Abstract
C1 chemistry has a central role in the efficient utilization of single-carbon molecules, contributing significantly to sustainability, innovation and economic growth across various sectors. In this study, we present an efficient and rapid method for synthesizing a variety of heteroannulated pyrimidones using cyanoacetamide-based multicomponent reaction (MCR) chemistry. By utilizing specific MCR-based scaffolds as precursors and employing the abundant and inexpensive formamide as a C1 feedstock under neat conditions, we were able to efficiently access substituted thieno-, quinolino- and indolopyrimidones without the need of column chromatography. Further, a single-crystal X-ray structure was obtained, revealing certain geometrical features.

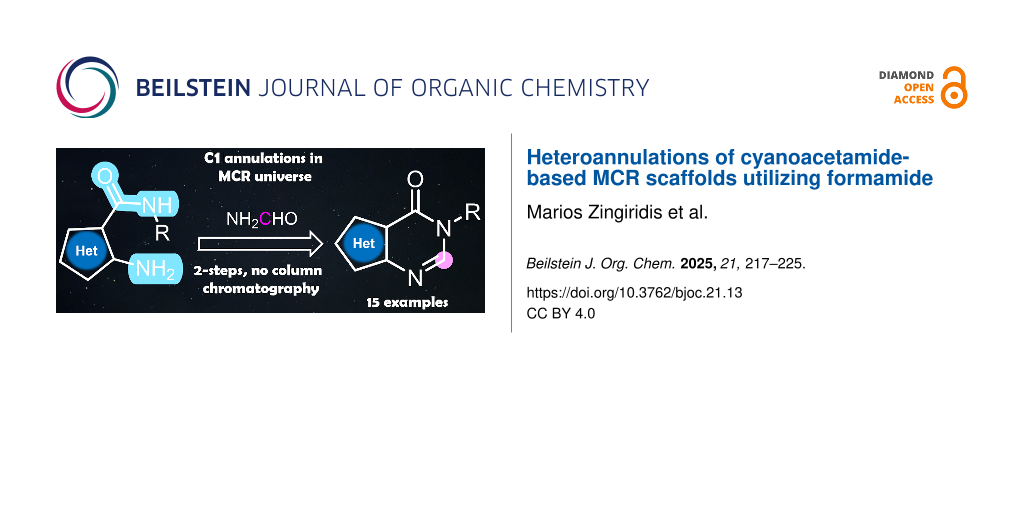
Graphical Abstract
Introduction
The term “net-zero carbon” is becoming increasingly common as we consider a future marked by a rising global temperature and severe weather patterns – a result of human-induced greenhouse gas emissions. The principle of net-zero revolves around the idea of using Earth’s carbon resources at a rate that does not exceed their natural replenishment. In 2015, the United Nations introduced the Sustainable Development Goals (SDGs), wherein the 7th goal focuses on ensuring access to affordable, renewable and clean energy [1]. Moreover, the European Union has committed to ambitious environmental targets as part of the European Green Deal [2].
Advancements in C1 chemistry are pivotal to achieving this equilibrium. As such, it is becoming increasingly essential for synthetic methods to align with a net-zero carbon future [2,3]. Therefore, advancing C1 chemistry remains a crucial endeavor for our group [4]. In synthetic organic chemistry, C1 compounds are usually installed using CO, CO2, HCO2H, CH3OH and CH4, which is mostly due to their presence in greenhouse emissions [5,6]. Although abundant and inexpensive, their valorization still remains problematic due to their thermodynamic stability and chemical inertness [7-15]. Multicomponent reaction (MCR) chemistry is a type of convergent chemistry characterized by diversity, complexity and efficiency. MCRs are compatible with C1 chemistry due to the generally great tolerance of different functional groups. They have been mostly employed in the synthesis of oxazolidinones and oxazinanones utilizing CO2 and CO [4,16-20]. In addition, carbonyldiimidazole-based heteroannulations via MCRs have been reported, giving access to drug like scaffolds [21-24]. However, their employment in C1 chemistry should and could be advanced.
Herein, we point out formamide as an alternative relevant building block for C1 chemistry by using specific, suitably functionalized MCR scaffolds. This positions formamides as versatile, synthetic hubs towards privileged scaffolds and high-end chemicals. Over the years, the reactivity of formamide has been widely explored in heterocyclic chemistry, but it has only recently started to be established as a C1 feedstock. [25]. Its high polarity and dielectric constant (it is miscible with water) [26], with the ability to solubilize a wide range of reagents, from salts to polymers, proteins and saccharides, renders formamide an excellent C1 building block [25,27]. Thus, our target under the umbrella of C1 chemistry was to provide a straightforward access to the privileged scaffold of fused heteroannulated pyrimidones, which demonstrate a broad range of biological activities [28-35], including use as emissive nucleoside analogues [36-38]. Moreover, they are used in a variety of commercially available drugs (Figure 1).
Figure 1: Heteroannulated pyrimidones in drug discovery: blockbuster drugs that are based on the privileged pyrimidine scaffold.
Figure 1: Heteroannulated pyrimidones in drug discovery: blockbuster drugs that are based on the privileged p...
Results and Discussion
Design and strategy
We envisioned applying the Niementowski quinazoline synthesis [25,39-41] (Scheme 1A) by employing three different heterocyclic systems as precursors, which have both an orthogonally installed amino group and a disubstituted amide group at the 2- and 3-positions, respectively, and reacting them with formamide (Scheme 1B). Those synthetic hubs can be rapidly accessed by cyanoacetamide-based MCRs, which is an interesting reaction type that gives access to privileged cores and has been utilized numerous times in medicinal chemistry campaigns as hits, leads and eventually even drugs, such as 2-aminothiophenes, -quinolines and -indoles [42-45].
Scheme 1: Strategies towards targeted adducts: A) Niementowski quinazoline synthesis utilizing anthranilic acid and B) access to heteroannulated pyrimidones by MCRs of suitably substituted heterocycles and formamide as C1 source.
Scheme 1: Strategies towards targeted adducts: A) Niementowski quinazoline synthesis utilizing anthranilic ac...
Synthetic exploitation
The synthesis of the key cyanoacetamide building blocks was our primary objective. In a parallel setup, a variety of primary amines was reacted with methyl cyanoacetate [46], giving rise to the corresponding cyanoacetamides 1 (Scheme 2). Subsequently, they were reacted accordingly to yield a variety of the targeted precursors, 2-aminothiophenes 2 [42], 2-aminoquinolines 3 [45] and 2-aminoindoles 4 [44], via Gewald three-component reactions. Our focus was to create a representative, diverse and structurally complex library of building blocks, covering a range of shapes and chemical spaces, to facilitate formamide-based heteroannulation for the synthesis of the desired adducts. Thus, we employed aliphatic and (hetero)aromatic, bulky and linear amines with different substitution patterns. The compounds 2–4 were purified by recrystallization and employed as such (Scheme 2).
Scheme 2: Access to the key building blocks 2–4 by employing three different nonisocyanide-based MCRs. Diversity and complexity were the essential features of our library of starting materials. The colored frames indicate the different scaffold types.
Scheme 2: Access to the key building blocks 2–4 by employing three different nonisocyanide-based MCRs. Divers...
To our great delight, the heterocycles 2–4 could successfully be subjected to refluxing formamide under neat conditions, instantly yielding the desired thienopyrimidones 5a–e, quinolinopyrimidones 6a–e and indolopyrimidones 7a–e, respectively, as reported in the literature [42,44]. In accordance with the reported mechanism, after the initial formylation of the amino group at position 2, an intramolecular nucleophilic attack by the NH moiety of the amide group resulted in pyrimidone annulation [41]. This was observed for the first time and completed the reported heteroannulation landscape utilizing the Niementowski reaction [25]. In general, the reactions had a rather broad scope as a great range of cyanoacetamides was compatible. The synthesis was efficient and rapid as the final adducts could be isolated only by precipitation. In addition, the reactions were performed in a parallel setup using custom-made metal blocks.
Specifically, thienopyrimidine and thienopyrimidone derivatives exhibit a range of biological activities, i.e., analgesia, anti-inflammation, antihypertension and many more (DB06889, DB07397, DB08777) [47-56]. Thienopyrimidone derivatives have also been used in the context of other Gewald-based MCRs in the past, but the reported diversity has been rather low [57-60]. The reaction of 2 with formamide was performed within only 3 h (Scheme 3), yielding the N-substituted thienopyrimidones 5a–e in 30–99% total yield (2 steps), as well with aliphatic and (hetero)aromatic substituents.
Scheme 3: Synthesis of N-substituted thienopyrimidones 5a–e by a Gewald three-component reaction employing 2-aminothiophenes 2a–e and formamide as C1 source. A characteristic fluorescence for compound 5a was reported (365 nm in DMSO). The reported yield refers to the overall two-step synthesis.
Scheme 3: Synthesis of N-substituted thienopyrimidones 5a–e by a Gewald three-component reaction employing 2-...
Quinoline derivatives are prevalent in nature, and many exhibit a range of biological activities, including antimalarial, antitumor, anthelmintic, antibacterial, antiasthmatic, and antiplatelet effects [61-63]. In particular, quinolinopyrimidine and pyrimidone derivatives have attracted a great interest due to their biological profile [64-69]. After some optimization, we determined that harsher reaction conditions than for the synthesis of 5 were required for the targeted adducts 6 to form. Additional treatment with DIPEA/DMF afforded the corresponding substituted quinolinopyrimidones 6a–e in 47–65% total yield (2 steps) within 12–16 h. Notably, the products carried a series of aliphatic and aromatic substituents (Scheme 4).
Scheme 4: Synthesis of N-substituted quinolinopyrimidones 6a–e from 2-aminoindoles 3a–e and formamide as C1 source. A characteristic fluorescence for compound 6c was reported (365 nm in DMSO). The reported yields refer to the overall two-step synthesis.
Scheme 4: Synthesis of N-substituted quinolinopyrimidones 6a–e from 2-aminoindoles 3a–e and formamide as C1 s...
Pyrimidines and pyrimidone-bearing indole derivatives are crucial in organic chemistry because of their extensive use as bioactive compounds with a wide array of significant biological activities (DB03074, DB03304, DB08131) [70-72]. In a similar fashion, substituted indolopyrimidone derivatives 7a–e were obtained within 3 h in 31–90% total yield (2 steps) upon heating with formamide (Scheme 5).
Scheme 5: Synthesis of N-substituted indolopyrimidones 7a–e from 2-aminoindoles 4a–e and formamide as C1 source. A characteristic fluorescence for compound 7b was reported (365 nm in DMSO). The reported yield refers to the overall two-step synthesis.
Scheme 5: Synthesis of N-substituted indolopyrimidones 7a–e from 2-aminoindoles 4a–e and formamide as C1 sour...
Next, we characterized certain physicochemical properties of the newly synthesized compounds. The absorbance and emission spectra of 5–7 were obtained, showing similar profiles across all the different scaffolds (Figure 2 and Supporting Information File 1). The compounds 5–7 exhibited a maximum absorbance (λmax) at 330 nm, 284 nm and 310 nm, respectively, which also corresponded to their excitation wavelength (λex). Upon excitation, fluorescence emission was observed at an average wavelength of 384 nm for 5a–e, 439 nm for 6a–e and 430 nm for 7a–e (see Supporting Information File 1 for detailed information).
![[1860-5397-21-13-2]](/bjoc/content/figures/1860-5397-21-13-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Representative fluorescence spectrum of compounds 5b (λex = 330 nm) and 6e (λex = 430 nm) at 0.2 M in DMSO.
Figure 2: Representative fluorescence spectrum of compounds 5b (λex = 330 nm) and 6e (λex = 430 nm) at 0.2 M ...
In support of the proposed scaffold 7b, we were able to solve its crystal structure (Figure 3). An intermolecular bifurcated hydrogen bond network of 2.0 Å was revealed, demonstrating potential of these derivatives in drug and material discovery.
Figure 3: Molecular geometry observed within the crystal structure of compound 7b (CCDC 2376493).
Figure 3: Molecular geometry observed within the crystal structure of compound 7b (CCDC 2376493).
Conclusion
In conclusion, we successfully combined the Niementowski quinazoline synthesis with nonisocyanide-based chemistry, thereby expanding and enhancing the scope of C1 MCR chemistry. That way, we obtained 15 diversely substituted heteroannulated pyrimidones, employing privileged thiophene, quinoline and indole scaffolds in a rapid fashion, without the need of column chromatography, in a parallel setup.
Supporting Information
| Supporting Information File 1: Experimental methods, synthetic procedures, analytical data and exemplary copies of NMR spectra of novel compounds. | ||
| Format: PDF | Size: 1.7 MB | Download |
| Supporting Information File 2: CheckCIF report for 7b. | ||
| Format: PDF | Size: 136.1 KB | Download |
| Supporting Information File 3: CIF file for 7b. | ||
| Format: CIF | Size: 447.9 KB | Download |
Data Availability Statement
Data generated and analyzed during this study is available from the corresponding author upon reasonable request.
References
-
United Nations Department of Economic and Social Affairs (UNDESA) and the Secretariat of the United Nations Framework Convention on Climate Change (UNFCCC). Third Global Conference on Strengthening Synergies Between the Paris Agreement and the 2030 Agenda For Sustainable Development, 2022.
Return to citation in text: [1] -
Wu, X.-F.; Dyson, P. J.; Arndtsen, B. A. J. Org. Chem. 2023, 88, 4891–4893. doi:10.1021/acs.joc.3c00626
Return to citation in text: [1] [2] -
Trost, B. Science 1991, 254, 1471–1477. doi:10.1126/science.1962206
Return to citation in text: [1] -
Fragkiadakis, M.; Anastasiou, P.-K.; Volyrakis, I.; Pantousas, A.; Stoumpos, C. C.; Neochoritis, C. G. Chem. Commun. 2023, 59, 14411–14414. doi:10.1039/d3cc04597h
Return to citation in text: [1] [2] -
Kerdphon, S.; Sanghong, P.; Chatwichien, J.; Choommongkol, V.; Rithchumpon, P.; Singh, T.; Meepowpan, P. Eur. J. Org. Chem. 2020, 2730–2734. doi:10.1002/ejoc.202000257
Return to citation in text: [1] -
Jacquet, O.; Das Neves Gomes, C.; Ephritikhine, M.; Cantat, T. ChemCatChem 2013, 5, 117–120. doi:10.1002/cctc.201200732
Return to citation in text: [1] -
Dabral, S.; Schaub, T. Adv. Synth. Catal. 2019, 361, 223–246. doi:10.1002/adsc.201801215
Return to citation in text: [1] -
Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Nat. Commun. 2015, 6, 5933. doi:10.1038/ncomms6933
Return to citation in text: [1] -
Sakakura, T.; Choi, J.-C.; Yasuda, H. Chem. Rev. 2007, 107, 2365–2387. doi:10.1021/cr068357u
Return to citation in text: [1] -
Mikkelsen, M.; Jørgensen, M.; Krebs, F. C. Energy Environ. Sci. 2010, 3, 43–81. doi:10.1039/b912904a
Return to citation in text: [1] -
Pinaka, A.; Vougioukalakis, G. C. Coord. Chem. Rev. 2015, 288, 69–97. doi:10.1016/j.ccr.2015.01.010
Return to citation in text: [1] -
Aresta, M.; Dibenedetto, A.; Angelini, A. Chem. Rev. 2014, 114, 1709–1742. doi:10.1021/cr4002758
Return to citation in text: [1] -
Wang, S.; Xi, C. Chem. Soc. Rev. 2019, 48, 382–404. doi:10.1039/c8cs00281a
Return to citation in text: [1] -
Pradhan, S.; Roy, S.; Sahoo, B.; Chatterjee, I. Chem. – Eur. J. 2021, 27, 2254–2269. doi:10.1002/chem.202003685
Return to citation in text: [1] -
Hang, W.; Li, D.; Zou, S.; Xi, C. J. Org. Chem. 2023, 88, 5007–5014. doi:10.1021/acs.joc.2c01840
Return to citation in text: [1] -
Niemi, T.; Perea‐Buceta, J. E.; Fernández, I.; Hiltunen, O.-M.; Salo, V.; Rautiainen, S.; Räisänen, M. T.; Repo, T. Chem. – Eur. J. 2016, 22, 10355–10359. doi:10.1002/chem.201602338
Return to citation in text: [1] -
Mei, C.; Zhao, Y.; Chen, Q.; Cao, C.; Pang, G.; Shi, Y. ChemCatChem 2018, 10, 3057–3068. doi:10.1002/cctc.201800142
Return to citation in text: [1] -
Wang, B.; Elageed, E. H. M.; Zhang, D.; Yang, S.; Wu, S.; Zhang, G.; Gao, G. ChemCatChem 2014, 6, 278–283. doi:10.1002/cctc.201300801
Return to citation in text: [1] -
Feng, L.; Li, X.; Xu, C.; Sadeghzadeh, S. M. Catal. Lett. 2020, 150, 1729–1740. doi:10.1007/s10562-019-03073-2
Return to citation in text: [1] -
Wang, L.; Qi, C.; Xiong, W.; Jiang, H. Chin. J. Catal. 2022, 43, 1598–1617. doi:10.1016/s1872-2067(21)64029-9
Return to citation in text: [1] -
Li, J.; Di Lorenzo, V.; Patil, P.; Ruiz-Moreno, A. J.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Velasco-Velázquez, M. A.; Dömling, A. ACS Comb. Sci. 2020, 22, 356–360. doi:10.1021/acscombsci.0c00072
Return to citation in text: [1] -
Eleftheriadis, N.; Poelman, H.; Leus, N. G. J.; Honrath, B.; Neochoritis, C. G.; Dolga, A.; Dömling, A.; Dekker, F. J. Eur. J. Med. Chem. 2016, 122, 786–801. doi:10.1016/j.ejmech.2016.07.010
Return to citation in text: [1] -
Xiao, Z.; Osipyan, A.; Song, S.; Chen, D.; Schut, R. A.; van Merkerk, R.; van der Wouden, P. E.; Cool, R. H.; Quax, W. J.; Melgert, B. N.; Poelarends, G. J.; Dekker, F. J. J. Med. Chem. 2022, 65, 2059–2077. doi:10.1021/acs.jmedchem.1c01598
Return to citation in text: [1] -
Xiao, Z.; Dekker, F. Substituted Thiophene Compounds as D-Dopachrome Tautomerase Inhibitors. WO Patent WO2023031246, March 9, 2023.
Return to citation in text: [1] -
Sacchelli, B. A. L.; Rocha, B. C.; Fantinel, M.; Andrade, L. H. Eur. J. Org. Chem. 2024, 27, e202300930. doi:10.1002/ejoc.202300930
Return to citation in text: [1] [2] [3] [4] -
Jiang, L.; Li, K.; Porter, W. N.; Wang, H.; Li, G.; Chen, J. G. J. Am. Chem. Soc. 2024, 146, 2857–2875. doi:10.1021/jacs.3c13374
Return to citation in text: [1] -
Xu, L.; Jiang, Y.; Ma, D. Org. Lett. 2012, 14, 1150–1153. doi:10.1021/ol300084v
Return to citation in text: [1] -
Abdellatif, K. R. A.; Bakr, R. B. Med. Chem. Res. 2021, 30, 31–49. doi:10.1007/s00044-020-02656-8
Return to citation in text: [1] -
Vyas, A.; Sahu, B.; Pathania, S.; Nandi, N. K.; Chauhan, G.; Asati, V.; Kumar, B. J. Heterocycl. Chem. 2023, 60, 1081–1121. doi:10.1002/jhet.4593
Return to citation in text: [1] -
Thakur, S.; Ansari, A. J.; Joshi, G. FDA approved fused pyrimidine-based drugs. In Fused Pyrimidine-Based Drug Discovery; Raj, K., Ed.; Elsevier: Amsterdam, Netherlands, 2023; pp 13–38. doi:10.1016/b978-0-443-18616-5.00004-1
Return to citation in text: [1] -
Xu, G.-F.; Song, B.-A.; Bhadury, P. S.; Yang, S.; Zhang, P.-Q.; Jin, L.-H.; Xue, W.; Hu, D.-Y.; Lu, P. Bioorg. Med. Chem. 2007, 15, 3768–3774. doi:10.1016/j.bmc.2007.03.037
Return to citation in text: [1] -
Long, C.; Li, P.; Chen, M.; Dong, L.; Hu, D.; Song, B. Eur. J. Med. Chem. 2015, 102, 639–647. doi:10.1016/j.ejmech.2015.08.029
Return to citation in text: [1] -
Kulkarni, S. S.; Singh, S.; Shah, J. R.; Low, W.-K.; Talele, T. T. Eur. J. Med. Chem. 2012, 50, 264–273. doi:10.1016/j.ejmech.2012.02.001
Return to citation in text: [1] -
Kargbo, R. B. ACS Med. Chem. Lett. 2024, 15, 1193–1195. doi:10.1021/acsmedchemlett.4c00327
Return to citation in text: [1] -
Cline, S. J.; Hodgson, D. J. J. Am. Chem. Soc. 1980, 102, 6285–6288. doi:10.1021/ja00540a018
Return to citation in text: [1] -
Sinkeldam, R. W.; McCoy, L. S.; Shin, D.; Tor, Y. Angew. Chem., Int. Ed. 2013, 52, 14026–14030. doi:10.1002/anie.201307064
Return to citation in text: [1] -
Ludford, P. T., III; Yang, S.; Bucardo, M. S.; Tor, Y. Chem. – Eur. J. 2022, 28, e202104472. doi:10.1002/chem.202104472
Return to citation in text: [1] -
Shin, D.; Sinkeldam, R. W.; Tor, Y. J. Am. Chem. Soc. 2011, 133, 14912–14915. doi:10.1021/ja206095a
Return to citation in text: [1] -
Bogert, M. T.; Gotthelf, A. H. J. Am. Chem. Soc. 1900, 22, 522–535. doi:10.1021/ja02046a008
Return to citation in text: [1] -
Von Niementowski, S. J. Prakt. Chem. 1895, 51, 564–572. doi:10.1002/prac.18950510150
Return to citation in text: [1] -
Meyer, J.; Wagner, E. C. J. Org. Chem. 1943, 08, 239–252. doi:10.1021/jo01191a005
Return to citation in text: [1] [2] -
Wang, K.; Kim, D.; Dömling, A. J. Comb. Chem. 2010, 12, 111–118. doi:10.1021/cc9001586
Return to citation in text: [1] [2] [3] -
Kok, T.; Wapenaar, H.; Wang, K.; Neochoritis, C. G.; Zarganes-Tzitzikas, T.; Proietti, G.; Eleftheriadis, N.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Cool, R. H.; Poelarends, G. J.; Dömling, A.; Dekker, F. J. Bioorg. Med. Chem. 2018, 26, 999–1005. doi:10.1016/j.bmc.2017.12.032
Return to citation in text: [1] -
Wang, K.; Herdtweck, E.; Dömling, A. ACS Comb. Sci. 2011, 13, 140–146. doi:10.1021/co100040z
Return to citation in text: [1] [2] [3] -
Wang, K.; Herdtweck, E.; Dömling, A. ACS Comb. Sci. 2012, 14, 316–322. doi:10.1021/co3000133
Return to citation in text: [1] [2] -
Wang, K.; Nguyen, K.; Huang, Y.; Dömling, A. J. Comb. Chem. 2009, 11, 920–927. doi:10.1021/cc9000778
Return to citation in text: [1] -
Ali, E. M. H.; Abdel-Maksoud, M. S.; Oh, C.-H. Bioorg. Med. Chem. 2019, 27, 1159–1194. doi:10.1016/j.bmc.2019.02.044
Return to citation in text: [1] -
Eissa, K. I.; Kamel, M. M.; Mohamed, L. W.; Doghish, A. S.; Alnajjar, R.; Al‐Karmalawy, A. A.; Kassab, A. E. Drug Dev. Res. 2023, 84, 937–961. doi:10.1002/ddr.22064
Return to citation in text: [1] -
Zhong, W.; Pasunooti, K. K.; Balamkundu, S.; Wong, Y. H.; Nah, Q.; Gadi, V.; Gnanakalai, S.; Chionh, Y. H.; McBee, M. E.; Gopal, P.; Lim, S. H.; Olivier, N.; Buurman, E. T.; Dick, T.; Liu, C. F.; Lescar, J.; Dedon, P. C. J. Med. Chem. 2019, 62, 7788–7805. doi:10.1021/acs.jmedchem.9b00582
Return to citation in text: [1] -
Tinney, F. J.; Cetenko, W. A.; Kerbleski, J. J.; Connor, D. T.; Sorenson, R. J.; Herzig, D. J. J. Med. Chem. 1981, 24, 878–882. doi:10.1021/jm00139a021
Return to citation in text: [1] -
Russo, F.; Romeo, G.; Santagati, N.; Caruso, A.; Cutuli, V.; Amore, D. Eur. J. Med. Chem. 1994, 29, 569–578. doi:10.1016/0223-5234(94)90149-x
Return to citation in text: [1] -
Modica, M.; Santagati, M.; Santagati, A.; Russo, F.; Cagnotto, A.; Goegan, M.; Mennini, T. Bioorg. Med. Chem. Lett. 2000, 10, 1089–1092. doi:10.1016/s0960-894x(00)00165-7
Return to citation in text: [1] -
Duval, E.; Case, A.; Stein, R. L.; Cuny, G. D. Bioorg. Med. Chem. Lett. 2005, 15, 1885–1889. doi:10.1016/j.bmcl.2005.02.005
Return to citation in text: [1] -
Miao, Z.; Sun, Y.-m.; Zhao, L.-y.; Li, Y.-s.; Wang, Y.-f.; Nan, J.-s.; Qiao, Z.-e.; Li, L.-l.; Yang, S.-y. Bioorg. Med. Chem. Lett. 2020, 30, 126966. doi:10.1016/j.bmcl.2020.126966
Return to citation in text: [1] -
Basile, L.; Álvarez, S.; Blanco, A.; Santagati, A.; Granata, G.; Di Pietro, P.; Guccione, S.; Muñoz-Fernández, M. Á. Eur. J. Med. Chem. 2012, 57, 149–161. doi:10.1016/j.ejmech.2012.09.005
Return to citation in text: [1] -
Zhu, W.; Chen, H.; Wang, Y.; Wang, J.; Peng, X.; Chen, X.; Gao, Y.; Li, C.; He, Y.; Ai, J.; Geng, M.; Zheng, M.; Liu, H. J. Med. Chem. 2017, 60, 6018–6035. doi:10.1021/acs.jmedchem.7b00076
Return to citation in text: [1] -
Bassetto, M.; Leyssen, P.; Neyts, J.; Yerukhimovich, M. M.; Frick, D. N.; Brancale, A. Eur. J. Med. Chem. 2016, 123, 31–47. doi:10.1016/j.ejmech.2016.07.035
Return to citation in text: [1] -
Salahuddin, M.; Kakad, S.; Shantakumar, S. M. E-J. Chem. 2009, 6, 801–808. doi:10.1155/2009/361282
Return to citation in text: [1] -
Pédeboscq, S.; Gravier, D.; Casadebaig, F.; Hou, G.; Gissot, A.; Rey, C.; Ichas, F.; De Giorgi, F.; Lartigue, L.; Pometan, J.-P. Bioorg. Med. Chem. 2012, 20, 6724–6731. doi:10.1016/j.bmc.2012.09.034
Return to citation in text: [1] -
Spooner, R. A.; Watson, P.; Smith, D. C.; Boal, F.; Amessou, M.; Johannes, L.; Clarkson, G. J.; Lord, J. M.; Stephens, D. J.; Roberts, L. M. Biochem. J. 2008, 414, 471–484. doi:10.1042/bj20080149
Return to citation in text: [1] -
Zishiri, V. K.; Joshi, M. C.; Hunter, R.; Chibale, K.; Smith, P. J.; Summers, R. L.; Martin, R. E.; Egan, T. J. J. Med. Chem. 2011, 54, 6956–6968. doi:10.1021/jm2009698
Return to citation in text: [1] -
Lilienkampf, A.; Mao, J.; Wan, B.; Wang, Y.; Franzblau, S. G.; Kozikowski, A. P. J. Med. Chem. 2009, 52, 2109–2118. doi:10.1021/jm900003c
Return to citation in text: [1] -
Swain, S. S.; Pati, S.; Hussain, T. Eur. J. Med. Chem. 2022, 232, 114173. doi:10.1016/j.ejmech.2022.114173
Return to citation in text: [1] -
El-Gazzar, A. B. A.; Hafez, H. N.; Nawwar, G. A. M. Eur. J. Med. Chem. 2009, 44, 1427–1436. doi:10.1016/j.ejmech.2008.09.030
Return to citation in text: [1] -
Ali, H. I.; Ashida, N.; Nagamatsu, T. Bioorg. Med. Chem. 2008, 16, 922–940. doi:10.1016/j.bmc.2007.10.014
Return to citation in text: [1] -
Joshi, A. A.; Viswanathan, C. L. Bioorg. Med. Chem. Lett. 2006, 16, 2613–2617. doi:10.1016/j.bmcl.2006.02.038
Return to citation in text: [1] -
Ibrahim, N. S. M.; Kadry, H. H.; Zaher, A. F.; Mohamed, K. O. Chem. Biol. Drug Des. 2023, 102, 996–1013. doi:10.1111/cbdd.14307
Return to citation in text: [1] -
Karthikeyan, C.; Lee, C.; Moore, J.; Mittal, R.; Suswam, E. A.; Abbott, K. L.; Pondugula, S. R.; Manne, U.; Narayanan, N. K.; Trivedi, P.; Tiwari, A. K. Bioorg. Med. Chem. 2015, 23, 602–611. doi:10.1016/j.bmc.2014.11.043
Return to citation in text: [1] -
Eleftheriadis, N.; Neochoritis, C. G.; Leus, N. G. J.; van der Wouden, P. E.; Dömling, A.; Dekker, F. J. J. Med. Chem. 2015, 58, 7850–7862. doi:10.1021/acs.jmedchem.5b01121
Return to citation in text: [1] -
Sayed, M.; Kamal El‐Dean, A. M.; Ahmed, M.; Hassanien, R. J. Chin. Chem. Soc. 2019, 66, 218–225. doi:10.1002/jccs.201800115
Return to citation in text: [1] -
Yang, Y.; Wu, S.; Zhao, C.; He, H.; Wu, Z.; Zhang, J.; Song, R. J. Agric. Food Chem. 2024, 72, 11331–11340. doi:10.1021/acs.jafc.3c08950
Return to citation in text: [1] -
Enriz, R. D.; Tosso, R. D.; Andújar, S. A.; Cabedo, N.; Cortés, D.; Nogueras, M.; Cobo, J.; Vargas, D. F.; Trilleras, J. Tetrahedron 2018, 74, 7047–7057. doi:10.1016/j.tet.2018.10.038
Return to citation in text: [1]
| 1. | United Nations Department of Economic and Social Affairs (UNDESA) and the Secretariat of the United Nations Framework Convention on Climate Change (UNFCCC). Third Global Conference on Strengthening Synergies Between the Paris Agreement and the 2030 Agenda For Sustainable Development, 2022. |
| 5. | Kerdphon, S.; Sanghong, P.; Chatwichien, J.; Choommongkol, V.; Rithchumpon, P.; Singh, T.; Meepowpan, P. Eur. J. Org. Chem. 2020, 2730–2734. doi:10.1002/ejoc.202000257 |
| 6. | Jacquet, O.; Das Neves Gomes, C.; Ephritikhine, M.; Cantat, T. ChemCatChem 2013, 5, 117–120. doi:10.1002/cctc.201200732 |
| 42. | Wang, K.; Kim, D.; Dömling, A. J. Comb. Chem. 2010, 12, 111–118. doi:10.1021/cc9001586 |
| 43. | Kok, T.; Wapenaar, H.; Wang, K.; Neochoritis, C. G.; Zarganes-Tzitzikas, T.; Proietti, G.; Eleftheriadis, N.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Cool, R. H.; Poelarends, G. J.; Dömling, A.; Dekker, F. J. Bioorg. Med. Chem. 2018, 26, 999–1005. doi:10.1016/j.bmc.2017.12.032 |
| 44. | Wang, K.; Herdtweck, E.; Dömling, A. ACS Comb. Sci. 2011, 13, 140–146. doi:10.1021/co100040z |
| 45. | Wang, K.; Herdtweck, E.; Dömling, A. ACS Comb. Sci. 2012, 14, 316–322. doi:10.1021/co3000133 |
| 4. | Fragkiadakis, M.; Anastasiou, P.-K.; Volyrakis, I.; Pantousas, A.; Stoumpos, C. C.; Neochoritis, C. G. Chem. Commun. 2023, 59, 14411–14414. doi:10.1039/d3cc04597h |
| 46. | Wang, K.; Nguyen, K.; Huang, Y.; Dömling, A. J. Comb. Chem. 2009, 11, 920–927. doi:10.1021/cc9000778 |
| 2. | Wu, X.-F.; Dyson, P. J.; Arndtsen, B. A. J. Org. Chem. 2023, 88, 4891–4893. doi:10.1021/acs.joc.3c00626 |
| 3. | Trost, B. Science 1991, 254, 1471–1477. doi:10.1126/science.1962206 |
| 36. | Sinkeldam, R. W.; McCoy, L. S.; Shin, D.; Tor, Y. Angew. Chem., Int. Ed. 2013, 52, 14026–14030. doi:10.1002/anie.201307064 |
| 37. | Ludford, P. T., III; Yang, S.; Bucardo, M. S.; Tor, Y. Chem. – Eur. J. 2022, 28, e202104472. doi:10.1002/chem.202104472 |
| 38. | Shin, D.; Sinkeldam, R. W.; Tor, Y. J. Am. Chem. Soc. 2011, 133, 14912–14915. doi:10.1021/ja206095a |
| 2. | Wu, X.-F.; Dyson, P. J.; Arndtsen, B. A. J. Org. Chem. 2023, 88, 4891–4893. doi:10.1021/acs.joc.3c00626 |
| 25. | Sacchelli, B. A. L.; Rocha, B. C.; Fantinel, M.; Andrade, L. H. Eur. J. Org. Chem. 2024, 27, e202300930. doi:10.1002/ejoc.202300930 |
| 39. | Bogert, M. T.; Gotthelf, A. H. J. Am. Chem. Soc. 1900, 22, 522–535. doi:10.1021/ja02046a008 |
| 40. | Von Niementowski, S. J. Prakt. Chem. 1895, 51, 564–572. doi:10.1002/prac.18950510150 |
| 41. | Meyer, J.; Wagner, E. C. J. Org. Chem. 1943, 08, 239–252. doi:10.1021/jo01191a005 |
| 25. | Sacchelli, B. A. L.; Rocha, B. C.; Fantinel, M.; Andrade, L. H. Eur. J. Org. Chem. 2024, 27, e202300930. doi:10.1002/ejoc.202300930 |
| 25. | Sacchelli, B. A. L.; Rocha, B. C.; Fantinel, M.; Andrade, L. H. Eur. J. Org. Chem. 2024, 27, e202300930. doi:10.1002/ejoc.202300930 |
| 27. | Xu, L.; Jiang, Y.; Ma, D. Org. Lett. 2012, 14, 1150–1153. doi:10.1021/ol300084v |
| 21. | Li, J.; Di Lorenzo, V.; Patil, P.; Ruiz-Moreno, A. J.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Velasco-Velázquez, M. A.; Dömling, A. ACS Comb. Sci. 2020, 22, 356–360. doi:10.1021/acscombsci.0c00072 |
| 22. | Eleftheriadis, N.; Poelman, H.; Leus, N. G. J.; Honrath, B.; Neochoritis, C. G.; Dolga, A.; Dömling, A.; Dekker, F. J. Eur. J. Med. Chem. 2016, 122, 786–801. doi:10.1016/j.ejmech.2016.07.010 |
| 23. | Xiao, Z.; Osipyan, A.; Song, S.; Chen, D.; Schut, R. A.; van Merkerk, R.; van der Wouden, P. E.; Cool, R. H.; Quax, W. J.; Melgert, B. N.; Poelarends, G. J.; Dekker, F. J. J. Med. Chem. 2022, 65, 2059–2077. doi:10.1021/acs.jmedchem.1c01598 |
| 24. | Xiao, Z.; Dekker, F. Substituted Thiophene Compounds as D-Dopachrome Tautomerase Inhibitors. WO Patent WO2023031246, March 9, 2023. |
| 28. | Abdellatif, K. R. A.; Bakr, R. B. Med. Chem. Res. 2021, 30, 31–49. doi:10.1007/s00044-020-02656-8 |
| 29. | Vyas, A.; Sahu, B.; Pathania, S.; Nandi, N. K.; Chauhan, G.; Asati, V.; Kumar, B. J. Heterocycl. Chem. 2023, 60, 1081–1121. doi:10.1002/jhet.4593 |
| 30. | Thakur, S.; Ansari, A. J.; Joshi, G. FDA approved fused pyrimidine-based drugs. In Fused Pyrimidine-Based Drug Discovery; Raj, K., Ed.; Elsevier: Amsterdam, Netherlands, 2023; pp 13–38. doi:10.1016/b978-0-443-18616-5.00004-1 |
| 31. | Xu, G.-F.; Song, B.-A.; Bhadury, P. S.; Yang, S.; Zhang, P.-Q.; Jin, L.-H.; Xue, W.; Hu, D.-Y.; Lu, P. Bioorg. Med. Chem. 2007, 15, 3768–3774. doi:10.1016/j.bmc.2007.03.037 |
| 32. | Long, C.; Li, P.; Chen, M.; Dong, L.; Hu, D.; Song, B. Eur. J. Med. Chem. 2015, 102, 639–647. doi:10.1016/j.ejmech.2015.08.029 |
| 33. | Kulkarni, S. S.; Singh, S.; Shah, J. R.; Low, W.-K.; Talele, T. T. Eur. J. Med. Chem. 2012, 50, 264–273. doi:10.1016/j.ejmech.2012.02.001 |
| 34. | Kargbo, R. B. ACS Med. Chem. Lett. 2024, 15, 1193–1195. doi:10.1021/acsmedchemlett.4c00327 |
| 35. | Cline, S. J.; Hodgson, D. J. J. Am. Chem. Soc. 1980, 102, 6285–6288. doi:10.1021/ja00540a018 |
| 4. | Fragkiadakis, M.; Anastasiou, P.-K.; Volyrakis, I.; Pantousas, A.; Stoumpos, C. C.; Neochoritis, C. G. Chem. Commun. 2023, 59, 14411–14414. doi:10.1039/d3cc04597h |
| 16. | Niemi, T.; Perea‐Buceta, J. E.; Fernández, I.; Hiltunen, O.-M.; Salo, V.; Rautiainen, S.; Räisänen, M. T.; Repo, T. Chem. – Eur. J. 2016, 22, 10355–10359. doi:10.1002/chem.201602338 |
| 17. | Mei, C.; Zhao, Y.; Chen, Q.; Cao, C.; Pang, G.; Shi, Y. ChemCatChem 2018, 10, 3057–3068. doi:10.1002/cctc.201800142 |
| 18. | Wang, B.; Elageed, E. H. M.; Zhang, D.; Yang, S.; Wu, S.; Zhang, G.; Gao, G. ChemCatChem 2014, 6, 278–283. doi:10.1002/cctc.201300801 |
| 19. | Feng, L.; Li, X.; Xu, C.; Sadeghzadeh, S. M. Catal. Lett. 2020, 150, 1729–1740. doi:10.1007/s10562-019-03073-2 |
| 20. | Wang, L.; Qi, C.; Xiong, W.; Jiang, H. Chin. J. Catal. 2022, 43, 1598–1617. doi:10.1016/s1872-2067(21)64029-9 |
| 7. | Dabral, S.; Schaub, T. Adv. Synth. Catal. 2019, 361, 223–246. doi:10.1002/adsc.201801215 |
| 8. | Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Nat. Commun. 2015, 6, 5933. doi:10.1038/ncomms6933 |
| 9. | Sakakura, T.; Choi, J.-C.; Yasuda, H. Chem. Rev. 2007, 107, 2365–2387. doi:10.1021/cr068357u |
| 10. | Mikkelsen, M.; Jørgensen, M.; Krebs, F. C. Energy Environ. Sci. 2010, 3, 43–81. doi:10.1039/b912904a |
| 11. | Pinaka, A.; Vougioukalakis, G. C. Coord. Chem. Rev. 2015, 288, 69–97. doi:10.1016/j.ccr.2015.01.010 |
| 12. | Aresta, M.; Dibenedetto, A.; Angelini, A. Chem. Rev. 2014, 114, 1709–1742. doi:10.1021/cr4002758 |
| 13. | Wang, S.; Xi, C. Chem. Soc. Rev. 2019, 48, 382–404. doi:10.1039/c8cs00281a |
| 14. | Pradhan, S.; Roy, S.; Sahoo, B.; Chatterjee, I. Chem. – Eur. J. 2021, 27, 2254–2269. doi:10.1002/chem.202003685 |
| 15. | Hang, W.; Li, D.; Zou, S.; Xi, C. J. Org. Chem. 2023, 88, 5007–5014. doi:10.1021/acs.joc.2c01840 |
| 26. | Jiang, L.; Li, K.; Porter, W. N.; Wang, H.; Li, G.; Chen, J. G. J. Am. Chem. Soc. 2024, 146, 2857–2875. doi:10.1021/jacs.3c13374 |
| 44. | Wang, K.; Herdtweck, E.; Dömling, A. ACS Comb. Sci. 2011, 13, 140–146. doi:10.1021/co100040z |
| 42. | Wang, K.; Kim, D.; Dömling, A. J. Comb. Chem. 2010, 12, 111–118. doi:10.1021/cc9001586 |
| 45. | Wang, K.; Herdtweck, E.; Dömling, A. ACS Comb. Sci. 2012, 14, 316–322. doi:10.1021/co3000133 |
| 64. | El-Gazzar, A. B. A.; Hafez, H. N.; Nawwar, G. A. M. Eur. J. Med. Chem. 2009, 44, 1427–1436. doi:10.1016/j.ejmech.2008.09.030 |
| 65. | Ali, H. I.; Ashida, N.; Nagamatsu, T. Bioorg. Med. Chem. 2008, 16, 922–940. doi:10.1016/j.bmc.2007.10.014 |
| 66. | Joshi, A. A.; Viswanathan, C. L. Bioorg. Med. Chem. Lett. 2006, 16, 2613–2617. doi:10.1016/j.bmcl.2006.02.038 |
| 67. | Ibrahim, N. S. M.; Kadry, H. H.; Zaher, A. F.; Mohamed, K. O. Chem. Biol. Drug Des. 2023, 102, 996–1013. doi:10.1111/cbdd.14307 |
| 68. | Karthikeyan, C.; Lee, C.; Moore, J.; Mittal, R.; Suswam, E. A.; Abbott, K. L.; Pondugula, S. R.; Manne, U.; Narayanan, N. K.; Trivedi, P.; Tiwari, A. K. Bioorg. Med. Chem. 2015, 23, 602–611. doi:10.1016/j.bmc.2014.11.043 |
| 69. | Eleftheriadis, N.; Neochoritis, C. G.; Leus, N. G. J.; van der Wouden, P. E.; Dömling, A.; Dekker, F. J. J. Med. Chem. 2015, 58, 7850–7862. doi:10.1021/acs.jmedchem.5b01121 |
| 70. | Sayed, M.; Kamal El‐Dean, A. M.; Ahmed, M.; Hassanien, R. J. Chin. Chem. Soc. 2019, 66, 218–225. doi:10.1002/jccs.201800115 |
| 71. | Yang, Y.; Wu, S.; Zhao, C.; He, H.; Wu, Z.; Zhang, J.; Song, R. J. Agric. Food Chem. 2024, 72, 11331–11340. doi:10.1021/acs.jafc.3c08950 |
| 72. | Enriz, R. D.; Tosso, R. D.; Andújar, S. A.; Cabedo, N.; Cortés, D.; Nogueras, M.; Cobo, J.; Vargas, D. F.; Trilleras, J. Tetrahedron 2018, 74, 7047–7057. doi:10.1016/j.tet.2018.10.038 |
| 57. | Bassetto, M.; Leyssen, P.; Neyts, J.; Yerukhimovich, M. M.; Frick, D. N.; Brancale, A. Eur. J. Med. Chem. 2016, 123, 31–47. doi:10.1016/j.ejmech.2016.07.035 |
| 58. | Salahuddin, M.; Kakad, S.; Shantakumar, S. M. E-J. Chem. 2009, 6, 801–808. doi:10.1155/2009/361282 |
| 59. | Pédeboscq, S.; Gravier, D.; Casadebaig, F.; Hou, G.; Gissot, A.; Rey, C.; Ichas, F.; De Giorgi, F.; Lartigue, L.; Pometan, J.-P. Bioorg. Med. Chem. 2012, 20, 6724–6731. doi:10.1016/j.bmc.2012.09.034 |
| 60. | Spooner, R. A.; Watson, P.; Smith, D. C.; Boal, F.; Amessou, M.; Johannes, L.; Clarkson, G. J.; Lord, J. M.; Stephens, D. J.; Roberts, L. M. Biochem. J. 2008, 414, 471–484. doi:10.1042/bj20080149 |
| 61. | Zishiri, V. K.; Joshi, M. C.; Hunter, R.; Chibale, K.; Smith, P. J.; Summers, R. L.; Martin, R. E.; Egan, T. J. J. Med. Chem. 2011, 54, 6956–6968. doi:10.1021/jm2009698 |
| 62. | Lilienkampf, A.; Mao, J.; Wan, B.; Wang, Y.; Franzblau, S. G.; Kozikowski, A. P. J. Med. Chem. 2009, 52, 2109–2118. doi:10.1021/jm900003c |
| 63. | Swain, S. S.; Pati, S.; Hussain, T. Eur. J. Med. Chem. 2022, 232, 114173. doi:10.1016/j.ejmech.2022.114173 |
| 25. | Sacchelli, B. A. L.; Rocha, B. C.; Fantinel, M.; Andrade, L. H. Eur. J. Org. Chem. 2024, 27, e202300930. doi:10.1002/ejoc.202300930 |
| 47. | Ali, E. M. H.; Abdel-Maksoud, M. S.; Oh, C.-H. Bioorg. Med. Chem. 2019, 27, 1159–1194. doi:10.1016/j.bmc.2019.02.044 |
| 48. | Eissa, K. I.; Kamel, M. M.; Mohamed, L. W.; Doghish, A. S.; Alnajjar, R.; Al‐Karmalawy, A. A.; Kassab, A. E. Drug Dev. Res. 2023, 84, 937–961. doi:10.1002/ddr.22064 |
| 49. | Zhong, W.; Pasunooti, K. K.; Balamkundu, S.; Wong, Y. H.; Nah, Q.; Gadi, V.; Gnanakalai, S.; Chionh, Y. H.; McBee, M. E.; Gopal, P.; Lim, S. H.; Olivier, N.; Buurman, E. T.; Dick, T.; Liu, C. F.; Lescar, J.; Dedon, P. C. J. Med. Chem. 2019, 62, 7788–7805. doi:10.1021/acs.jmedchem.9b00582 |
| 50. | Tinney, F. J.; Cetenko, W. A.; Kerbleski, J. J.; Connor, D. T.; Sorenson, R. J.; Herzig, D. J. J. Med. Chem. 1981, 24, 878–882. doi:10.1021/jm00139a021 |
| 51. | Russo, F.; Romeo, G.; Santagati, N.; Caruso, A.; Cutuli, V.; Amore, D. Eur. J. Med. Chem. 1994, 29, 569–578. doi:10.1016/0223-5234(94)90149-x |
| 52. | Modica, M.; Santagati, M.; Santagati, A.; Russo, F.; Cagnotto, A.; Goegan, M.; Mennini, T. Bioorg. Med. Chem. Lett. 2000, 10, 1089–1092. doi:10.1016/s0960-894x(00)00165-7 |
| 53. | Duval, E.; Case, A.; Stein, R. L.; Cuny, G. D. Bioorg. Med. Chem. Lett. 2005, 15, 1885–1889. doi:10.1016/j.bmcl.2005.02.005 |
| 54. | Miao, Z.; Sun, Y.-m.; Zhao, L.-y.; Li, Y.-s.; Wang, Y.-f.; Nan, J.-s.; Qiao, Z.-e.; Li, L.-l.; Yang, S.-y. Bioorg. Med. Chem. Lett. 2020, 30, 126966. doi:10.1016/j.bmcl.2020.126966 |
| 55. | Basile, L.; Álvarez, S.; Blanco, A.; Santagati, A.; Granata, G.; Di Pietro, P.; Guccione, S.; Muñoz-Fernández, M. Á. Eur. J. Med. Chem. 2012, 57, 149–161. doi:10.1016/j.ejmech.2012.09.005 |
| 56. | Zhu, W.; Chen, H.; Wang, Y.; Wang, J.; Peng, X.; Chen, X.; Gao, Y.; Li, C.; He, Y.; Ai, J.; Geng, M.; Zheng, M.; Liu, H. J. Med. Chem. 2017, 60, 6018–6035. doi:10.1021/acs.jmedchem.7b00076 |
| 42. | Wang, K.; Kim, D.; Dömling, A. J. Comb. Chem. 2010, 12, 111–118. doi:10.1021/cc9001586 |
| 44. | Wang, K.; Herdtweck, E.; Dömling, A. ACS Comb. Sci. 2011, 13, 140–146. doi:10.1021/co100040z |
| 41. | Meyer, J.; Wagner, E. C. J. Org. Chem. 1943, 08, 239–252. doi:10.1021/jo01191a005 |
© 2025 Zingiridis et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.