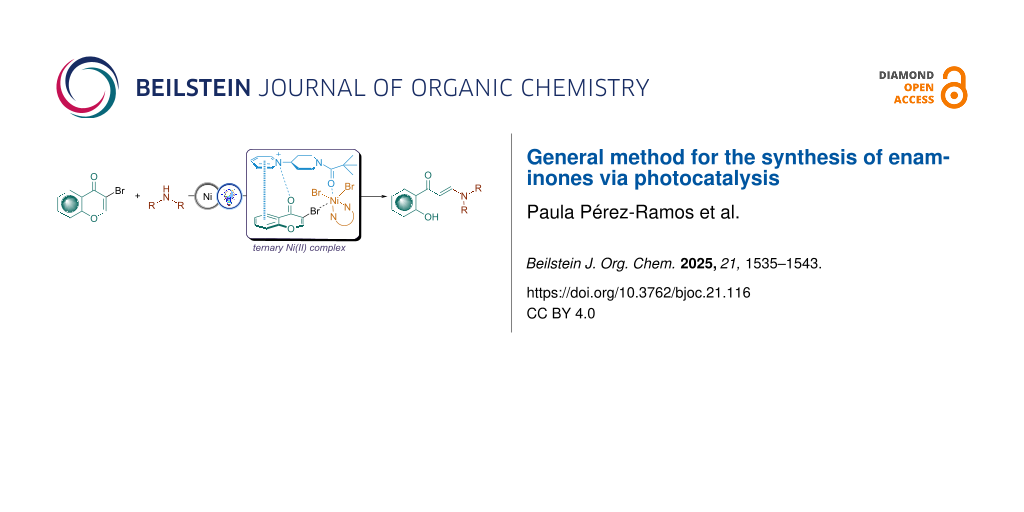
Abstract
Enaminones are key intermediates in the synthesis of several derivatives with important applications in medicinal chemistry. Furthermore, many marketed drugs feature the enaminone structural moiety. In this context, we have developed a photoredox and nickel catalytic system to rapidly forge the enaminone scaffold from 3-bromochromones via a Michael reaction of an amine with an electron-deficient alkene moiety and subsequent photocatalyzed debromination. With this dual catalytic system, a range of structurally diverse enaminone derivatives have been achieved in good yields with total trans selectivity. Mechanistic studies indicate that the key to the success of this process is the formation of an unexplored ternary Ni-complex with 3-bromochromone and a pyridinium salt, which is crucial for the effective activation of the α,β-unsaturated system towards the nucleophilic addition.
Graphical Abstract
Introduction
Enaminones are relevant intermediates in organic chemistry and the pharmaceutical industry [1-6]. These enamines have a carbonyl group conjugated to a carbon–carbon double bond, owing its great versatility in organic synthesis to its ability to act as both electrophiles and nucleophiles [7]. This makes enaminones very reactive, providing an excellent scaffold for organic synthesis. Thus, enaminones are valuable building blocks in the preparation of several carbocyclic [8-11], heterocyclic [12-18] as well as acyclic compounds [19-23]. Furthermore, the enaminone structural moiety represents the key framework of many drug classes, including antibiotic (1) [24], anti-inflammatory (2) [25], antinociceptive (3) [26], anticonvulsant (4) [27], antitubercular (5) [28], and antitumor (6) [29] agents (Figure 1).
Figure 1: Examples of compounds with medicinal effects containing an enaminone structural moiety.
Figure 1: Examples of compounds with medicinal effects containing an enaminone structural moiety.
In view of the important biological roles of enaminones and their relevance as synthetic intermediates, it is not surprising that there has been a continuous focus on developing general, straightforward, and efficient strategies for their synthesis. Enaminones are usually accessed by condensation of 1,3-dicarbonyl compounds with amines [30]. While this approach is simple and straightforward, it often leads to a mixture of constitutional isomers in which the two different α-positions of the ketone have been functionalized. Therefore, the development of novel methods for the synthesis of enaminones has attracted much attention over the past decades.
Kuwano’s group described the synthesis of enaminones from ethyl ketones via a nickel-catalyzed selective β-amination (Scheme 1A) [31]. The preparation of enaminones can also be achieved by the reaction of aldehydes and calcium carbide in the presence of amines and CuI as catalyst, as reported by Zhang and co-workers (Scheme 1B) [32]. On the other hand, Li et al. disclosed a silver-catalyzed amination of propargyl alcohols to afford enaminones (Scheme 1C) [33].
Although these new methods provide a wide variety of enaminones, there are limitations such as expensive and unavailable reagents, long reaction times and drastic reaction conditions. Furthermore, the increasing emphasis on economic and environmental factors has highlighted the limitations of traditional methods for enaminone synthesis to align with the modern understanding of organic chemistry.
With the increasing concern on the environmental impact of organic synthesis, photocatalysis emerged as a powerful synthetic tool in organic chemistry, offering new ways to deliver diverse organic products via mild, easy to handle, and environmentally benign operations [34-36]. Thus, the use of visible light as an energy source provides more efficient chemical transformations and minimize the use of harmful reagents, the generation of waste and the consumption of energy, fulfilling several principles of Green Chemistry and promoting greener opportunities for organic synthesis [37,38]. In this context, the reactivity of enaminones under visible-light-mediated reaction conditions has attracted significant attention [39]. However, it is rather surprising that a photocatalytic approach for the synthesis of enaminones has yet to be explored.
Herein, we report the first light-mediated reaction for the synthesis of enaminones from 3-bromochromones (Scheme 1D). Initially, a Ni(II)-catalyzed hydroamination protocol affords the intermediate 2-amino-3-bromochromanones, which upon photocatalytic dehalogenation and subsequent opening of the heterocyclic ring provide the corresponding enaminones. This transformation is simple, straightforward, and proceeds under mild conditions.
Results and Discussion
The initial challenge in achieving the desired reactivity was the activation of the unsaturated system towards the nucleophilic addition of the amine. The most common strategy to increase the reactivity of unsaturated esters towards an aza-Michael addition is the use of transition metal complexes as catalysts/promoters [40-42]. Considering this background, we reasoned that Ni(II) could be a suitable catalyst for the amination of unsaturated systems.
Initial investigations were carried out by using 3-bromochromone (7a) and morpholine (8a) as model substrates in the presence of Ni(II) salt (5 mol %) and ligand (5 mol %), a pyridinium salt (1 equiv) and a photocatalyst (1 mol %) under 427 nm blue LEDs. After carefully screening of the reaction parameters (Table 1 and Tables S1–S7, Supporting Information File 1), we found that a combination of 1-[1-(tert-butoxycarbonyl)piperidin-4-yl]-2,4,6-triphenylpyridin-1-ium (PS1), NiBr2·diglyme, 4,4'-dimethoxy-2,2'-bipyridine (dmbpy), and 10-(3,5-dimethoxyphenyl)-9-mesityl-1,3,6,8-tetramethoxyacridin-10-ium tetrafluoroborate (PC1) in N,N-dimethylformamide (DMF) at 20 °C afforded the best results, giving the desired product 9a in 70% isolated yield (Table 1, entry 1). Changing NiBr2·diglyme to other nickel salts, such as Ni(OTf)2 and NiCl2·diglyme led to lower yields (Table 1, entries 2 and 3). Similarly, changing the ligand for dtbbpy or phenantroline also resulted in a decrease in the efficiency of the process (Table 1, entries 4 and 5). The pyridinium salt has also a significant effect on reactivity; thus, when 1-benzyl-2,4,6-triphenylpyridin-1-ium (PS2) was used, enaminone 9a was isolated in only 34% yield (Table 1, entry 6). Replacing DMF by DME, DMSO or acetone diminished the product yields (Table 1, entries 7–9). The reactivity of acridinium PC1 was superior to that of other photocatalysts, including 4-CzlPN (PC2) and [Ir(dF(CF3)ppy)2(dtbbpy)]PF6 (PC3) (Table 1, entries 10 and 11).
Table 1: Optimization of reaction conditions.
|
|
||
| Entry | Deviation from the standard conditionsa | Yield (%)b |
| 1 | none | 70 |
| 2 | Ni(OTf)2 instead NiBr2·diglyme | 62 |
| 3 | NiCl2·diglyme instead NiBr2·diglyme | 49 |
| 4 | dtbbpy instead dmbpy | 66 |
| 5 | phenantroline instead dmbpy | 49 |
| 6 | PS2 instead PS1 | 34 |
| 7 | acetone instead DMF | 51 |
| 8 | DMSO instead DMF | 55 |
| 9 | DME instead DMF | 57 |
| 10 | PC2 instead PC1 | 46 |
| 11 | PC3 instead PC1 | 57 |
| 12 | 40 °C instead 20 °C | traces |
| 13 | 16 h instead 2 h | 18 |
| 14 | no PS | 33 |
| 15 | no Ni salt and ligand | 30 |
| 16 | no PC | n.r.c |
| 17 | no light | n.r. |
a3-Bromochromone 7a (0.2 mmol), morpholine 8a (0.3 mmol), PC1 (1.0 mol %), NiBr2·diglyme (5.0 mol %), dmbpy (5.0 mol %) and PS1 (1.0 equiv) in DMF under N2, irradiation with a 427 nm LED lamp at 20 °C for 2 hours. bIsolated yield of 9a after flash column chromatography. cNo reaction.
Attempts to increase the temperature of the process resulted in a complex reaction mixture in which only traces of the desired enaminone 9a were detected (Table 1, entry 12). Longer reaction times also led to significant degradation, isolating the desired enaminone in only 18% yield (Table 1, entry 13). The reaction with 3-chlorochromone gives lower yield while 3-iodochromone failed to provide the desired enaminone 9a (Table S7, Supporting Information File 1).
Control experiments including the reaction in the absence of visible-light or photocatalyst, showed no product formation (Table 1, entries 16 and 17). Interestingly, the yield of 9a dropped to 30% in the absence of Ni salt and 33% in the absence of the pyridinium salt (Table 1, entries 14 and 15).
With the optimal reaction conditions, we first studied the substrate scope of 3-bromochromones 7 (Scheme 2).
It was determined that the process was compatible with various halochromones bearing electron-withdrawing (F, Cl, Br) or electron-donating (Me, OMe) groups at different positions of the aromatic ring, affording the corresponding enaminone products 9a−h in moderate to good yields (45−70%). Of particular relevance is the effective formation of enaminones 9d and 9e containing bromo and chloro moieties, respectively, since they could enable further chemical transformations. Unfortunately, when 2-methyl-3-bromochromone was employed as the substrate, the process failed to afford the desired enaminone product, which was possibly caused by a combination of increased steric hindrance and decreased electrophilicity of the β-carbon due to the electron-donating nature of the methyl group.
Subsequently, we turned our attention to investigate a range of amine derivatives 8 under the standard conditions. When morpholine was replaced by piperidine, the expected enaminone 9g was provided, albeit in lower yield. Similarly, 4-methylpiperidine and N-tert-butoxycarbonylpiperazine afforded the corresponding enaminones 9i and 9j, respectively, in moderate yields. Gratifyingly, 1-(pyridin-2-yl)piperazine was also tolerated, providing the expected product 9h in 46% yield. Considering that pyridine is one of the core components of drug derivative formulations, present in more than 7,000 active pharmaceutical compounds [43], the possibility of introducing a pyridine ring into the enaminone structure could be useful for the development of new drug candidates.
When pyrrolidine was used as amine reagent, the target enaminone 9k was obtained, albeit in low yield. Regrettably, this transformation failed to provide the corresponding enaminone products by replacing alicyclic amines by diisopropylamine, probably due to steric hindrance. Steric factors could also explain the lack of reactivity of seven-membered azepane. In view of these results, it seems evident that six-membered cyclic amines have the optimal ring size for the photocatalyzed enaminone formation process. For enaminones 9b–k the remaining mass balance comprised mainly unreacted starting materials.
The scalability of the process was demonstrated in semipreparative scale for the reaction of 3-bromochromone (7a, 5.0 mmol) to afford enaminone 9a in a 68% isolated yield (Scheme 3).
Scheme 3: Scale-up synthesis of enaminone 9a.
Scheme 3: Scale-up synthesis of enaminone 9a.
In terms of the reaction mechanism, TEMPO completely inhibited the reaction, implying the possibility of a radical intermediate in the reaction (Scheme 4A). Moreover, the TEMPO adduct 10 was identified using GC–MS (Figure S1, Supporting Information File 1). When the reaction was performed under air-equilibrated conditions, the intended product 9a was obtained in a 31% yield, indicating that air influenced the interaction between the Ni-catalyst and the α,β-unsaturated carbonyl function (Scheme 4B). Furthermore, when the reaction of chromone 10 was carried out under standard conditions, the starting material was recovered unaltered, evidencing that the photocatalyzed dehalogenation step is crucial to enable the ring opening (Scheme 4C).
In order to determine the role of Ni(II) in this process, UV–vis studies were carried out. Thus, upon addition of PS1 and 7a to a solution of NiBr2-dmbpy, a charge transfer (CT) band at 688 nm was visible in the UV–vis spectrum (Figure S2, Supporting Information File 1). This observation is consistent with the formation of a six-coordinate, pseudo-octahedral Ni(II) complex [44] involving the carbonyl groups of PS1 and 7a, which is further activated on aggregation of the pyridinium salt and the chromone aromatic ring through π−π stacking.
Then, PC1 was submitted to Stern–Volmer quenching experiments. Whereas no interaction occurred between the excited form of PC1 and 3-bromochromone (7a), a direct interaction occurred between PC1* and morpholine (8a, Figure S3, Supporting Information File 1). Based on these results, the most plausible scenario might be that the reaction starts with the excitation of PC1 at 427 nm to generate the highly oxidative state PC1* that interacts with morpholine (8a) to generate the corresponding aminium radical cation.
To gain a better understanding of the process, the formation of the enaminone product 9a was monitored overtime by 1H NMR, which confirmed that the the reaction was complete within 2 h. Furthermore, the rate of the reaction is independent of the concentration of the 3-bromochromone substrate 7a and amine 8a (Figure S4, Supporting Information File 1).
Based on the above experiments and prior work on the photocatalytic reductive halogenation using acridinium photocatalysts [45,46], a possible mechanism for this reaction is proposed in Scheme 5.
A ternary complex is initially formed upon complexation of 3-bromochromone (7a) with NiBr2-dmbpy. By virtue of being coordinated to a Ni-center, the β-carbon is activated toward nucleophilic attack of the amine, furnishing 2-amino-3-bromochromenone I. Simultaneously, acridinium photocatalyst PC1 absorbed energy and transitioned from the ground state to excited state under visible-light irradiation. This excited state PC1* is quenched by the amine, generating the amine radical cation and PC1 radical via a single-electron transfer (SET) process. Then, the C−Br bond of I is cleaved by PC1•, generating a C-centered radical II, which can be further reduced to give an enolate III, that ultimately evolves to the more stable anion IV and undergoes protonation to afford the final enaminone product 9a.
Conclusion
In summary, a simple and effective nickel-assisted photocatalytic protocol for the direct formation of enaminones from 3-bromochromones is reported. The process involves a Ni(II)-catalyzed hydroamination, followed by photocatalytic reductive debromination. Mechanistic studies suggest that the key step of this transformation is the complexation of the starting 3-bromochromones to Ni(II) and a pyridinium salt, giving rise to a ternary Ni-complex which activates the α,β-unsaturated carbonyl compound towards nucleophilic addition. The presented method is operationally simple and can be conducted using low catalyst loadings of a Ni-catalyst and an inexpensive organic photocatalyst.
Supporting Information
| Supporting Information File 1: Additional optimization details, mechanistic studies, experimental details, characterization data and NMR spectra for enaminones 9. | ||
| Format: PDF | Size: 1.7 MB | Download |
Acknowledgements
We would like to thank Prof. Jose Manuel Costa-Fernández for his help with the spectroscopic studies.
Funding
This work has received financial support from MCI (PID2022-137893OB-I00), Principado de Asturias (SEK-25-GRU-GIC-24-054) and INGENIUM Alliance of European Universities (ERASMUS-EDU-2022-EUR-UNIV-2, Project number 101090042). P.P.R. thanks “Programa Investigo” for a predoctoral contract (AYUD/2022/9313, EU Next Generation).
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Cao, S.; Jing, Y.; Liu, Y.; Wan, J. Chin. J. Org. Chem. 2014, 34, 876–885. doi:10.6023/cjoc201312016
Return to citation in text: [1] -
Chattopadhyay, A. K.; Hanessian, S. Chem. Commun. 2015, 51, 16450–16467. doi:10.1039/c5cc05892a
Return to citation in text: [1] -
Huang, J.; Yu, F. Synthesis 2021, 53, 587–610. doi:10.1055/s-0040-1707328
Return to citation in text: [1] -
Wang, Y.; Zhang, C.; Li, S.; Liu, L.; Feng, X. ChemistrySelect 2022, 7, e202103345. doi:10.1002/slct.202103345
Return to citation in text: [1] -
Chen, X. Y.; Zhang, X.; Wan, J.-P. Org. Biomol. Chem. 2022, 20, 2356–2369. doi:10.1039/d2ob00126h
Return to citation in text: [1] -
Wang, Z.; Zhao, B.; Liu, Y.; Wan, J.-P. Adv. Synth. Catal. 2022, 364, 1508–1521. doi:10.1002/adsc.202200144
Return to citation in text: [1] -
Amaye, I. J.; Haywood, R. D.; Mandzo, E. M.; Wirick, J. J.; Jackson-Ayotunde, P. L. Tetrahedron 2021, 83, 131984. doi:10.1016/j.tet.2021.131984
Return to citation in text: [1] -
Zhou, S.; Wang, J.; Wang, L.; Song, C.; Chen, K.; Zhu, J. Angew. Chem., Int. Ed. 2016, 55, 9384–9388. doi:10.1002/anie.201603943
Return to citation in text: [1] -
Yang, L.; Wei, L.; Wan, J.-P. Chem. Commun. 2018, 54, 7475–7478. doi:10.1039/c8cc03514h
Return to citation in text: [1] -
Liu, M.; Yan, K.; Wen, J.; Liu, W.; Wang, M.; Wang, L.; Wang, X. Adv. Synth. Catal. 2022, 364, 512–517. doi:10.1002/adsc.202101181
Return to citation in text: [1] -
Fang, Z.; Ma, Y.; Dong, J. Tetrahedron Lett. 2022, 102, 153944. doi:10.1016/j.tetlet.2022.153944
Return to citation in text: [1] -
Liang, X.; Guo, P.; Yang, W.; Li, M.; Jiang, C.; Sun, W.; Loh, T.-P.; Jiang, Y. Chem. Commun. 2020, 56, 2043–2046. doi:10.1039/c9cc08582c
Return to citation in text: [1] -
Zheng, X.; Liu, Y.; Wan, J.-P. Chin. J. Org. Chem. 2021, 41, 2700–2706. doi:10.6023/cjoc202104053
Return to citation in text: [1] -
Wang, F.; Fu, R.; Chen, J.; Rong, J.; Wang, E.; Zhang, J.; Zhang, Z.; Jiang, Y. Chem. Commun. 2022, 58, 3477–3480. doi:10.1039/d2cc00383j
Return to citation in text: [1] -
Dattatri; Singam, M. K. R.; Nanubolu, J. B.; Reddy, M. S. Org. Biomol. Chem. 2022, 20, 6363–6367. doi:10.1039/d2ob00839d
Return to citation in text: [1] -
Ying, J.; Liu, T.; Liu, Y.; Wan, J.-P. Org. Lett. 2022, 24, 2404–2408. doi:10.1021/acs.orglett.2c00671
Return to citation in text: [1] -
Guo, Y.; Liu, Y.; Wan, J.-P. Chin. Chem. Lett. 2022, 33, 855–858. doi:10.1016/j.cclet.2021.08.003
Return to citation in text: [1] -
Liang, Y.; Wang, R. Tetrahedron Lett. 2023, 114, 154287. doi:10.1016/j.tetlet.2022.154287
Return to citation in text: [1] -
Duan, X.; Li, H.; Li, W.; Wang, J.; Liu, N. ChemistrySelect 2021, 6, 6478–6482. doi:10.1002/slct.202101726
Return to citation in text: [1] -
Liu, T.; Wei, L.; Zhao, B.; Liu, Y.; Wan, J.-P. J. Org. Chem. 2021, 86, 9861–9868. doi:10.1021/acs.joc.1c00862
Return to citation in text: [1] -
Lu, F.; Zhang, K.; Yao, Y.; Yin, Y.; Chen, J.; Zhang, X.; Wang, Y.; Lu, L.; Gao, Z.; Lei, A. Green Chem. 2021, 23, 763–766. doi:10.1039/d0gc03590d
Return to citation in text: [1] -
Yu, Q.; Liu, Y.; Wan, J.-P. Chin. Chem. Lett. 2021, 32, 3514–3517. doi:10.1016/j.cclet.2021.04.037
Return to citation in text: [1] -
Zheng, Y.; Liu, Z.-W.; Li, T.; Li, X.; Li, S.-H. Org. Lett. 2022, 24, 7533–7537. doi:10.1021/acs.orglett.2c02824
Return to citation in text: [1] -
Cindrić, M.; Rubčić, M.; Hrenar, T.; Pisk, J.; Cvijanović, D.; Lovrić, J.; Vrdoljak, V. J. Mol. Struct. 2018, 1154, 636–642. doi:10.1016/j.molstruc.2017.10.078
Return to citation in text: [1] -
Kumar, R.; Saha, N.; Purohit, P.; Garg, S. K.; Seth, K.; Meena, V. S.; Dubey, S.; Dave, K.; Goyal, R.; Sharma, S. S.; Banerjee, U. C.; Chakraborti, A. K. Eur. J. Med. Chem. 2019, 182, 111601. doi:10.1016/j.ejmech.2019.111601
Return to citation in text: [1] -
Masocha, W.; Kombian, S. B.; Edafiogho, I. O. Sci. Rep. 2016, 6, 21582. doi:10.1038/srep21582
Return to citation in text: [1] -
Amaye, I. J.; Heinbockel, T.; Woods, J.; Wang, Z.; Martin-Caraballo, M.; Jackson-Ayotunde, P. Int. J. Environ. Res. Public Health 2018, 15, 1784. doi:10.3390/ijerph15081784
Return to citation in text: [1] -
Bangalore, P. K.; Vagolu, S. K.; Bollikanda, R. K.; Veeragoni, D. K.; Choudante, P. C.; Misra, S.; Sriram, D.; Sridhar, B.; Kantevari, S. J. Nat. Prod. 2020, 83, 26–35. doi:10.1021/acs.jnatprod.9b00475
Return to citation in text: [1] -
Ghorab, M. M.; Ragab, F. A.; Heiba, H. I.; El-Gazzar, M. G.; El-Gazzar, M. G. M. Bioorg. Med. Chem. Lett. 2018, 28, 1464–1470. doi:10.1016/j.bmcl.2018.03.089
Return to citation in text: [1] -
Liu, J.-Y.; Cao, G.-E.; Xu, W.; Cao, J.; Wang, W.-L. Appl. Organomet. Chem. 2010, 24, 685–691. doi:10.1002/aoc.1667
Return to citation in text: [1] -
Ueno, S.; Shimizu, R.; Kuwano, R. Angew. Chem., Int. Ed. 2009, 48, 4543–4545. doi:10.1002/anie.200900892
Return to citation in text: [1] -
Yu, D.; Sum, Y. N.; Ean, A. C. C.; Chin, M. P.; Zhang, Y. Angew. Chem., Int. Ed. 2013, 52, 5125–5128. doi:10.1002/anie.201301019
Return to citation in text: [1] -
Li, M.; Fang, D.; Geng, F.; Dai, X. Tetrahedron Lett. 2017, 58, 4747–4749. doi:10.1016/j.tetlet.2017.09.054
Return to citation in text: [1] -
Marzo, L.; Pagire, S. K.; Reiser, O.; König, B. Angew. Chem., Int. Ed. 2018, 57, 10034–10072. doi:10.1002/anie.201709766
Return to citation in text: [1] -
Shaw, M. H.; Twilton, J.; MacMillan, D. W. C. J. Org. Chem. 2016, 81, 6898–6926. doi:10.1021/acs.joc.6b01449
Return to citation in text: [1] -
Narayanam, J. M. R.; Stephenson, C. R. J. Chem. Soc. Rev. 2011, 40, 102–113. doi:10.1039/b913880n
Return to citation in text: [1] -
Crisenza, G. E. M.; Melchiorre, P. Nat. Commun. 2020, 11, 803. doi:10.1038/s41467-019-13887-8
Return to citation in text: [1] -
Yoon, T. P.; Ischay, M. A.; Du, J. Nat. Chem. 2010, 2, 527–532. doi:10.1038/nchem.687
Return to citation in text: [1] -
Han, Y.; Zhou, L.; Wang, C.; Feng, S.; Ma, R.; Wan, J.-P. Chin. Chem. Lett. 2024, 35, 108977. doi:10.1016/j.cclet.2023.108977
Return to citation in text: [1] -
Kim, S.; Kang, S.; Kim, G.; Lee, Y. J. Org. Chem. 2016, 81, 4048–4057. doi:10.1021/acs.joc.6b00341
Return to citation in text: [1] -
Lapointe, S.; Zargarian, D. Dalton Trans. 2016, 45, 15800–15810. doi:10.1039/c6dt02105k
Return to citation in text: [1] -
Yamuna, P.; Philip, R. M.; Anilkumar, G. Tetrahedron 2022, 122, 132936. doi:10.1016/j.tet.2022.132936
Return to citation in text: [1] -
De, S.; Kumar S K, A.; Shah, S. K.; Kazi, S.; Sarkar, N.; Banerjee, S.; Dey, S. RSC Adv. 2022, 12, 15385–15406. doi:10.1039/d2ra01571d
Return to citation in text: [1] -
Goldcamp, M. J.; Edison, S. E.; Squires, L. N.; Rosa, D. T.; Vowels, N. K.; Coker, N. L.; Krause Bauer, J. A.; Baldwin, M. J. Inorg. Chem. 2003, 42, 717–728. doi:10.1021/ic025860q
Return to citation in text: [1] -
Maji, T.; Karmakar, A.; Reiser, O. J. Org. Chem. 2011, 76, 736–739. doi:10.1021/jo102239x
Return to citation in text: [1] -
Nguyen, J. D.; D'Amato, E. M.; Narayanam, J. M. R.; Stephenson, C. R. J. Nat. Chem. 2012, 4, 854–859. doi:10.1038/nchem.1452
Return to citation in text: [1]
| 40. | Kim, S.; Kang, S.; Kim, G.; Lee, Y. J. Org. Chem. 2016, 81, 4048–4057. doi:10.1021/acs.joc.6b00341 |
| 41. | Lapointe, S.; Zargarian, D. Dalton Trans. 2016, 45, 15800–15810. doi:10.1039/c6dt02105k |
| 42. | Yamuna, P.; Philip, R. M.; Anilkumar, G. Tetrahedron 2022, 122, 132936. doi:10.1016/j.tet.2022.132936 |
| 37. | Crisenza, G. E. M.; Melchiorre, P. Nat. Commun. 2020, 11, 803. doi:10.1038/s41467-019-13887-8 |
| 38. | Yoon, T. P.; Ischay, M. A.; Du, J. Nat. Chem. 2010, 2, 527–532. doi:10.1038/nchem.687 |
| 39. | Han, Y.; Zhou, L.; Wang, C.; Feng, S.; Ma, R.; Wan, J.-P. Chin. Chem. Lett. 2024, 35, 108977. doi:10.1016/j.cclet.2023.108977 |
| 1. | Cao, S.; Jing, Y.; Liu, Y.; Wan, J. Chin. J. Org. Chem. 2014, 34, 876–885. doi:10.6023/cjoc201312016 |
| 2. | Chattopadhyay, A. K.; Hanessian, S. Chem. Commun. 2015, 51, 16450–16467. doi:10.1039/c5cc05892a |
| 3. | Huang, J.; Yu, F. Synthesis 2021, 53, 587–610. doi:10.1055/s-0040-1707328 |
| 4. | Wang, Y.; Zhang, C.; Li, S.; Liu, L.; Feng, X. ChemistrySelect 2022, 7, e202103345. doi:10.1002/slct.202103345 |
| 5. | Chen, X. Y.; Zhang, X.; Wan, J.-P. Org. Biomol. Chem. 2022, 20, 2356–2369. doi:10.1039/d2ob00126h |
| 6. | Wang, Z.; Zhao, B.; Liu, Y.; Wan, J.-P. Adv. Synth. Catal. 2022, 364, 1508–1521. doi:10.1002/adsc.202200144 |
| 19. | Duan, X.; Li, H.; Li, W.; Wang, J.; Liu, N. ChemistrySelect 2021, 6, 6478–6482. doi:10.1002/slct.202101726 |
| 20. | Liu, T.; Wei, L.; Zhao, B.; Liu, Y.; Wan, J.-P. J. Org. Chem. 2021, 86, 9861–9868. doi:10.1021/acs.joc.1c00862 |
| 21. | Lu, F.; Zhang, K.; Yao, Y.; Yin, Y.; Chen, J.; Zhang, X.; Wang, Y.; Lu, L.; Gao, Z.; Lei, A. Green Chem. 2021, 23, 763–766. doi:10.1039/d0gc03590d |
| 22. | Yu, Q.; Liu, Y.; Wan, J.-P. Chin. Chem. Lett. 2021, 32, 3514–3517. doi:10.1016/j.cclet.2021.04.037 |
| 23. | Zheng, Y.; Liu, Z.-W.; Li, T.; Li, X.; Li, S.-H. Org. Lett. 2022, 24, 7533–7537. doi:10.1021/acs.orglett.2c02824 |
| 33. | Li, M.; Fang, D.; Geng, F.; Dai, X. Tetrahedron Lett. 2017, 58, 4747–4749. doi:10.1016/j.tetlet.2017.09.054 |
| 12. | Liang, X.; Guo, P.; Yang, W.; Li, M.; Jiang, C.; Sun, W.; Loh, T.-P.; Jiang, Y. Chem. Commun. 2020, 56, 2043–2046. doi:10.1039/c9cc08582c |
| 13. | Zheng, X.; Liu, Y.; Wan, J.-P. Chin. J. Org. Chem. 2021, 41, 2700–2706. doi:10.6023/cjoc202104053 |
| 14. | Wang, F.; Fu, R.; Chen, J.; Rong, J.; Wang, E.; Zhang, J.; Zhang, Z.; Jiang, Y. Chem. Commun. 2022, 58, 3477–3480. doi:10.1039/d2cc00383j |
| 15. | Dattatri; Singam, M. K. R.; Nanubolu, J. B.; Reddy, M. S. Org. Biomol. Chem. 2022, 20, 6363–6367. doi:10.1039/d2ob00839d |
| 16. | Ying, J.; Liu, T.; Liu, Y.; Wan, J.-P. Org. Lett. 2022, 24, 2404–2408. doi:10.1021/acs.orglett.2c00671 |
| 17. | Guo, Y.; Liu, Y.; Wan, J.-P. Chin. Chem. Lett. 2022, 33, 855–858. doi:10.1016/j.cclet.2021.08.003 |
| 18. | Liang, Y.; Wang, R. Tetrahedron Lett. 2023, 114, 154287. doi:10.1016/j.tetlet.2022.154287 |
| 34. | Marzo, L.; Pagire, S. K.; Reiser, O.; König, B. Angew. Chem., Int. Ed. 2018, 57, 10034–10072. doi:10.1002/anie.201709766 |
| 35. | Shaw, M. H.; Twilton, J.; MacMillan, D. W. C. J. Org. Chem. 2016, 81, 6898–6926. doi:10.1021/acs.joc.6b01449 |
| 36. | Narayanam, J. M. R.; Stephenson, C. R. J. Chem. Soc. Rev. 2011, 40, 102–113. doi:10.1039/b913880n |
| 8. | Zhou, S.; Wang, J.; Wang, L.; Song, C.; Chen, K.; Zhu, J. Angew. Chem., Int. Ed. 2016, 55, 9384–9388. doi:10.1002/anie.201603943 |
| 9. | Yang, L.; Wei, L.; Wan, J.-P. Chem. Commun. 2018, 54, 7475–7478. doi:10.1039/c8cc03514h |
| 10. | Liu, M.; Yan, K.; Wen, J.; Liu, W.; Wang, M.; Wang, L.; Wang, X. Adv. Synth. Catal. 2022, 364, 512–517. doi:10.1002/adsc.202101181 |
| 11. | Fang, Z.; Ma, Y.; Dong, J. Tetrahedron Lett. 2022, 102, 153944. doi:10.1016/j.tetlet.2022.153944 |
| 31. | Ueno, S.; Shimizu, R.; Kuwano, R. Angew. Chem., Int. Ed. 2009, 48, 4543–4545. doi:10.1002/anie.200900892 |
| 7. | Amaye, I. J.; Haywood, R. D.; Mandzo, E. M.; Wirick, J. J.; Jackson-Ayotunde, P. L. Tetrahedron 2021, 83, 131984. doi:10.1016/j.tet.2021.131984 |
| 32. | Yu, D.; Sum, Y. N.; Ean, A. C. C.; Chin, M. P.; Zhang, Y. Angew. Chem., Int. Ed. 2013, 52, 5125–5128. doi:10.1002/anie.201301019 |
| 27. | Amaye, I. J.; Heinbockel, T.; Woods, J.; Wang, Z.; Martin-Caraballo, M.; Jackson-Ayotunde, P. Int. J. Environ. Res. Public Health 2018, 15, 1784. doi:10.3390/ijerph15081784 |
| 29. | Ghorab, M. M.; Ragab, F. A.; Heiba, H. I.; El-Gazzar, M. G.; El-Gazzar, M. G. M. Bioorg. Med. Chem. Lett. 2018, 28, 1464–1470. doi:10.1016/j.bmcl.2018.03.089 |
| 45. | Maji, T.; Karmakar, A.; Reiser, O. J. Org. Chem. 2011, 76, 736–739. doi:10.1021/jo102239x |
| 46. | Nguyen, J. D.; D'Amato, E. M.; Narayanam, J. M. R.; Stephenson, C. R. J. Nat. Chem. 2012, 4, 854–859. doi:10.1038/nchem.1452 |
| 26. | Masocha, W.; Kombian, S. B.; Edafiogho, I. O. Sci. Rep. 2016, 6, 21582. doi:10.1038/srep21582 |
| 30. | Liu, J.-Y.; Cao, G.-E.; Xu, W.; Cao, J.; Wang, W.-L. Appl. Organomet. Chem. 2010, 24, 685–691. doi:10.1002/aoc.1667 |
| 25. | Kumar, R.; Saha, N.; Purohit, P.; Garg, S. K.; Seth, K.; Meena, V. S.; Dubey, S.; Dave, K.; Goyal, R.; Sharma, S. S.; Banerjee, U. C.; Chakraborti, A. K. Eur. J. Med. Chem. 2019, 182, 111601. doi:10.1016/j.ejmech.2019.111601 |
| 43. | De, S.; Kumar S K, A.; Shah, S. K.; Kazi, S.; Sarkar, N.; Banerjee, S.; Dey, S. RSC Adv. 2022, 12, 15385–15406. doi:10.1039/d2ra01571d |
| 24. | Cindrić, M.; Rubčić, M.; Hrenar, T.; Pisk, J.; Cvijanović, D.; Lovrić, J.; Vrdoljak, V. J. Mol. Struct. 2018, 1154, 636–642. doi:10.1016/j.molstruc.2017.10.078 |
| 28. | Bangalore, P. K.; Vagolu, S. K.; Bollikanda, R. K.; Veeragoni, D. K.; Choudante, P. C.; Misra, S.; Sriram, D.; Sridhar, B.; Kantevari, S. J. Nat. Prod. 2020, 83, 26–35. doi:10.1021/acs.jnatprod.9b00475 |
| 44. | Goldcamp, M. J.; Edison, S. E.; Squires, L. N.; Rosa, D. T.; Vowels, N. K.; Coker, N. L.; Krause Bauer, J. A.; Baldwin, M. J. Inorg. Chem. 2003, 42, 717–728. doi:10.1021/ic025860q |
© 2025 Pérez-Ramos et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.