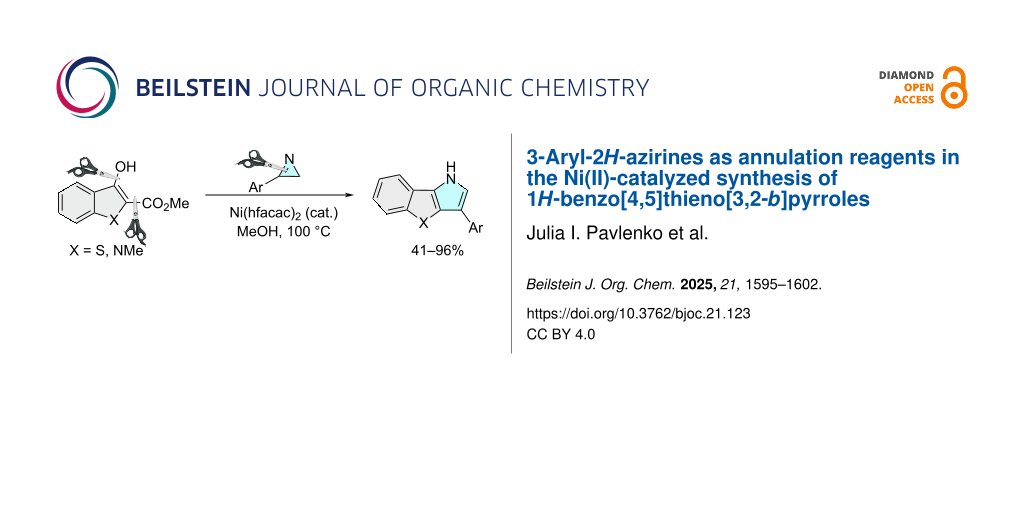
Abstract
A high-yielding method for the synthesis of 3-arylbenzo[4,5]thieno[3,2-b]pyrroles has been developed via pyrrole ring annulation to the aromatic benzo[b]thiophene system, using 3-arylazirines as a N‒C=C synthon. The reaction is catalyzed by Ni(hfacac)2 and proceeds through the azirine ring opening across the N=C3 bond. Azirines with both electron-donating and electron-withdrawing C3-aryl substituents tolerate the reaction conditions. The reaction of the N-methylindole analog also provides the annulation product but in moderate yield. The described synthesis is the first example of a dealkoxycarbonylative annulation reaction, in which 2H-azirines act as the annulation reagent.
Graphical Abstract
Introduction
2H-Azirines represent a valuable class of nitrogen heterocycles that are widely used as versatile building blocks in organic synthesis. In particular, the unique ability of these compounds to undergo selective opening of the three-membered ring at either of the two nitrogen–carbon bonds under certain conditions and to be incorporated into the target molecule as a N‒C‒C synthon is successfully used to obtain a variety of heterocyclic and acyclic nitrogen-containing compounds [1-3]. Such reactions can be initiated by electrophilic, nucleophilic or radical reagents, photoirradiation or proceed under acid-, metal-, or photocatalytic conditions. This strategy of azirine ring expansion is applicable to the synthesis of a variety of 4‒9-membered N-heterocycles, differing in the nature and number of heteroatoms and their mutual arrangement. These also include ortho-fused heterocycles, which are obtained from functionalized cyclic systems and azirines as annulation reagents. Significant progress has been achieved in the synthesis of pyrrolo-fused systems by the reactions of intermolecular annulation of a variety of non-aromatic five- [4-6] and six-membered carbo- and heterocycles [7-9] with azirines, which occur under transition metal catalysis or photocatalysis. Annulation of the pyrrole ring to an aromatic system is limited to reactions of functionalized arenes with azirines. These include the [3 + 2] cycloaddition of azirines to arynes (Scheme 1, reaction 1) [10-12], Pd-catalyzed reaction of azirines with iodoarenes (Scheme 1, reaction 2) [13], and reaction of 2-aroylazirines with naphthols (Scheme 1, reaction 3) [8]. The [3 + 2] annulation reactions of heteroaromatics with azirines are known only for 2-chloro- and 2-sulfanylpyridines [14,15], which result in the formation of imidazo[1,2-a]pyridines (Scheme 1, reactions 4 and 5). To the best of our knowledge, no successful methods enabling the fusion of a pyrrole or azole ring to a 5-membered heteroaromatic system have been reported to date. The formation of dihydrobenzofuro[3,2-b]pyrrole cycloadducts as by-products has been observed in the synthesis of aziridines by the transition metal-catalyzed reaction of benzofurans with 3-arylazirines [16].
Scheme 1: Synthesis of fused pyrroles and azoles by [3 + 2] annulation reactions of azirines.
Scheme 1: Synthesis of fused pyrroles and azoles by [3 + 2] annulation reactions of azirines.
This paper presents the use of azirines as annulation reagents for the preparation of 3-aryl-substituted benzo[4,5]thieno[3,2-b]pyrroles by the annulation of the 1H-pyrrole ring to the benzo[b]thiophene system (Scheme 1, reaction 6). The behavior of indoles as aza-analogs of the benzo[b]thiophenes under the identified catalytic annulation conditions is also discussed.
Results and Discussion
Guided by the known fact that 3-arylazirines in the presence of transition metal compounds are sensitive to the enol form of 1,3-dicarbonyls [5,17-19], we chose for our study methyl 3-hydroxybenzo[b]thiophene-2-carboxylate (1) as an aromatic substrate having a 1,3-dicarbonyl tautomeric form. Initially, it was shown that compound 1 does not react with 3-(p-tolyl)-2H-azirine (2a) when heated at 100 °C in methanol (Table 1, entry 1). No reaction was observed either in the presence of Rh(I), Mn(III), Fe(II), and Cu(II) compounds (Table 1, entries 2‒5). The Cu(I) compounds tested also did not promote the reaction between 1 and 2a, but some of them were found to catalyze the dimerization of azirine 2a to 2H-imidazole 4 (Table 1, entries 6‒9). Imidazole 4 is a known compound that is formed in low yield upon treatment of azirine 2a with FeCl2 in MeCN [20]. To our delight, the reaction carried out in the presence of 5 mol % of NiSO4 gave not only dimer 4, but also the annulation product, compound 3a. Further optimization of the reaction conditions aimed at suppressing the formation of dimer 4 showed that nickel chelates exhibit enhanced selectivity in catalyzing the annulation reaction. The highest yield of 3a (85%) was achieved by treating 1 with 3.2 equiv of the azirine in MeOH at 100 °С with 50 mol % of Ni(hfacac)2 (Table 1, entry 13). Lowering the temperature and the catalyst loading resulted in a slight deterioration in the yield and a significant slowing of the reaction, while changing the solvent gave completely unsatisfactory results.
Table 1: Optimization of 3a synthesis a.
|
|
|||||
| Entry | Catalyst (mol %) | Azirine (equiv)b | Time (h) | Yield of 3a (%) | Yield of 4 (%)c |
| 1 | ‒ | 3.2 | 25 | 0 | 0 |
| 2 | RhCl(PPh3)3 (5) | 1.6 | 12 | 0 | 0 |
| 3 | Mn(OAc)3 (5) | 1.6 | 12 | 0 | 0 |
| 4 | FeCl2 (5) | 3.2 | 2 | 0 | 0 |
| 5 | Cu(acac)2 (5) | 1.6 | 12 | 0 | 0 |
| 6 | CuBr(PPh3)3 (5) | 3.2 | 1.5 | 0 | 42 |
| 7 | IPrCuCl (5) | 1.6 | 1.0 | 0 | traces |
| 8 | CuI (5) | 3.2 | 2 | 0 | 41 |
| 9 | CuTC (5) | 3.2 | 2 | 0 | 45 |
| 10 | NiSO4 (5) | 3.2 | 8 | 32 | 12 |
| 11 | Ni(acac)2d (5) | 3.2 | 2 | 74 | 16 |
| 12 | Ni(tfacac)2e (5) | 3.2 | 6.5 | 29 | 13 |
| 13 | Ni(hfacac)2f (50) | 3.2 | 1.5 | 85 | 0 |
| 14 | Ni(hfacac)2 (25) | 3.2 | 2 | 83 | 0 |
| 15 | Ni(hfacac)2 (25) | 3 | 3 | 70 | 0 |
| 16 | Ni(hfacac)2 (25) | 2.5 | 3 | 61 | 0 |
| 17 | Ni(hfacac)2 (50)g | 3.2 | 2 | 0 | 0 |
aBenzothiophene 1 loading 0.4 mmol. bIn portions of 1.6 equiv. cIsolated yields. dacac = acetylacetonate. etfacac = trifluoroacetylacetonate. fhfacac = hexafluoroacetylacetonate. gIsopropyl alcohol or 1,2-dichloroethane was used as a solvent.
The absence of the methoxycarbonyl group in the obtained product 3a was unexpected, since there are no examples in the literature of the annulation reactions of cyclic substrates with azirines which are accompanied by the loss of an ester group. In our case, the demethoxycarbonylative annulation enables the formation of aromatic tricyclic benzo[4,5]thieno[3,2-b]pyrrole system which is encountered in compounds with antitumor activity [21,22] as well as in compounds exhibiting fluorescent properties [22,23]. Since general methods for the synthesis of benzo[4,5]thieno[3,2-b]pyrroles are lacking and obtaining their derivatives remains challenging [24,25], the reaction we discovered could significantly facilitate access to compounds of this type.
To test the generality and usefulness of the reaction, we investigated a variety of 3-aryl-2H-azirines with different substitution patterns of the aryl group (Scheme 2). As the presented data show, the reaction is insensitive to the electronic effects of substituents in the aryl group and, in the majority of cases, gives very high yields of annulation products. The introduction of an ortho-substituent into the benzene ring also has no effect on the synthesis efficiency (compound 3f). A slight decrease in yield was observed only for the naphthyl-substituted annulation product 3k.
Scheme 2: Synthesis of benzo[4,5]thieno[3,2-b]pyrroles 3.
Scheme 2: Synthesis of benzo[4,5]thieno[3,2-b]pyrroles 3.
The plausible mechanism of the reaction involves the nucleophilic addition of the Ni-enolate of 1 to the azirine C=N bond, followed by cyclization and the aziridine ring opening into the [3 + 2] cycloaddition product 5 (Scheme 3). It is noteworthy that the annulation proceeds via the azirine N‒C3 bond cleavage. Elimination of the methoxycarbonyl group most likely occurs under the action of methanol. The driving force of this process is the formation of an aromatic thiophene system.
Scheme 3: Plausible mechanism for the formation of compounds 3.
Scheme 3: Plausible mechanism for the formation of compounds 3.
To demonstrate the practicability and synthetic utility of this method for the synthesis of benzo[4,5]thieno[3,2-b]pyrrole derivatives, several transformations of compound 3b were carried out (Scheme 4). Benzothienopyrrole 3b was methylated using the MeI/NaH system into 1-methyl-substituted derivative 6 in 81% yield. With the same efficiency, when compound 3b was treated with di-tert-butyl dicarbonate in the presence of 4-(dimethylamino)pyridine (DMAP), Boc-derivative 7 was obtained. Finally, the formylation of 3b under the Vilsmeier–Haack reaction conditions provided aldehyde 8 in high yield.
Scheme 4: Post-modifications of 1H-benzo[4,5]thieno[3,2-b]pyrrole (3b).
Scheme 4: Post-modifications of 1H-benzo[4,5]thieno[3,2-b]pyrrole (3b).
To further assess the scope and limitations of the developed annulation protocol, we investigated the reactivity of aza-analogs of ester 1, indoles 9a‒c, toward azirine 2a under the same conditions. The reaction of N-methylindole 9a with 2a, carried out in the presence of Ni(hfacac)2 (50 mol %), unfortunately, did not give any identifiable products. Experiments with the catalysts presented in Table 1 were also unsuccessful. Nevertheless, favorable outcomes were achieved through a substantial decrease in catalyst loading. Heating of indole 9a with azirine 2a in the presence of 5 mol % of Ni(hfacac)2 afforded the desired annulation product 10 in 41% yield (Scheme 5).
Scheme 5: Synthesis of pyrrolo[3,2-b]indole 10.
Scheme 5: Synthesis of pyrrolo[3,2-b]indole 10.
Unfortunately, we were unable to find conditions that allowed a similar reaction of NH-indole 9b to occur. At the same time, when Ni(hfacac)2 was replaced with the NHC-complex, IPrCuCl, the formation of cycloadduct 11 was detected, which was isolated in 10% yield (Scheme 6). The formation of this compound implies the cleavage of the azirine N−C2 bond, indicating that the IPrCuCl-catalyzed reaction proceeds by a different mechanism, likely involving the intermediate formation of free radical species [9]. A similar reaction of N-acetyl substituted indole 9c produced [3 + 2] cycloaddition products 12 in even lower yield (4%). However, the main reaction product turned out to be unstable compound 13, which, nevertheless, was isolated in 40% yield as a single diastereomer and characterized by NMR and HRMS data. Compound 13 is likely formed from aziridine 14 via the N→N acetyl group transfer and subsequent isomerization of the acetylaziridinyl substituent.
Scheme 6: IPrCuCl-catalyzed reactions of indoles 9b,c with azirine 2a.
Scheme 6: IPrCuCl-catalyzed reactions of indoles 9b,c with azirine 2a.
The constitutional isomer of indole 9a, indole 15, having a nucleophilic reaction center in the β-position of the indole system, does not give annulation products either with Ni(hfacac)2 or with IPrCuCl. In both cases, the main product is aziridine 16 (Scheme 7). It is noteworthy that the IPrCuCl-catalyzed reaction produces aziridine 16 with a high prevalence of one of the diastereomers, while the Ni(hfacac)2-catalyzed reaction proceeds with low diastereoselectivity. The significant difference in selectivity can be explained by the fact that, in contrast to the Ni(II)-catalyzed reaction depicted in Scheme 3, the copper(I) catalysis involves the coordination by the metal of both reaction partners, and the formation of the C–C bond between the rings of the reacting molecules occurs in the coordination metal sphere as an intra-, rather than intermolecular, process. The formation of compound 17, the product of oxidative dimerization of indole-based enol 15, in the second reaction also points to different mechanisms of the formation of azirindine 16 under Ni(II)- and Cu(I)-catalysis. Oxidative dimerization of non-aromatic cyclic enols has been previously observed in their reactions with 3-arylazirines catalyzed by Cu(I) and Cu(II) complexes and was attributed to the recombination of intermediate free radicals [4].
Scheme 7: Ni(II)- and Cu(I)-catalyzed reactions of indole 15 with azirine 2a.
Scheme 7: Ni(II)- and Cu(I)-catalyzed reactions of indole 15 with azirine 2a.
Conclusion
In summary, a protocol for the efficient construction of the benzo[4,5]thieno[3,2-b]pyrrole skeleton by the annulation reaction of the aromatic system, 3-hydroxybenzo[b]thiophene-3-carboxylic ester, with 3-arylazirines has been developed. The annulation is catalyzed by Ni(hfacac)2 and proceeds through azirine ring opening across the N–C3 bond. Azirines with both electron-donating and electron-withdrawing C3-aryl substituents tolerate the reaction conditions, and give the annulation products in high yields. The synthesized 3-aryl-1H-benzo[4,5]thieno[3,2-b]pyrroles can be effectively modified at the positions 1 and 2. The N-methylaza analog of the benzo[b]thiophene (N-methylindole) reacts similarly to provide the annulation product in moderate yield. The described reaction is the first example of a dealkoxycarbonylative annulation reaction using 2H-azirines as annulation reagent.
Supporting Information
Deposition number 2402772 (compound 7) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre.
| Supporting Information File 1: Full experimental details, characterization data and copies of NMR spectra for all new compounds. | ||
| Format: PDF | Size: 3.9 MB | Download |
Acknowledgements
This research was carried out using resources of the Centre for Magnetic Resonance, the Research Centre for X-ray Diffraction Studies, the Centre for Chemical Analysis and Materials, and the Centre for Optical and Laser Materials Research of the Science Park of St. Petersburg State University.
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Xu, F.; Zeng, F.-W.; Luo, W.-J.; Zhang, S.-Y.; Huo, J.-Q.; Li, Y.-P. Eur. J. Org. Chem. 2024, 27, e202301292. doi:10.1002/ejoc.202301292
Return to citation in text: [1] -
Charushin, V. N.; Verbitskiy, E. V.; Chupakhin, O. N.; Vorobyeva, D. V.; Gribanov, P. S.; Osipov, S. N.; Ivanov, A. V.; Martynovskaya, S. V.; Sagitova, E. F.; Dyachenko, V. D.; Dyachenko, I. V.; Krivokolylsko, S. G.; Dotsenko, V. V.; Aksenov, A. V.; Aksenov, D. A.; Aksenov, N. A.; Larin, A. A.; Fershtat, L. L.; Muzalevskiy, V. M.; Nenajdenko, V. G.; Gulevskaya, A. V.; Pozharskii, A. F.; Filatova, E. A.; Belyaeva, K. V.; Trofimov, B. A.; Balova, I. A.; Danilkina, N. A.; Govdi, A. I.; Tikhomirov, A. S.; Shchekotikhin, A. E.; Novikov, M. S.; Rostovskii, N. V.; Khlebnikov, A. F.; Klimochkin, Y. N.; Leonova, M. V.; Tkachenko, I. M.; Mamedov, V. A. O.; Mamedova, V. L.; Zhukova, N. A.; Semenov, V. E.; Sinyashin, O. G.; Borshchev, O. V.; Luponosov, Y. N.; Ponomarenko, S. A.; Fisyuk, A. S.; Kostyuchenko, A. S.; Ilkin, V. G.; Beryozkina, T. V.; Bakulev, V. A.; Gazizov, A. S.; Zagidullin, A. A.; Karasik, A. A.; Kukushkin, M. E.; Beloglazkina, E. K.; Golantsov, N. E.; Festa, A. A.; Voskresenskii, L. G.; Moshkin, V. S.; Buev, E. M.; Sosnovskikh, V. Y.; Mironova, I. A.; Postnikov, P. S.; Zhdankin, V. V.; Yusubov, M. S. O.; Yaremenko, I. A.; Vil', V. A.; Krylov, I. B.; Terent'ev, A. O.; Gorbunova, Y. G.; Martynov, A. G.; Tsivadze, A. Y.; Stuzhin, P. A.; Ivanova, S. S.; Koifman, O. I.; Burov, O. N.; Kletskii, M. E.; Kurbatov, S. V.; Yarovaya, O. I.; Volcho, K. P.; Salakhutdinov, N. F.; Panova, M. A.; Burgart, Y. V.; Saloutin, V. I.; Sitdikova, A. R.; Shchegravina, E. S.; Fedorov, A. Y. Russ. Chem. Rev. 2024, 93, RCR5125. doi:10.59761/rcr5125
Return to citation in text: [1] -
Khlebnikov, A. F.; Novikov, M. S.; Rostovskii, N. V. Tetrahedron 2019, 75, 2555–2624. doi:10.1016/j.tet.2019.03.040
Return to citation in text: [1] -
Rostovskii, N. V.; Sakharov, P. A.; Novikov, M. S.; Khlebnikov, A. F.; Starova, G. L. Org. Lett. 2015, 17, 4148–4151. doi:10.1021/acs.orglett.5b01883
Return to citation in text: [1] [2] -
Xie, F.; Sun, Y.; Song, H.; Dong, S.; Zhao, Q.; Liu, J.; Miao, Y. Org. Lett. 2022, 24, 268–273. doi:10.1021/acs.orglett.1c03933
Return to citation in text: [1] [2] -
Vélez del Burgo, A.; Ochoa de Retana, A. M.; de los Santos, J. M.; Palacios, F. J. Org. Chem. 2016, 81, 100–108. doi:10.1021/acs.joc.5b02347
Return to citation in text: [1] -
Sakharov, P. A.; Rostovskii, N. V.; Khlebnikov, A. F.; Panikorovskii, T. L.; Novikov, M. S. Org. Lett. 2019, 21, 3615–3619. doi:10.1021/acs.orglett.9b01043
Return to citation in text: [1] -
Borra, S.; Chandrasekhar, D.; Newar, U. D.; Maurya, R. A. J. Org. Chem. 2019, 84, 1042–1052. doi:10.1021/acs.joc.8b02459
Return to citation in text: [1] [2] -
Sakharov, P. A.; Rostovskii, N. V.; Khlebnikov, A. F.; Novikov, M. S. Molecules 2022, 27, 5681. doi:10.3390/molecules27175681
Return to citation in text: [1] [2] -
Nair, V.; Kim, K. H. J. Org. Chem. 1975, 40, 3784–3786. doi:10.1021/jo00913a039
Return to citation in text: [1] -
Thangaraj, M.; Bhojgude, S. S.; Jain, S.; Gonnade, R. G.; Biju, A. T. J. Org. Chem. 2016, 81, 8604–8611. doi:10.1021/acs.joc.6b01472
Return to citation in text: [1] -
Grosso, C.; Alves, C.; Sase, T. J.; Alves, N. G.; Cardoso, A. L.; Lemos, A.; Pinho e Melo, T. M. V. D. ACS Omega 2024, 9, 29282–29289. doi:10.1021/acsomega.4c00305
Return to citation in text: [1] -
Candito, D. A.; Lautens, M. Org. Lett. 2010, 12, 3312–3315. doi:10.1021/ol100975b
Return to citation in text: [1] -
Vuillermet, F.; Bourret, J.; Pelletier, G. J. Org. Chem. 2021, 86, 388–402. doi:10.1021/acs.joc.0c02148
Return to citation in text: [1] -
Biswas, S.; Roy, A.; Duari, S.; Maity, S.; Elsharif, A. M.; Biswas, S. Org. Biomol. Chem. 2024, 22, 4697–4703. doi:10.1039/d4ob00410h
Return to citation in text: [1] -
Sakharov, P. A.; Rostovskii, N. V.; Khlebnikov, A. F.; Khoroshilova, O. V.; Novikov, M. S. Adv. Synth. Catal. 2019, 361, 3359–3372. doi:10.1002/adsc.201900366
Return to citation in text: [1] -
Wu, W.; Wen, S.; Zhang, X.; Lin, Q.; Weng, Z. Org. Lett. 2021, 23, 6352–6356. doi:10.1021/acs.orglett.1c02136
Return to citation in text: [1] -
Hu, H.; Wang, C.; Lai, H.; Wang, S.; Ni, H.; Yu, W.; Cao, P. Org. Chem. Front. 2020, 7, 3686–3691. doi:10.1039/d0qo00951b
Return to citation in text: [1] -
Zhao, M.-N.; Ning, G.-W.; Yang, D.-S.; Gao, P.; Fan, M.-J.; Zhao, L.-F. Tetrahedron Lett. 2020, 61, 152319. doi:10.1016/j.tetlet.2020.152319
Return to citation in text: [1] -
Auricchio, S.; Grassi, S.; Malpezzi, L.; Sarzi Sartori, A.; Truscello, A. M. Eur. J. Org. Chem. 2001, 1183–1187. doi:10.1002/1099-0690(200103)2001:6<1183::aid-ejoc1183>3.0.co;2-g
Return to citation in text: [1] -
Parmentier, J.-G.; Portevin, B.; Golsteyn, R. M.; Pierré, A.; Hickman, J.; Gloanec, P.; De Nanteuil, G. Bioorg. Med. Chem. Lett. 2009, 19, 841–844. doi:10.1016/j.bmcl.2008.12.001
Return to citation in text: [1] -
Castanheira, E. M. S.; Abreu, A. S.; Queiroz, M.-J. R. P.; Ferreira, P. M. T.; Coutinho, P. J. G.; Nazareth, N.; Nascimento, M. S.-J. J. Photochem. Photobiol., A 2009, 206, 220–226. doi:10.1016/j.jphotochem.2009.07.007
Return to citation in text: [1] [2] -
Chen, J.; Burghart, A.; Derecskei-Kovacs, A.; Burgess, K. J. Org. Chem. 2000, 65, 2900–2906. doi:10.1021/jo991927o
Return to citation in text: [1] -
Shimkin, A. A.; Mailian, A. K.; Shirinian, V. Z.; Krayushkin, M. M. Synthesis 2007, 2706–2710. doi:10.1055/s-2007-983821
Return to citation in text: [1] -
El‐Atawy, M. A.; Ferretti, F.; Ragaini, F. Eur. J. Org. Chem. 2017, 1902–1910. doi:10.1002/ejoc.201700165
Return to citation in text: [1]
| 1. | Xu, F.; Zeng, F.-W.; Luo, W.-J.; Zhang, S.-Y.; Huo, J.-Q.; Li, Y.-P. Eur. J. Org. Chem. 2024, 27, e202301292. doi:10.1002/ejoc.202301292 |
| 2. | Charushin, V. N.; Verbitskiy, E. V.; Chupakhin, O. N.; Vorobyeva, D. V.; Gribanov, P. S.; Osipov, S. N.; Ivanov, A. V.; Martynovskaya, S. V.; Sagitova, E. F.; Dyachenko, V. D.; Dyachenko, I. V.; Krivokolylsko, S. G.; Dotsenko, V. V.; Aksenov, A. V.; Aksenov, D. A.; Aksenov, N. A.; Larin, A. A.; Fershtat, L. L.; Muzalevskiy, V. M.; Nenajdenko, V. G.; Gulevskaya, A. V.; Pozharskii, A. F.; Filatova, E. A.; Belyaeva, K. V.; Trofimov, B. A.; Balova, I. A.; Danilkina, N. A.; Govdi, A. I.; Tikhomirov, A. S.; Shchekotikhin, A. E.; Novikov, M. S.; Rostovskii, N. V.; Khlebnikov, A. F.; Klimochkin, Y. N.; Leonova, M. V.; Tkachenko, I. M.; Mamedov, V. A. O.; Mamedova, V. L.; Zhukova, N. A.; Semenov, V. E.; Sinyashin, O. G.; Borshchev, O. V.; Luponosov, Y. N.; Ponomarenko, S. A.; Fisyuk, A. S.; Kostyuchenko, A. S.; Ilkin, V. G.; Beryozkina, T. V.; Bakulev, V. A.; Gazizov, A. S.; Zagidullin, A. A.; Karasik, A. A.; Kukushkin, M. E.; Beloglazkina, E. K.; Golantsov, N. E.; Festa, A. A.; Voskresenskii, L. G.; Moshkin, V. S.; Buev, E. M.; Sosnovskikh, V. Y.; Mironova, I. A.; Postnikov, P. S.; Zhdankin, V. V.; Yusubov, M. S. O.; Yaremenko, I. A.; Vil', V. A.; Krylov, I. B.; Terent'ev, A. O.; Gorbunova, Y. G.; Martynov, A. G.; Tsivadze, A. Y.; Stuzhin, P. A.; Ivanova, S. S.; Koifman, O. I.; Burov, O. N.; Kletskii, M. E.; Kurbatov, S. V.; Yarovaya, O. I.; Volcho, K. P.; Salakhutdinov, N. F.; Panova, M. A.; Burgart, Y. V.; Saloutin, V. I.; Sitdikova, A. R.; Shchegravina, E. S.; Fedorov, A. Y. Russ. Chem. Rev. 2024, 93, RCR5125. doi:10.59761/rcr5125 |
| 3. | Khlebnikov, A. F.; Novikov, M. S.; Rostovskii, N. V. Tetrahedron 2019, 75, 2555–2624. doi:10.1016/j.tet.2019.03.040 |
| 13. | Candito, D. A.; Lautens, M. Org. Lett. 2010, 12, 3312–3315. doi:10.1021/ol100975b |
| 4. | Rostovskii, N. V.; Sakharov, P. A.; Novikov, M. S.; Khlebnikov, A. F.; Starova, G. L. Org. Lett. 2015, 17, 4148–4151. doi:10.1021/acs.orglett.5b01883 |
| 10. | Nair, V.; Kim, K. H. J. Org. Chem. 1975, 40, 3784–3786. doi:10.1021/jo00913a039 |
| 11. | Thangaraj, M.; Bhojgude, S. S.; Jain, S.; Gonnade, R. G.; Biju, A. T. J. Org. Chem. 2016, 81, 8604–8611. doi:10.1021/acs.joc.6b01472 |
| 12. | Grosso, C.; Alves, C.; Sase, T. J.; Alves, N. G.; Cardoso, A. L.; Lemos, A.; Pinho e Melo, T. M. V. D. ACS Omega 2024, 9, 29282–29289. doi:10.1021/acsomega.4c00305 |
| 7. | Sakharov, P. A.; Rostovskii, N. V.; Khlebnikov, A. F.; Panikorovskii, T. L.; Novikov, M. S. Org. Lett. 2019, 21, 3615–3619. doi:10.1021/acs.orglett.9b01043 |
| 8. | Borra, S.; Chandrasekhar, D.; Newar, U. D.; Maurya, R. A. J. Org. Chem. 2019, 84, 1042–1052. doi:10.1021/acs.joc.8b02459 |
| 9. | Sakharov, P. A.; Rostovskii, N. V.; Khlebnikov, A. F.; Novikov, M. S. Molecules 2022, 27, 5681. doi:10.3390/molecules27175681 |
| 24. | Shimkin, A. A.; Mailian, A. K.; Shirinian, V. Z.; Krayushkin, M. M. Synthesis 2007, 2706–2710. doi:10.1055/s-2007-983821 |
| 25. | El‐Atawy, M. A.; Ferretti, F.; Ragaini, F. Eur. J. Org. Chem. 2017, 1902–1910. doi:10.1002/ejoc.201700165 |
| 4. | Rostovskii, N. V.; Sakharov, P. A.; Novikov, M. S.; Khlebnikov, A. F.; Starova, G. L. Org. Lett. 2015, 17, 4148–4151. doi:10.1021/acs.orglett.5b01883 |
| 5. | Xie, F.; Sun, Y.; Song, H.; Dong, S.; Zhao, Q.; Liu, J.; Miao, Y. Org. Lett. 2022, 24, 268–273. doi:10.1021/acs.orglett.1c03933 |
| 6. | Vélez del Burgo, A.; Ochoa de Retana, A. M.; de los Santos, J. M.; Palacios, F. J. Org. Chem. 2016, 81, 100–108. doi:10.1021/acs.joc.5b02347 |
| 9. | Sakharov, P. A.; Rostovskii, N. V.; Khlebnikov, A. F.; Novikov, M. S. Molecules 2022, 27, 5681. doi:10.3390/molecules27175681 |
| 5. | Xie, F.; Sun, Y.; Song, H.; Dong, S.; Zhao, Q.; Liu, J.; Miao, Y. Org. Lett. 2022, 24, 268–273. doi:10.1021/acs.orglett.1c03933 |
| 17. | Wu, W.; Wen, S.; Zhang, X.; Lin, Q.; Weng, Z. Org. Lett. 2021, 23, 6352–6356. doi:10.1021/acs.orglett.1c02136 |
| 18. | Hu, H.; Wang, C.; Lai, H.; Wang, S.; Ni, H.; Yu, W.; Cao, P. Org. Chem. Front. 2020, 7, 3686–3691. doi:10.1039/d0qo00951b |
| 19. | Zhao, M.-N.; Ning, G.-W.; Yang, D.-S.; Gao, P.; Fan, M.-J.; Zhao, L.-F. Tetrahedron Lett. 2020, 61, 152319. doi:10.1016/j.tetlet.2020.152319 |
| 21. | Parmentier, J.-G.; Portevin, B.; Golsteyn, R. M.; Pierré, A.; Hickman, J.; Gloanec, P.; De Nanteuil, G. Bioorg. Med. Chem. Lett. 2009, 19, 841–844. doi:10.1016/j.bmcl.2008.12.001 |
| 22. | Castanheira, E. M. S.; Abreu, A. S.; Queiroz, M.-J. R. P.; Ferreira, P. M. T.; Coutinho, P. J. G.; Nazareth, N.; Nascimento, M. S.-J. J. Photochem. Photobiol., A 2009, 206, 220–226. doi:10.1016/j.jphotochem.2009.07.007 |
| 16. | Sakharov, P. A.; Rostovskii, N. V.; Khlebnikov, A. F.; Khoroshilova, O. V.; Novikov, M. S. Adv. Synth. Catal. 2019, 361, 3359–3372. doi:10.1002/adsc.201900366 |
| 22. | Castanheira, E. M. S.; Abreu, A. S.; Queiroz, M.-J. R. P.; Ferreira, P. M. T.; Coutinho, P. J. G.; Nazareth, N.; Nascimento, M. S.-J. J. Photochem. Photobiol., A 2009, 206, 220–226. doi:10.1016/j.jphotochem.2009.07.007 |
| 23. | Chen, J.; Burghart, A.; Derecskei-Kovacs, A.; Burgess, K. J. Org. Chem. 2000, 65, 2900–2906. doi:10.1021/jo991927o |
| 14. | Vuillermet, F.; Bourret, J.; Pelletier, G. J. Org. Chem. 2021, 86, 388–402. doi:10.1021/acs.joc.0c02148 |
| 15. | Biswas, S.; Roy, A.; Duari, S.; Maity, S.; Elsharif, A. M.; Biswas, S. Org. Biomol. Chem. 2024, 22, 4697–4703. doi:10.1039/d4ob00410h |
| 8. | Borra, S.; Chandrasekhar, D.; Newar, U. D.; Maurya, R. A. J. Org. Chem. 2019, 84, 1042–1052. doi:10.1021/acs.joc.8b02459 |
| 20. | Auricchio, S.; Grassi, S.; Malpezzi, L.; Sarzi Sartori, A.; Truscello, A. M. Eur. J. Org. Chem. 2001, 1183–1187. doi:10.1002/1099-0690(200103)2001:6<1183::aid-ejoc1183>3.0.co;2-g |
© 2025 Pavlenko et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.