Abstract
A new method for the synthesis of heterocyclic systems containing tetrazole and tetrahydroisoquinoline is developed via the performance of one-pot Ugi-azide and Heck cyclization reactions. The integration of the multicomponent and post-condensation reactions in one-pot maximizes the pot-, atom-, and step-economy (PASE).
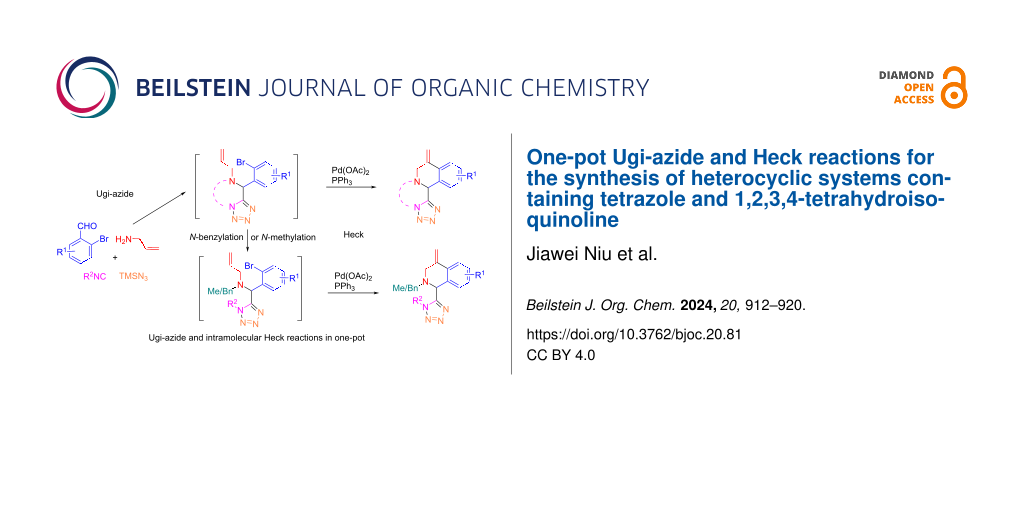
Graphical Abstract
Introduction
Tetrazole is a privileged heterocycle existing in a range of biological and medicinally interesting compounds [1,2] with antifungal [3,4], antibacterial [5], anticancer [6,7], antiparasitic [8], and antihypertensive properties [9] including FDA approved drugs such as valsartan and cefmetazole [10,11] (Figure 1). The tetrazole ring can also be found in functional materials for photography, imaging, and military applications [12-17]. The hydroisoquinoline core, such as 1,2,3,4-tetrahydroisoquinoline and pyrazino[2,1-a]isoquinolinone, is also a privileged heterocycle which can be found in natural products and synthetic compounds with antitumor, anti-HIV, antibiotic, antifungal, antivirus, and anti-inflammatory activities [18-21]. The antischistosomal drug praziquantel (PZQ), a tetrahydroisoquinoline derivative, is a commercialized drug for the treatment of schistosomiasis [22-25]. The combination of the privileged heterocycles tetrazole and tetrahydroisoquinoline in one molecule generates new molecules which could have biological activities.
Figure 1: Representative bioactive tetrazole- and tetrahydroisoquinoline-containing compounds.
Figure 1: Representative bioactive tetrazole- and tetrahydroisoquinoline-containing compounds.
A standard Ugi four-component reaction (Ugi-4CR) of an aldehyde, amine, isocyanide, and a carboxylic acid produces highly diverse peptidic structures A with up to four points of substitution (Scheme 1) [26,27]. By replacing the carboxylic acid with a nucleophilic azide reagent XN3 (generally TMSN3), the Ugi-azide four-component reaction (UA-4CR) of an aldehyde, amine, isocyanide, and azide gives 1,5-disubstituted 1H-tetrazoles (1,5-DS-1H-Ts) B. The performance of post-condensation reactions of UA-4CR adducts has resulted in various 1,5-DS-1H-Ts containing heterocyclic compounds [28-32], such as bis-heterocyclic lactam-tetrazoles [33,34], 2-tetrazolylmethyl-2,3,4,9-tetrahydro-1H-β-carbolines [35], ketopiperazinetetrazoles [36], imidazotetrazolodiazepinones [37], tetracyclic tetrazolylpyridoimidazoquinolines [38], bis-heterocyclic 1,5-disubstituted tetrazoleindolizines [39] and (E)-12-tetrazolyl-5H-quinazolino[3,2-a]quinazolines [40]. Among them, the Hulme group reported a UA-4CR/post-condensation sequence to give fused imidazotetrazolodiazepinones (Scheme 2A) [37]. The Gámez-Montaño group introduced a one-pot synthesis of Ugi-azide/N-acylation/Diels–Alder/dehydration reactions for isoindolin-1-one and 1,5-DS-T in a linked manner (Scheme 2B) [41]. The Ding group developed sequential Ugi-azide/Ag-catalyzed oxidative cycloisomerization reactions for the synthesis of 2-tetrazolyl-substituted 3-acylpyrroles (Scheme 2C) [42]. The Ding group also reported sequential Ugi-azide/Staudinger/aza-Wittig/addition/Ag-catalyzed cyclization reactions for obtaining 12-tetrazolyl-substituted (E)-5H-quinazolino[3,2-a]quinazolines (Scheme 2D) [40].
Scheme 1: The Ugi and Ugi-azide reactions.
Scheme 1: The Ugi and Ugi-azide reactions.
Scheme 2: Ugi-azide and post-condensation reactions for the synthesis of various heterocyclic scaffolds.
Scheme 2: Ugi-azide and post-condensation reactions for the synthesis of various heterocyclic scaffolds.
There are numbers of Ugi and subsequential Heck (or reductive Heck) reactions that have been developed for the synthesis of poly-heterocyclic compounds [43-51]. Reported in this paper is a one-pot Ugi-azide reaction followed by an intramolecular Heck reaction for the synthesis of tetrazolyl-1,2,3,4-tetrahydroisoquinoline scaffolds 6 and 8 (Scheme 3). The first step is the Ugi-azide reaction of a 2-bromobenzoaldehyde 1, allylamine hydrochloride (2), azidotrimethylsilane (TMSN3, 3), and an isocyanide 4 affording tetrazoles 5. If ethyl isocyanoacetate is used as the isocyanide source, the Ugi-azide reaction gives rise to ring-fused tetrazolo[1,5-a]pyrazin-6(5H)-one adducts 5. The subsequent Pd-catalyzed intramolecular Heck reaction of compounds 5 or 7 then affords 1,2,3,4-tetrohydroisoquinolines 6 and 8, respectively.
Scheme 3: One-pot synthesis of tetrazolyl-1,2,3,4-tetrahydroisoquinoline.
Scheme 3: One-pot synthesis of tetrazolyl-1,2,3,4-tetrahydroisoquinoline.
Results and Discussion
Following the reported procedures [41], the Ugi-azide reaction of 2-bromobenzaldehyde (1a, 1 mmol), allylamine hydrochloride (2, 1 mmol), trimethylsilyl azide (3, 1 mmol) and tert-butyl isocyanide (4a, 1 mmol) in MeOH at 40 °C for 24 h afforded 1,5-DS-1H-T 5a in 92% yield after chromatography purification. Our effort was then focused on the optimization of the intramolecular Heck reaction of 5a for making 1,2,3,4-tetrahydroisoquinoline 6a. A systematic evaluation of different catalysts and ligands, solvents, bases, as well as reaction temperatures and times was conducted (Table 1). The Heck reaction of 5a was first examined by using 10 mol % Pd(OAc)2, 20 mol % PPh3, 2 equiv of Et3N in CH3CN or DMF at 105 °C for 24 h under N2 atmosphere. However, the reactions failed under these conditions (Table 1, entries 1 and 2). When K2CO3 was used as a base to replace Et3N, the reactions in either CH3CN or DMF for 3 h both gave cyclized product 6a in 70% yield (Table 1, entries 3 and 4). An increase of the reaction time to 12 h did not improve the yield (Table 1, entry 5). The reaction was further evaluated in the absence of ligand which afforded the product in 35% yield (Table 1, entry 6). Screening of ligands, e.g., PCy3 and P(o-tol)3 reduced the yield of the desired product 6a (Table 1, entries 7 and 8). Lowering the amount of Pd(OAc)2 or changing the reaction temperatures resulted low yields of 6a (Table 1, entries 9–11). Similar results were observed from the reactions using other bases, such as K3PO4, NaOAc, and Cs2CO3 (Table 1, entries 12–14). Investigating other Pd catalysts, suche as PdCl2 and Pd(dba)2 also gave low yields (Table 1, entries 15 and 16). Since CH3CN is a more favorable solvent than DMF in green chemistry consideration [52,53], the optimal reaction conditions for the Heck reaction were to use 1 mmol of 5a with 10 mol % Pd(OAc)2 and 20 mol % PPh3, 2 equiv of K2CO3 in 3 mL CH3CN at 105 °C for 3 h under N2 atmosphere which afforded product 6a in 70% yield (Table 1, entry 3).
Table 1: Conditions for one-pot Ugi-azide and Heck reactions.a
|
|
|||||||
| Entry | Catalyst | Ligand | Solvent | Base | Temp (°C) | Time (h) | Yield (%)b |
| 1 | Pd(OAc)2 | PPh3 | MeCN | Et3N | 105 | 24 | – |
| 2 | Pd(OAc)2 | PPh3 | DMF | Et3N | 105 | 24 | – |
| 3 | Pd(OAc)2 | PPh3 | MeCN | K2CO3 | 105 | 3 | 70 |
| 4 | Pd(OAc)2 | PPh3 | DMF | K2CO3 | 105 | 3 | 70 |
| 5 | Pd(OAc)2 | PPh3 | MeCN | K2CO3 | 105 | 12 | 65 |
| 6 | Pd(OAc)2 | – | MeCN | K2CO3 | 105 | 6 | 35 |
| 7 | Pd(OAc)2 | PCy3 | MeCN | K2CO3 | 105 | 6 | 46 |
| 8 | Pd(OAc)2 | P(o-tol)3 | MeCN | K2CO3 | 105 | 6 | 56 |
| 9c | Pd(OAc)2 | PPh3 | MeCN | K2CO3 | 105 | 3 | 58 |
| 10 | Pd(OAc)2 | PPh3 | MeCN | K2CO3 | 70 | 8 | 60 |
| 11 | Pd(OAc)2 | PPh3 | MeCN | K2CO3 | 120 | 3 | 62 |
| 12 | Pd(OAc)2 | PPh3 | MeCN | K3PO4 | 105 | 3 | 39 |
| 13 | Pd(OAc)2 | PPh3 | MeCN | NaOAc | 105 | 3 | 62 |
| 14 | Pd(OAc)2 | PPh3 | MeCN | Cs2CO3 | 105 | 3 | 56 |
| 15 | PdCl2 | PPh3 | MeCN | K2CO3 | 105 | 5 | 53 |
| 16 | Pd(dba)2 | PPh3 | MeCN | K2CO3 | 105 | 6 | 61 |
| 17d | Pd(OAc)2 | PPh3 | MeCN | K2CO3 | 105 | 3 | 60 |
aReaction conditions: Ugi-azide step, 2-bromobenzaldehyde (1a, 1 mmol), allylamine hydrochloride (2, 1 mmol), trimethylsilyl azide (3, 1 mmol) and tert-butyl isocyanide (4a, 1 mmol), Et3N (1.2 mmol) in 5 mL MeOH, 40 °C for 24 h. Heck reaction step, catalyst (10 mol %), ligand (20 mol %), solvent (3 mL), base (2 equiv), nitrogen atmosphere. bIsolated yield. cPd(OAc)2 5 mol %, PPh3 10 mol %. dReaction was carried out in one-pot, starting compound is 1a (1 mmol), first Ugi-azide reaction followed by the Heck reaction.
The combination of an initial multicomponent reaction with post-condensation reactions in one-pot is a good strategy to develop high pot, atom and step economy (PASE) syntheses [54-58]. We then made the effort to integrate the Ugi and Heck reactions in one-pot for making tetrazolyl-1,2,3,4-tetrahydroisoquinolines 6. Thus, a mixture of 2-bromobenzaldehyde (1a, 1 mmol), allylamine hydrochloride (2, 1 mmol), trimethylsilyl azide (3, 1 mmol), and tert-butyl isocyanide (4a, 1 mmol) was stirred in MeOH at 40 °C for 24 h, and after the reaction was completed, the solvent was evaporated under vacuum to give crude Ugi adduct 5a which was used for the intramolecular Heck reaction without further purification. Thus, to the solution of crude 5a dissolved in MeCN (3 mL) were added 10 mol % of Pd(OAc)2, 20 mol % of PPh3, and 2 equiv of K2CO3 and the mixture stirred for 3 h at 105 °C under N2 atmosphere to give the desired product 6a in 60% isolated yield (entry 17 in Table 1).
With the optimized one-pot reactions in hands, we next evaluated the substrate scope by synthesizing 11 derivatives (Scheme 4) using nine benzaldehydes 1, two isonitriles or ethyl isocyanoacetate 4, allylamine hydrochloride (2), and trimethylsilyl azide (3) for the initial Ugi-azide reaction. Among them, products 6a and 6b from the reaction of isonitriles were synthesized in moderate yields (58–60%). For the Ugi reaction involving isocyanoacetate, lactamination occurred spontaneously to provide the ring-fused tetrazolo[1,5-a]pyrazin-6(5H)-one adducts 5 which after intramolecular Heck reaction gave functionalized tetracyclic tetrazolo-pyrazino[2,1-a]isoquinolin-6(5H)-ones 6c–k in 73–79% yields. The presence of electron-donating or electron-withdrawing groups on the aromatic ring did not show significant effects on the Heck reaction.
Scheme 4: One-pot synthesis of tetrazolo-pyrazino[2,1-a]isoquinolin-6(5H)-ones 6.
Scheme 4: One-pot synthesis of tetrazolo-pyrazino[2,1-a]isoquinolin-6(5H)-ones 6.
Products 6c–k were obtained in higher yields than products 6a,b. We believe that the secondary amine in the Ugi reaction products 5 could affect the yield of the Heck reaction. To address the issue, compounds 5 were N-alkylated to afford intermediates 7 which were used in the subsequent Heck reaction step. Thus, an alternative one-pot Ugi-azide/N-alkylation/Heck reaction procedure was developed (Scheme 5). A mixture of 2-bromobenzaldehyde (1a, 1 mmol), allylamine hydrochloride (2, 1 mmol), trimethylsilyl azide (3, 1 mmol) and benzyl isocyanide (1 mmol) in MeOH was reacted at 40 °C for 24 h. After evaporating the solvent, 3 mL CH3CN were added to the crude 1,5-DS-1H-T 5a followed by the addition of 1 equiv of benzyl bromide and 2 equiv of K2CO3 for the alkylation reaction at 80 °C for 3 h to give N-benzylated compound 7a. Finally, 10 mol % of Pd(OAc)2, 20 mol % of PPh3, 2 equiv of K2CO3 were added to the reaction mixture for the Heck reaction at 105 °C for 3 h under N2 atmosphere to afford tetrazolyl-1,2,3,4-tetrahydroisoquinoline 8a in 74% isolated yield which is higher than the one-pot Ugi/Hecke reaction to give product 6b (58%). Under the alternative one-pot reaction conditions involving an N-alkylation step, the substrate scope was explored by the preparation of 10 derivatives 8a–j (Scheme 5) using seven benzaldehydes 1, two isonitriles 4, and allylamine hydrochloride (2) with trimethylsilyl azide (3) for the Ugi-azide reaction. The N-alkylations were conducted using benzyl bromide and iodomethane, respectively. The final products 8b–j were obtained in 66–74% yields.
Scheme 5: One-pot synthesis for tetrazolyl-1,2,3,4-tetrahydroisoquinolines 8.
Scheme 5: One-pot synthesis for tetrazolyl-1,2,3,4-tetrahydroisoquinolines 8.
To evaluate the scalability of the two-step one-pot reaction protocol, we performed the synthesis of tetracyclic tetrazolo-pyrazino[2,1-a]isoquinolin-6(5H)-one 6c in gram quantity from 10 mmol of 1a which led to the formation of product 6c in a satisfactory yield 77% (Scheme 6).
Scheme 6: Gram-scale two-step one-pot synthesis of 6c.
Scheme 6: Gram-scale two-step one-pot synthesis of 6c.
The products 6 and 8 were characterized by 1H and 13C NMR, and HRMS analysis. In addition, single crystals of compound 6d and 8c were obtained for X-ray analysis to confirm the structures (Figure 2).
![[1860-5397-20-81-2]](/bjoc/content/figures/1860-5397-20-81-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: ORTEP diagrams of compound 6d (left) [CCDC: 2164364] and 8c (right) [CCDC: 2321622].
Figure 2: ORTEP diagrams of compound 6d (left) [CCDC: 2164364] and 8c (right) [CCDC: 2321622].
Conclusion
In conclusion, we have developed a one-pot synthesis with two or three steps for making tetrazolo-pyrazino[2,1-a]isoquinolin-6(5H)-ones. The initial Ugi-azide four-component reaction constructs the tetrazole motif while the subsequent intramolecular Heck reaction assembles the tetrahydroisoquinoline. The one-pot reaction avoids the intermediate purification which has favorable PASE in the synthesis of heterocyclic compounds.
Experimental
General procedure for the synthesis of Ugi-azide adduct 5a
A solution of 2-bromobenzaldehyde 1 (1 mmol, 1 equiv), allylamine hydrochloride (2, 1 mmol, 1 equiv), trimethylsilyl azide (3, 1 mmol, 1 equiv) and tert-butyl isocyanide 4a (1 mmol, 1 equiv) in MeOH (5 mL) with Et3N (1.5 mmol) was heated at 40 °C for 24 h in a sealed vial. Upon completion of the reaction, the reaction mixture was filtered and evaporated under vacuum to give crude products 5a. Further purification was conducted by flash chromatography with 1:6 petroleum ether/EtOAc to afford 5a in 92% yields. The adduct was confirmed by NMR.
General procedure for the Heck reaction; synthesis of product 6a
A mixture of Ugi-azide adduct 5a (1 mmol), Pd(OAc)2 (0.1 mmol), PPh3 (0.2 mmol), K2CO3 (2 mmol) or NaOAc (2 mmol) in MeCN (3 mL) was stirred at 105 °C for 3 h under nitrogen atmosphere. After aqueous work-up, the crude product was purified by flash chromatography with 1:4 ethyl acetate/petroleum ether to afford product 6a.
General procedure for the one-pot synthesis of tetrazole-containing 1,2,3,4-tetrahydroisoquinolines 6
A mixture of 2-bromobenzaldehyde 1 (1 mmol), allylamine hydrochloride (2, 1 mmol), trimethylsilyl azide (3, 1 mmol) and isocyanide 4 (1 mmol) in MeOH was stirred at 40 °C for 24 h. After the reaction was complete, the solvent was evaporated under vacuum to give the crude Ugi adduct 5, which was used in the Heck reaction without further purification. To a solution of the crude intermediate 5 in MeCN (3 mL) was added 10 mol % of Pd(OAc)2, 20 mol % of PPh3, 2 equiv of K2CO3 and the mixture stirred for 3 h at 105 °C under N2 atmosphere. After aqueous work-up, the crude product was purified by flash chromatography with 1:3 ethyl acetate/petroleum ether to afford products 6.
General procedure for the one-pot synthesis of tetrazolyl-1,2,3,4-tetrahydroisoquinolines 8
A mixture of 2-bromobenzaldehyde 1 (1 mmol), allylamine hydrochloride (2, 1 mmol), trimethylsilyl azide (3, 1 mmol) and isocyanide 4 (1 mmol) in MeOH was reacted at 40 °C for 24 h. After evaporating the solvent, 3 mL CH3CN were added to the crude 1,5-DS-1H-T 5 followed by the addition of 1 equiv of benzyl bromide or iodomethane and 2 equiv of K2CO3 for the alkylation reaction at 80 °C for 3 h to give N-alkylated compounds 7. Finally, 10 mol % of Pd(OAc)2, 20 mol % of PPh3, 2 equiv of K2CO3 were added to the reaction mixture for the Heck reaction at 105 °C for 3 h under N2 atmosphere. After aqueous work-up, the crude products were purified by flash chromatography with 1:4 ethyl acetate/petroleum ether to afford products 8.
Supporting Information
| Supporting Information File 1: General reaction procedures, compound characterization data, and copies of NMR spectra. | ||
| Format: PDF | Size: 3.3 MB | Download |
| Supporting Information File 2: Crystallographic information file for compound 6d. | ||
| Format: CIF | Size: 247.4 KB | Download |
| Supporting Information File 3: Crystallographic information file for compound 8c. | ||
| Format: CIF | Size: 432.6 KB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information to this article. Data generated and analyzed during this study is openly available in CCDC. The data of CCDC-2164364 can be obtained free of charge at doi: https://doi.org/10.5517/ccdc.csd.cc2bn66f; the data of CCDC- 2321622: can be obtained free of charge at doi: https://doi.org/10.5517/ccdc.csd.cc2hxv1b.
References
-
Neochoritis, C. G.; Zhao, T.; Dömling, A. Chem. Rev. 2019, 119, 1970–2042. doi:10.1021/acs.chemrev.8b00564
Return to citation in text: [1] -
Ostrovskii, V. A.; Popova, E. A.; Trifonov, R. E. Tetrazoles. In Comprehensive Heterocyclic Chemistry IV; StC. Black, D.; Cossy, J.; Stevens, C. V., Eds.; Elsevier: Amsterdam, Netherlands, 2022; Vol. 6, pp 182–232. doi:10.1016/b978-0-12-818655-8.00131-1
Return to citation in text: [1] -
Vandecruys, P.; Baldewijns, S.; Sillen, M.; Van Genechten, W.; Van Dijck, P. Expert Rev. Anti-Infect. Ther. 2023, 21, 799–812. doi:10.1080/14787210.2023.2233696
Return to citation in text: [1] -
Staniszewska, M.; Zdrojewski, T.; Gizińska, M.; Rogalska, M.; Kuryk, Ł.; Kowalkowska, A.; Łukowska-Chojnacka, E. Eur. J. Med. Chem. 2022, 230, 114060. doi:10.1016/j.ejmech.2021.114060
Return to citation in text: [1] -
Kritchenkov, A. S.; Lipkan, N. A.; Kurliuk, A. V.; Shakola, T. V.; Egorov, A. R.; Volkova, O. V.; Meledina, T. V.; Suchkova, E. P.; Zabodalova, L. A.; Dysin, A. P. Pharm. Chem. J. 2020, 54, 138–141. doi:10.1007/s11094-020-02180-4
Return to citation in text: [1] -
Cortes-García, C. J.; Islas-Jácome, A.; Rentería-Gómez, A.; Gámez-Montaño, R. Monatsh. Chem. 2016, 147, 1277–1290. doi:10.1007/s00706-016-1686-x
Return to citation in text: [1] -
Vanam, N. R.; Anireddy, J. S. Chem. Data Collect. 2023, 48, 101092. doi:10.1016/j.cdc.2023.101092
Return to citation in text: [1] -
Cano, P. A.; Islas-Jácome, A.; González-Marrero, J.; Yépez-Mulia, L.; Calzada, F.; Gámez-Montaño, R. Bioorg. Med. Chem. 2014, 22, 1370–1376. doi:10.1016/j.bmc.2013.12.069
Return to citation in text: [1] -
Toplak Časar, R.; Časar, Z. Bioorg. Med. Chem. 2018, 26, 4348–4359. doi:10.1016/j.bmc.2018.06.036
Return to citation in text: [1] -
Prieto, C.; Evtoski, Z.; Pardo-Figuerez, M.; Hrakovsky, J.; Lagaron, J. M. Mol. Pharmaceutics 2021, 18, 2947–2958. doi:10.1021/acs.molpharmaceut.1c00098
Return to citation in text: [1] -
Li, L.-H.; Yen, M.-Y.; Ho, C.-C.; Wu, P.; Wang, C.-C.; Maurya, P. K.; Chen, P.-S.; Chen, W.; Hsieh, W.-Y.; Chen, H.-W. PLoS One 2013, 8, e64794. doi:10.1371/journal.pone.0064794
Return to citation in text: [1] -
Wei, C.-X.; Bian, M.; Gong, G.-H. Molecules 2015, 20, 5528–5553. doi:10.3390/molecules20045528
Return to citation in text: [1] -
Frija, L. M. T.; Ismael, A.; Cristiano, M. L. S. Molecules 2010, 15, 3757–3774. doi:10.3390/molecules15053757
Return to citation in text: [1] -
Myznikov, L. V.; Hrabalek, A.; Koldobskii, G. I. Chem. Heterocycl. Compd. (N. Y., NY, U. S.) 2007, 43, 1–9. doi:10.1007/s10593-007-0001-5
Return to citation in text: [1] -
Lv, F.; Liu, Y.; Zou, J.; Zhang, D.; Yao, Z. Dyes Pigm. 2006, 68, 211–216. doi:10.1016/j.dyepig.2004.07.017
Return to citation in text: [1] -
Song, W.; Wang, Y.; Qu, J.; Madden, M. M.; Lin, Q. Angew. Chem., Int. Ed. 2008, 47, 2832–2835. doi:10.1002/anie.200705805
Return to citation in text: [1] -
Shmatova, O. I.; Nenajdenko, V. G. J. Org. Chem. 2013, 78, 9214–9222. doi:10.1021/jo401428q
Return to citation in text: [1] -
Shang, X.-F.; Yang, C.-J.; Morris‐Natschke, S. L.; Li, J.-C.; Yin, X.-D.; Liu, Y.-Q.; Guo, X.; Peng, J.-W.; Goto, M.; Zhang, J.-Y.; Lee, K.-H. Med. Res. Rev. 2020, 40, 2212–2289. doi:10.1002/med.21703
Return to citation in text: [1] -
Li, D.-D.; Yu, P.; Xiao, W.; Wang, Z.-Z.; Zhao, L.-G. Curr. Top. Med. Chem. 2020, 20, 2634–2647. doi:10.2174/1568026620666200908165913
Return to citation in text: [1] -
Qing, Z.-X.; Yang, P.; Tang, Q.; Cheng, P.; Liu, X.-B.; Zheng, Y.-J.; Liu, Y.-S.; Zeng, J.-G. Curr. Org. Chem. 2017, 21, 1920–1934. doi:10.2174/1385272821666170207114214
Return to citation in text: [1] -
Maiti, M.; Kumar, G. S. J. Nucleic Acids 2010, 2010, 593408. doi:10.4061/2010/593408
Return to citation in text: [1] -
Wang, Z.-x.; Chen, J.-l.; Qiao, C. Chem. Biol. Drug Des. 2013, 82, 216–225. doi:10.1111/cbdd.12153
Return to citation in text: [1] -
Liu, H.; William, S.; Herdtweck, E.; Botros, S.; Dömling, A. Chem. Biol. Drug Des. 2012, 79, 470–477. doi:10.1111/j.1747-0285.2011.01288.x
Return to citation in text: [1] -
Dömling, A.; Khoury, K. ChemMedChem 2010, 5, 1420–1434. doi:10.1002/cmdc.201000202
Return to citation in text: [1] -
Waechtler, A.; Cezanne, B.; Maillard, D.; Sun, R.; Wang, S.; Wang, J.; Harder, A. ChemMedChem 2023, 18, e202300154. doi:10.1002/cmdc.202300154
Return to citation in text: [1] -
Dömling, A.; Ugi, I. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. doi:10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u
Return to citation in text: [1] -
Hooshmand, S. E.; Zhang, W. Molecules 2023, 28, 1642. doi:10.3390/molecules28041642
Return to citation in text: [1] -
Maleki, A.; Sarvary, A. RSC Adv. 2015, 5, 60938–60955. doi:10.1039/c5ra11531k
Return to citation in text: [1] -
Mohammadkhani, L.; Heravi, M. M. Mol. Diversity 2020, 24, 841–853. doi:10.1007/s11030-019-09972-1
Return to citation in text: [1] -
Barreto, A. d. F. S.; Santos, V. A. d.; Andrade, C. K. Z. Beilstein J. Org. Chem. 2017, 13, 2596–2602. doi:10.3762/bjoc.13.256
Return to citation in text: [1] -
Foley, C.; Shaw, A.; Hulme, C. Org. Lett. 2018, 20, 1275–1278. doi:10.1021/acs.orglett.7b03977
Return to citation in text: [1] -
Wang, Y.; Patil, P.; Kurpiewska, K.; Kalinowska-Tluscik, J.; Dömling, A. Org. Lett. 2019, 21, 3533–3537. doi:10.1021/acs.orglett.9b00778
Return to citation in text: [1] -
Gunawan, S.; Petit, J.; Hulme, C. ACS Comb. Sci. 2012, 14, 160–163. doi:10.1021/co200209a
Return to citation in text: [1] -
Gunawan, S.; Hulme, C. Org. Biomol. Chem. 2013, 11, 6036. doi:10.1039/c3ob40900g
Return to citation in text: [1] -
Cárdenas-Galindo, L. E.; Islas-Jácome, A.; Alvarez-Rodríguez, N. V.; El Kaim, L.; Gámez-Montaño, R. Synthesis 2014, 46, 49–56. doi:10.1055/s-0033-1340051
Return to citation in text: [1] -
Zarganes‐Tzitzikas, T.; Patil, P.; Khoury, K.; Herdtweck, E.; Dömling, A. Eur. J. Org. Chem. 2015, 51–55. doi:10.1002/ejoc.201403401
Return to citation in text: [1] -
Medda, F.; Martinez-Ariza, G.; Hulme, C. Tetrahedron Lett. 2015, 56, 5295–5298. doi:10.1016/j.tetlet.2015.07.083
Return to citation in text: [1] [2] -
Saha, D.; Kharbanda, A.; Essien, N.; Zhang, L.; Cooper, R.; Basak, D.; Kendrick, S.; Frett, B.; Li, H.-y. Org. Chem. Front. 2019, 6, 2234–2239. doi:10.1039/c9qo00389d
Return to citation in text: [1] -
Niño‐Pantoja, I.; Gallardo‐Alfonzo, A.; Solis‐Santos, M.; Ordoñez, M.; Contreras‐Celedón, C.; Islas‐Jácome, A.; Chacón‐García, L.; Cortés‐García, C. J. Eur. J. Org. Chem. 2022, e202200230. doi:10.1002/ejoc.202200230
Return to citation in text: [1] -
Yang, M.-L.; Zhao, L.; Chen, H.-R.; Ding, M.-W. J. Org. Chem. 2023, 88, 1898–1906. doi:10.1021/acs.joc.2c02621
Return to citation in text: [1] [2] -
Rentería-Gómez, A.; Islas-Jácome, A.; Cruz-Jiménez, A. E.; Manzano-Velázquez, J. C.; Rojas-Lima, S.; Jiménez-Halla, J. O. C.; Gámez-Montaño, R. ACS Omega 2016, 1, 943–951. doi:10.1021/acsomega.6b00281
Return to citation in text: [1] [2] -
Kong, H.-H.; Pan, H.-L.; Ding, M.-W. J. Org. Chem. 2018, 83, 12921–12930. doi:10.1021/acs.joc.8b01984
Return to citation in text: [1] -
Amador-Sánchez, Y. A.; Hernández-Vázquez, E.; González-Mojica, N.; Ramírez-Apan, M. T.; Miranda, L. D. Tetrahedron 2020, 76, 131383. doi:10.1016/j.tet.2020.131383
Return to citation in text: [1] -
Janatian Ghazvini, H.; Müller, T. J. J.; Rominger, F.; Balalaie, S. J. Org. Chem. 2019, 84, 10740–10748. doi:10.1021/acs.joc.9b01269
Return to citation in text: [1] -
García-González, M. C.; Hernández-Vázquez, E.; Vengochea-Gómez, F. A.; Miranda, L. D. Tetrahedron Lett. 2018, 59, 848–852. doi:10.1016/j.tetlet.2018.01.058
Return to citation in text: [1] -
Balalaie, S.; Ramezani Kejani, R.; Ghabraie, E.; Darvish, F.; Rominger, F.; Hamdan, F.; Bijanzadeh, H. R. J. Org. Chem. 2017, 82, 12141–12152. doi:10.1021/acs.joc.7b01855
Return to citation in text: [1] -
Hernández-Vázquez, E.; Miranda, L. D. Org. Biomol. Chem. 2016, 14, 4875–4884. doi:10.1039/c6ob00431h
Return to citation in text: [1] -
Vachhani, D. D.; Butani, H. H.; Sharma, N.; Bhoya, U. C.; Shah, A. K.; Van der Eycken, E. V. Chem. Commun. 2015, 51, 14862–14865. doi:10.1039/c5cc05193b
Return to citation in text: [1] -
Moni, L.; Denißen, M.; Valentini, G.; Müller, T. J. J.; Riva, R. Chem. – Eur. J. 2015, 21, 753–762. doi:10.1002/chem.201404209
Return to citation in text: [1] -
Rasouli, M. A.; Miri, S.; Rashidi Ranjbar, P. J. Heterocycl. Chem. 2015, 52, 1864–1870. doi:10.1002/jhet.2270
Return to citation in text: [1] -
Peshkov, A. A.; Peshkov, V. A.; Pereshivko, O. P.; Van der Eycken, E. V. Tetrahedron 2015, 71, 3863–3871. doi:10.1016/j.tet.2015.04.022
Return to citation in text: [1] -
Alfonsi, K.; Colberg, J.; Dunn, P. J.; Fevig, T.; Jennings, S.; Johnson, T. A.; Kleine, H. P.; Knight, C.; Nagy, M. A.; Perry, D. A.; Stefaniak, M. Green Chem. 2008, 10, 31–36. doi:10.1039/b711717e
Return to citation in text: [1] -
Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C. R.; Abou-Shehada, S.; Dunn, P. J. Green Chem. 2016, 18, 288–296. doi:10.1039/c5gc01008j
Return to citation in text: [1] -
Zhang, W.; Yi, W.-B. Pot, Atom, and Step Economy (PASE) Synthesis; SpringerBriefs in Molecular Science; Springer International Publishing: Cham, Switzerland, 2019. doi:10.1007/978-3-030-22596-4
Return to citation in text: [1] -
Ma, X.; Zhang, W. iScience 2022, 25, 105005. doi:10.1016/j.isci.2022.105005
Return to citation in text: [1] -
Hayashi, Y. Chem. Sci. 2016, 7, 866–880. doi:10.1039/c5sc02913a
Return to citation in text: [1] -
Hayashi, Y. Acc. Chem. Res. 2021, 54, 1385–1398. doi:10.1021/acs.accounts.0c00803
Return to citation in text: [1] -
Biesen, L.; Müller, T. J. J. Adv. Synth. Catal. 2021, 363, 980–1006. doi:10.1002/adsc.202001219
Return to citation in text: [1]
| 1. | Neochoritis, C. G.; Zhao, T.; Dömling, A. Chem. Rev. 2019, 119, 1970–2042. doi:10.1021/acs.chemrev.8b00564 |
| 2. | Ostrovskii, V. A.; Popova, E. A.; Trifonov, R. E. Tetrazoles. In Comprehensive Heterocyclic Chemistry IV; StC. Black, D.; Cossy, J.; Stevens, C. V., Eds.; Elsevier: Amsterdam, Netherlands, 2022; Vol. 6, pp 182–232. doi:10.1016/b978-0-12-818655-8.00131-1 |
| 8. | Cano, P. A.; Islas-Jácome, A.; González-Marrero, J.; Yépez-Mulia, L.; Calzada, F.; Gámez-Montaño, R. Bioorg. Med. Chem. 2014, 22, 1370–1376. doi:10.1016/j.bmc.2013.12.069 |
| 36. | Zarganes‐Tzitzikas, T.; Patil, P.; Khoury, K.; Herdtweck, E.; Dömling, A. Eur. J. Org. Chem. 2015, 51–55. doi:10.1002/ejoc.201403401 |
| 6. | Cortes-García, C. J.; Islas-Jácome, A.; Rentería-Gómez, A.; Gámez-Montaño, R. Monatsh. Chem. 2016, 147, 1277–1290. doi:10.1007/s00706-016-1686-x |
| 7. | Vanam, N. R.; Anireddy, J. S. Chem. Data Collect. 2023, 48, 101092. doi:10.1016/j.cdc.2023.101092 |
| 37. | Medda, F.; Martinez-Ariza, G.; Hulme, C. Tetrahedron Lett. 2015, 56, 5295–5298. doi:10.1016/j.tetlet.2015.07.083 |
| 5. | Kritchenkov, A. S.; Lipkan, N. A.; Kurliuk, A. V.; Shakola, T. V.; Egorov, A. R.; Volkova, O. V.; Meledina, T. V.; Suchkova, E. P.; Zabodalova, L. A.; Dysin, A. P. Pharm. Chem. J. 2020, 54, 138–141. doi:10.1007/s11094-020-02180-4 |
| 33. | Gunawan, S.; Petit, J.; Hulme, C. ACS Comb. Sci. 2012, 14, 160–163. doi:10.1021/co200209a |
| 34. | Gunawan, S.; Hulme, C. Org. Biomol. Chem. 2013, 11, 6036. doi:10.1039/c3ob40900g |
| 3. | Vandecruys, P.; Baldewijns, S.; Sillen, M.; Van Genechten, W.; Van Dijck, P. Expert Rev. Anti-Infect. Ther. 2023, 21, 799–812. doi:10.1080/14787210.2023.2233696 |
| 4. | Staniszewska, M.; Zdrojewski, T.; Gizińska, M.; Rogalska, M.; Kuryk, Ł.; Kowalkowska, A.; Łukowska-Chojnacka, E. Eur. J. Med. Chem. 2022, 230, 114060. doi:10.1016/j.ejmech.2021.114060 |
| 35. | Cárdenas-Galindo, L. E.; Islas-Jácome, A.; Alvarez-Rodríguez, N. V.; El Kaim, L.; Gámez-Montaño, R. Synthesis 2014, 46, 49–56. doi:10.1055/s-0033-1340051 |
| 18. | Shang, X.-F.; Yang, C.-J.; Morris‐Natschke, S. L.; Li, J.-C.; Yin, X.-D.; Liu, Y.-Q.; Guo, X.; Peng, J.-W.; Goto, M.; Zhang, J.-Y.; Lee, K.-H. Med. Res. Rev. 2020, 40, 2212–2289. doi:10.1002/med.21703 |
| 19. | Li, D.-D.; Yu, P.; Xiao, W.; Wang, Z.-Z.; Zhao, L.-G. Curr. Top. Med. Chem. 2020, 20, 2634–2647. doi:10.2174/1568026620666200908165913 |
| 20. | Qing, Z.-X.; Yang, P.; Tang, Q.; Cheng, P.; Liu, X.-B.; Zheng, Y.-J.; Liu, Y.-S.; Zeng, J.-G. Curr. Org. Chem. 2017, 21, 1920–1934. doi:10.2174/1385272821666170207114214 |
| 21. | Maiti, M.; Kumar, G. S. J. Nucleic Acids 2010, 2010, 593408. doi:10.4061/2010/593408 |
| 26. | Dömling, A.; Ugi, I. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. doi:10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u |
| 27. | Hooshmand, S. E.; Zhang, W. Molecules 2023, 28, 1642. doi:10.3390/molecules28041642 |
| 12. | Wei, C.-X.; Bian, M.; Gong, G.-H. Molecules 2015, 20, 5528–5553. doi:10.3390/molecules20045528 |
| 13. | Frija, L. M. T.; Ismael, A.; Cristiano, M. L. S. Molecules 2010, 15, 3757–3774. doi:10.3390/molecules15053757 |
| 14. | Myznikov, L. V.; Hrabalek, A.; Koldobskii, G. I. Chem. Heterocycl. Compd. (N. Y., NY, U. S.) 2007, 43, 1–9. doi:10.1007/s10593-007-0001-5 |
| 15. | Lv, F.; Liu, Y.; Zou, J.; Zhang, D.; Yao, Z. Dyes Pigm. 2006, 68, 211–216. doi:10.1016/j.dyepig.2004.07.017 |
| 16. | Song, W.; Wang, Y.; Qu, J.; Madden, M. M.; Lin, Q. Angew. Chem., Int. Ed. 2008, 47, 2832–2835. doi:10.1002/anie.200705805 |
| 17. | Shmatova, O. I.; Nenajdenko, V. G. J. Org. Chem. 2013, 78, 9214–9222. doi:10.1021/jo401428q |
| 28. | Maleki, A.; Sarvary, A. RSC Adv. 2015, 5, 60938–60955. doi:10.1039/c5ra11531k |
| 29. | Mohammadkhani, L.; Heravi, M. M. Mol. Diversity 2020, 24, 841–853. doi:10.1007/s11030-019-09972-1 |
| 30. | Barreto, A. d. F. S.; Santos, V. A. d.; Andrade, C. K. Z. Beilstein J. Org. Chem. 2017, 13, 2596–2602. doi:10.3762/bjoc.13.256 |
| 31. | Foley, C.; Shaw, A.; Hulme, C. Org. Lett. 2018, 20, 1275–1278. doi:10.1021/acs.orglett.7b03977 |
| 32. | Wang, Y.; Patil, P.; Kurpiewska, K.; Kalinowska-Tluscik, J.; Dömling, A. Org. Lett. 2019, 21, 3533–3537. doi:10.1021/acs.orglett.9b00778 |
| 10. | Prieto, C.; Evtoski, Z.; Pardo-Figuerez, M.; Hrakovsky, J.; Lagaron, J. M. Mol. Pharmaceutics 2021, 18, 2947–2958. doi:10.1021/acs.molpharmaceut.1c00098 |
| 11. | Li, L.-H.; Yen, M.-Y.; Ho, C.-C.; Wu, P.; Wang, C.-C.; Maurya, P. K.; Chen, P.-S.; Chen, W.; Hsieh, W.-Y.; Chen, H.-W. PLoS One 2013, 8, e64794. doi:10.1371/journal.pone.0064794 |
| 9. | Toplak Časar, R.; Časar, Z. Bioorg. Med. Chem. 2018, 26, 4348–4359. doi:10.1016/j.bmc.2018.06.036 |
| 22. | Wang, Z.-x.; Chen, J.-l.; Qiao, C. Chem. Biol. Drug Des. 2013, 82, 216–225. doi:10.1111/cbdd.12153 |
| 23. | Liu, H.; William, S.; Herdtweck, E.; Botros, S.; Dömling, A. Chem. Biol. Drug Des. 2012, 79, 470–477. doi:10.1111/j.1747-0285.2011.01288.x |
| 24. | Dömling, A.; Khoury, K. ChemMedChem 2010, 5, 1420–1434. doi:10.1002/cmdc.201000202 |
| 25. | Waechtler, A.; Cezanne, B.; Maillard, D.; Sun, R.; Wang, S.; Wang, J.; Harder, A. ChemMedChem 2023, 18, e202300154. doi:10.1002/cmdc.202300154 |
| 40. | Yang, M.-L.; Zhao, L.; Chen, H.-R.; Ding, M.-W. J. Org. Chem. 2023, 88, 1898–1906. doi:10.1021/acs.joc.2c02621 |
| 38. | Saha, D.; Kharbanda, A.; Essien, N.; Zhang, L.; Cooper, R.; Basak, D.; Kendrick, S.; Frett, B.; Li, H.-y. Org. Chem. Front. 2019, 6, 2234–2239. doi:10.1039/c9qo00389d |
| 39. | Niño‐Pantoja, I.; Gallardo‐Alfonzo, A.; Solis‐Santos, M.; Ordoñez, M.; Contreras‐Celedón, C.; Islas‐Jácome, A.; Chacón‐García, L.; Cortés‐García, C. J. Eur. J. Org. Chem. 2022, e202200230. doi:10.1002/ejoc.202200230 |
| 52. | Alfonsi, K.; Colberg, J.; Dunn, P. J.; Fevig, T.; Jennings, S.; Johnson, T. A.; Kleine, H. P.; Knight, C.; Nagy, M. A.; Perry, D. A.; Stefaniak, M. Green Chem. 2008, 10, 31–36. doi:10.1039/b711717e |
| 53. | Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C. R.; Abou-Shehada, S.; Dunn, P. J. Green Chem. 2016, 18, 288–296. doi:10.1039/c5gc01008j |
| 54. | Zhang, W.; Yi, W.-B. Pot, Atom, and Step Economy (PASE) Synthesis; SpringerBriefs in Molecular Science; Springer International Publishing: Cham, Switzerland, 2019. doi:10.1007/978-3-030-22596-4 |
| 55. | Ma, X.; Zhang, W. iScience 2022, 25, 105005. doi:10.1016/j.isci.2022.105005 |
| 56. | Hayashi, Y. Chem. Sci. 2016, 7, 866–880. doi:10.1039/c5sc02913a |
| 57. | Hayashi, Y. Acc. Chem. Res. 2021, 54, 1385–1398. doi:10.1021/acs.accounts.0c00803 |
| 58. | Biesen, L.; Müller, T. J. J. Adv. Synth. Catal. 2021, 363, 980–1006. doi:10.1002/adsc.202001219 |
| 43. | Amador-Sánchez, Y. A.; Hernández-Vázquez, E.; González-Mojica, N.; Ramírez-Apan, M. T.; Miranda, L. D. Tetrahedron 2020, 76, 131383. doi:10.1016/j.tet.2020.131383 |
| 44. | Janatian Ghazvini, H.; Müller, T. J. J.; Rominger, F.; Balalaie, S. J. Org. Chem. 2019, 84, 10740–10748. doi:10.1021/acs.joc.9b01269 |
| 45. | García-González, M. C.; Hernández-Vázquez, E.; Vengochea-Gómez, F. A.; Miranda, L. D. Tetrahedron Lett. 2018, 59, 848–852. doi:10.1016/j.tetlet.2018.01.058 |
| 46. | Balalaie, S.; Ramezani Kejani, R.; Ghabraie, E.; Darvish, F.; Rominger, F.; Hamdan, F.; Bijanzadeh, H. R. J. Org. Chem. 2017, 82, 12141–12152. doi:10.1021/acs.joc.7b01855 |
| 47. | Hernández-Vázquez, E.; Miranda, L. D. Org. Biomol. Chem. 2016, 14, 4875–4884. doi:10.1039/c6ob00431h |
| 48. | Vachhani, D. D.; Butani, H. H.; Sharma, N.; Bhoya, U. C.; Shah, A. K.; Van der Eycken, E. V. Chem. Commun. 2015, 51, 14862–14865. doi:10.1039/c5cc05193b |
| 49. | Moni, L.; Denißen, M.; Valentini, G.; Müller, T. J. J.; Riva, R. Chem. – Eur. J. 2015, 21, 753–762. doi:10.1002/chem.201404209 |
| 50. | Rasouli, M. A.; Miri, S.; Rashidi Ranjbar, P. J. Heterocycl. Chem. 2015, 52, 1864–1870. doi:10.1002/jhet.2270 |
| 51. | Peshkov, A. A.; Peshkov, V. A.; Pereshivko, O. P.; Van der Eycken, E. V. Tetrahedron 2015, 71, 3863–3871. doi:10.1016/j.tet.2015.04.022 |
| 41. | Rentería-Gómez, A.; Islas-Jácome, A.; Cruz-Jiménez, A. E.; Manzano-Velázquez, J. C.; Rojas-Lima, S.; Jiménez-Halla, J. O. C.; Gámez-Montaño, R. ACS Omega 2016, 1, 943–951. doi:10.1021/acsomega.6b00281 |
| 42. | Kong, H.-H.; Pan, H.-L.; Ding, M.-W. J. Org. Chem. 2018, 83, 12921–12930. doi:10.1021/acs.joc.8b01984 |
| 40. | Yang, M.-L.; Zhao, L.; Chen, H.-R.; Ding, M.-W. J. Org. Chem. 2023, 88, 1898–1906. doi:10.1021/acs.joc.2c02621 |
| 37. | Medda, F.; Martinez-Ariza, G.; Hulme, C. Tetrahedron Lett. 2015, 56, 5295–5298. doi:10.1016/j.tetlet.2015.07.083 |
| 41. | Rentería-Gómez, A.; Islas-Jácome, A.; Cruz-Jiménez, A. E.; Manzano-Velázquez, J. C.; Rojas-Lima, S.; Jiménez-Halla, J. O. C.; Gámez-Montaño, R. ACS Omega 2016, 1, 943–951. doi:10.1021/acsomega.6b00281 |
© 2024 Niu et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.