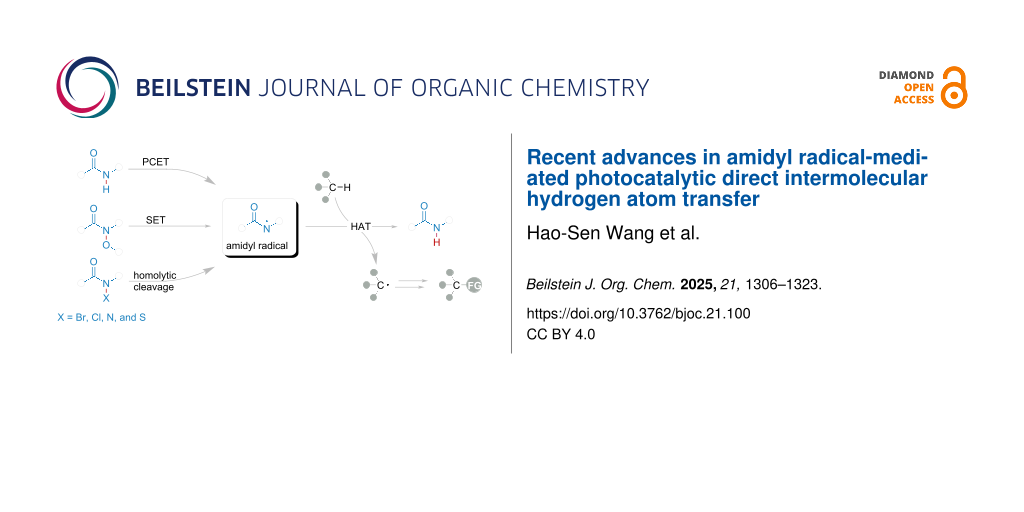
Abstract
In recent years, amidyl radicals have emerged as highly efficient and versatile reagents for hydrogen atom transfer (HAT) in photocatalytic reactions. These radicals display exceptional selectivity and efficiency in abstracting hydrogen atoms from C–H, Si–H, B–H, and Ge–H, positioning them as invaluable tools in synthetic chemistry. This review summarizes the latest advancements in the photocatalyzed generation of amidyl radicals as HAT reagents, with a particular emphasis on their role in the intermolecular HAT process. We highlight key developments, mechanistic insights, and emerging strategies that harness the unique reactivity of amidyl radicals in the selective functionalization of a variety of substrates.
Graphical Abstract
Introduction
C–H bonds are the predominant chemical bonds in organic compounds, and their direct conversion can rapidly and efficiently increase the complexity and functionality of organic molecules. On the other hand, C–H bonds exhibit low reactivity due to their relatively high bond dissociation energy (BDE) (Figure 1a). Therefore, the direct functionalization of C–H bonds is extremely challenging [1-5].
Figure 1: (a) BDE of C–H. (b) Direct functionalization of C–H catalyzed by transition-metal. (c) Direct functionalization of C–H via HAT process.
Figure 1: (a) BDE of C–H. (b) Direct functionalization of C–H catalyzed by transition-metal. (c) Direct funct...
In recent decades, transition-metal-catalyzed C–H bond functionalization demonstrated a decent methodology of organic synthesis. These elegant strategies presented powerful C–H bond transformation toolkits (Figure 1b) [6-8]. One of the exceptions to the perfection is the pre-functionalization of substrates. Current catalytic methodologies predominantly rely on substrate prefunctionalization through directing group (DG) incorporation, inevitably necessitating covalent DG-metal coordinative anchoring. This prerequisite fundamentally compromises both atomic efficiency and synthetic practicality, thereby imposing fundamental constraints on the catalytic system's intrinsic sustainability and operational scalability [9,10]. Moreover, a high temperature and additive oxidants are generally required, which would limit the substrate scope.
The hydrogen atom transfer (HAT) process has emerged as a powerful avenue for addressing these challenges, leveraging the HAT reagents to selectively abstract hydrogen atoms from these C–H bonds and directly functionalize these bonds via radical reactions (Figure 1c) [11-18]. This approach involves HAT reagents abstracting hydrogen atoms from C–H bonds to generate highly reactive C-centered radicals, which can subsequently form C–C or C–heteroatom bonds. The incorporation of HAT strategies into the functionalization of C–H bonds represents a significant advancement in synthetic organic chemistry for their high atom economy and step economy.
HAT reagents (HR), including alkoxyl, acyloxyl, halogen radicals, and amidyl (Figure 1c) [19-27], serve as key species for the HAT process. These HR were generated from different HAT reagent precursors (HRP) in a variety of strategies. Among these, amidyl radical HRPs have gained significant attention in recent years due to their ease of HRP synthesis and the relatively green chemistry of generating amidyl radicals. Amidyl radicals offer several advantages that enhance their applicability in organic synthesis:
1) The BDE of amidyl N–H bond is more than 105 kcal/mol, relative to the bond (C–H, Si–H, B–H, and Ge–H) which BDE is lower than 100 kcal/mol (Figure 2a) [28-30]. Almost 5 kcal/mol difference between two species could spontaneously undergo a HAT process. That also justifies the selectivity and efficiency of amidyl radical serving as HAT reagent.
Figure 2: (a) Amidyl radical-enabled hydrogen atom transfer. (b) Substituent effects to amidyl radical properties. (c) This review: generation of amidyl radical and transformation.
Figure 2: (a) Amidyl radical-enabled hydrogen atom transfer. (b) Substituent effects to amidyl radical proper...
2) Recent research indicated a critical correlation between electronic effects and activation energy modulation during transition state formation. Specifically, donor/acceptor electronic configurations in the substrate could either stabilize or destabilize the transient hybrid state, thereby thermodynamically governing the energy barrier for intermolecular HAT progression. When the partial positive and negative charges of two species can be stabilized by the electronic effects, these species are considered to be polarity matched during the HAT process. Conversely, when there is a polarity mismatch, the HAT process is likely to be impeded (Figure 2a). C–H bonds predominantly prefer to be nucleophilic, which smoothly facilitates the HAT process with amidyl radical. This effect is also called a polarity match [31-39].
3) Considering the electronic effect, modifying the substituent of the N atom could tune the property of HAT capability (Figure 2b) [40-43]. The electron-withdrawing groups could stabilize the charge of the N-centered radical during the HAT process by decreasing the charge density [44]. Notably, the BDE of N–H in the corresponding amide might be too low to ensure a spontaneous HAT process due to the electronic effect of the substituent. When introducing electron-donating groups to address this contradiction, another vital impact arises, the intramolecular HAT would take place. The amidyl radical would abstract a hydrogen atom from the nearest C–H, i.e., 1,2-HAT. Taken all these together, the substituent group should be decently modified.
In recent years, photocatalysis has been widely adopted due to its green and efficient nature [45-51]. The generation of amidyl radical is implemented by HRP. Six different methods (Figure 2c), which have been developed for visible-light mediated reactions, could generate amidyl radicals from HRP: (a) direct single-electron oxidation of amide HRP in the presence of photocatalyst and a base via a proton-coupled electron transfer (PCET) process by the cleavage of the N–H bond; (b) single-electron reduction of HRP catalyzed by photocatalyst via a single-electron transfer (SET) process by the cleavage of the N–O bond; (c) direct homolytic cleavage of weak N–S or N–X bonds in HRP initiated in the presence of visible light; (d) the intersystem crossing (ISC) of S1 to T1 state directly from the amide anion. This review is organized by bond cleavage type, offering a deep insight in the development of novel methods for amidyl radical-mediated photocatalytic direct intermolecular hydrogen atom transfer.
Although, amidyl radicals employed in many reactions as HAT reagents via heating conditions have been summarized in several studies [52-58]. To advance the research of direct functionalization via HAT processes and the development of green chemistry in photocatalysis, this review will focus on the generation of amidyl radicals and reaction mechanisms and highlight the photocatalyzed reaction characteristics. This review aims to provide researchers with a systematic understanding and strategic toolkit, thereby propelling the development of direct functionalization of C–H, B–H, Si–H, and Ge–H techniques in modern organic synthesis. Most of the photocatalysts used in this review are listed in Figure 3.
Figure 3: Representative photocatalysts discussed in this review.
Figure 3: Representative photocatalysts discussed in this review.
Review
Amidyl radical from N–H bond cleavage
N-Alkylbenzamide constitutes the primary structural unit of this class of compounds. The structures of these compounds are relatively simple and readily synthesizable. In these photocatalytic systems, direct single-electron oxidation of the amide HRP occurs in the presence of a photoredox catalyst and a base via a proton-coupled electron transfer process [59-69]. Following this process, the corresponding amidyl radical abstracts a hydrogen atom from the substrate, resulting in the conversion of the amidyl radical back to N-alkylbenzamide. This pathway creates a complete cycle in synchrony with the photocatalytic cycle, thereby allowing these HRPs to be consistently employed for catalytic equivalence.
In 2016, Knowles’ group independently developed an oxidative photocatalytic system capable of directly generating amidyl radicals from N-ethyl-4-methoxybenzamide, utilizing the photocatalyst [Ir(dF(CF3)ppy)2(4,4'-d(CF3)bpy)]PF6 in combination with a base (NBu4OP(O)(OBu)2) (Scheme 1) [59]. The generation of amidyl radical 5 involved a stepwise PCET process catalyzed by the combined effect, in the presence of photocatalyst and the base. Subsequently, amidyl radical 5 abstracted a hydrogen atom from substrate 1. This HAT process returned the amidyl radical 5 to HRP-1, enabling the continuation of the HAT cycle in synchronization with the photocatalytic cycle. The resulting radical 4 then underwent Giese addition with activated alkenes, leading to the formation of products 8, 9, and 10 with 59%, 60%, and 69% yields. This powerful and efficient toolkit effectively overcame the limitations of intramolecular HAT processes.
Scheme 1: Alkylation of C(sp3)–H catalyzed by amidyl radical under visible light.
Scheme 1: Alkylation of C(sp3)–H catalyzed by amidyl radical under visible light.
Building on this strategy, Kanai’s group reported a novel HAT method employing a new radical precursor in an oxidative photocatalytic system in 2018 (Scheme 2) [70]. Through a similar oxidative pathway, amidyl radical 13 was generated directly from the amide HRP-2, facilitating a smooth HAT process with substrate 1 while simultaneously regenerating HRP-2. The resulting radical 4 then participated in an addition reaction with radical anion 15. The radical anion 15 was reduced by the photocatalyst Ir(Fppy)3 from the reagent 11. The resulting anion 14 underwent aromatization to release a nitrile anion, subsequently yielding product 12. This strategy also successfully produced products 16 and 17 with yields of 85% and 56%, respectively, from cycloalkenes and alcohols.
Scheme 2: Direct heteroarylation of C(sp3)–H catalyzed by amidyl radical under visible light.
Scheme 2: Direct heteroarylation of C(sp3)–H catalyzed by amidyl radical under visible light.
To eliminate the need for noble metal photocatalysts in the system, Duan’s group employed 2,4,5,6-tetra-9H-carbazol-9-yl-1,3-benzenedicarbonitrile (4CzIPN) as a metal-free photocatalyst (Scheme 3) [71]. This system initiated the formation of amidyl radical 20 from HRP-3 through a PCET process, involving the oxidation of excited 4CzIPN* and deprotonation by a base. The resulting amidyl radical 20 smoothly abstracted a hydrogen atom from the substrate via a HAT process, generating a radical 4. This C-centered radical subsequently underwent Giese addition with activated alkenes, resulting in the formation of radical 21. Radical 21 then oxidized the photocatalyst radical anion to its ground state while simultaneously generating anion 22. Ultimately, anion 22 yielded product 19 through protonation. This system demonstrated good applicability, achieving yields of 53% to 60% for products 23, 24, and 25.
Scheme 3: Alkylation of C(sp3)–H catalyzed by amidyl radical and metal-free photocatalyst under visible light.
Scheme 3: Alkylation of C(sp3)–H catalyzed by amidyl radical and metal-free photocatalyst under visible light....
To further investigate the scope of substrates, Selvakumar’s group employed HRP-4 in combination with 4CzIPN (Scheme 4) [72]. This system examined the applicability of Si–H and Ge–H bonds through a HAT process. As seen in previous strategies, HRP-4 was converted into amidyl radical 29 in the presence of 4CzIPN and a base via a PCET process. Radical 29 subsequently engaged in a HAT process with substrate 26, generating either a Si radical or a Ge radical 28. Following this, radical 28 underwent Giese addition with activated alkenes. The reduction of species 31 was efficiently promoted by the PC radical anion. The resulting anion 32 ultimately produced product 27 through protonation. This system demonstrated the significant HAT capability of amidyl radical 29, as evidenced by the synthesis of products 33, 34, and 35 with yields reaching 70% to 78%.
Scheme 4: Alkylation of C(sp3)–H, Si–H, and Ge–H catalyzed by amidyl radical under visible light.
Scheme 4: Alkylation of C(sp3)–H, Si–H, and Ge–H catalyzed by amidyl radical under visible light.
Amidyl radical from N–N bond cleavage
N-Amidopyridinium salts are known to undergo SET reduction, leading to the formation of amidyl radicals. Hong’s group has made significant advances in the cleavage of N–N bonds in recent years. Through SET reduction of N-amidopyridinium salts to generate amidyl radicals, Hong’s group has accomplished various remote functionalizations of C–H bonds via 1,5-hydrogen atom transfer processes [73-76].
In 2021, Hong’s group reported a HAT combined with a reverse hydrogen atom transfer (rHAT) system (Scheme 5) [77]. By utilizing anthraquinone (AQ) as the HAT photocatalyst, activated AQ was able to abstract a hydrogen atom from substrate 1. The addition of the corresponding radical 4 to HRP-5 facilitated the release of amidyl radical 36, which simultaneously produced the final product 35. Amidyl radical 36 was capable of abstracting hydrogen atoms from both substrate 1 and AQ–H. The HAT process between substrate 1 and amidyl radical 36 initiated a chain reaction pathway leading to the formation of product 35. Conversely, the rHAT process between amidyl radical 36 and AQ–H allowed for the regeneration of the photocatalyst and the completion of the catalytic cycle. Amidyl radical 36 played a central role in this photocatalytic system. This strategy demonstrated good chemical selectivity for the functionalization of pyridine and alkanes, resulting in 55% to 86% yields of products 38, 39, 40, and 41, respectively.
Scheme 5: Direct heteroarylation of C(sp3)–H catalyzed by synergistic promotion of amidyl radical and photocatalyst, under visible light.
Scheme 5: Direct heteroarylation of C(sp3)–H catalyzed by synergistic promotion of amidyl radical and photoca...
Amidyl radical from N–O bond cleavage
In 2022, Alexanian’s group demonstrated the homolytic cleavage of the N–O bond using N-(tert-butyl)-O-(1-phenylvinyl)-phenylhydroxyamide as a HAT reagent [78,79]. This compound was capable of initiating the formation of amidyl radicals through visible light activation. Although their controlled experiments showed that this method was effective, the use of heating conditions resulted in a higher yield of the corresponding products. This advancement prompted scientists to explore alternative pathways for generating amidyl radicals, as an alternative to the traditional SET reduction of the N–O bond [80-82]. The SET reduction is able to produce amidyl radicals and oxygen anions in the presence of photocatalysts activated by visible light. Two representative cases illustrating this approach were reported in 2023.
Building upon the experiments conducted by Alexanian’s group, Yan’s group extended the applicability of carborane as a HAT substrate (Scheme 6) [83]. Initially, under optimized conditions, HRP-6 was employed to generate amidyl radical 45, which subsequently participates in the HAT process with the carborane substrate. This process results in the formation of borone radical 47, accompanied by amide 46. The resultant radical 47 can be intercepted by species 43, simultaneously releasing radical 48 and product 44. Radical 48 reacts with HRP-6, leading to the regeneration of amidyl radical 45, the release of byproduct 49, and the initiation of a chain reaction pathway. Notably, this system could give rise to the formation of the highly applied value products 50, 51, and 52, with the 39% to 60% yields. The work by Yan demonstrated the HAT capabilities of amidyl radical 45 and significantly broadened the substrate scope of amidyl radical-enhanced photocatalytic systems.
Scheme 6: Direct B–H functionalization of icosahedral carboranes catalyzed by amidyl radical under visible light.
Scheme 6: Direct B–H functionalization of icosahedral carboranes catalyzed by amidyl radical under visible li...
The reduction of the N–O bond through traditional SET processes is effectively illustrated by N-(acyloxy)phthalimides [84]. These compounds preferentially undergo SET reduction, resulting in the cleavage of the N–O bond. Typically, this cleavage generates an amidyl anion and an O radical [5,85-88]. Conversely, it is also possible for the N–O bond to produce an amidyl radical alongside an O anion.
In 2023, Doyle’s group reported a novel system initiated by an off-cycle reductive quenching of the activated CF3-4CzIPN* species, leading to the generation of a ground state photocatalyst radical anion (Scheme 7) [89]. This radical anion subsequently underwent SET reduction of HRP-7, resulting in the liberation of amidyl radical 45. The amidyl radical 45 efficiently abstracted a hydrogen atom from substrate 1, yielding radical 4 and byproduct amide 46. Furthermore, the resultant radical 4 was oxidized by the excited photocatalyst, resulting in the concurrent generation of the carbon cation 55. This cation was subsequently trapped by a nucleophile, leading to the formation of product 54. This system demonstrated a broad applicability for the general nucleophilic amination of benzylic C–H bonds. The substrate's scope and selectivity were exemplified by the satisfactory yields of products 55–57, and 58, which achieved yields of 43–85%.
Scheme 7: Nucleophilic amination of C(sp3)–H enabled by amidyl radical under visible light.
Scheme 7: Nucleophilic amination of C(sp3)–H enabled by amidyl radical under visible light.
Inspired by these previous work, Yu’s group devised a new photocatalyzed system catalyzed by a newly designed photocatalyst Br-5CzBN. This robust strategy implements direct heteroarylation of C(sp3)–H and C(sp3)–H without the presence of strong bases, acids, or oxidants (Scheme 8) [90]. The reaction is initiated by SET reduction of HRP-8 via excited photocatalyst Br-5CzBN*, subsequently generating HAT reagent 45, O-anion 64, and Br-5CzBN+•. HAT reagent 45 engages a HAT event with the substrate, converting it into the byproduct 46 and generating a carbon-centered radical 62. Species 62 is trapped by heteroarene 60, leading to the formation of the intermediate 63. This intermediate 63 undergoes SET and proton transfer with the assistance of O-anion 64 and the Br-5CzBN+• radical cation, delivering the final product 61 and regenerating photocatalyst Br-5CzBN. HRP-8 functions as an oxidizing agent, facilitating the generation of a highly active HAT reagent, while the O-anion 64 serves as a base. This elucidates the rationale behind the self-sufficiency of this photocatalytic system, obviating the need for supplementary oxidants and bases, thereby enabling broad substrate adaptability. This streamlined approach demonstrates significant potential for extended utility in pharmaceutical late-stage functionalization (LSF), particularly evidenced by the synthetically valuable yields (60–80%) obtained for structurally diversified target molecules 65–68 under optimized reaction conditions. In these cases, the high polarity of the radical intermediates of CF3CH2OH and 4-methylbenzonitrile, combined with the poor solubility of adamantane, may explain the possible reasons behind the reaction process [91].
Scheme 8: Direct heteroarylation of C(sp3)–H and C(sp3)–H without the presence of strong bases, acids, or oxidants.
Scheme 8: Direct heteroarylation of C(sp3)–H and C(sp3)–H without the presence of strong bases, acids, or oxi...
Amidyl radical from N–S bond cleavage
The work of Alexanian’s group has significantly advanced the field of organic synthesis over recent years, particularly in the area of N–S bond homolytic cleavage [92-94]. Initial studies demonstrated that high-temperature conditions were required to facilitate this reaction; however, such extreme conditions limited the practical applicability of the reactions. To address this limitation, Alexanian's research has shifted toward the principles of green chemistry, utilizing visible light to achieve mild reaction conditions. This approach has not only enhanced the feasibility of the reactions but has also led to the establishment of a comprehensive platform for C–H functionalization through the introduction of the highly versatile xanthyl functional group.
In 2016, Alexanian’s group successfully implemented a method for the direct xanthylation of C–H bonds with high selectivity and efficiency (Scheme 9) [95]. This process is initiated through a visible light-triggered chain reaction, involving the homolytic cleavage of HRP-9. The liberation of amidyl radical 45 facilitates hydrogen atom abstraction from the substrate, resulting in the formation of radical 4 and the concurrent generation of byproduct 46. Subsequent trapping of radical 4 by HRP-9 leads to the generation of product 69. This methodology demonstrates a broad substrate scope and exhibits significant synthetic utility, particularly for the generation of products 70, 71, and 72 with yields ranging from 54% to 59%.
Scheme 9: Xanthylation of C(sp3)–H addressed by amidyl radical under visible light.
Scheme 9: Xanthylation of C(sp3)–H addressed by amidyl radical under visible light.
In an effort to evaluate the practicality of visible light-promoted xanthylation, Alexanian’s group conducted a regioselective C–H xanthylation of polyolefins (Scheme 10) [96]. Building on previous findings, the HRP-9 undergoes homolytic N–S cleavage, yielding amidyl radical 45. This radical effectively abstracts a hydrogen atom from the polyolefin substrate, demonstrating notable regioselectivity. The xanthylation reaction preferentially generates main products 76, and byproducts (77 and 78). Furthermore, the successful implementation of this methodology facilitates the production of a diverse range of functionalized polyolefins, showcasing the applicability of this xanthylated polyolefin in various reactions, including trifluoromethylthiolation, polymer grafting, Michael addition, and epoxide opening.
Scheme 10: Xanthylation of C(sp3)–H in polyolefins addressed by amidyl radical under visible light.
Scheme 10: Xanthylation of C(sp3)–H in polyolefins addressed by amidyl radical under visible light.
Amidyl radical from N–X bond cleavage
Direct halogenation of C–H bonds is of significant value in organic synthesis. Introducing a bromine or chlorine atom into aliphatic C–H bonds with high site selectivity and efficiency poses a formidable challenge. Traditional strategies for the halogenation of aliphatic C–H bonds typically rely on biomimetic iron-catalyzed oxidation systems that require electrophilic heterocycles. These limitations hinder the broader application of such systems.
Alexanian’s group modified amidyl radical precursors by incorporating halogen atoms, transforming them into bifunctional reagents. The HAT component of amidyl radical precursors was facilitated by amidyl radicals, while halogenation was promoted by the introduced halogen atom [26].
In 2014, Alexanian’s group reported a site-selective aliphatic C–H bromination utilizing modified HRP as both the bromination reagent and HAT reagent (Scheme 11) [25]. Initiated by visible light, HRP-10 underwent homolytic cleavage of the N–Br bond, generating amidyl radical 45. This amidyl radical subsequently participated in a HAT process with the aliphatic substrate 1, leading to the formation of radical 4 and byproduct amide 46. The corresponding radical 4 was then trapped by HRP-10, thereby triggering a chain reaction that regenerated amidyl radical 45. This system effectively examined the site selectivity of aliphatic C–H bromination, yielding products 80–82, and 83 with 54% to 63% yields at selective positions. This bromination system provided a mild reaction environment suitable for aliphatic C–H bonds.
Scheme 11: Site-selective C(sp3)–H bromination implemented by amidyl radical under visible light.
Scheme 11: Site-selective C(sp3)–H bromination implemented by amidyl radical under visible light.
In 2016, Alexanian’s group reported a chlorination method for aliphatic C–H bonds with high site selectivity to expand the halogenation capabilities and applicability of their system (Scheme 12) [97]. Consistent with previous experiments, the reaction was initiated by visible light, generating amidyl radical 45 from HRP-11. The resulting radical 45 abstracted a hydrogen atom from substrate 1, simultaneously generating radical 4. Subsequently, radical 4 was trapped by HRP-11, leading to the formation of chlorinated product 84. In this system, monochlorinated products were obtained with good selectivity, evidenced by yields of 69% and 54% for products 85, and 86, respectively. Notably, the natural product sclareolide underwent chlorination with an impressive selectivity, achieving an 82% yield for the product 87.
Scheme 12: Site-selective chlorination of C(sp3)–H in natural products implemented by amidyl radical under visible light.
Scheme 12: Site-selective chlorination of C(sp3)–H in natural products implemented by amidyl radical under vis...
Amidyl radical from amide anion
In 2024, Ooi and colleagues established a pivotal advancement in catalytic methodology through the rational design of zwitterionic acridinium amidates. These photoactive amidyl radical precursors demonstrated exceptional HAT reactivity, enabling efficient functionalization of unactivated C–H bonds under mild irradiation conditions (Scheme 13) [98]. The mechanistic pathway initiates with ground-state complexation 90 between HRP-12 and HFIP via hydrogen bonding. Following visible-light excitation of 90, intersystem crossing (ISC) from the S1(LE) to T2 state generates the catalytically competent triplet excited state 91. This N-centered radical species subsequently abstracts a hydrogen atom from substrate 1 through HAT, producing an α-amido-acridinyl radical intermediate 92 and a substrate-derived carbon-centered radical 4. Radical 4 undergoes regioselective addition to the acceptor, forming transient radical adduct 93. A concomitant SET from 92 to 93 generates a carbanion, which undergoes either solvent-mediated protonation or direct proton transfer from the acridiniumamide, ultimately delivering product 89 while regenerating the zwitterionic HRP-12 catalyst.
Scheme 13: Alkylation of C(sp3)–H catalyzed by amidyl radical photocatalyst under visible light.
Scheme 13: Alkylation of C(sp3)–H catalyzed by amidyl radical photocatalyst under visible light.
This catalytic platform demonstrated exceptional site selectivity in aliphatic C–H bromination under ambient temperature and visible-light irradiation, achieving site-selective bromination (products 94–96) in 51–99% yields across electronically differentiated positions. The system's operational mildness and functional group tolerance highlight its suitability for late-stage functionalization of complex aliphatic architectures.
Conclusion
In this review, we highlight recent advances in the use of visible light to enhance amidyl radical-mediated direct intermolecular HAT for the functionalization of C–H, Si–H, Ge–H, and B–H bonds. These robust strategies hold the promise of the direct functionalization of these bonds through high selectivity, efficiency, and a stepwise approach.
In the presence of amidyl radicals, hydrogen atoms are directly abstracted from C–H, Si–H, Ge–H, and B–H bonds, leading to the formation of corresponding radicals. We summarize and emphasize notable pioneering experiments in this area. Switchable amidyl radicals provide an effective toolkit for completing hydrogen atom transfer processes. Transitioning from noble metal photocatalysts to organic photocatalysts and from HAT reagents to bifunctional reagents, these remarkable photocatalytic systems have inspired innovations across various fields of organic synthesis methodology.
Despite significant research achievements of amidyl radicals in the photocatalytic transformation of C(sp³)–H, C(sp²)–H, S–H, Ge–H, and B–H bonds, they still face considerable challenges in the application of hydrogen abstraction from electron-deficient C–H bonds due to their inherent polarity. Furthermore, amidyl radicals encounter difficulties in regioselectivity when applied to structurally complex C–H substrates, which limits their utility in modifying intricate molecular architectures. Future advancements are anticipated through structural modifications of amidyl radicals aimed at optimizing their polarity via electronic effects, which may enhance their effectiveness in hydrogen abstraction from electron-deficient substrates. Additionally, strategies to optimize steric effects could improve their regioselectivity in hydrogen abstraction from complex substrates.
Crucially, structural optimization of HRP components could potentially overcome current mechanistic limitations, establishing a generalized platform for hydrogen atom transfer (HAT)-enabled direct functionalization. This advanced methodology would demonstrate unprecedented versatility across diverse bond activation challenges, particularly in C(sp³)–H, C(sp²)–H, S–H, Ge–H, and B–H bond transformations. The proposed system architecture emphasizes synergistic reagent cooperation rather than isolated component performance, representing a paradigm shift in photoredox catalysis design principles.
Funding
We acknowledge the financial support from the National Natural Science Foundation of China (21971224 and 22171249), the Science & Technology Innovation Talents in Universities of Henan Province (23HASTIT003), Science and Technology Research and Development Plan Joint Fund of Henan Province (242301420006).
Data Availability Statement
Data sharing is not applicable as no new data was generated or analyzed in this study.
References
-
Pratley, C.; Fenner, S.; Murphy, J. A. Chem. Rev. 2022, 122, 8181–8260. doi:10.1021/acs.chemrev.1c00831
Return to citation in text: [1] -
Xiong, T.; Zhang, Q. Chem. Soc. Rev. 2016, 45, 3069–3087. doi:10.1039/c5cs00852b
Return to citation in text: [1] -
Yu, X.-Y.; Chen, J.-R.; Xiao, W.-J. Chem. Rev. 2021, 121, 506–561. doi:10.1021/acs.chemrev.0c00030
Return to citation in text: [1] -
Zhang, J.; Huan, X.-D.; Wang, X.; Li, G.-Q.; Xiao, W.-J.; Chen, J.-R. Chem. Commun. 2024, 60, 6340–6361. doi:10.1039/d4cc01969e
Return to citation in text: [1] -
Guo, Y.; Lin, G.; Zhang, M.; Xu, J.; Song, Q. Nat. Commun. 2024, 15, 7313. doi:10.1038/s41467-024-51334-5
Return to citation in text: [1] [2] -
Hartwig, J. F. J. Am. Chem. Soc. 2016, 138, 2–24. doi:10.1021/jacs.5b08707
Return to citation in text: [1] -
Liu, B.; Romine, A. M.; Rubel, C. Z.; Engle, K. M.; Shi, B.-F. Chem. Rev. 2021, 121, 14957–15074. doi:10.1021/acs.chemrev.1c00519
Return to citation in text: [1] -
Mishra, A. A.; Subhedar, D.; Bhanage, B. M. Chem. Rec. 2019, 19, 1829–1857. doi:10.1002/tcr.201800093
Return to citation in text: [1] -
He, C.; Whitehurst, W. G.; Gaunt, M. J. Chem 2019, 5, 1031–1058. doi:10.1016/j.chempr.2018.12.017
Return to citation in text: [1] -
Zhang, Y.; Qi, Z.-H.; Ruan, G.-Y.; Zhang, Y.; Liu, W.; Wang, Y. RSC Adv. 2015, 5, 71586–71592. doi:10.1039/c5ra11488h
Return to citation in text: [1] -
Wu, X.; Zhu, C. CCS Chem. 2020, 2, 813–828. doi:10.31635/ccschem.020.202000234
Return to citation in text: [1] -
Maity, B.; Dutta, S.; Cavallo, L. Chem. Soc. Rev. 2023, 52, 5373–5387. doi:10.1039/d2cs00960a
Return to citation in text: [1] -
Wang, X.; He, J.; Wang, Y.-N.; Zhao, Z.; Jiang, K.; Yang, W.; Zhang, T.; Jia, S.; Zhong, K.; Niu, L.; Lan, Y. Chem. Rev. 2024, 124, 10192–10280. doi:10.1021/acs.chemrev.4c00188
Return to citation in text: [1] -
Hu, X.; Cheng-Sánchez, I.; Kong, W.; Molander, G. A.; Nevado, C. Nat. Catal. 2024, 7, 655–665. doi:10.1038/s41929-024-01153-0
Return to citation in text: [1] -
Li, Q.-Y.; Cheng, S.; Ye, Z.; Huang, T.; Yang, F.; Lin, Y.-M.; Gong, L. Nat. Commun. 2023, 14, 6366. doi:10.1038/s41467-023-42191-9
Return to citation in text: [1] -
Gong, L. Nat. Synth. 2022, 1, 915–916. doi:10.1038/s44160-022-00174-6
Return to citation in text: [1] -
Li, B.; Qin, H.; Yan, K.; Ma, J.; Yang, J.; Wen, J. Org. Chem. Front. 2022, 9, 6861–6868. doi:10.1039/d2qo01498j
Return to citation in text: [1] -
Yan, T.; Yang, J.; Yan, K.; Wang, Z.; Li, B.; Wen, J. Angew. Chem., Int. Ed. 2024, 63, e202405186. doi:10.1002/anie.202405186
Return to citation in text: [1] -
Kawasaki, T.; Ishida, N.; Murakami, M. J. Am. Chem. Soc. 2020, 142, 3366–3370. doi:10.1021/jacs.9b13920
Return to citation in text: [1] -
Ishida, N.; Masuda, Y.; Imamura, Y.; Yamazaki, K.; Murakami, M. J. Am. Chem. Soc. 2019, 141, 19611–19615. doi:10.1021/jacs.9b12529
Return to citation in text: [1] -
Wang, Z.; Ji, X.; Han, T.; Deng, G.-J.; Huang, H. Adv. Synth. Catal. 2019, 361, 5643–5647. doi:10.1002/adsc.201901168
Return to citation in text: [1] -
Treacy, S. M.; Rovis, T. J. Am. Chem. Soc. 2021, 143, 2729–2735. doi:10.1021/jacs.1c00687
Return to citation in text: [1] -
Tu, J.-L.; Hu, A.-M.; Guo, L.; Xia, W. J. Am. Chem. Soc. 2023, 145, 7600–7611. doi:10.1021/jacs.3c01082
Return to citation in text: [1] -
Shen, Y.; Gu, Y.; Martin, R. J. Am. Chem. Soc. 2018, 140, 12200–12209. doi:10.1021/jacs.8b07405
Return to citation in text: [1] -
Schmidt, V. A.; Quinn, R. K.; Brusoe, A. T.; Alexanian, E. J. J. Am. Chem. Soc. 2014, 136, 14389–14392. doi:10.1021/ja508469u
Return to citation in text: [1] [2] -
Carestia, A. M.; Ravelli, D.; Alexanian, E. J. Chem. Sci. 2018, 9, 5360–5365. doi:10.1039/c8sc01756e
Return to citation in text: [1] [2] -
Mao, R.; Bera, S.; Turla, A. C.; Hu, X. J. Am. Chem. Soc. 2021, 143, 14667–14675. doi:10.1021/jacs.1c05874
Return to citation in text: [1] -
Huang, C.; Qin, Y.-S.; Wang, C.-L.; Xiao, P.; Tang, S.; Liu, H.-J.; Wei, Z.; Cai, H. Chem. Commun. 2024, 60, 2669–2672. doi:10.1039/d3cc06210d
Return to citation in text: [1] -
Sonawane, S. C.; Gourkhede, R.; Saini, P.; Ramakrishnan, S.; Balakrishna, M. S. Chem. Commun. 2024, 60, 6055–6058. doi:10.1039/d4cc01119h
Return to citation in text: [1] -
Sharma, A. K.; Maseras, F. Inorg. Chem. 2024, 63, 13801–13806. doi:10.1021/acs.inorgchem.4c01763
Return to citation in text: [1] -
Roberts, B. P. Chem. Soc. Rev. 1999, 28, 25–35. doi:10.1039/a804291h
Return to citation in text: [1] -
Cao, J.; Wang, G.; Gao, L.; Cheng, X.; Li, S. Chem. Sci. 2018, 9, 3664–3671. doi:10.1039/c7sc05225a
Return to citation in text: [1] -
Bell, J. D.; Murphy, J. A. Chem. Soc. Rev. 2021, 50, 9540–9685. doi:10.1039/d1cs00311a
Return to citation in text: [1] -
Zhang, Y.; Li, K.-D.; Zhou, C.-Q.; Xing, Z.-X.; Huang, H.-M. Green Chem. 2024, 26, 10434–10440. doi:10.1039/d4gc02879a
Return to citation in text: [1] -
Pannwitz, A.; Wenger, O. S. Chem. Commun. 2019, 55, 4004–4014. doi:10.1039/c9cc00821g
Return to citation in text: [1] -
Latrache, M.; Hoffmann, N. Chem. Soc. Rev. 2021, 50, 7418–7435. doi:10.1039/d1cs00196e
Return to citation in text: [1] -
Zhou, R.; Li, J.; Cheo, H. W.; Chua, R.; Zhan, G.; Hou, Z.; Wu, J. Chem. Sci. 2019, 10, 7340–7344. doi:10.1039/c9sc02818h
Return to citation in text: [1] -
Su, Y.; Li, Y.; Ganguly, R.; Kinjo, R. Chem. Sci. 2017, 8, 7419–7423. doi:10.1039/c7sc03528d
Return to citation in text: [1] -
Zhu, X.; Li, P.; Shi, Q.; Wang, L. Green Chem. 2016, 18, 6373–6379. doi:10.1039/c6gc01487a
Return to citation in text: [1] -
Tierney, M. M.; Crespi, S.; Ravelli, D.; Alexanian, E. J. J. Org. Chem. 2019, 84, 12983–12991. doi:10.1021/acs.joc.9b01774
Return to citation in text: [1] -
Nanjo, T.; Matsumoto, A.; Oshita, T.; Takemoto, Y. J. Am. Chem. Soc. 2023, 145, 19067–19075. doi:10.1021/jacs.3c06931
Return to citation in text: [1] -
Lee, H.; He, T.; Cook, S. P. Org. Lett. 2023, 25, 1–4. doi:10.1021/acs.orglett.2c02864
Return to citation in text: [1] -
Gonzalez, M. I.; Gygi, D.; Qin, Y.; Zhu, Q.; Johnson, E. J.; Chen, Y.-S.; Nocera, D. G. J. Am. Chem. Soc. 2022, 144, 1464–1472. doi:10.1021/jacs.1c13333
Return to citation in text: [1] -
Li, N.; Li, J.; Qin, M.; Li, J.; Han, J.; Zhu, C.; Li, W.; Xie, J. Nat. Commun. 2022, 13, 4224. doi:10.1038/s41467-022-31956-3
Return to citation in text: [1] -
Wu, Y.; Liu, Z.; Li, Y.; Chen, J.; Zhu, X.; Na, P. Chin. J. Catal. 2019, 40, 60–69. doi:10.1016/s1872-2067(18)63170-5
Return to citation in text: [1] -
Liu, D.; Chen, S.; Zhang, Y.; Li, R.; Peng, T. Appl. Catal., B 2023, 333, 122805. doi:10.1016/j.apcatb.2023.122805
Return to citation in text: [1] -
Wei, Y.; Shahid, M. Z.; Lyu, S.; Sun, W.; Lyu, S. RSC Adv. 2021, 11, 22618–22624. doi:10.1039/d1ra02958d
Return to citation in text: [1] -
Ouyang, W.-T.; Jiang, J.; Jiang, Y.-F.; Li, T.; Liu, Y.-Y.; Ji, H.-T.; Ou, L.-J.; He, W.-M. Chin. Chem. Lett. 2024, 35, 110038. doi:10.1016/j.cclet.2024.110038
Return to citation in text: [1] -
Xu, Y.-D.; Xing, Y.-M.; Ji, H.-T.; Ou, L.-J.; He, W.-B.; Peng, J.; Wang, J.-S.; Jiang, J.; He, W.-M. J. Org. Chem. 2024, 89, 17701–17707. doi:10.1021/acs.joc.4c02445
Return to citation in text: [1] -
Hou, J.-C.; Jiang, J.; Dai, H.; Wang, J.-S.; Li, T.; Chen, X.; He, W.-M. Sci. China: Chem. 2025, 68, 1945–1951. doi:10.1007/s11426-024-2496-5
Return to citation in text: [1] -
Zhang, Q.; Zhao, Q.; Wu, X.; Wang, L.; Shen, K.; Hua, Y.; Gao, C.; Zhang, Y.; Peng, M.; Zhao, K. Chin. Chem. Lett. 2025, 36, 110167. doi:10.1016/j.cclet.2024.110167
Return to citation in text: [1] -
Guo, J.-J.; Hu, A.; Zuo, Z. Tetrahedron Lett. 2018, 59, 2103–2111. doi:10.1016/j.tetlet.2018.04.060
Return to citation in text: [1] -
Chen, H.; Yu, S. Org. Biomol. Chem. 2020, 18, 4519–4532. doi:10.1039/d0ob00854k
Return to citation in text: [1] -
Davies, J.; Morcillo, S. P.; Douglas, J. J.; Leonori, D. Chem. – Eur. J. 2018, 24, 12154–12163. doi:10.1002/chem.201801655
Return to citation in text: [1] -
Gao, S.; Li, F. Adv. Funct. Mater. 2023, 33, 2304291. doi:10.1002/adfm.202304291
Return to citation in text: [1] -
Lasso, J. D.; Castillo-Pazos, D. J.; Li, C.-J. Chem. Soc. Rev. 2021, 50, 10955–10982. doi:10.1039/d1cs00380a
Return to citation in text: [1] -
Liang, Y.-F.; Bilal, M.; Tang, L.-Y.; Wang, T.-Z.; Guan, Y.-Q.; Cheng, Z.; Zhu, M.; Wei, J.; Jiao, N. Chem. Rev. 2023, 123, 12313–12370. doi:10.1021/acs.chemrev.3c00219
Return to citation in text: [1] -
Bellotti, P.; Huang, H.-M.; Faber, T.; Glorius, F. Chem. Rev. 2023, 123, 4237–4352. doi:10.1021/acs.chemrev.2c00478
Return to citation in text: [1] -
Choi, G. J.; Zhu, Q.; Miller, D. C.; Gu, C. J.; Knowles, R. R. Nature 2016, 539, 268–271. doi:10.1038/nature19811
Return to citation in text: [1] [2] -
Chu, J. C. K.; Rovis, T. Nature 2016, 539, 272–275. doi:10.1038/nature19810
Return to citation in text: [1] -
Li, W.; Sun, B.; Zhang, L.; Mo, F. Green Chem. 2023, 25, 5030–5034. doi:10.1039/d3gc01426f
Return to citation in text: [1] -
Wang, C.; Chen, Z.; Sun, J.; Tong, L.; Wang, W.; Song, S.; Li, J. Nat. Commun. 2024, 15, 5087. doi:10.1038/s41467-024-49337-3
Return to citation in text: [1] -
Stateman, L. M.; Dare, R. M.; Paneque, A. N.; Nagib, D. A. Chem 2022, 8, 210–224. doi:10.1016/j.chempr.2021.10.022
Return to citation in text: [1] -
Ding, W.-W.; Zhou, Y.; Song, S.; Han, Z.-Y. Org. Lett. 2022, 24, 7350–7354. doi:10.1021/acs.orglett.2c02877
Return to citation in text: [1] -
Deng, Z.; Zhao, Z.; He, G.; Chen, G. Org. Lett. 2021, 23, 3631–3635. doi:10.1021/acs.orglett.1c01020
Return to citation in text: [1] -
Shu, W.; Zhang, H.; Huang, Y. Org. Lett. 2019, 21, 6107–6111. doi:10.1021/acs.orglett.9b02255
Return to citation in text: [1] -
Herron, A. N.; Hsu, C.-P.; Yu, J.-Q. Org. Lett. 2022, 24, 3652–3656. doi:10.1021/acs.orglett.2c01261
Return to citation in text: [1] -
Chen, Z.; Zhu, W.; Wang, C.; Xu, N.; Jin, Q.; Huang, X.; Song, S.; Li, J. Org. Chem. Front. 2023, 10, 4709–4717. doi:10.1039/d3qo00931a
Return to citation in text: [1] -
Fu, X.; Tian, J.; Zhang, M.; Jing, Y.; Liu, Y.; Song, H.; Wang, Q. Adv. Sci. 2025, 12, 2411744. doi:10.1002/advs.202411744
Return to citation in text: [1] -
Tanaka, H.; Sakai, K.; Kawamura, A.; Oisaki, K.; Kanai, M. Chem. Commun. 2018, 54, 3215–3218. doi:10.1039/c7cc09457d
Return to citation in text: [1] -
Ma, Z.-Y.; Li, M.; Guo, L.-N.; Liu, L.; Wang, D.; Duan, X.-H. Org. Lett. 2021, 23, 474–479. doi:10.1021/acs.orglett.0c03992
Return to citation in text: [1] -
Ram Bajya, K.; Kumar, M.; Ansari, A.; Selvakumar, S. Adv. Synth. Catal. 2023, 365, 976–982. doi:10.1002/adsc.202300040
Return to citation in text: [1] -
Kim, J.; Kim, Y.-E.; Hong, S. Angew. Chem., Int. Ed. 2024, 63, e202409561. doi:10.1002/anie.202409561
Return to citation in text: [1] -
Jung, S.; Lee, H.; Moon, Y.; Jung, H.-Y.; Hong, S. ACS Catal. 2019, 9, 9891–9896. doi:10.1021/acscatal.9b03367
Return to citation in text: [1] -
Shin, S.; Lee, S.; Choi, W.; Kim, N.; Hong, S. Angew. Chem., Int. Ed. 2021, 60, 7873–7879. doi:10.1002/anie.202016156
Return to citation in text: [1] -
Vellakkaran, M.; Kim, T.; Hong, S. Angew. Chem., Int. Ed. 2022, 61, e202113658. doi:10.1002/anie.202113658
Return to citation in text: [1] -
Lee, W.; Jung, S.; Kim, M.; Hong, S. J. Am. Chem. Soc. 2021, 143, 3003–3012. doi:10.1021/jacs.1c00549
Return to citation in text: [1] -
Fazekas, T. J.; Alty, J. W.; Neidhart, E. K.; Miller, A. S.; Leibfarth, F. A.; Alexanian, E. J. Science 2022, 375, 545–550. doi:10.1126/science.abh4308
Return to citation in text: [1] -
Liang, D.; Chen, J.-R.; Tan, L.-P.; He, Z.-W.; Xiao, W.-J. J. Am. Chem. Soc. 2022, 144, 6040–6049. doi:10.1021/jacs.2c01116
Return to citation in text: [1] -
Zhang, B.; Erb, F. R.; Vasilopoulos, A.; Voight, E. A.; Alexanian, E. J. J. Am. Chem. Soc. 2023, 145, 26540–26544. doi:10.1021/jacs.3c10751
Return to citation in text: [1] -
Miller, A. S.; Alexanian, E. J. Chem. Sci. 2022, 13, 11878–11882. doi:10.1039/d2sc04605a
Return to citation in text: [1] -
Lyu, X.-L.; Huang, S.-S.; Song, H.-J.; Liu, Y.-X.; Wang, Q.-M. Org. Lett. 2019, 21, 5728–5732. doi:10.1021/acs.orglett.9b02105
Return to citation in text: [1] -
Ren, H.; Zhang, P.; Xu, J.; Ma, W.; Tu, D.; Lu, C.-s.; Yan, H. J. Am. Chem. Soc. 2023, 145, 7638–7647. doi:10.1021/jacs.3c01314
Return to citation in text: [1] -
Fu, M.-C.; Shang, R.; Zhao, B.; Wang, B.; Fu, Y. Science 2019, 363, 1429–1434. doi:10.1126/science.aav3200
Return to citation in text: [1] -
Qi, X.-K.; Zheng, M.-J.; Yang, C.; Zhao, Y.; Guo, L.; Xia, W. J. Am. Chem. Soc. 2023, 145, 16630–16641. doi:10.1021/jacs.3c04073
Return to citation in text: [1] -
Feng, L.; Guo, L.; Yang, C.; Zhou, J.; Xia, W. Org. Lett. 2020, 22, 3964–3968. doi:10.1021/acs.orglett.0c01267
Return to citation in text: [1] -
Zhu, Y.; Gao, H.; Tu, J.-L.; Yang, C.; Guo, L.; Zhao, Y.; Xia, W. Org. Chem. Front. 2024, 11, 1729–1735. doi:10.1039/d3qo01822a
Return to citation in text: [1] -
Mu, S.; Guo, Y.; Huang, X.; Luo, Y.; Chen, M.; Xu, J.; Song, Q. Org. Chem. Front. 2023, 10, 3259–3263. doi:10.1039/d3qo00537b
Return to citation in text: [1] -
Ruos, M. E.; Kinney, R. G.; Ring, O. T.; Doyle, A. G. J. Am. Chem. Soc. 2023, 145, 18487–18496. doi:10.1021/jacs.3c04912
Return to citation in text: [1] -
Wang, H.-S.; Li, H.-C.; Yuan, X.-Y.; Sun, K.; Chen, X.-L.; Qu, L.; Yu, B. Green Chem. 2025, 27, 4655–4663. doi:10.1039/d4gc06209d
Return to citation in text: [1] -
Garwood, J. J. A.; Chen, A. D.; Nagib, D. A. J. Am. Chem. Soc. 2024, 146, 28034–28059. doi:10.1021/jacs.4c06774
Return to citation in text: [1] -
Na, C. G.; Ravelli, D.; Alexanian, E. J. J. Am. Chem. Soc. 2020, 142, 44–49. doi:10.1021/jacs.9b10825
Return to citation in text: [1] -
Williamson, J. B.; Na, C. G.; Johnson, R. R., III; Daniel, W. F. M.; Alexanian, E. J.; Leibfarth, F. A. J. Am. Chem. Soc. 2019, 141, 12815–12823. doi:10.1021/jacs.9b05799
Return to citation in text: [1] -
Margrey, K. A.; Czaplyski, W. L.; Nicewicz, D. A.; Alexanian, E. J. J. Am. Chem. Soc. 2018, 140, 4213–4217. doi:10.1021/jacs.8b00592
Return to citation in text: [1] -
Czaplyski, W. L.; Na, C. G.; Alexanian, E. J. J. Am. Chem. Soc. 2016, 138, 13854–13857. doi:10.1021/jacs.6b09414
Return to citation in text: [1] -
Williamson, J. B.; Czaplyski, W. L.; Alexanian, E. J.; Leibfarth, F. A. Angew. Chem., Int. Ed. 2018, 57, 6261–6265. doi:10.1002/anie.201803020
Return to citation in text: [1] -
Quinn, R. K.; Könst, Z. A.; Michalak, S. E.; Schmidt, Y.; Szklarski, A. R.; Flores, A. R.; Nam, S.; Horne, D. A.; Vanderwal, C. D.; Alexanian, E. J. J. Am. Chem. Soc. 2016, 138, 696–702. doi:10.1021/jacs.5b12308
Return to citation in text: [1] -
Entgelmeier, L.-M.; Mori, S.; Sendo, S.; Yamaguchi, R.; Suzuki, R.; Yanai, T.; García Mancheño, O.; Ohmatsu, K.; Ooi, T. Angew. Chem., Int. Ed. 2024, 63, e202404890. doi:10.1002/anie.202404890
Return to citation in text: [1]
| 95. | Czaplyski, W. L.; Na, C. G.; Alexanian, E. J. J. Am. Chem. Soc. 2016, 138, 13854–13857. doi:10.1021/jacs.6b09414 |
| 96. | Williamson, J. B.; Czaplyski, W. L.; Alexanian, E. J.; Leibfarth, F. A. Angew. Chem., Int. Ed. 2018, 57, 6261–6265. doi:10.1002/anie.201803020 |
| 26. | Carestia, A. M.; Ravelli, D.; Alexanian, E. J. Chem. Sci. 2018, 9, 5360–5365. doi:10.1039/c8sc01756e |
| 1. | Pratley, C.; Fenner, S.; Murphy, J. A. Chem. Rev. 2022, 122, 8181–8260. doi:10.1021/acs.chemrev.1c00831 |
| 2. | Xiong, T.; Zhang, Q. Chem. Soc. Rev. 2016, 45, 3069–3087. doi:10.1039/c5cs00852b |
| 3. | Yu, X.-Y.; Chen, J.-R.; Xiao, W.-J. Chem. Rev. 2021, 121, 506–561. doi:10.1021/acs.chemrev.0c00030 |
| 4. | Zhang, J.; Huan, X.-D.; Wang, X.; Li, G.-Q.; Xiao, W.-J.; Chen, J.-R. Chem. Commun. 2024, 60, 6340–6361. doi:10.1039/d4cc01969e |
| 5. | Guo, Y.; Lin, G.; Zhang, M.; Xu, J.; Song, Q. Nat. Commun. 2024, 15, 7313. doi:10.1038/s41467-024-51334-5 |
| 19. | Kawasaki, T.; Ishida, N.; Murakami, M. J. Am. Chem. Soc. 2020, 142, 3366–3370. doi:10.1021/jacs.9b13920 |
| 20. | Ishida, N.; Masuda, Y.; Imamura, Y.; Yamazaki, K.; Murakami, M. J. Am. Chem. Soc. 2019, 141, 19611–19615. doi:10.1021/jacs.9b12529 |
| 21. | Wang, Z.; Ji, X.; Han, T.; Deng, G.-J.; Huang, H. Adv. Synth. Catal. 2019, 361, 5643–5647. doi:10.1002/adsc.201901168 |
| 22. | Treacy, S. M.; Rovis, T. J. Am. Chem. Soc. 2021, 143, 2729–2735. doi:10.1021/jacs.1c00687 |
| 23. | Tu, J.-L.; Hu, A.-M.; Guo, L.; Xia, W. J. Am. Chem. Soc. 2023, 145, 7600–7611. doi:10.1021/jacs.3c01082 |
| 24. | Shen, Y.; Gu, Y.; Martin, R. J. Am. Chem. Soc. 2018, 140, 12200–12209. doi:10.1021/jacs.8b07405 |
| 25. | Schmidt, V. A.; Quinn, R. K.; Brusoe, A. T.; Alexanian, E. J. J. Am. Chem. Soc. 2014, 136, 14389–14392. doi:10.1021/ja508469u |
| 26. | Carestia, A. M.; Ravelli, D.; Alexanian, E. J. Chem. Sci. 2018, 9, 5360–5365. doi:10.1039/c8sc01756e |
| 27. | Mao, R.; Bera, S.; Turla, A. C.; Hu, X. J. Am. Chem. Soc. 2021, 143, 14667–14675. doi:10.1021/jacs.1c05874 |
| 71. | Ma, Z.-Y.; Li, M.; Guo, L.-N.; Liu, L.; Wang, D.; Duan, X.-H. Org. Lett. 2021, 23, 474–479. doi:10.1021/acs.orglett.0c03992 |
| 11. | Wu, X.; Zhu, C. CCS Chem. 2020, 2, 813–828. doi:10.31635/ccschem.020.202000234 |
| 12. | Maity, B.; Dutta, S.; Cavallo, L. Chem. Soc. Rev. 2023, 52, 5373–5387. doi:10.1039/d2cs00960a |
| 13. | Wang, X.; He, J.; Wang, Y.-N.; Zhao, Z.; Jiang, K.; Yang, W.; Zhang, T.; Jia, S.; Zhong, K.; Niu, L.; Lan, Y. Chem. Rev. 2024, 124, 10192–10280. doi:10.1021/acs.chemrev.4c00188 |
| 14. | Hu, X.; Cheng-Sánchez, I.; Kong, W.; Molander, G. A.; Nevado, C. Nat. Catal. 2024, 7, 655–665. doi:10.1038/s41929-024-01153-0 |
| 15. | Li, Q.-Y.; Cheng, S.; Ye, Z.; Huang, T.; Yang, F.; Lin, Y.-M.; Gong, L. Nat. Commun. 2023, 14, 6366. doi:10.1038/s41467-023-42191-9 |
| 16. | Gong, L. Nat. Synth. 2022, 1, 915–916. doi:10.1038/s44160-022-00174-6 |
| 17. | Li, B.; Qin, H.; Yan, K.; Ma, J.; Yang, J.; Wen, J. Org. Chem. Front. 2022, 9, 6861–6868. doi:10.1039/d2qo01498j |
| 18. | Yan, T.; Yang, J.; Yan, K.; Wang, Z.; Li, B.; Wen, J. Angew. Chem., Int. Ed. 2024, 63, e202405186. doi:10.1002/anie.202405186 |
| 72. | Ram Bajya, K.; Kumar, M.; Ansari, A.; Selvakumar, S. Adv. Synth. Catal. 2023, 365, 976–982. doi:10.1002/adsc.202300040 |
| 9. | He, C.; Whitehurst, W. G.; Gaunt, M. J. Chem 2019, 5, 1031–1058. doi:10.1016/j.chempr.2018.12.017 |
| 10. | Zhang, Y.; Qi, Z.-H.; Ruan, G.-Y.; Zhang, Y.; Liu, W.; Wang, Y. RSC Adv. 2015, 5, 71586–71592. doi:10.1039/c5ra11488h |
| 59. | Choi, G. J.; Zhu, Q.; Miller, D. C.; Gu, C. J.; Knowles, R. R. Nature 2016, 539, 268–271. doi:10.1038/nature19811 |
| 6. | Hartwig, J. F. J. Am. Chem. Soc. 2016, 138, 2–24. doi:10.1021/jacs.5b08707 |
| 7. | Liu, B.; Romine, A. M.; Rubel, C. Z.; Engle, K. M.; Shi, B.-F. Chem. Rev. 2021, 121, 14957–15074. doi:10.1021/acs.chemrev.1c00519 |
| 8. | Mishra, A. A.; Subhedar, D.; Bhanage, B. M. Chem. Rec. 2019, 19, 1829–1857. doi:10.1002/tcr.201800093 |
| 70. | Tanaka, H.; Sakai, K.; Kawamura, A.; Oisaki, K.; Kanai, M. Chem. Commun. 2018, 54, 3215–3218. doi:10.1039/c7cc09457d |
| 44. | Li, N.; Li, J.; Qin, M.; Li, J.; Han, J.; Zhu, C.; Li, W.; Xie, J. Nat. Commun. 2022, 13, 4224. doi:10.1038/s41467-022-31956-3 |
| 52. | Guo, J.-J.; Hu, A.; Zuo, Z. Tetrahedron Lett. 2018, 59, 2103–2111. doi:10.1016/j.tetlet.2018.04.060 |
| 53. | Chen, H.; Yu, S. Org. Biomol. Chem. 2020, 18, 4519–4532. doi:10.1039/d0ob00854k |
| 54. | Davies, J.; Morcillo, S. P.; Douglas, J. J.; Leonori, D. Chem. – Eur. J. 2018, 24, 12154–12163. doi:10.1002/chem.201801655 |
| 55. | Gao, S.; Li, F. Adv. Funct. Mater. 2023, 33, 2304291. doi:10.1002/adfm.202304291 |
| 56. | Lasso, J. D.; Castillo-Pazos, D. J.; Li, C.-J. Chem. Soc. Rev. 2021, 50, 10955–10982. doi:10.1039/d1cs00380a |
| 57. | Liang, Y.-F.; Bilal, M.; Tang, L.-Y.; Wang, T.-Z.; Guan, Y.-Q.; Cheng, Z.; Zhu, M.; Wei, J.; Jiao, N. Chem. Rev. 2023, 123, 12313–12370. doi:10.1021/acs.chemrev.3c00219 |
| 58. | Bellotti, P.; Huang, H.-M.; Faber, T.; Glorius, F. Chem. Rev. 2023, 123, 4237–4352. doi:10.1021/acs.chemrev.2c00478 |
| 98. | Entgelmeier, L.-M.; Mori, S.; Sendo, S.; Yamaguchi, R.; Suzuki, R.; Yanai, T.; García Mancheño, O.; Ohmatsu, K.; Ooi, T. Angew. Chem., Int. Ed. 2024, 63, e202404890. doi:10.1002/anie.202404890 |
| 40. | Tierney, M. M.; Crespi, S.; Ravelli, D.; Alexanian, E. J. J. Org. Chem. 2019, 84, 12983–12991. doi:10.1021/acs.joc.9b01774 |
| 41. | Nanjo, T.; Matsumoto, A.; Oshita, T.; Takemoto, Y. J. Am. Chem. Soc. 2023, 145, 19067–19075. doi:10.1021/jacs.3c06931 |
| 42. | Lee, H.; He, T.; Cook, S. P. Org. Lett. 2023, 25, 1–4. doi:10.1021/acs.orglett.2c02864 |
| 43. | Gonzalez, M. I.; Gygi, D.; Qin, Y.; Zhu, Q.; Johnson, E. J.; Chen, Y.-S.; Nocera, D. G. J. Am. Chem. Soc. 2022, 144, 1464–1472. doi:10.1021/jacs.1c13333 |
| 59. | Choi, G. J.; Zhu, Q.; Miller, D. C.; Gu, C. J.; Knowles, R. R. Nature 2016, 539, 268–271. doi:10.1038/nature19811 |
| 60. | Chu, J. C. K.; Rovis, T. Nature 2016, 539, 272–275. doi:10.1038/nature19810 |
| 61. | Li, W.; Sun, B.; Zhang, L.; Mo, F. Green Chem. 2023, 25, 5030–5034. doi:10.1039/d3gc01426f |
| 62. | Wang, C.; Chen, Z.; Sun, J.; Tong, L.; Wang, W.; Song, S.; Li, J. Nat. Commun. 2024, 15, 5087. doi:10.1038/s41467-024-49337-3 |
| 63. | Stateman, L. M.; Dare, R. M.; Paneque, A. N.; Nagib, D. A. Chem 2022, 8, 210–224. doi:10.1016/j.chempr.2021.10.022 |
| 64. | Ding, W.-W.; Zhou, Y.; Song, S.; Han, Z.-Y. Org. Lett. 2022, 24, 7350–7354. doi:10.1021/acs.orglett.2c02877 |
| 65. | Deng, Z.; Zhao, Z.; He, G.; Chen, G. Org. Lett. 2021, 23, 3631–3635. doi:10.1021/acs.orglett.1c01020 |
| 66. | Shu, W.; Zhang, H.; Huang, Y. Org. Lett. 2019, 21, 6107–6111. doi:10.1021/acs.orglett.9b02255 |
| 67. | Herron, A. N.; Hsu, C.-P.; Yu, J.-Q. Org. Lett. 2022, 24, 3652–3656. doi:10.1021/acs.orglett.2c01261 |
| 68. | Chen, Z.; Zhu, W.; Wang, C.; Xu, N.; Jin, Q.; Huang, X.; Song, S.; Li, J. Org. Chem. Front. 2023, 10, 4709–4717. doi:10.1039/d3qo00931a |
| 69. | Fu, X.; Tian, J.; Zhang, M.; Jing, Y.; Liu, Y.; Song, H.; Wang, Q. Adv. Sci. 2025, 12, 2411744. doi:10.1002/advs.202411744 |
| 31. | Roberts, B. P. Chem. Soc. Rev. 1999, 28, 25–35. doi:10.1039/a804291h |
| 32. | Cao, J.; Wang, G.; Gao, L.; Cheng, X.; Li, S. Chem. Sci. 2018, 9, 3664–3671. doi:10.1039/c7sc05225a |
| 33. | Bell, J. D.; Murphy, J. A. Chem. Soc. Rev. 2021, 50, 9540–9685. doi:10.1039/d1cs00311a |
| 34. | Zhang, Y.; Li, K.-D.; Zhou, C.-Q.; Xing, Z.-X.; Huang, H.-M. Green Chem. 2024, 26, 10434–10440. doi:10.1039/d4gc02879a |
| 35. | Pannwitz, A.; Wenger, O. S. Chem. Commun. 2019, 55, 4004–4014. doi:10.1039/c9cc00821g |
| 36. | Latrache, M.; Hoffmann, N. Chem. Soc. Rev. 2021, 50, 7418–7435. doi:10.1039/d1cs00196e |
| 37. | Zhou, R.; Li, J.; Cheo, H. W.; Chua, R.; Zhan, G.; Hou, Z.; Wu, J. Chem. Sci. 2019, 10, 7340–7344. doi:10.1039/c9sc02818h |
| 38. | Su, Y.; Li, Y.; Ganguly, R.; Kinjo, R. Chem. Sci. 2017, 8, 7419–7423. doi:10.1039/c7sc03528d |
| 39. | Zhu, X.; Li, P.; Shi, Q.; Wang, L. Green Chem. 2016, 18, 6373–6379. doi:10.1039/c6gc01487a |
| 25. | Schmidt, V. A.; Quinn, R. K.; Brusoe, A. T.; Alexanian, E. J. J. Am. Chem. Soc. 2014, 136, 14389–14392. doi:10.1021/ja508469u |
| 28. | Huang, C.; Qin, Y.-S.; Wang, C.-L.; Xiao, P.; Tang, S.; Liu, H.-J.; Wei, Z.; Cai, H. Chem. Commun. 2024, 60, 2669–2672. doi:10.1039/d3cc06210d |
| 29. | Sonawane, S. C.; Gourkhede, R.; Saini, P.; Ramakrishnan, S.; Balakrishna, M. S. Chem. Commun. 2024, 60, 6055–6058. doi:10.1039/d4cc01119h |
| 30. | Sharma, A. K.; Maseras, F. Inorg. Chem. 2024, 63, 13801–13806. doi:10.1021/acs.inorgchem.4c01763 |
| 45. | Wu, Y.; Liu, Z.; Li, Y.; Chen, J.; Zhu, X.; Na, P. Chin. J. Catal. 2019, 40, 60–69. doi:10.1016/s1872-2067(18)63170-5 |
| 46. | Liu, D.; Chen, S.; Zhang, Y.; Li, R.; Peng, T. Appl. Catal., B 2023, 333, 122805. doi:10.1016/j.apcatb.2023.122805 |
| 47. | Wei, Y.; Shahid, M. Z.; Lyu, S.; Sun, W.; Lyu, S. RSC Adv. 2021, 11, 22618–22624. doi:10.1039/d1ra02958d |
| 48. | Ouyang, W.-T.; Jiang, J.; Jiang, Y.-F.; Li, T.; Liu, Y.-Y.; Ji, H.-T.; Ou, L.-J.; He, W.-M. Chin. Chem. Lett. 2024, 35, 110038. doi:10.1016/j.cclet.2024.110038 |
| 49. | Xu, Y.-D.; Xing, Y.-M.; Ji, H.-T.; Ou, L.-J.; He, W.-B.; Peng, J.; Wang, J.-S.; Jiang, J.; He, W.-M. J. Org. Chem. 2024, 89, 17701–17707. doi:10.1021/acs.joc.4c02445 |
| 50. | Hou, J.-C.; Jiang, J.; Dai, H.; Wang, J.-S.; Li, T.; Chen, X.; He, W.-M. Sci. China: Chem. 2025, 68, 1945–1951. doi:10.1007/s11426-024-2496-5 |
| 51. | Zhang, Q.; Zhao, Q.; Wu, X.; Wang, L.; Shen, K.; Hua, Y.; Gao, C.; Zhang, Y.; Peng, M.; Zhao, K. Chin. Chem. Lett. 2025, 36, 110167. doi:10.1016/j.cclet.2024.110167 |
| 97. | Quinn, R. K.; Könst, Z. A.; Michalak, S. E.; Schmidt, Y.; Szklarski, A. R.; Flores, A. R.; Nam, S.; Horne, D. A.; Vanderwal, C. D.; Alexanian, E. J. J. Am. Chem. Soc. 2016, 138, 696–702. doi:10.1021/jacs.5b12308 |
| 78. | Fazekas, T. J.; Alty, J. W.; Neidhart, E. K.; Miller, A. S.; Leibfarth, F. A.; Alexanian, E. J. Science 2022, 375, 545–550. doi:10.1126/science.abh4308 |
| 79. | Liang, D.; Chen, J.-R.; Tan, L.-P.; He, Z.-W.; Xiao, W.-J. J. Am. Chem. Soc. 2022, 144, 6040–6049. doi:10.1021/jacs.2c01116 |
| 73. | Kim, J.; Kim, Y.-E.; Hong, S. Angew. Chem., Int. Ed. 2024, 63, e202409561. doi:10.1002/anie.202409561 |
| 74. | Jung, S.; Lee, H.; Moon, Y.; Jung, H.-Y.; Hong, S. ACS Catal. 2019, 9, 9891–9896. doi:10.1021/acscatal.9b03367 |
| 75. | Shin, S.; Lee, S.; Choi, W.; Kim, N.; Hong, S. Angew. Chem., Int. Ed. 2021, 60, 7873–7879. doi:10.1002/anie.202016156 |
| 76. | Vellakkaran, M.; Kim, T.; Hong, S. Angew. Chem., Int. Ed. 2022, 61, e202113658. doi:10.1002/anie.202113658 |
| 77. | Lee, W.; Jung, S.; Kim, M.; Hong, S. J. Am. Chem. Soc. 2021, 143, 3003–3012. doi:10.1021/jacs.1c00549 |
| 91. | Garwood, J. J. A.; Chen, A. D.; Nagib, D. A. J. Am. Chem. Soc. 2024, 146, 28034–28059. doi:10.1021/jacs.4c06774 |
| 92. | Na, C. G.; Ravelli, D.; Alexanian, E. J. J. Am. Chem. Soc. 2020, 142, 44–49. doi:10.1021/jacs.9b10825 |
| 93. | Williamson, J. B.; Na, C. G.; Johnson, R. R., III; Daniel, W. F. M.; Alexanian, E. J.; Leibfarth, F. A. J. Am. Chem. Soc. 2019, 141, 12815–12823. doi:10.1021/jacs.9b05799 |
| 94. | Margrey, K. A.; Czaplyski, W. L.; Nicewicz, D. A.; Alexanian, E. J. J. Am. Chem. Soc. 2018, 140, 4213–4217. doi:10.1021/jacs.8b00592 |
| 89. | Ruos, M. E.; Kinney, R. G.; Ring, O. T.; Doyle, A. G. J. Am. Chem. Soc. 2023, 145, 18487–18496. doi:10.1021/jacs.3c04912 |
| 90. | Wang, H.-S.; Li, H.-C.; Yuan, X.-Y.; Sun, K.; Chen, X.-L.; Qu, L.; Yu, B. Green Chem. 2025, 27, 4655–4663. doi:10.1039/d4gc06209d |
| 84. | Fu, M.-C.; Shang, R.; Zhao, B.; Wang, B.; Fu, Y. Science 2019, 363, 1429–1434. doi:10.1126/science.aav3200 |
| 5. | Guo, Y.; Lin, G.; Zhang, M.; Xu, J.; Song, Q. Nat. Commun. 2024, 15, 7313. doi:10.1038/s41467-024-51334-5 |
| 85. | Qi, X.-K.; Zheng, M.-J.; Yang, C.; Zhao, Y.; Guo, L.; Xia, W. J. Am. Chem. Soc. 2023, 145, 16630–16641. doi:10.1021/jacs.3c04073 |
| 86. | Feng, L.; Guo, L.; Yang, C.; Zhou, J.; Xia, W. Org. Lett. 2020, 22, 3964–3968. doi:10.1021/acs.orglett.0c01267 |
| 87. | Zhu, Y.; Gao, H.; Tu, J.-L.; Yang, C.; Guo, L.; Zhao, Y.; Xia, W. Org. Chem. Front. 2024, 11, 1729–1735. doi:10.1039/d3qo01822a |
| 88. | Mu, S.; Guo, Y.; Huang, X.; Luo, Y.; Chen, M.; Xu, J.; Song, Q. Org. Chem. Front. 2023, 10, 3259–3263. doi:10.1039/d3qo00537b |
| 80. | Zhang, B.; Erb, F. R.; Vasilopoulos, A.; Voight, E. A.; Alexanian, E. J. J. Am. Chem. Soc. 2023, 145, 26540–26544. doi:10.1021/jacs.3c10751 |
| 81. | Miller, A. S.; Alexanian, E. J. Chem. Sci. 2022, 13, 11878–11882. doi:10.1039/d2sc04605a |
| 82. | Lyu, X.-L.; Huang, S.-S.; Song, H.-J.; Liu, Y.-X.; Wang, Q.-M. Org. Lett. 2019, 21, 5728–5732. doi:10.1021/acs.orglett.9b02105 |
| 83. | Ren, H.; Zhang, P.; Xu, J.; Ma, W.; Tu, D.; Lu, C.-s.; Yan, H. J. Am. Chem. Soc. 2023, 145, 7638–7647. doi:10.1021/jacs.3c01314 |
© 2025 Wang et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.