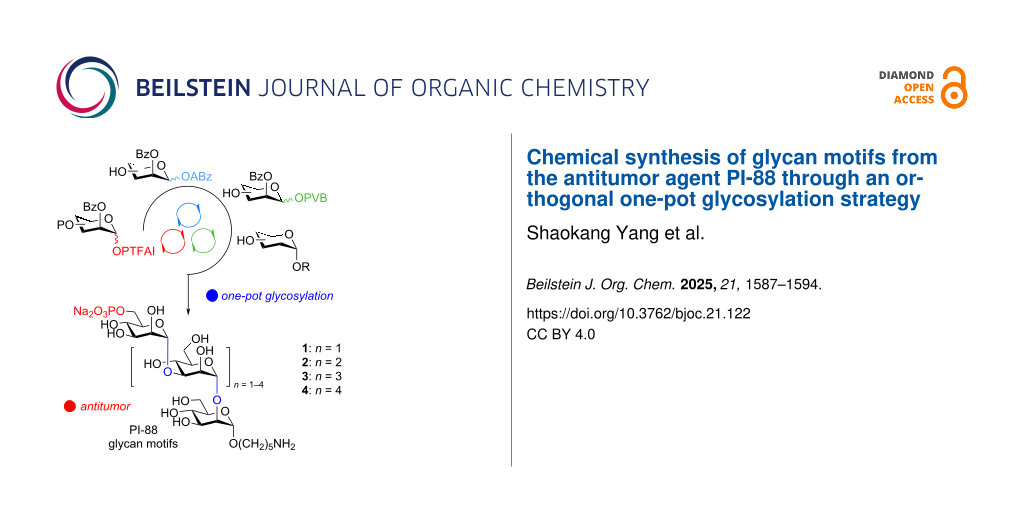
Abstract
Chemical synthesis of monophosphorylated glycan motifs from the antitumor agent PI-88 has been achieved through an orthogonal one-pot glycosylation strategy on the basis of glycosyl ortho-(1-phenylvinyl)benzoates, which not only accelerated synthesis, but also precluded the potential issues inherent to one-pot glycan assembly associated with thioglycosides. The following aspects were featured in synthetic approaches: 1) synthesis of trisaccharide and tetrasaccharide PI-88 glycans via [1 + 1 + 1] and [1 + 1 + 1 + 1] one-pot orthogonal glycosylation, respectively; 2) synthesis of PI-88 glycan motif pentasaccharide via [1 + 1 + 1] and [1 + 1 + 3] one-pot orthogonal glycosylation; 3) synthesis of hexasaccharide via [1 + 1 + 1] and [1 + 1 + 1 + 3] one-pot assembly.
Graphical Abstract
Introduction
Carbohydrates as one of four essential biomolecules have been widely recognized as important targets for the development of carbohydrate-based therapeutics [1-18]. The example in point is the antitumor agent PI-88 (muparfostat), which retards tumor growth via inhibiting angiogenesis in two ways: 1) interaction with pro-angiogenic growth factors such as vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) and 2) by prevention of the release of angiogenic growth factors from the extracellular matrix (ECM) via inhibition of heparanase [19-22]. PI-88 is a complex mixture of monophosphorylated, highly sulfated mannose glycans derived from the extracellular phosphomannan of Pichia holstii NRRL Y-2448 yeast [23-25], which had progressed to phase III clinical trials for post-resection hepatocellular carcinoma [26]. Interestingly, Ferro and co-workers revised the structure of PI-88 to I and II in 2017 via successful separation of oligosaccharide phosphate fractions by preparative ion-exchange chromatography (Scheme 1A) [27]. Besides the major components α(1→3)/α(1→2)-linked pentasaccharide (≈60%) and tetrasaccharide (≈30%) in I, the minor components of all α(1→3)-linked mannosides were also present in II.
Scheme 1: (A) Glycan structures of PI-88 and (B) retrosynthetic analysis of PI-88 glycan motifs 1–4.
Scheme 1: (A) Glycan structures of PI-88 and (B) retrosynthetic analysis of PI-88 glycan motifs 1–4.
During the past two decades, several strategies have been developed to synthesize glycan motifs from PI-88 [28-36]. In comparison with previous, traditional, and time-consuming synthesis of PI-88 glycan components, the one-pot glycan assembly strategy has some advantages, including: 1) acceleration of glycan synthesis, 2) avoidance of purification of intermediates during glycosylation intervals, and 3) reduction of chemical waste [37-42]. Recently, we introduced a new one-pot glycosylation strategy on the basis of recently developed glycosyl ortho-(1-phenylvinyl)benzoate (PVB) [43-45] donors from our group, which has been successfully applied to the streamline synthesis of various glycans from oligosaccharides to polysaccharides such as mannose-capped lipoarabinomannan motifs up to 101-mer from the Mycobacterium tuberculosis cell wall, nona-decasaccharide motif from Ganoderma sinense, and tridesaccharide motif from Bacteroides vulgatus lipopolysaccharides [46-56]. Here, we report the chemical synthesis of monophosphorylated glycan motifs 1–4 from PI-88 through an orthogonal one-pot glycosylation strategy via strategic combinations of glycosyl N-phenyltrifluoroacetimidates (PTFAI) [57,58], glycosyl ortho-(alkynylbenzoates) [59,60] (ABz), and glycosyl PVB, which precluded the potential issues inherent to one-pot glycosylation based on thioglycosides such as aglycone transfer [43-45,61].
Results and Discussion
Retrosynthetic analysis
Retrosynthetically, we envisaged that glycans 1–4 could be derived from monosaccharide building blocks Man PTFAI 5 and 6, Man ABz 7, Man PVB 8, and Man 9 through orthogonal one-pot glycosylation strategy (Scheme 1B). The 2-O-Bz group in 5–8 served as the neighboring participating group for the stereoselective construction of 1,2-trans-mannosidic bonds, while the 3-O-Lev group in 6 was the temporary protecting group for (1→3)-branching. The C6–OH group in 5 was protected as TBDPS group, which could be selectively replaced by the destined phosphate residue.
One-pot synthesis of glycans 1 and 2
We commenced with the synthesis of monophosphorylated trisaccharide 1 (Scheme 2A). Glycosylation of mannosyl PTFAI 5 (1.2 equiv) with 3-OH in mannosyl PVB 8 (1.0 equiv) in the presence of TMSOTf as catalyst proceeded smoothly at 0 °C to room temperature, affording the α-Man-(1→3)-Man PVB disaccharide. The further coupling of the above PVB disaccharide with the poorly reactive 2-OH in mannoside 9 (0.9 equiv) under activation with NIS and TMSOTf at 0 °C to room temperature, successfully furnished the desired α-Man-(1→3)-α-Man-(1→3)-α-Man trisaccharide 10 in 87% yield in a one pot manner. Removal of TBDPS group in 10 with 70% HF·pyridine and subsequent phosphitylation of the resulting free alcohol with phosphoramidite 11 provided the desired phosphite, which was further oxidized by 3-chloroperoxybenzoic aicd (mCPBA) at −78 °C to 0 °C, producing the desired phosphorylated fully protected trisaccharide 12 in 79% overall yield over three steps. Removal of all protecting groups in trisaccharide 12 is a challenging task due to the presence of polar groups, including phosphoryl acid and amine groups [62]. After several optimizations, the following sequence was adopted to remove all Bn, Bz, and Cbz groups: 1) global hydrogenolysis of Bn and Cbz groups in 12 with Pd(OH)2/C in a mixed solvent (THF/MeOH/AcOH/H2O) and 2) saponification of all Bz groups with 1 M NaOH (dioxane/MeOH/H2O, room temperature). The monophosphorylated trisaccharide 1 was obtained in 60% overall yield over two steps from 12 after purification over a SephadexTM LH-20 column. It was noted that the switch of deprotection sequences (first Bz groups, second Bn and Cbz groups) failed to efficiently produce trisaccharide 1.
Scheme 2: One-pot synthesis of glycans 1 and 2.
Scheme 2: One-pot synthesis of glycans 1 and 2.
The synthesis of monophosphorylated tetrasaccharide 2 was next investigated (Scheme 2B). TMSOTf was used to activate Man PTFAI 5 (1.1 equiv) in the presence of mannosyl ABz 7 (1.0 equiv) at 0 °C to room temperature, readily producing the α-Man-(1→3)-Man ABz disaccharide. Yu glycosylation of the above ABz disaccharide with 3-OH in Man PVB 8 (0.9 equiv) under the catalysis of PhP3AuOTf at room temperature successfully gave α-Man-(1→3)-α-Man-(1→3)-Man PVB trisaccharide, which was further coupled with the poorly reactive C2–OH in mannoside 9 (0.8 equiv) in the presence of NIS and TMSOTf at 0 °C to rt, uneventfully furnishing the desired tetrasaccharide α-Man-(1→3)-α-Man-(1→3)-α-Man-(1→2)-α-Man 13 in 69% yield in the same flask. The TBDPS-protected 13 was readily converted to phosphorylated protected tetrasaccharide 14 in 89% ovall yield over the following steps: 1) deprotection of the TBDPS group, 2) phosphitylation of the free alcohol with phosphoramidite 11 in the presence of 1H-tetrazole and 4 Å MS, and 3) oxidation of the phosphite by mCPBA. Hydrogenolysis of Bn and Cbz groups in 14 with Pd(OH)2/C and subsequent saponification of all Bz groups with 1 M NaOH successfully produced monophosphorylated tetrasaccharide 2 in 63% overall yield.
One-pot synthesis of glycans 3 and 4
Furthermore, we investigated the synthesis of monophosphorylated pentasaccharide 3 (Scheme 3). Orthogonal one-pot glycosylation of Man PTFAI 6 (1.2 equiv), Man PVB 8 (1.0 equiv), and mannoside 9 (0.9 equiv) readily generated α-Man-(1→3)-α-Man-(1→2)-α-Man trisaccharide 15 with 86% yield in one pot. The further sequential [1 + 1 + 3] one-pot orthogonal glycosylation of Man PTFAI 5 (1.1 equiv), Man PVB 8 (1.0 equiv), and trisaccharide 16 (0.9 equiv) derived from 15 via selective removal of the Lev group with NH2NH2·H2O successfully generated the desired pentasaccharide α-Man-(1→3)-α-Man-(1→3)-α-Man -(1→3)-α-Man-(1→2)-α-Man 17 in 83% yield in a one-pot manner, which was readily converted to the phosphorylated protected pentasaccharide 18 in 92% overall yield via the switch of the TBDPS group with the phosphate group. First global deprotection of Bn and Cbz groups in 18 with Pd(OH)2/C, followed by saponifications of all Bz groups with 1 M NaOH provided the desired monophosphorylated pentasaccharide 3 in 56% overall yield, which is the major glycan motif from PI-88.
Finally, the synthesis of the monophosphorylated hexasaccharide 4 was studied (Scheme 4). Orthogonal one-pot coupling of Man PTFAI 5 (1.1 equiv), Man ABz 7 (1.0 equiv), PVB 8 (0.9 equiv), and α-Man-(1→3)-α-Man-(1→2)-α-Man trisaccharide 16 (0.8 equiv) proceeded uneventfully, successfully producing the desired α-Man-(1→3)-α-Man-(1→3)-α-Man-(1→3)-α-Man-(1→3)-α-Man-(1→2)-α-Man hexasaccharide 19 in 66% yield in the same flask. The TBDPS group in 19 was readily converted to a phosphate group in 20 with 88% overall yield over three steps. The desired monophosphorylated hexasaccharide 4 was obtained in 60% overall yield from 20 via sequential global deprotection of the Bn, Cbz, and Bz groups.
The structures of the synthetic glycan motifs 1–4 were supported by their 1H and 13C NMR spectra and MALDI–TOF as well as ESI mass spectra. In particular, the anomeric proton signals of 1–4 were highlighted in the 1H NMR spectra of synthetic glycans motifs 1–4 (see Supporting Information File 1).
Conclusion
In summary, the monophosphorylated glycan motifs 1–4 from PI-88 have been collectively synthesized via a one-pot orthogonal glycosylation strategy on the basis of glycosyl PVB, which avoids such issues as aglycon transfer inherent to one-pot glycosylations based on thioglycosides. Specifically, the following features were highlighted in our synthetic approach: 1) [1 + 1 + 1] one-pot orthogonal glycosylation for the synthesis of trisaccharide 1; 2) [1 + 1 + 1 + 1] orthogonal one-pot glycosylation for the synthesis of tetrasaccharide 2; 3) [1 + 1 + 1] and [1 + 1 + 3] orthogonal one-pot assembly of pentasaccharide 3; 4) [1 + 1 + 1] and [1 + 1 + 1 + 3] orthogonal one-pot assembly of hexasaccharide 4.
Supporting Information
| Supporting Information File 1: Experimental procedures and spectral data for all new compounds including 1H NMR, 13C NMR, and HRMS. | ||
| Format: PDF | Size: 6.7 MB | Download |
Funding
The financial support from the National Natural Science Foundations of China (22322110), the Yunnan Revitalization Talent Support Program: Yunling Scholar Project, the Yunnan Fundamental Research Projects (grant NO. 202501AV070010), the Young Talents Project of High-level Talent Introduction Program of Yunnan Province and the Yunnan Province Science and Technology Department (202305AH340005) are greatly acknowledged.
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article
References
-
Ramadan, S.; Mayieka, M.; Pohl, N. L. B.; Liu, J.; Hsieh-Wilson, L. C.; Huang, X. Curr. Opin. Chem. Biol. 2024, 80, 102455. doi:10.1016/j.cbpa.2024.102455
Return to citation in text: [1] -
Qin, C.; Tian, G.; Hu, J.; Zou, X.; Yin, J. Curr. Opin. Chem. Biol. 2024, 78, 102424. doi:10.1016/j.cbpa.2023.102424
Return to citation in text: [1] -
Wang, X.; Xiao, G. Curr. Opin. Chem. Biol. 2023, 77, 102387. doi:10.1016/j.cbpa.2023.102387
Return to citation in text: [1] -
Shang, W.; Niu, D. Acc. Chem. Res. 2023, 56, 2473–2488. doi:10.1021/acs.accounts.3c00374
Return to citation in text: [1] -
Wang, S.; Yang, Y.; Zhu, Q.; Lin, G.-Q.; Yu, B. Curr. Opin. Chem. Biol. 2022, 69, 102154. doi:10.1016/j.cbpa.2022.102154
Return to citation in text: [1] -
Del Bino, L.; Østerlid, K. E.; Wu, D.-Y.; Nonne, F.; Romano, M. R.; Codée, J.; Adamo, R. Chem. Rev. 2022, 122, 15672–15716. doi:10.1021/acs.chemrev.2c00021
Return to citation in text: [1] -
Li, J.; Nguyen, H. M. Acc. Chem. Res. 2022, 55, 3738–3751. doi:10.1021/acs.accounts.2c00636
Return to citation in text: [1] -
Di Lorenzo, F.; Duda, K. A.; Lanzetta, R.; Silipo, A.; De Castro, C.; Molinaro, A. Chem. Rev. 2022, 122, 15767–15821. doi:10.1021/acs.chemrev.0c01321
Return to citation in text: [1] -
Seeberger, P. H. Chem. Rev. 2021, 121, 3598–3626. doi:10.1021/acs.chemrev.0c01210
Return to citation in text: [1] -
Krasnova, L.; Wong, C.-H. J. Am. Chem. Soc. 2019, 141, 3735–3754. doi:10.1021/jacs.8b11005
Return to citation in text: [1] -
Kulkarni, S. S.; Wang, C.-C.; Sabbavarapu, N. M.; Podilapu, A. R.; Liao, P.-H.; Hung, S.-C. Chem. Rev. 2018, 118, 8025–8104. doi:10.1021/acs.chemrev.8b00036
Return to citation in text: [1] -
Bennett, C. S.; Galan, M. C. Chem. Rev. 2018, 118, 7931–7985. doi:10.1021/acs.chemrev.7b00731
Return to citation in text: [1] -
Panza, M.; Pistorio, S. G.; Stine, K. J.; Demchenko, A. V. Chem. Rev. 2018, 118, 8105–8150. doi:10.1021/acs.chemrev.8b00051
Return to citation in text: [1] -
Leng, W.-L.; Yao, H.; He, J.-X.; Liu, X.-W. Acc. Chem. Res. 2018, 51, 628–639. doi:10.1021/acs.accounts.7b00449
Return to citation in text: [1] -
Peng, P.; Schmidt, R. R. Acc. Chem. Res. 2017, 50, 1171–1183. doi:10.1021/acs.accounts.6b00518
Return to citation in text: [1] -
Danishefsky, S. J.; Shue, Y.-K.; Chang, M. N.; Wong, C.-H. Acc. Chem. Res. 2015, 48, 643–652. doi:10.1021/ar5004187
Return to citation in text: [1] -
Astronomo, R. D.; Burton, D. R. Nat. Rev. Drug Discovery 2010, 9, 308–324. doi:10.1038/nrd3012
Return to citation in text: [1] -
Boltje, T. J.; Buskas, T.; Boons, G.-J. Nat. Chem. 2009, 1, 611–622. doi:10.1038/nchem.399
Return to citation in text: [1] -
Kudchadkar, R.; Gonzalez, R.; Lewis, K. D. Expert Opin. Invest. Drugs 2008, 17, 1769–1776. doi:10.1517/13543784.17.11.1769
Return to citation in text: [1] -
Khachigian, L. M.; Parish, C. R. Cardiovasc. Drug Rev. 2004, 22, 1–6. doi:10.1111/j.1527-3466.2004.tb00127.x
Return to citation in text: [1] -
Chhabra, M.; Ferro, V. PI-88 and Related Heparan Sulfate Mimetics. Heparanase; Advances in Experimental Medicine and Biology, Vol. 1221; Springer: Cham, Switzerland, 2020; pp 473–491. doi:10.1007/978-3-030-34521-1_19
Return to citation in text: [1] -
Ferro, V.; Dredge, K.; Liu, L.; Hammond, E.; Bytheway, I.; Li, C.; Johnstone, K.; Karoli, T.; Davis, K.; Copeman, E.; Gautam, A. Semin. Thromb. Hemostasis 2007, 33, 557–568. doi:10.1055/s-2007-982088
Return to citation in text: [1] -
Ferro, V.; Fewings, K.; Palermo, M. C.; Li, C. Carbohydr. Res. 2001, 332, 183–189. doi:10.1016/s0008-6215(01)00061-1
Return to citation in text: [1] -
Yu, G.; Gunay, N. S.; Linhardt, R. J.; Toida, T.; Fareed, J.; Hoppensteadt, D. A.; Shadid, H.; Ferro, V.; Li, C.; Fewings, K.; Palermo, M. C.; Podger, D. Eur. J. Med. Chem. 2002, 37, 783–791. doi:10.1016/s0223-5234(02)01347-8
Return to citation in text: [1] -
Elli, S.; Stancanelli, E.; Handley, P. N.; Carroll, A.; Urso, E.; Guerrini, M.; Ferro, V. Glycobiology 2018, 28, 731–740. doi:10.1093/glycob/cwy068
Return to citation in text: [1] -
Chen, P.-J.; Lee, P.-H.; Han, K.-H.; Fan, J.; Cheung, T. T.; Hu, R.-H.; Paik, S. W.; Lee, W.-C.; Chau, G.-Y.; Jeng, L.-B.; Wang, H. J.; Choi, J. Y.; Chen, C.-L.; Cho, M.; Ho, M.-C.; Wu, C.-C.; Lee, K. S.; Mao, Y.; Hu, F.-C.; Lai, K.-L. Ann. Oncol. 2017, 28, v213. doi:10.1093/annonc/mdx369.008
Return to citation in text: [1] -
Handley, P. N.; Carroll, A.; Ferro, V. Carbohydr. Res. 2017, 446-447, 68–75. doi:10.1016/j.carres.2017.05.008
Return to citation in text: [1] -
Ventura, J.; Uriel, C.; Gómez, A. M.; López, J. C. Carbohydr. Res. 2022, 516, 108557. doi:10.1016/j.carres.2022.108557
Return to citation in text: [1] -
Mong, K.-K. T.; Shiau, K.-S.; Lin, Y. H.; Cheng, K.-C.; Lin, C.-H. Org. Biomol. Chem. 2015, 13, 11550–11560. doi:10.1039/c5ob01786f
Return to citation in text: [1] -
Liu, L.; Johnstone, K. D.; Fairweather, J. K.; Dredge, K.; Ferro, V. Aust. J. Chem. 2009, 62, 546. doi:10.1071/ch09015
Return to citation in text: [1] -
Valerio, S.; Pastore, A.; Adinolfi, M.; Iadonisi, A. J. Org. Chem. 2008, 73, 4496–4503. doi:10.1021/jo8003953
Return to citation in text: [1] -
Fairweather, J. K.; Hammond, E.; Johnstone, K. D.; Ferro, V. Bioorg. Med. Chem. 2008, 16, 699–709. doi:10.1016/j.bmc.2007.10.044
Return to citation in text: [1] -
Namme, R.; Mitsugi, T.; Takahashi, H.; Ikegami, S. Tetrahedron Lett. 2005, 46, 3033–3036. doi:10.1016/j.tetlet.2005.03.016
Return to citation in text: [1] -
Gu, G.; Wei, G.; Du, Y. Carbohydr. Res. 2004, 339, 1155–1162. doi:10.1016/j.carres.2004.01.020
Return to citation in text: [1] -
Fairweather, J. K.; Karoli, T.; Ferro, V. Bioorg. Med. Chem. 2004, 12, 6063–6075. doi:10.1016/j.bmc.2004.09.005
Return to citation in text: [1] -
Zhou, J.; Lv, S.; Zhang, D.; Xia, F.; Hu, W. J. Org. Chem. 2017, 82, 2599–2621. doi:10.1021/acs.joc.6b03017
Return to citation in text: [1] -
Hu, C.; Wu, S.; He, F.; Cai, D.; Xu, Z.; Ma, W.; Liu, Y.; Wei, B.; Li, T.; Ding, K. Angew. Chem., Int. Ed. 2022, 61, e202202554. doi:10.1002/anie.202202554
Return to citation in text: [1] -
Xiao, X.; Zeng, J.; Fang, J.; Sun, J.; Li, T.; Song, Z.; Cai, L.; Wan, Q. J. Am. Chem. Soc. 2020, 142, 5498–5503. doi:10.1021/jacs.0c00447
Return to citation in text: [1] -
Cheng, C.-W.; Wu, C.-Y.; Hsu, W.-L.; Wong, C.-H. Biochemistry 2020, 59, 3078–3088. doi:10.1021/acs.biochem.9b00613
Return to citation in text: [1] -
Huang, X.; Huang, L.; Wang, H.; Ye, X.-S. Angew. Chem., Int. Ed. 2004, 43, 5221–5224. doi:10.1002/anie.200460176
Return to citation in text: [1] -
Zhang, Y.; Xiang, G.; He, S.; Hu, Y.; Liu, Y.; Xu, L.; Xiao, G. Org. Lett. 2019, 21, 2335–2339. doi:10.1021/acs.orglett.9b00617
Return to citation in text: [1] -
Zhang, Y.; Chen, Z.; Huang, Y.; He, S.; Yang, X.; Wu, Z.; Wang, X.; Xiao, G. Angew. Chem., Int. Ed. 2020, 59, 7576–7584. doi:10.1002/anie.202000992
Return to citation in text: [1] -
Li, P.; He, H.; Zhang, Y.; Yang, R.; Xu, L.; Chen, Z.; Huang, Y.; Bao, L.; Xiao, G. Nat. Commun. 2020, 11, 405. doi:10.1038/s41467-020-14295-z
Return to citation in text: [1] [2] -
He, H.; Xu, L.; Sun, R.; Zhang, Y.; Huang, Y.; Chen, Z.; Li, P.; Yang, R.; Xiao, G. Chem. Sci. 2021, 12, 5143–5151. doi:10.1039/d0sc06815b
Return to citation in text: [1] [2] -
Xiao, G. Acc. Chem. Res. 2025, 58, 2350–2363. doi:10.1021/acs.accounts.5c00387
Return to citation in text: [1] [2] -
Ma, Y.; Zhang, Y.; Huang, Y.; Chen, Z.; Xian, Q.; Su, R.; Jiang, Q.; Wang, X.; Xiao, G. J. Am. Chem. Soc. 2024, 146, 4112–4122. doi:10.1021/jacs.3c12815
Return to citation in text: [1] -
Chen, Z.; Xiao, G. J. Am. Chem. Soc. 2024, 146, 17446–17455. doi:10.1021/jacs.4c05188
Return to citation in text: [1] -
Zhang, Y.; Wang, L.; Zhou, Q.; Li, Z.; Li, D.; Yin, C.; Wang, X.; Xiao, G. Angew. Chem., Int. Ed. 2023, 62, e202301351. doi:10.1002/anie.202301351
Return to citation in text: [1] -
Zhang, Y.; He, H.; Chen, Z.; Huang, Y.; Xiang, G.; Li, P.; Yang, X.; Lu, G.; Xiao, G. Angew. Chem., Int. Ed. 2021, 60, 12597–12606. doi:10.1002/anie.202103826
Return to citation in text: [1] -
Zhang, Y.; Hu, Y.; Liu, S.; He, H.; Sun, R.; Lu, G.; Xiao, G. Chem. Sci. 2022, 13, 7755–7764. doi:10.1039/d2sc02176e
Return to citation in text: [1] -
Shou, K.; Zhang, Y.; Ji, Y.; Liu, B.; Zhou, Q.; Tan, Q.; Li, F.; Wang, X.; Lu, G.; Xiao, G. Chem. Sci. 2024, 15, 6552–6561. doi:10.1039/d4sc01348d
Return to citation in text: [1] -
Li, P.; Fan, H.; Tan, Q.; Xiao, G. Org. Lett. 2023, 25, 2788–2792. doi:10.1021/acs.orglett.3c00670
Return to citation in text: [1] -
Sun, X.; Chen, Z.; Yang, R.; Wang, M.; Wang, X.; Zhang, Q.; Xiao, G. Org. Lett. 2023, 25, 7364–7368. doi:10.1021/acs.orglett.3c02842
Return to citation in text: [1] -
Chen, Z.; Xiao, G. Org. Lett. 2023, 25, 7395–7399. doi:10.1021/acs.orglett.3c02898
Return to citation in text: [1] -
Ma, Y.; Jiang, Q.; Wang, X.; Xiao, G. Org. Lett. 2022, 24, 7950–7954. doi:10.1021/acs.orglett.2c03081
Return to citation in text: [1] -
Shou, K.; Liu, S.; Zhang, Y.; Xiao, G. Chin. J. Chem. 2024, 42, 1593–1598. doi:10.1002/cjoc.202400121
Return to citation in text: [1] -
Yu, B.; Sun, J. Chem. Commun. 2010, 46, 4668. doi:10.1039/c0cc00563k
Return to citation in text: [1] -
Yu, B.; Tao, H. Tetrahedron Lett. 2001, 42, 2405–2407. doi:10.1016/s0040-4039(01)00157-5
Return to citation in text: [1] -
Li, Y.; Yang, Y.; Yu, B. Tetrahedron Lett. 2008, 49, 3604–3608. doi:10.1016/j.tetlet.2008.04.017
Return to citation in text: [1] -
Yu, B. Acc. Chem. Res. 2018, 51, 507–516. doi:10.1021/acs.accounts.7b00573
Return to citation in text: [1] -
Christensen, H. M.; Oscarson, S.; Jensen, H. H. Carbohydr. Res. 2015, 408, 51–95. doi:10.1016/j.carres.2015.02.007
Return to citation in text: [1] -
Zhu, Q.; Shen, Z.; Chiodo, F.; Nicolardi, S.; Molinaro, A.; Silipo, A.; Yu, B. Nat. Commun. 2020, 11, 4142. doi:10.1038/s41467-020-17992-x
Return to citation in text: [1]
| 1. | Ramadan, S.; Mayieka, M.; Pohl, N. L. B.; Liu, J.; Hsieh-Wilson, L. C.; Huang, X. Curr. Opin. Chem. Biol. 2024, 80, 102455. doi:10.1016/j.cbpa.2024.102455 |
| 2. | Qin, C.; Tian, G.; Hu, J.; Zou, X.; Yin, J. Curr. Opin. Chem. Biol. 2024, 78, 102424. doi:10.1016/j.cbpa.2023.102424 |
| 3. | Wang, X.; Xiao, G. Curr. Opin. Chem. Biol. 2023, 77, 102387. doi:10.1016/j.cbpa.2023.102387 |
| 4. | Shang, W.; Niu, D. Acc. Chem. Res. 2023, 56, 2473–2488. doi:10.1021/acs.accounts.3c00374 |
| 5. | Wang, S.; Yang, Y.; Zhu, Q.; Lin, G.-Q.; Yu, B. Curr. Opin. Chem. Biol. 2022, 69, 102154. doi:10.1016/j.cbpa.2022.102154 |
| 6. | Del Bino, L.; Østerlid, K. E.; Wu, D.-Y.; Nonne, F.; Romano, M. R.; Codée, J.; Adamo, R. Chem. Rev. 2022, 122, 15672–15716. doi:10.1021/acs.chemrev.2c00021 |
| 7. | Li, J.; Nguyen, H. M. Acc. Chem. Res. 2022, 55, 3738–3751. doi:10.1021/acs.accounts.2c00636 |
| 8. | Di Lorenzo, F.; Duda, K. A.; Lanzetta, R.; Silipo, A.; De Castro, C.; Molinaro, A. Chem. Rev. 2022, 122, 15767–15821. doi:10.1021/acs.chemrev.0c01321 |
| 9. | Seeberger, P. H. Chem. Rev. 2021, 121, 3598–3626. doi:10.1021/acs.chemrev.0c01210 |
| 10. | Krasnova, L.; Wong, C.-H. J. Am. Chem. Soc. 2019, 141, 3735–3754. doi:10.1021/jacs.8b11005 |
| 11. | Kulkarni, S. S.; Wang, C.-C.; Sabbavarapu, N. M.; Podilapu, A. R.; Liao, P.-H.; Hung, S.-C. Chem. Rev. 2018, 118, 8025–8104. doi:10.1021/acs.chemrev.8b00036 |
| 12. | Bennett, C. S.; Galan, M. C. Chem. Rev. 2018, 118, 7931–7985. doi:10.1021/acs.chemrev.7b00731 |
| 13. | Panza, M.; Pistorio, S. G.; Stine, K. J.; Demchenko, A. V. Chem. Rev. 2018, 118, 8105–8150. doi:10.1021/acs.chemrev.8b00051 |
| 14. | Leng, W.-L.; Yao, H.; He, J.-X.; Liu, X.-W. Acc. Chem. Res. 2018, 51, 628–639. doi:10.1021/acs.accounts.7b00449 |
| 15. | Peng, P.; Schmidt, R. R. Acc. Chem. Res. 2017, 50, 1171–1183. doi:10.1021/acs.accounts.6b00518 |
| 16. | Danishefsky, S. J.; Shue, Y.-K.; Chang, M. N.; Wong, C.-H. Acc. Chem. Res. 2015, 48, 643–652. doi:10.1021/ar5004187 |
| 17. | Astronomo, R. D.; Burton, D. R. Nat. Rev. Drug Discovery 2010, 9, 308–324. doi:10.1038/nrd3012 |
| 18. | Boltje, T. J.; Buskas, T.; Boons, G.-J. Nat. Chem. 2009, 1, 611–622. doi:10.1038/nchem.399 |
| 27. | Handley, P. N.; Carroll, A.; Ferro, V. Carbohydr. Res. 2017, 446-447, 68–75. doi:10.1016/j.carres.2017.05.008 |
| 26. | Chen, P.-J.; Lee, P.-H.; Han, K.-H.; Fan, J.; Cheung, T. T.; Hu, R.-H.; Paik, S. W.; Lee, W.-C.; Chau, G.-Y.; Jeng, L.-B.; Wang, H. J.; Choi, J. Y.; Chen, C.-L.; Cho, M.; Ho, M.-C.; Wu, C.-C.; Lee, K. S.; Mao, Y.; Hu, F.-C.; Lai, K.-L. Ann. Oncol. 2017, 28, v213. doi:10.1093/annonc/mdx369.008 |
| 23. | Ferro, V.; Fewings, K.; Palermo, M. C.; Li, C. Carbohydr. Res. 2001, 332, 183–189. doi:10.1016/s0008-6215(01)00061-1 |
| 24. | Yu, G.; Gunay, N. S.; Linhardt, R. J.; Toida, T.; Fareed, J.; Hoppensteadt, D. A.; Shadid, H.; Ferro, V.; Li, C.; Fewings, K.; Palermo, M. C.; Podger, D. Eur. J. Med. Chem. 2002, 37, 783–791. doi:10.1016/s0223-5234(02)01347-8 |
| 25. | Elli, S.; Stancanelli, E.; Handley, P. N.; Carroll, A.; Urso, E.; Guerrini, M.; Ferro, V. Glycobiology 2018, 28, 731–740. doi:10.1093/glycob/cwy068 |
| 62. | Zhu, Q.; Shen, Z.; Chiodo, F.; Nicolardi, S.; Molinaro, A.; Silipo, A.; Yu, B. Nat. Commun. 2020, 11, 4142. doi:10.1038/s41467-020-17992-x |
| 19. | Kudchadkar, R.; Gonzalez, R.; Lewis, K. D. Expert Opin. Invest. Drugs 2008, 17, 1769–1776. doi:10.1517/13543784.17.11.1769 |
| 20. | Khachigian, L. M.; Parish, C. R. Cardiovasc. Drug Rev. 2004, 22, 1–6. doi:10.1111/j.1527-3466.2004.tb00127.x |
| 21. | Chhabra, M.; Ferro, V. PI-88 and Related Heparan Sulfate Mimetics. Heparanase; Advances in Experimental Medicine and Biology, Vol. 1221; Springer: Cham, Switzerland, 2020; pp 473–491. doi:10.1007/978-3-030-34521-1_19 |
| 22. | Ferro, V.; Dredge, K.; Liu, L.; Hammond, E.; Bytheway, I.; Li, C.; Johnstone, K.; Karoli, T.; Davis, K.; Copeman, E.; Gautam, A. Semin. Thromb. Hemostasis 2007, 33, 557–568. doi:10.1055/s-2007-982088 |
| 46. | Ma, Y.; Zhang, Y.; Huang, Y.; Chen, Z.; Xian, Q.; Su, R.; Jiang, Q.; Wang, X.; Xiao, G. J. Am. Chem. Soc. 2024, 146, 4112–4122. doi:10.1021/jacs.3c12815 |
| 47. | Chen, Z.; Xiao, G. J. Am. Chem. Soc. 2024, 146, 17446–17455. doi:10.1021/jacs.4c05188 |
| 48. | Zhang, Y.; Wang, L.; Zhou, Q.; Li, Z.; Li, D.; Yin, C.; Wang, X.; Xiao, G. Angew. Chem., Int. Ed. 2023, 62, e202301351. doi:10.1002/anie.202301351 |
| 49. | Zhang, Y.; He, H.; Chen, Z.; Huang, Y.; Xiang, G.; Li, P.; Yang, X.; Lu, G.; Xiao, G. Angew. Chem., Int. Ed. 2021, 60, 12597–12606. doi:10.1002/anie.202103826 |
| 50. | Zhang, Y.; Hu, Y.; Liu, S.; He, H.; Sun, R.; Lu, G.; Xiao, G. Chem. Sci. 2022, 13, 7755–7764. doi:10.1039/d2sc02176e |
| 51. | Shou, K.; Zhang, Y.; Ji, Y.; Liu, B.; Zhou, Q.; Tan, Q.; Li, F.; Wang, X.; Lu, G.; Xiao, G. Chem. Sci. 2024, 15, 6552–6561. doi:10.1039/d4sc01348d |
| 52. | Li, P.; Fan, H.; Tan, Q.; Xiao, G. Org. Lett. 2023, 25, 2788–2792. doi:10.1021/acs.orglett.3c00670 |
| 53. | Sun, X.; Chen, Z.; Yang, R.; Wang, M.; Wang, X.; Zhang, Q.; Xiao, G. Org. Lett. 2023, 25, 7364–7368. doi:10.1021/acs.orglett.3c02842 |
| 54. | Chen, Z.; Xiao, G. Org. Lett. 2023, 25, 7395–7399. doi:10.1021/acs.orglett.3c02898 |
| 55. | Ma, Y.; Jiang, Q.; Wang, X.; Xiao, G. Org. Lett. 2022, 24, 7950–7954. doi:10.1021/acs.orglett.2c03081 |
| 56. | Shou, K.; Liu, S.; Zhang, Y.; Xiao, G. Chin. J. Chem. 2024, 42, 1593–1598. doi:10.1002/cjoc.202400121 |
| 59. | Li, Y.; Yang, Y.; Yu, B. Tetrahedron Lett. 2008, 49, 3604–3608. doi:10.1016/j.tetlet.2008.04.017 |
| 60. | Yu, B. Acc. Chem. Res. 2018, 51, 507–516. doi:10.1021/acs.accounts.7b00573 |
| 43. | Li, P.; He, H.; Zhang, Y.; Yang, R.; Xu, L.; Chen, Z.; Huang, Y.; Bao, L.; Xiao, G. Nat. Commun. 2020, 11, 405. doi:10.1038/s41467-020-14295-z |
| 44. | He, H.; Xu, L.; Sun, R.; Zhang, Y.; Huang, Y.; Chen, Z.; Li, P.; Yang, R.; Xiao, G. Chem. Sci. 2021, 12, 5143–5151. doi:10.1039/d0sc06815b |
| 45. | Xiao, G. Acc. Chem. Res. 2025, 58, 2350–2363. doi:10.1021/acs.accounts.5c00387 |
| 43. | Li, P.; He, H.; Zhang, Y.; Yang, R.; Xu, L.; Chen, Z.; Huang, Y.; Bao, L.; Xiao, G. Nat. Commun. 2020, 11, 405. doi:10.1038/s41467-020-14295-z |
| 44. | He, H.; Xu, L.; Sun, R.; Zhang, Y.; Huang, Y.; Chen, Z.; Li, P.; Yang, R.; Xiao, G. Chem. Sci. 2021, 12, 5143–5151. doi:10.1039/d0sc06815b |
| 45. | Xiao, G. Acc. Chem. Res. 2025, 58, 2350–2363. doi:10.1021/acs.accounts.5c00387 |
| 61. | Christensen, H. M.; Oscarson, S.; Jensen, H. H. Carbohydr. Res. 2015, 408, 51–95. doi:10.1016/j.carres.2015.02.007 |
| 37. | Hu, C.; Wu, S.; He, F.; Cai, D.; Xu, Z.; Ma, W.; Liu, Y.; Wei, B.; Li, T.; Ding, K. Angew. Chem., Int. Ed. 2022, 61, e202202554. doi:10.1002/anie.202202554 |
| 38. | Xiao, X.; Zeng, J.; Fang, J.; Sun, J.; Li, T.; Song, Z.; Cai, L.; Wan, Q. J. Am. Chem. Soc. 2020, 142, 5498–5503. doi:10.1021/jacs.0c00447 |
| 39. | Cheng, C.-W.; Wu, C.-Y.; Hsu, W.-L.; Wong, C.-H. Biochemistry 2020, 59, 3078–3088. doi:10.1021/acs.biochem.9b00613 |
| 40. | Huang, X.; Huang, L.; Wang, H.; Ye, X.-S. Angew. Chem., Int. Ed. 2004, 43, 5221–5224. doi:10.1002/anie.200460176 |
| 41. | Zhang, Y.; Xiang, G.; He, S.; Hu, Y.; Liu, Y.; Xu, L.; Xiao, G. Org. Lett. 2019, 21, 2335–2339. doi:10.1021/acs.orglett.9b00617 |
| 42. | Zhang, Y.; Chen, Z.; Huang, Y.; He, S.; Yang, X.; Wu, Z.; Wang, X.; Xiao, G. Angew. Chem., Int. Ed. 2020, 59, 7576–7584. doi:10.1002/anie.202000992 |
| 28. | Ventura, J.; Uriel, C.; Gómez, A. M.; López, J. C. Carbohydr. Res. 2022, 516, 108557. doi:10.1016/j.carres.2022.108557 |
| 29. | Mong, K.-K. T.; Shiau, K.-S.; Lin, Y. H.; Cheng, K.-C.; Lin, C.-H. Org. Biomol. Chem. 2015, 13, 11550–11560. doi:10.1039/c5ob01786f |
| 30. | Liu, L.; Johnstone, K. D.; Fairweather, J. K.; Dredge, K.; Ferro, V. Aust. J. Chem. 2009, 62, 546. doi:10.1071/ch09015 |
| 31. | Valerio, S.; Pastore, A.; Adinolfi, M.; Iadonisi, A. J. Org. Chem. 2008, 73, 4496–4503. doi:10.1021/jo8003953 |
| 32. | Fairweather, J. K.; Hammond, E.; Johnstone, K. D.; Ferro, V. Bioorg. Med. Chem. 2008, 16, 699–709. doi:10.1016/j.bmc.2007.10.044 |
| 33. | Namme, R.; Mitsugi, T.; Takahashi, H.; Ikegami, S. Tetrahedron Lett. 2005, 46, 3033–3036. doi:10.1016/j.tetlet.2005.03.016 |
| 34. | Gu, G.; Wei, G.; Du, Y. Carbohydr. Res. 2004, 339, 1155–1162. doi:10.1016/j.carres.2004.01.020 |
| 35. | Fairweather, J. K.; Karoli, T.; Ferro, V. Bioorg. Med. Chem. 2004, 12, 6063–6075. doi:10.1016/j.bmc.2004.09.005 |
| 36. | Zhou, J.; Lv, S.; Zhang, D.; Xia, F.; Hu, W. J. Org. Chem. 2017, 82, 2599–2621. doi:10.1021/acs.joc.6b03017 |
| 57. | Yu, B.; Sun, J. Chem. Commun. 2010, 46, 4668. doi:10.1039/c0cc00563k |
| 58. | Yu, B.; Tao, H. Tetrahedron Lett. 2001, 42, 2405–2407. doi:10.1016/s0040-4039(01)00157-5 |
© 2025 Yang et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.