59. | Dahiya, A.; Gevondian, A. G.; Schoenebeck, F. J. Am. Chem. Soc. 2023, 145, 7729–7735. doi:10.1021/jacs.3c01081 |
| 60. | Dahiya, A.; Fricke, C.; Schoenebeck, F. J. Am. Chem. Soc. 2020, 142, 7754–7759. doi:10.1021/jacs.0c02860 |
| 61. | Sherborne, G. J.; Gevondian, A. G.; Funes‐Ardoiz, I.; Dahiya, A.; Fricke, C.; Schoenebeck, F. Angew. Chem., Int. Ed. 2020, 59, 15543–15548. doi:10.1002/anie.202005066 |
| 62. | Fricke, C.; Deckers, K.; Schoenebeck, F. Angew. Chem., Int. Ed. 2020, 59, 18717–18722. doi:10.1002/anie.202008372 |
| 63. | Kaithal, A.; Sasmal, H. S.; Dutta, S.; Schäfer, F.; Schlichter, L.; Glorius, F. J. Am. Chem. Soc. 2023, 145, 4109–4118. doi:10.1021/jacs.2c12062 |
| 57. | Fricke, C.; Sherborne, G. J.; Funes‐Ardoiz, I.; Senol, E.; Guven, S.; Schoenebeck, F. Angew. Chem., Int. Ed. 2019, 58, 17788–17795. doi:10.1002/anie.201910060 |
| 58. | Dahiya, A.; Schoetz, M. D.; Schoenebeck, F. Angew. Chem., Int. Ed. 2023, 62, e202310380. doi:10.1002/anie.202310380 |
| 59. | Dahiya, A.; Gevondian, A. G.; Schoenebeck, F. J. Am. Chem. Soc. 2023, 145, 7729–7735. doi:10.1021/jacs.3c01081 |
| 60. | Dahiya, A.; Fricke, C.; Schoenebeck, F. J. Am. Chem. Soc. 2020, 142, 7754–7759. doi:10.1021/jacs.0c02860 |
| 61. | Sherborne, G. J.; Gevondian, A. G.; Funes‐Ardoiz, I.; Dahiya, A.; Fricke, C.; Schoenebeck, F. Angew. Chem., Int. Ed. 2020, 59, 15543–15548. doi:10.1002/anie.202005066 |
| 62. | Fricke, C.; Deckers, K.; Schoenebeck, F. Angew. Chem., Int. Ed. 2020, 59, 18717–18722. doi:10.1002/anie.202008372 |
| 63. | Kaithal, A.; Sasmal, H. S.; Dutta, S.; Schäfer, F.; Schlichter, L.; Glorius, F. J. Am. Chem. Soc. 2023, 145, 4109–4118. doi:10.1021/jacs.2c12062 |
| 64. | Luo, Y.; Tian, T.; Nishihara, Y.; Lv, L.; Li, Z. Chem. Commun. 2021, 57, 9276–9279. doi:10.1039/d1cc03907e |
| 65. | Xu, Q.-H.; Xiao, B. Org. Chem. Front. 2022, 9, 7016–7027. doi:10.1039/d2qo01467j |
| 66. | Li, W.-F.; Xu, Q.-H.; Miao, Q.-Y.; Xiao, B. J. Org. Chem. 2024, 89, 16269–16281. doi:10.1021/acs.joc.3c02348 |
| 67. | Han, A.-C.; Xiao, L.-J.; Zhou, Q.-L. J. Am. Chem. Soc. 2024, 146, 5643–5649. doi:10.1021/jacs.3c14386 |
| 43. | Lam, P. Y. S.; Deudon, S.; Hauptman, E.; Clark, C. G. Tetrahedron Lett. 2001, 42, 2427–2429. doi:10.1016/s0040-4039(01)00203-9 |
| 44. | Denmark, S. E.; Smith, R. C.; Chang, W.-T. T.; Muhuhi, J. M. J. Am. Chem. Soc. 2009, 131, 3104–3118. doi:10.1021/ja8091449 |
| 45. | Denmark, S. E.; Regens, C. S. Acc. Chem. Res. 2008, 41, 1486–1499. doi:10.1021/ar800037p |
| 46. | Hirabayashi, K.; Mori, A.; Kawashima, J.; Suguro, M.; Nishihara, Y.; Hiyama, T. J. Org. Chem. 2000, 65, 5342–5349. doi:10.1021/jo000679p |
| 47. | Nakao, Y.; Takeda, M.; Matsumoto, T.; Hiyama, T. Angew. Chem., Int. Ed. 2010, 49, 4447–4450. doi:10.1002/anie.201000816 |
| 48. | Hagiwara, E.; Gouda, K.-i.; Hatanaka, Y.; Hiyama, T. Tetrahedron Lett. 1997, 38, 439–442. doi:10.1016/s0040-4039(96)02320-9 |
| 49. | Hatanaka, Y.; Hiyama, T. J. Org. Chem. 1988, 53, 918–920. doi:10.1021/jo00239a056 |
| 50. | Denmark, S. E.; Wehrli, D. Org. Lett. 2000, 2, 565–568. doi:10.1021/ol005565e |
| 51. | Denmark, S. E.; Choi, J. Y. J. Am. Chem. Soc. 1999, 121, 5821–5822. doi:10.1021/ja9908117 |
| 52. | Yamamoto, K.; Kanezashi, M.; Tsuru, T.; Ohshita, J. Polym. J. 2017, 49, 401–406. doi:10.1038/pj.2016.128 |
| 53. | Venkatesh, G. B.; Hari Prasad, S. Phosphorus, Sulfur Silicon Relat. Elem. 2015, 190, 335–341. doi:10.1080/10426507.2014.947405 |
| 54. | Li, L.; Shang, T.; Ma, X.; Guo, H.; Zhu, A.; Zhang, G. Synlett 2015, 26, 695–699. doi:10.1055/s-0034-1379970 |
© 2024 Halford-McGuff et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.