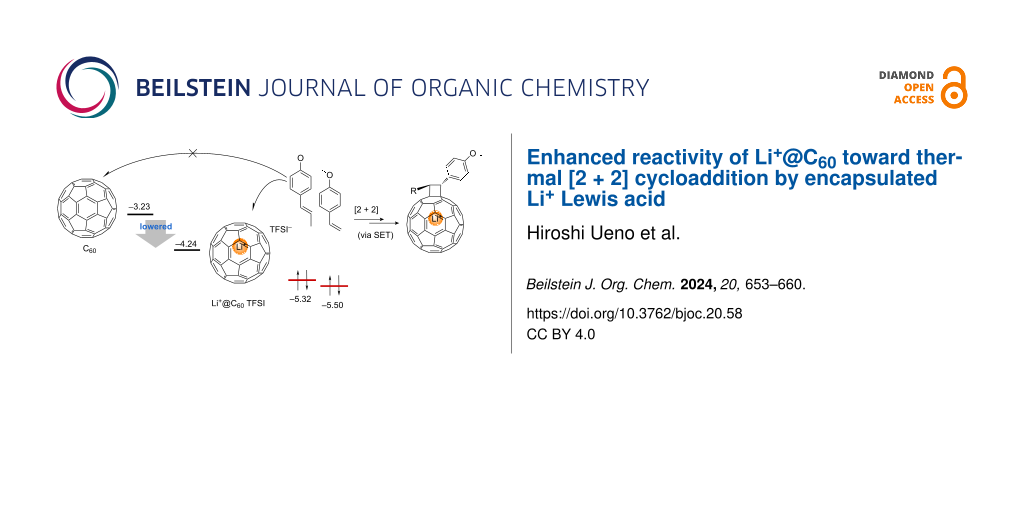
Abstract
Lithium ion-endohedral fullerene (Li+@C60), a member of the burgeoning family of ion-endohedral fullerenes, holds substantial promise for diverse applications owing to its distinctive ionic properties. Despite the high demand for precise property tuning through chemical modification, there have been only a few reports detailing synthetic protocols for the derivatization of this novel material. In this study, we report the synthesis of Li+@C60 derivatives via the thermal [2 + 2] cycloaddition reaction of styrene derivatives, achieving significantly higher yields of monofunctionalized Li+@C60 compared to previously reported reactions. Furthermore, by combining experimental and theoretical approaches, we clarified the range of applicable substrates for the thermal [2 + 2] cycloaddition of Li+@C60, highlighting the expanded scope of this straightforward and selective functionalization method.
Graphical Abstract
Introduction
Chemical functionalization of fullerenes is a fascinating and extensively studied approach, playing a pivotal role in fullerene-based materials science to introduce various characteristic functionalities [1-7]. Significant progress in synthetic procedures has contributed to diversifying their properties, enabling widespread and interdisciplinary applications in various research fields, such as biomedicine, photovoltaic devices, and materials chemistry.
Meanwhile, lithium ion-endohedral fullerenes (Li+@C60) [8], the first member of the emerging ion-endohedral fullerene family, have attracted significant attention owing to the distinctive ionic properties originating from the ion pair structure consisting of a cationic endohedral fullerene core and an external counter anion. Despite being a relatively recent addition, Li+@C60 has been the focus of intensive studies in chemistry, physics, and related interdisciplinary fields over the past 13 years [9]. A noteworthy discovery during these investigations is the significant enhancement of reactivity arising from the encapsulated Li+. Both experimental and theoretical approaches have diligently explored the details of reaction kinetics, quantitatively elucidating the impact of encapsulated Li+ on the reactivity of the outer fullerene cage as a specialized “encapsulated” Lewis acid catalyst [10,11]. While previous studies have revealed valuable insights, such as accelerated 1,3-dipolar and Diels–Alder reactions [12,13], it is noteworthy that the anticipated diverse properties resulting from the derivatization of Li+@C60 have not yet been fully realized. To further leverage the unique properties of the novel ion-endohedral fullerene, achieving diverse property tuning through chemical modification has been in high demand for its further applications, which is similar to what has been developed during the recent empty fullerene sciences.
As a continuation of our studies on the synthesis of Li+@C60 derivatives, we herein focus on the modification of Li+@C60 through thermal [2 + 2] cycloaddition. The [2 + 2] cycloaddition reactions of empty C60 have been known to proceed with unsaturated substrates having HOMO levels suitable for the thermal or photoinduced single-electron-transfer (SET) process (Scheme 1) [14-23]. Although the thermal [2 + 2] reactions are generally simple and scalable, the reactions are scarcely applied for the derivatization of fullerenes due to the limitation in the variety of possible substrates. Considering the electronic effect of the encapsulated Li+ on the outer C60 cage, Li+@C60 can react with a wider range of unsaturated substrates having a relatively lower HOMO level.
Scheme 1: Reaction mechanisms of thermal and photoinduced [2 + 2] cycloaddition on C60 [19,22,23].
Scheme 1: Reaction mechanisms of thermal and photoinduced [2 + 2] cycloaddition on C60 [19,22,23].
With the previously uncovered reactivity of Li+@C60 in hand, we synthesized Li+@C60 derivatives in this study through the thermal [2 + 2] cycloaddition of styrene derivatives, which do not react with empty C60 through the same reaction pathway. Although a major issue in the derivatization of Li+@C60 is the formation of multifunctionalized byproducts, it was significantly prevented in the reaction, leading to a much better yield of the target monofunctionalized Li+@C60 derivatives [24]. Additionally, we investigated the range of the HOMO level of the reactants suitable for the thermal [2 + 2] cycloaddition of Li+@C60 using both experimental and theoretical approaches. This study clearly demonstrated the significantly improved reactivity of Li+@C60 in the thermal [2 + 2] cycloaddition reaction, highlighting the expanded scope of this straightforward and selective reaction for Li+@C60.
Results and Discussion
We began by performing density functional theory (DFT) calculations to screen the substrates with suitable HOMO levels for the thermal [2 + 2] cycloaddition with Li+@C60. The structures of several kinds of possible reactants were optimized at the B3LYP/6-31G(d) level of theory. The calculated HOMO levels are summarized in Figure 1 along with the LUMO levels of Li+@C60 and empty C60 computed at the same level of theory. Among the computed substrates having a carbon–carbon unsaturated bond, thermal [2 + 2] cycloaddition of N,N,N',N'-tetraethylethynediamine and 1-morpholino-1-cyclopentene with empty C60 has been reported [17,23], while electron-rich styrene derivatives 1 and 2 can react with empty C60 only through a photoinduced SET pathway [19,22]. From these results, the energy gap between the HOMO of the alkene substrate and the LUMO of the fullerene acceptor, where the thermal SET reaction is presumed to occur, is estimated to be approximately less than 1.84 eV. Taking these results into consideration, Li+@C60 with a LUMO level of −4.24 eV is expected to undergo thermal [2 + 2] cycloaddition with reactants having a HOMO level of −6.08 eV or higher, such as styrene derivatives 1, 2, and 3.
Figure 1: HOMO levels of unsaturated substrates and LUMO levels of fullerenes computed at the B3LYP/6-31G(d) level of theory.
Figure 1: HOMO levels of unsaturated substrates and LUMO levels of fullerenes computed at the B3LYP/6-31G(d) ...
Based on the results of theoretical calculations, styrene derivatives 1, 2, and 3 were selected as the reactants for the thermal [2 + 2] cycloaddition with Li+@C60. For comparison, we also investigated the reaction of reactant 4, which has a larger energy gap between its HOMO and LUMO of Li+@C60 (1.92 eV). All reactions were conducted in the dark to avoid photoinduced SET reactions (Scheme 2). First, the reactivity was assessed by monitoring the reaction progress using a previously developed electrolyte-added HPLC technique [25]. As expected, both substrates 1 and 2 reacted with Li+@C60 at room temperature and exhibited HPLC signals assignable to the desirable monoadducts 5a and 5b (Figure 2). It is noteworthy that the reaction of 2 proceeded faster than that of 1, although 2 has a lower HOMO level than 1. This is likely due to the steric effect caused by the methyl group directly connected to the alkenyl C=C bond in reactant 1. After optimizing the reaction conditions, compounds 5a and 5b were isolated in 71% and 53% yields, respectively. Importantly, the generation of multiadducts in the thermal [2 + 2] cycloaddition was significantly prevented, even under conditions with an excess amount of reactant, resulting in much better yields of the target products compared to other reported reactions of Li+@C60. It should also be mentioned that while these products were stable at ambient temperature in the dark, photoirradiation triggered the elimination of the addends, reforming the starting Li+@C60 (Figure 3). No other insoluble or undetectable products by HPLC were identified during the study. On the other hand, the reactions of 3 and 4 with Li+@C60 did not proceed significantly even under higher temperature reaction conditions (5c: 1.6% and 5d: 0.5% in HPLC yields, Figures S1 and S2 in Supporting Information File 1). This result indicates that the HOMO levels of compounds 3 and 4 are around the threshold HOMO level for the thermal reaction with Li+@C60. The slightly higher reactivity of 3 than 4 can be simply explained by the higher HOMO level of 3 compared to that of 4.
Scheme 2: Thermal [2 + 2] reaction of Li+@C60 TFSI− with substrates 1–4. a100 equiv for the reaction screening, 20 equiv for the synthesis of 5a, and 40 equiv for the synthesis of 5b. bRoom temperature for the reaction screening, 50 °C for the synthesis. cIsolated yield. dHPLC yield.
Scheme 2: Thermal [2 + 2] reaction of Li+@C60 TFSI− with substrates 1–4. a100 equiv for the reaction screenin...
![[1860-5397-20-58-2]](/bjoc/content/figures/1860-5397-20-58-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: HPLC profiles of themal [2 + 2]reaction of Li+@C60 with substrate 1 (a) and 2 (b) in o-dichlorobenzene at room temperature.
Figure 2: HPLC profiles of themal [2 + 2]reaction of Li+@C60 with substrate 1 (a) and 2 (b) in o-dichlorobenz...
![[1860-5397-20-58-3]](/bjoc/content/figures/1860-5397-20-58-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: HPLC profiles of 5a (a) and 5b (b) before and after photoirradiation at room temperature.
Figure 3: HPLC profiles of 5a (a) and 5b (b) before and after photoirradiation at room temperature.
The products were characterized by spectroscopic and spectrometric analyses (Figures S3–S11 in Supporting Information File 1). 1H, and 13C NMR spectra clearly indicated the formation of [2 + 2] monoadducts. 7Li NMR spectra showed a sharp singlet signal at −12.4 (5a) and −13.5 ppm (5b), which clearly indicated that the Li+ was encapsulated in the highly shielded inner space of the fullerene cage. The observed chemical shift was almost identical to that of reported Li+@C60 derivatives [10,12,24]. Although the product 5a may have stereoisomers, only the E-isomer was observed, as confirmed by 1H-1H 2D-NOESY NMR spectrum (Figure 4). This is not surprising because similar stereoselectivity has been reported in the photoinduced [2 + 2] cycloaddition reaction of empty C60, where the E-isomer is most thermodynamically stable [19,22]. The positive mode high-resolution matrix-assisted laser desorption ionization mass spectra showed the formation of the monoadducts at m/z 875.10431 (5a) and 861.08866 (5b), which were assigned to each molecular ion ([M]+ calcd for C70H12OLi (5a): 875.10427 and C69H10OLi (5b): 861.08862, respectively). The UV–vis absorption spectra showed broad absorption in the visible region with an absorption maximum at 711 nm, which was known to show a characteristic pattern of functionalized fullerene having an addend on a [6,6] bond [26].
![[1860-5397-20-58-4]](/bjoc/content/figures/1860-5397-20-58-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: 1H-1H 2D-NOESY NMR spectrum (600 MHz, CD2Cl2) of 5a (a) and NOE correlations between two protons. The spectrum (700 MHz, CD2Cl2) of 5b is shown in (b).
Figure 4: 1H-1H 2D-NOESY NMR spectrum (600 MHz, CD2Cl2) of 5a (a) and NOE correlations between two protons. T...
As mentioned above, a distinctive feature of this reaction is the significantly lower yield of multiadducts compared to previously reported functionalizations of Li+@C60. The reason can be explained by the difference in electron-accepting ability between the monoadduct and pristine Li+@C60 investigated by cyclic voltammetry (Figure 5). Both products exhibited reversible first and second redox waves, with subsequent reduction resulting in an irreversible electrochemical response. The first reduction potentials of 5a and 5b were measured at −0.51 V and −0.52 V (vs Fc/Fc+), respectively, which were more negative than that of pristine Li+@C60 (E1/2red1 = −0.39 V). While the detailed reasons for the irreversible redox properties after the second reduction process have not been thoroughly investigated, the observed phenomena could potentially be attributed to ring opening or simple decomposition under the conditions. From these results, the LUMO levels of the compounds were estimated according to the following equation [27]: ELUMO (eV) = −[4.80 + E1/2red1 (V vs Fc/Fc+)], and the results were summarized in Table 1. The monoadducts with a higher LUMO level are expected to have lower reactivity in the thermal [2 + 2] cycloaddition than pristine Li+@C60. Moreover, it is plausible that unreacted Li+@C60 serves as an oxidant for the reduced monoadducts potentially generated by SET from reactants to monoadducts. These factors contribute to the suppression of multiadduct formation, resulting in the selective generation of the target monoadducts. Specifically, Li+@C60, influenced by the electronic effects of the encapsulated Li+ Lewis acid, commonly exhibits significantly higher reactivity compared to empty C60. The much-enhanced reactivity often leads to the formation of multiadducts more notably than in the case of empty fullerenes, and hence, achieving the selective monofunctionalization of Li+@C60 has been a major challenge. The approach we developed in this study proves highly advantageous for the selective formation of monofunctionalized Li+@C60 derivatives, holding great promise for the design, properties tuning, and synthesis of Li+@C60-based materials for future applications.
![[1860-5397-20-58-5]](/bjoc/content/figures/1860-5397-20-58-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Cyclic voltammograms of 5a, 5b, and Li+@C60 TFSI− with the potentials relative to the ferrocene/ferrocenium (Fc/Fc+) reference couple. Working electrode: Pt, counter electrode: Pt, reference electrode: Ag/Ag+ in acetonitrile, solvent: o-dichlorobenzene, supporting electrolyte: 50 mM TBAPF6.
Figure 5: Cyclic voltammograms of 5a, 5b, and Li+@C60 TFSI− with the potentials relative to the ferrocene/fer...
Conclusion
In summary, we successfully synthesized Li+@C60 derivatives through the thermal [2 + 2] cycloaddition of styrene derivatives. Due to the lower-lying LUMO of Li+@C60, the styrene reactant, which did not react with empty C60 through the same pathway, underwent a reaction with Li+@C60, yielding the target monofunctionalized products. The results underscore the significantly enhanced reactivity of Li+@C60 in the thermal [2 + 2] cycloaddition reaction due to the electronic effect of the encapsulated Li+ Lewis acid. Moreover, the formation of undesirable bis- and multiadducts was notably suppressed, resulting in much better yields of the target monoadducts. Electrochemical measurements revealed that the functionalization raised the LUMO level of Li+@C60, leading to lower reactivity for the second addition. With this facile, selective, and high-yield approach for the derivatization of ion-endohedral fullerene, the design and synthesis of novel Li+@C60 derivatives for further application in various research fields are currently underway.
Experimental
General procedure
Unless otherwise noted, all chemicals, including anhydrous solvents, were obtained from commercial suppliers (FUJIFILM Wako Pure Chemical Corp., TCI, Sigma-Aldrich) and used as received without further purification. Li+@C60TFSI− was purchased as PF6− salt from Idea International Corp., and then its counter anion was exchanged to TFSI− according to reported procedures [9].
NMR spectra were recorded on a JEOL JNM-ECZ400S (1H: 400 MHz, 7Li: 155 MHz, 13C: 100 MHz), a Bruker ADVANCE III (1H: 600 MHz) and a Bruker ADVANCE III 700 (1H: 700 MHz, 7Li: 272 MHz, 13C: 176 MHz) spectrometer. Chemical shifts (δ) were reported in parts per million (ppm) relative to residual proton of solvent for 1H (5.32 ppm, CDHCl2), LiCl in D2O for 7Li (0 ppm, external standard), and carbon of the solvent for 13C (53.84 ppm, CD2Cl2). High-resolution matrix-assisted laser desorption ionization (HR-MALDI) mass spectra were obtained on a Bruker solariX 12T mass spectrometer with dithranol as a matrix. UV–vis absorption spectra were measured on a JASCO V-670 and a Shimadzu UV-1800 spectrophotometer. Cyclic voltammograms were recorded on a BAS ALS 600A and a BAS ALS 620D apparatus with a three-electrode system.
Reactivitiy comparison of Li+@C60 TFSI− and styrenes
In an Ar-filled glove box, Li+@C60 TFSI− and styrenes 1–4 were dissolved in anhydrous chlorobenzene. 100 µL of Li+@C60 TFSI− solution (2.0 mM) and 100 μL of each styrene solution (200 mM) were mixed, respectively. The solutions were stirred in glove box for the indicated reaction time. At the time, 20 μL of solution was divided, taken out from glove box, frozen by liq. N2, and stored in a freezer until HPLC measurement.
The solutions were subjected to analytical HPLC. HPLC profiles are shown in Supporting Information File 1. HPLC conditions: column: Buckyprep ø 4.6 × (10 + 250) mm; mobile phase: chlorobenzene/acetonitrile 95:5 containing 30 mM LiTFSI; flow rate: 1.5 mL/min; temperature: 50 °C; detector: UV 337 nm; injection sample volume: 5 µL.
Synthesis of Li+@C60{(4-MeOC6H4)CH=CHMe} TFSI− (5a)
To 2.5 mL of a chlorobenzene/acetonitrile 1:1 (v/v) solution containing Li+@C60 TFSI− (8.5 mg, 8.4 µmol) was added trans-anethole (25 µL, 24.7 mg, 0.17 mmol). The solution was stirred at 50 °C for 15 hours. The resulted solution was subjected to preparative HPLC. Condition: solvent: chlorobenzene/acetonitrile 4:11 (v/v) containing 2 mM LiTFSI; column: Inertsil CX (GL Sciences), ø 4.6 × 250 mm. The fraction containing Li+@C60{(4-MeOC6H4)CH=CHMe} was collected and evaporated under reduced pressure. The resulting solid was washed with diethyl ether and dissolved in dichloromethane. The desired monoadduct Li+@C60{(4-MeOC6H4)CH=CHMe} TFSI− (5a, 6.9 mg, 6.0 µmol, 71%) was afforded from the solution by vapor-diffusion recrystallization with diethyl ether.
1H NMR (400 MHz, CD2Cl2) δ 2.23 (d, J = 6.9 Hz, 3H), 3.88 (s, 3H), 5.07 (qd, J = 6.9 8.7 Hz, 1H), 5.38 (d, J = 8.7 Hz, 1H), 7.12 (d, J = 8.7 Hz, 2H), 7.92 (d, J = 8.8 Hz, 2H); 13C NMR (100 MHz, CD2Cl2) δ 19.83, 47.39, 55.80, 58.72, 70.58, 73.03, 115.10, 119.84 (q, JCF = 320 Hz, CF3), 129.96, 130.56, 136.89, 136.93, 139.24, 139.66, 140.23, 140.50, 141.45, 141.62, 141.70, 141.79, 141.82, 141.85, 142.02, 142.34, 142.50, 142.53, 142.76, 142.84, 142.85, 142.92, 142.96, 143.00, 143.19, 144.04, 144.24, 144.32, 144.80, 144.89, 144.93, 145.02, 145.20, 145.23, 145.35, 145.39, 145.50, 145.57, 145.62, 145.79, 145.82, 145.91, 145.96, 145.99, 146.05, 146.12, 146.66, 146.72, 147.48, 147.85, 153.12, 153.33, 156.27, 156.48, 160.24; 7Li NMR (155 MHz, CD2Cl2) δ −12.4; HRMS–MALDI–TOF, positive ion mode, dithranol (m/z): [M]+ calcd for C70H12OLi, 875.10427; found, 875.10431.
Synthesis of Li+@C60{(4-MeOC6H4)CH=CH2} TFSI− (5b)
To 2.5 mL of a chlorobenzene/acetonitrile 1:1 (v/v) solution containing Li+@C60 TFSI− (9.4 mg, 9.3 µmol) was added 4-methoxystyrene (50.1 µL, 50.1 mg, 0.37 mmol). The solution was stirred at 50 °C for 45 min. The resulted solution was subjected to preparative HPLC. Conditions: solvent: chlorobenzene/acetonitrile 95:5 (v/v) containing 30 mM LiTFSI; column: Buckyprep (Nacalai tesque), ø 10 × (20 + 250) mm. The fraction containing Li+@C60{(4-MeOC6H4)CH=CH2} was concentrated under reduced pressure. The desired monoadduct Li+@C60{(4-MeOC6H4)CH=CH2} TFSI− (5b, 5.6 mg, 4.9 µmol, 53%) was afforded by precipitation with diethyl ether and filtration.
1H NMR (700 MHz, CD2Cl2) δ 3.87 (s, 3H), 4.59 (dd, J = 10.8 Hz, 13.8 Hz, 1H), 4.69 (dd, J = 8.6 Hz, 13.8 Hz, 1H), 5.89 (dd, J = 8.6, 10.7 Hz, 1H), 7.12 (d, J = 8.8 Hz, 2H), 7.95 (d, J = 8.7 Hz, 2H); 13C NMR (176 MHz, CD2Cl2) δ 37.94, 49.75, 55.65, 64.70, 74.90, 114.85, 120.07 (q, JCF = 321 Hz, CF3), 129.69, 131.42, 136.98, 137.32, 137.89 ,138.88, 139.99, 140.14, 140.24, 140.27, 141.24, 141.34, 141.55, 141.57, 141.60, 141.66, 141.68, 141.71, 142.18, 142.23, 142.35, 142.37, 142.61, 142.65, 142.66, 142.73, 142.76, 142.80, 143.01, 143.90, 144.02, 144.06, 144.09, 144.73, 144.83, 144.90, 144.94, 145.02, 145.14, 145.22, 145.29, 145.43, 145.57, 145.61, 145.63, 145.67, 145.72, 145.77, 145.82, 145.90, 146.51, 146.69, 146.85, 153.12, 155.45, 155.75, 155.85, 159.84; 7Li NMR (272 MHz, CD2Cl2) δ −13.3; HRMS–MALDI–TOF, positive ion mode, dithranol (m/z): [M]+ calcd for C69H10OLi, 861.08862; found, 861.08866.
Supporting Information
| Supporting Information File 1: HPLC profiles, NMR, HRMS, UV–vis absorption spectra, and computational details. | ||
| Format: PDF | Size: 845.7 KB | Download |
Acknowledgements
We thank to Dr. N. Ikuma for a fruitful discussion. Generous support from the FRIS CoRE, Tohoku University, which is a shared research environment, is also acknowledged.
Funding
This work was partly supported by Grants-in-Aid for Scientific Research (21H01737 to H. U.) from JSPS, Japan, and financial supports from Fukamatsugumi Co., Ltd., Sendai, Japan (to Endowed Research Laboratory of Dimensional Integrated Nanomaterials, Graduate school of Science, Tohoku University).
Data Availability Statement
The data that supports the findings of this study is available from the corresponding author upon reasonable request.
References
-
Taylor, R. Lecture notes on fullerene chemistry. A handbook for chemists; Imperial College Press: London, UK, 1999. doi:10.1142/9781848160675
Return to citation in text: [1] -
Hirsch, A.; Brettreich, M. Fullerenes: Chemistry and Reactions; Wiley-VCH: Weinheim, Germany, 2005. doi:10.1002/3527603492
Return to citation in text: [1] -
Nakamura, E.; Isobe, H. Acc. Chem. Res. 2003, 36, 807–815. doi:10.1021/ar030027y
Return to citation in text: [1] -
Matsuo, Y. Chem. Lett. 2012, 41, 754–759. doi:10.1246/cl.2012.754
Return to citation in text: [1] -
Li, C.-Z.; Yip, H.-L.; Jen, A. K.-Y. J. Mater. Chem. 2012, 22, 4161–4177. doi:10.1039/c2jm15126j
Return to citation in text: [1] -
Li, Y. Chem. – Asian J. 2013, 8, 2316–2328. doi:10.1002/asia.201300600
Return to citation in text: [1] -
Liu, J.; Qiu, L.; Shao, S. J. Mater. Chem. C 2021, 9, 16143–16163. doi:10.1039/d1tc04038c
Return to citation in text: [1] -
Aoyagi, S.; Nishibori, E.; Sawa, H.; Sugimoto, K.; Takata, M.; Miyata, Y.; Kitaura, R.; Shinohara, H.; Okada, H.; Sakai, T.; Ono, Y.; Kawachi, K.; Yokoo, K.; Ono, S.; Omote, K.; Kasama, Y.; Ishikawa, S.; Komuro, T.; Tobita, H. Nat. Chem. 2010, 2, 678–683. doi:10.1038/nchem.698
Return to citation in text: [1] -
Matsuo, Y.; Okada, H.; Ueno, H. Endohedral Lithium-containing Fullerenes: Preparation, Derivatization, and Application; Springer: Berlin, Germany, 2017. doi:10.1007/978-981-10-5004-6
Return to citation in text: [1] [2] -
Ueno, H.; Kawakami, H.; Nakagawa, K.; Okada, H.; Ikuma, N.; Aoyagi, S.; Kokubo, K.; Matsuo, Y.; Oshima, T. J. Am. Chem. Soc. 2014, 136, 11162–11167. doi:10.1021/ja505952y
Return to citation in text: [1] [2] -
Ma, Y.; Ueno, H.; Okada, H.; Manzhos, S.; Matsuo, Y. Org. Lett. 2020, 22, 7239–7243. doi:10.1021/acs.orglett.0c02570
Return to citation in text: [1] -
Matsuo, Y.; Okada, H.; Maruyama, M.; Sato, H.; Tobita, H.; Ono, Y.; Omote, K.; Kawachi, K.; Kasama, Y. Org. Lett. 2012, 14, 3784–3787. doi:10.1021/ol301671n
Return to citation in text: [1] [2] -
Fujiki, S.; Takada, T.; Nagasawa, S.; Okada, H.; Sasano, Y.; Kwon, E.; Matsuo, Y.; Iwabuchi, Y. Chem. Commun. 2023, 59, 1237–1240. doi:10.1039/d2cc06301h
Return to citation in text: [1] -
Wilson, S. R.; Kaprinidis, N.; Wu, Y.; Schuster, D. I. J. Am. Chem. Soc. 1993, 115, 8495–8496. doi:10.1021/ja00071a088
Return to citation in text: [1] -
Zhang, X.; Romero, A.; Foote, C. S. J. Am. Chem. Soc. 1993, 115, 11024–11025. doi:10.1021/ja00076a084
Return to citation in text: [1] -
Wilson, S. R.; Wu, Y.; Kaprinidis, N. A.; Schuster, D. I.; Welch, C. J. J. Org. Chem. 1993, 58, 6548–6549. doi:10.1021/jo00076a007
Return to citation in text: [1] -
Zhang, X.; Foote, C. S. J. Am. Chem. Soc. 1995, 117, 4271–4275. doi:10.1021/ja00120a007
Return to citation in text: [1] [2] -
Zhang, X.; Fan, A.; Foote, C. S. J. Org. Chem. 1996, 61, 5456–5461. doi:10.1021/jo9602231
Return to citation in text: [1] -
Vassilikogiannakis, G.; Orfanopoulos, M. Tetrahedron Lett. 1997, 38, 4323–4326. doi:10.1016/s0040-4039(97)00891-5
Return to citation in text: [1] [2] [3] [4] -
Vassilikogiannakis, G.; Chronakis, N.; Orfanopoulos, M. J. Am. Chem. Soc. 1998, 120, 9911–9920. doi:10.1021/ja981377w
Return to citation in text: [1] -
Vassilikogiannakis, G.; Orfanopoulos, M. J. Org. Chem. 1999, 64, 3392–3393. doi:10.1021/jo982486w
Return to citation in text: [1] -
Vassilikogiannakis, G.; Hatzimarinaki, M.; Orfanopoulos, M. J. Org. Chem. 2000, 65, 8180–8187. doi:10.1021/jo0006223
Return to citation in text: [1] [2] [3] [4] -
Mikie, T.; Asahara, H.; Nagao, K.; Ikuma, N.; Kokubo, K.; Oshima, T. Org. Lett. 2011, 13, 4244–4247. doi:10.1021/ol201590a
Return to citation in text: [1] [2] [3] -
Ueno, H.; Okada, H.; Aoyagi, S.; Matsuo, Y. J. Org. Chem. 2017, 82, 11631–11635. doi:10.1021/acs.joc.7b01893
Return to citation in text: [1] [2] -
For the details of electrolyte-added HPLC techniques, see refences [9-11,24].
Return to citation in text: [1] -
Smith, A. B., III; Strongin, R. M.; Brard, L.; Furst, G. T.; Romanow, W. J.; Owens, K. G.; King, R. C. J. Am. Chem. Soc. 1993, 115, 5829–5830. doi:10.1021/ja00066a063
Return to citation in text: [1] -
Wong, W.-Y.; Wang, X.-Z.; He, Z.; Djurišić, A. B.; Yip, C.-T.; Cheung, K.-Y.; Wang, H.; Mak, C. S. K.; Chan, W.-K. Nat. Mater. 2007, 6, 521–527. doi:10.1038/nmat1909
Return to citation in text: [1]
| 9. | Matsuo, Y.; Okada, H.; Ueno, H. Endohedral Lithium-containing Fullerenes: Preparation, Derivatization, and Application; Springer: Berlin, Germany, 2017. doi:10.1007/978-981-10-5004-6 |
| 10. | Ueno, H.; Kawakami, H.; Nakagawa, K.; Okada, H.; Ikuma, N.; Aoyagi, S.; Kokubo, K.; Matsuo, Y.; Oshima, T. J. Am. Chem. Soc. 2014, 136, 11162–11167. doi:10.1021/ja505952y |
| 11. | Ma, Y.; Ueno, H.; Okada, H.; Manzhos, S.; Matsuo, Y. Org. Lett. 2020, 22, 7239–7243. doi:10.1021/acs.orglett.0c02570 |
| 24. | Ueno, H.; Okada, H.; Aoyagi, S.; Matsuo, Y. J. Org. Chem. 2017, 82, 11631–11635. doi:10.1021/acs.joc.7b01893 |
| 1. | Taylor, R. Lecture notes on fullerene chemistry. A handbook for chemists; Imperial College Press: London, UK, 1999. doi:10.1142/9781848160675 |
| 2. | Hirsch, A.; Brettreich, M. Fullerenes: Chemistry and Reactions; Wiley-VCH: Weinheim, Germany, 2005. doi:10.1002/3527603492 |
| 3. | Nakamura, E.; Isobe, H. Acc. Chem. Res. 2003, 36, 807–815. doi:10.1021/ar030027y |
| 4. | Matsuo, Y. Chem. Lett. 2012, 41, 754–759. doi:10.1246/cl.2012.754 |
| 5. | Li, C.-Z.; Yip, H.-L.; Jen, A. K.-Y. J. Mater. Chem. 2012, 22, 4161–4177. doi:10.1039/c2jm15126j |
| 6. | Li, Y. Chem. – Asian J. 2013, 8, 2316–2328. doi:10.1002/asia.201300600 |
| 7. | Liu, J.; Qiu, L.; Shao, S. J. Mater. Chem. C 2021, 9, 16143–16163. doi:10.1039/d1tc04038c |
| 12. | Matsuo, Y.; Okada, H.; Maruyama, M.; Sato, H.; Tobita, H.; Ono, Y.; Omote, K.; Kawachi, K.; Kasama, Y. Org. Lett. 2012, 14, 3784–3787. doi:10.1021/ol301671n |
| 13. | Fujiki, S.; Takada, T.; Nagasawa, S.; Okada, H.; Sasano, Y.; Kwon, E.; Matsuo, Y.; Iwabuchi, Y. Chem. Commun. 2023, 59, 1237–1240. doi:10.1039/d2cc06301h |
| 27. | Wong, W.-Y.; Wang, X.-Z.; He, Z.; Djurišić, A. B.; Yip, C.-T.; Cheung, K.-Y.; Wang, H.; Mak, C. S. K.; Chan, W.-K. Nat. Mater. 2007, 6, 521–527. doi:10.1038/nmat1909 |
| 10. | Ueno, H.; Kawakami, H.; Nakagawa, K.; Okada, H.; Ikuma, N.; Aoyagi, S.; Kokubo, K.; Matsuo, Y.; Oshima, T. J. Am. Chem. Soc. 2014, 136, 11162–11167. doi:10.1021/ja505952y |
| 11. | Ma, Y.; Ueno, H.; Okada, H.; Manzhos, S.; Matsuo, Y. Org. Lett. 2020, 22, 7239–7243. doi:10.1021/acs.orglett.0c02570 |
| 9. | Matsuo, Y.; Okada, H.; Ueno, H. Endohedral Lithium-containing Fullerenes: Preparation, Derivatization, and Application; Springer: Berlin, Germany, 2017. doi:10.1007/978-981-10-5004-6 |
| 9. | Matsuo, Y.; Okada, H.; Ueno, H. Endohedral Lithium-containing Fullerenes: Preparation, Derivatization, and Application; Springer: Berlin, Germany, 2017. doi:10.1007/978-981-10-5004-6 |
| 19. | Vassilikogiannakis, G.; Orfanopoulos, M. Tetrahedron Lett. 1997, 38, 4323–4326. doi:10.1016/s0040-4039(97)00891-5 |
| 22. | Vassilikogiannakis, G.; Hatzimarinaki, M.; Orfanopoulos, M. J. Org. Chem. 2000, 65, 8180–8187. doi:10.1021/jo0006223 |
| 8. | Aoyagi, S.; Nishibori, E.; Sawa, H.; Sugimoto, K.; Takata, M.; Miyata, Y.; Kitaura, R.; Shinohara, H.; Okada, H.; Sakai, T.; Ono, Y.; Kawachi, K.; Yokoo, K.; Ono, S.; Omote, K.; Kasama, Y.; Ishikawa, S.; Komuro, T.; Tobita, H. Nat. Chem. 2010, 2, 678–683. doi:10.1038/nchem.698 |
| 26. | Smith, A. B., III; Strongin, R. M.; Brard, L.; Furst, G. T.; Romanow, W. J.; Owens, K. G.; King, R. C. J. Am. Chem. Soc. 1993, 115, 5829–5830. doi:10.1021/ja00066a063 |
| 17. | Zhang, X.; Foote, C. S. J. Am. Chem. Soc. 1995, 117, 4271–4275. doi:10.1021/ja00120a007 |
| 23. | Mikie, T.; Asahara, H.; Nagao, K.; Ikuma, N.; Kokubo, K.; Oshima, T. Org. Lett. 2011, 13, 4244–4247. doi:10.1021/ol201590a |
| 25. | For the details of electrolyte-added HPLC techniques, see refences [9-11,24]. |
| 24. | Ueno, H.; Okada, H.; Aoyagi, S.; Matsuo, Y. J. Org. Chem. 2017, 82, 11631–11635. doi:10.1021/acs.joc.7b01893 |
| 10. | Ueno, H.; Kawakami, H.; Nakagawa, K.; Okada, H.; Ikuma, N.; Aoyagi, S.; Kokubo, K.; Matsuo, Y.; Oshima, T. J. Am. Chem. Soc. 2014, 136, 11162–11167. doi:10.1021/ja505952y |
| 12. | Matsuo, Y.; Okada, H.; Maruyama, M.; Sato, H.; Tobita, H.; Ono, Y.; Omote, K.; Kawachi, K.; Kasama, Y. Org. Lett. 2012, 14, 3784–3787. doi:10.1021/ol301671n |
| 24. | Ueno, H.; Okada, H.; Aoyagi, S.; Matsuo, Y. J. Org. Chem. 2017, 82, 11631–11635. doi:10.1021/acs.joc.7b01893 |
| 19. | Vassilikogiannakis, G.; Orfanopoulos, M. Tetrahedron Lett. 1997, 38, 4323–4326. doi:10.1016/s0040-4039(97)00891-5 |
| 22. | Vassilikogiannakis, G.; Hatzimarinaki, M.; Orfanopoulos, M. J. Org. Chem. 2000, 65, 8180–8187. doi:10.1021/jo0006223 |
| 23. | Mikie, T.; Asahara, H.; Nagao, K.; Ikuma, N.; Kokubo, K.; Oshima, T. Org. Lett. 2011, 13, 4244–4247. doi:10.1021/ol201590a |
| 14. | Wilson, S. R.; Kaprinidis, N.; Wu, Y.; Schuster, D. I. J. Am. Chem. Soc. 1993, 115, 8495–8496. doi:10.1021/ja00071a088 |
| 15. | Zhang, X.; Romero, A.; Foote, C. S. J. Am. Chem. Soc. 1993, 115, 11024–11025. doi:10.1021/ja00076a084 |
| 16. | Wilson, S. R.; Wu, Y.; Kaprinidis, N. A.; Schuster, D. I.; Welch, C. J. J. Org. Chem. 1993, 58, 6548–6549. doi:10.1021/jo00076a007 |
| 17. | Zhang, X.; Foote, C. S. J. Am. Chem. Soc. 1995, 117, 4271–4275. doi:10.1021/ja00120a007 |
| 18. | Zhang, X.; Fan, A.; Foote, C. S. J. Org. Chem. 1996, 61, 5456–5461. doi:10.1021/jo9602231 |
| 19. | Vassilikogiannakis, G.; Orfanopoulos, M. Tetrahedron Lett. 1997, 38, 4323–4326. doi:10.1016/s0040-4039(97)00891-5 |
| 20. | Vassilikogiannakis, G.; Chronakis, N.; Orfanopoulos, M. J. Am. Chem. Soc. 1998, 120, 9911–9920. doi:10.1021/ja981377w |
| 21. | Vassilikogiannakis, G.; Orfanopoulos, M. J. Org. Chem. 1999, 64, 3392–3393. doi:10.1021/jo982486w |
| 22. | Vassilikogiannakis, G.; Hatzimarinaki, M.; Orfanopoulos, M. J. Org. Chem. 2000, 65, 8180–8187. doi:10.1021/jo0006223 |
| 23. | Mikie, T.; Asahara, H.; Nagao, K.; Ikuma, N.; Kokubo, K.; Oshima, T. Org. Lett. 2011, 13, 4244–4247. doi:10.1021/ol201590a |
| 19. | Vassilikogiannakis, G.; Orfanopoulos, M. Tetrahedron Lett. 1997, 38, 4323–4326. doi:10.1016/s0040-4039(97)00891-5 |
| 22. | Vassilikogiannakis, G.; Hatzimarinaki, M.; Orfanopoulos, M. J. Org. Chem. 2000, 65, 8180–8187. doi:10.1021/jo0006223 |
© 2024 Ueno et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.