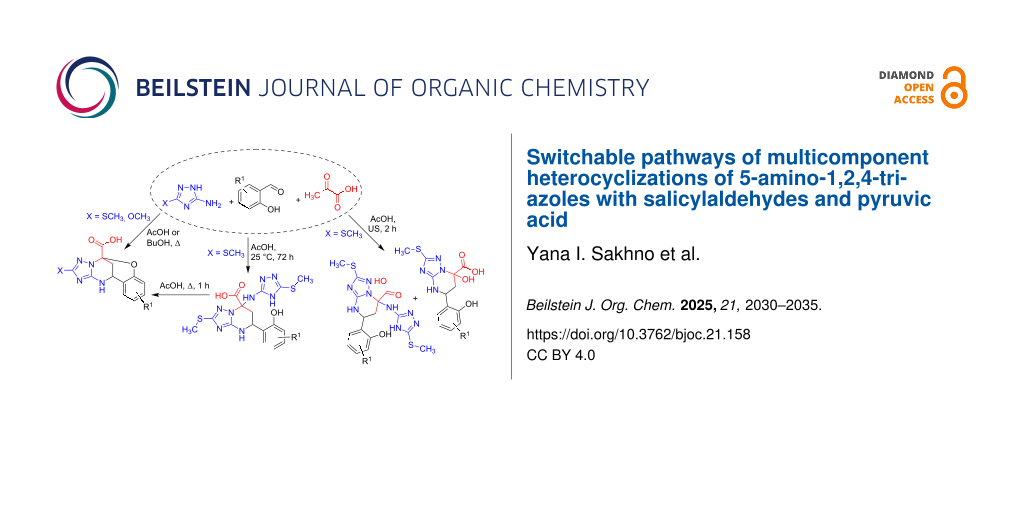
Abstract
Switchable multicomponent reactions involving 3-substituted-5-amino-1,2,4-triazoles, pyruvic acid, and salicylaldehydes were studied under different conditions. Upon conventional heating, benzotriazolooxadiazocine-5-carboxylic acids were formed in the three-component reactions as single reaction products. Upon ultrasonic activation or mechanical stirring at room temperature, the multicomponent reaction of the same starting materials led to the formation of only tetrahydrotriazolopyrimidine derivatives.
Graphical Abstract
Introduction
Multicomponent reactions (MCRs) are a powerful tool for the formation of heterocyclic systems in a minimal number of steps and offer several ways to vary substituents and they are widely used in drug discovery and development, as well as in diversity-oriented synthesis [1-4]. In addition, the application of condition-based divergence strategy [5] and the concept of multicomponent-switched reactions [6] allows to tune the selectivity of MCRs and direct them into several different pathways. That is why the study of multicomponent heterocyclizations of aromatic aldehydes, compounds with an active methylene group and aminoazoles, for example with 3-amino-1,2,4-triazoles, is important because the formation of different chemotypes of final heterocyclic compounds are possible depending on the structure of the reagents, the solvents and the catalysts, and type of activation methods [7-9].
MCRs of aminotriazoles, methylene-active compounds, and substituted salicylaldehydes are particularly interesting because of the possibility of additional reactions and post-cyclizations involving the o-hydroxy group. It was demonstrated [10-12] that the multicomponent reaction of substituted 3-amino-1,2,4-triazoles, various salicylaldehydes, and acetone depending on the conditions, can either be limited to a Biginelli condensation with the formation of hydroxytetrahydropyrimidines or proceed with further post-cyclization to form oxygen-bridged triazolobenzoxadiazocine derivatives. Furthermore, a multicomponent synthesis of oxygen-bridged pyrimidine systems also was described in several other publications [13-15]. On the other hand, three-component reactions of aminoazoles, salicylaldehyde, and esters (or amides) of acetoacetic acid with isolation of other types of heterocylic compounds were described in some papers [16,17].
Our early works were devoted to the study of the reactions of aminoazoles, pyruvic acid and its derivatives with salicylaldehydes and it was found that depending on the conditions (reaction time, temperature, and method of process activation, in particular ultrasound and microwave irradiation), different types of heterocycles I–VI were formed (Scheme 1) [8,18,19].
Scheme 1: Diversity of heterocyclization products from reaction of aminoazoles with salicylaldehydes, and pyruvic acids.
Scheme 1: Diversity of heterocyclization products from reaction of aminoazoles with salicylaldehydes, and pyr...
It is known that substituents in aminoazoles can significantly influence their behavior in multicomponent heterocyclization reactions [4,8,20,21]. However, the reactions of 5-amino-1,2,4-triazoles containing substituents in position 3, which can also affect their chemical behaviour, with pyruvic acid, and salicylaldehydes remain undescribed. The present paper is intended to fill this gap and complete the description of this type of switchable multicomponent reactions.
Results and Discussion
Therefore, the MCRs between some 3-substituted 5-amino-1,2,4-triazoles, salicylaldehydes, and pyruvic acid were studied under different reaction conditions. In particular, it was found that the three-component reaction of an equimolar mixture of 5-amino-3-methylthio(methoxy)-1,2,4-triazole 1a,b, salicylaldehydes 2a–f, and pyruvic acid (3) under conventional heating at reflux in acetic acid for 3 h for aminotriazole 1a or in n-BuOH for 7 h in case of aminotriazole 1b led to the formation of oxygen-bridged 2-(methylthio or methoxy)-11,12-dihydro-5H-5,11-methanobenzo[g][1,2,4]triazolo[1,5-c][1,3,5]oxadiazocine-5-carboxylic acids 4a–j (Scheme 2, Table 1). It should be noted that this MCR involving unsubstituted 5-amino-1H-1,2,4-triazole under various conditions never proceeds towards the formation of oxygen-bridged compounds [18]. Thus, there is indeed a significant influence of the substituent in position 3 of 5-amino-1H-1,2,4-triazoles on their chemical behavior in this MCR.
Scheme 2: MCRs of 3-amino-5-methylthio-1,2,4-triazole (1a) and 3-amino-5-methoxy-1,2,4-triazole (1b) with salicylaldehydes 2a–f, and pyruvic acid (3).
Scheme 2: MCRs of 3-amino-5-methylthio-1,2,4-triazole (1a) and 3-amino-5-methoxy-1,2,4-triazole (1b) with sal...
Table 1: Synthesis of compounds 4a–j.
| Entry | Starting materials | Reaction time | Product | Yield | |||
| Compd. | R | Compd. | R1 | (hours) | (%) | ||
| 1 | 1a | SCH3 | 2a | H | 3 | 4a | 45 |
| 2 | 1a | SCH3 | 2b | 3-CH3O | 3 | 4b | 60 |
| 3 | 1a | SCH3 | 2c | 5-Br | 3 | 4c | 42 |
| 4 | 1a | SCH3 | 2d | 5-NO2 | 3 | 4d | 35 |
| 5 | 1a | SCH3 | 2e | 5-F | 3 | 4e | 32 |
| 6 | 1a | SCH3 | 2f | 5-Cl | 3 | 4f | 42 |
| 7 | 1b | OCH3 | 2b | 3-CH3O | 7 | 4g | 46 |
| 8 | 1b | OCH3 | 2c | 5-Br | 7 | 4h | 41 |
| 9 | 1b | OCH3 | 2e | 5-F | 7 | 4i | 35 |
| 10 | 1b | OCH3 | 2f | 5-Cl | 7 | 4j | 68 |
In the case of 3-carbomethoxy- and 3-(trifluoromethyl)-5-amino-1,2,4-triazoles the treatment with pyruvic acid and salicylaldehydes under thermal heating in different solvents (AcOH, DMF, n-butanol), instead of oxygen-bridged compounds gave a mixture of unidentified products with trace amounts of Schiff bases between amino-1,2,4-triazole and salicylaldehyde as well as with impurities of the reagents. This is most likely due to the electron-withdrawing nature of the substituent at C(3) in these 5-amino-1,2,4-triazoles that do not favor the proceeding of the MCR in contrast to 5-aminotriazoles 1a,b having pronounced electron-donating substituents (methylthio- and methoxy groups).
On the other hand, the MCR of the reagents 1a, 2a–c and 3 under ultrasonic irradiation for 2 h at room temperature (25 °C) (Scheme 3) afforded a mixture of 5-(2-hydroxyphenyl)-2-(methylthio)-7-((3-(methylthio)-4H-1,2,4-triazol-5-yl)amino)-4,5,6,7-tetrahydro[1,2,4]triazolo[1,5-a]pyrimidine-7-carboxylic acids 5a–d and 5-(2-hydroxyphenyl)-7-hydroxy-2-(methylthio)-4,5,6,7-tetrahydro[1,2,4]triazolo[1,5-a]pyrimidine-7-carboxylic acids 6a–d (in a ratio of ca. 80:20 for compounds 5a–c and 6a–c; and 15:85 for compounds 5d and 6d). Unfortunately, all attempts to separate these mixtures were unsuccessful.
Scheme 3: MCRs of 3-amino-5-methylthio-1,2,4-triazole (1a), salicylaldehydes 2a–c,f, and pyruvic acid (3) under different conditions.
Scheme 3: MCRs of 3-amino-5-methylthio-1,2,4-triazole (1a), salicylaldehydes 2a–c,f, and pyruvic acid (3) und...
However, repeating the reaction under mechanical stirring at room temperature for 72 h yielded tetrahydrotriazolopyrimidine-7-carboxylic acids 5a–c as a mixture of two diastereomers (Scheme 3, Table 2). In the case of aldehyde 2b, the MCR always led to the formation of a mixture of products 5d and 6d. Attempts to synthesize 5d and 6d as individual compounds under various conditions were unsuccessful.
In addition, it was found that compounds 5 can be converted into oxygen-bridged heterocycles 4 after 1 hour of conventional heating at reflux in acetic acid (Scheme 3). In contrast, all attempts to transform 7-triazolylamino derivatives of pyrimidinecarboxylic acids 5 into their 7-hydroxy derivatives 6 were unsuccessful. It also failed to perform the reverse conversion. The same situation was observed for similar compounds based on 5-amino-1H-pyrazole-4-carbonitrile [22].
It should be noted that diazocines 4 were stable and did not undergo transformations when heated or irradiated with ultrasound. This may indicate that pyrimidine-7-carboxylic acids 5 and 6 are formed in parallel processes under kinetic control of the reaction, while oxygen-bridged heterocycles 4 are formed under thermodynamic control.
The purity and structure of compounds 4 and 5 were established by elemental analysis, mass spectrometry, 1H and 13C NMR spectroscopy, and X-ray diffraction study (Figure 1).
![[1860-5397-21-158-1]](/bjoc/content/figures/1860-5397-21-158-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Molecular structure of compound 4c according to X-ray diffraction data. Thermal ellipsoids are shown at 50% probability level.
Figure 1: Molecular structure of compound 4c according to X-ray diffraction data. Thermal ellipsoids are show...
For example, the 1H NMR spectra of products 4a–f exhibit signals for proton of the pyrimidine NH group (8.38–8.53 ppm), signals for aromatic protons (6.58–8.34 ppm), multiplet for the proton of the CH group at 4.64–4.92 ppm, a singlet for the protons for the triazole SCH3 group at 2.37–2.38 ppm as well as signals for other substituents. The peaks of the proton of the carboxyl group is not recognized, probably due to exchange processes. The signals of the CH2 group in the range around δ = 2.5 ppm are not always clearly identifiable, as they partially or completely overlap with the remaining DMSO signal. Compounds 4g–j are characterized by the following signals in the 1H NMR spectra: a proton singlet of the pyrimidine NH group at 8.30–8.31 ppm, a multiplet of aromatic protons in the region of 6.95–7.51 ppm, a proton multiplet of the CH group at 4.65–4.68 ppm and singlet for protons for the triazole CH3O group at 3.7 ppm.
The 1H NMR spectra of heterocyclic acids 5a–c exhibited a broad singlet of proton of the carboxyl group at 11.88–14.02 ppm, a singlet of proton of the hydroxy group at 9.41–9.86 ppm, a singlet of proton of the pyrimidine NH group at 7.72–7.91 ppm, a singlet of proton of the triazolylamine NH group at 7.44–7.57 ppm, peaks for aromatic protons at 6.70–7.52 ppm, a multiplet of proton of the CH group at 5.0–5.19 ppm and two signals for diastereotopic protons of CH2 group (about 2.89–3.11 and 2.28–2.35 ppm) and protons for the triazole SCH3 group at 2.38–2.46 ppm. The doubling of some signals in the 1H NMR spectra probably indicates the formation of both possible diastereomers. The structure of compounds 6a–d was confirmed from the 1H NMR spectra and the mass spectra of mixture by comparison with literature data for similar pyrimidines [17,18].
Conclusion
In summary, the multicomponent reaction of 3-amino-5-methylthio(methoxy)-1,2,4-triazoles with salicylaldehydes, and pyruvic acid can be switched between two different directions using conventional thermal heating, mechanical stirring at room temperature and ultrasonication at room temperature. The treatment under reflux conditions leads to the formation of oxygen-bridged benzo[g][1,2,4]triazolo[1,5-c][1,3,5]oxadiazocine-5-carboxylic acids exclusively while the reaction under mechanical stirring gives 5-(2-hydroxyphenyl)-2-(methylthio)-7-((3-(methylthio)-4H-1,2,4-triazol-5-yl)amino)-4,5,6,7-tetrahydro[1,2,4]triazolo[1,5-a]pyrimidine-7-carboxylic acids. The reaction under ultrasonication at room temperature yields a mixture of the latter with 5-(2-hydroxyphenyl)-7-hydroxy-2-(methylthio)-4,5,6,7-tetrahydro[1,2,4]triazolo[1,5-a]pyrimidine-7-carboxylic acids.
Supporting Information
| Supporting Information File 1: Experimental procedures, product characterization, and copies of NMR spectra. | ||
| Format: PDF | Size: 2.2 MB | Download |
Data Availability Statement
Data generated and analyzed during this study is available from the corresponding author upon reasonable request.
References
-
Graebin, C. S.; Ribeiro, F. V.; Rogério, K. R.; Kümmerle, A. E. Curr. Org. Synth. 2019, 16, 855–899. doi:10.2174/1570179416666190718153703
Return to citation in text: [1] -
John, S. E.; Gulati, S.; Shankaraiah, N. Org. Chem. Front. 2021, 8, 4237–4287. doi:10.1039/d0qo01480j
Return to citation in text: [1] -
Graziano, G.; Stefanachi, A.; Contino, M.; Prieto-Díaz, R.; Ligresti, A.; Kumar, P.; Scilimati, A.; Sotelo, E.; Leonetti, F. Int. J. Mol. Sci. 2023, 24, 6581. doi:10.3390/ijms24076581
Return to citation in text: [1] -
Murlykina, M. V.; Morozova, A. D.; Zviagin, I. M.; Sakhno, Y. I.; Desenko, S. M.; Chebanov, V. A. Front. Chem. (Lausanne, Switz.) 2018, 6, 527. doi:10.3389/fchem.2018.00527
Return to citation in text: [1] [2] -
Scheffelaar, R.; Ruijter, E.; Orru, R. V. A. Top. Heterocycl. Chem. 2010, 25, 95–126. doi:10.1007/7081_2010_44
Return to citation in text: [1] -
Chebanov, V. A.; Desenko, S. M.; Lipson, V. V.; Gorobets, N. Y. Multicomponent‐Switched Reactions in Synthesis of Heterocycles. In Multicomponent Reactions towards Heterocycles; Van der Eycken, E.; Sharma, U. K., Eds.; Wiley-VCH: Weinheim, Germany, 2022; pp 287–338. doi:10.1002/9783527832439.ch8
Return to citation in text: [1] -
Damera, T.; Pagadala, R.; Rana, S.; Jonnalagadda, S. B. Catalysts 2023, 13, 1034. doi:10.3390/catal13071034
Return to citation in text: [1] -
Desenko, S. M.; Gorobets, M. Y.; Lipson, V. V.; Sakhno, Y. I.; Chebanov, V. A. Chem. Rec. 2024, 24, e202300244. doi:10.1002/tcr.202300244
Return to citation in text: [1] [2] [3] -
Sakhno, Y. I.; Mykhailenko, M. V.; Kolosov, M. A.; Shvets, E. H.; Musatov, V. I.; Chorna, N. V.; Desenko, S. M.; Chebanov, V. A. Ukr. Bioorg. Acta 2020, 15, 22–26. doi:10.15407/bioorganica2020.02.022
Return to citation in text: [1] -
Doğan, İ. S.; Gümüş, M. K.; Gorobets, N. Y.; Reis, R.; Orak, D.; Sipahi, H.; Sari, S.; Chebanov, V. A. Pharm. Chem. J. 2022, 56, 769–776. doi:10.1007/s11094-022-02708-w
Return to citation in text: [1] -
Zeng, M.; Xue, Y.; Qin, Y.; Peng, F.; Li, Q.; Zeng, M.-H. Chin. Chem. Lett. 2022, 33, 4891–4895. doi:10.1016/j.cclet.2022.02.075
Return to citation in text: [1] -
Gorobets, N. Y.; Sedash, Y. V.; Ostras, K. S.; Zaremba, O. V.; Shishkina, S. V.; Baumer, V. N.; Shishkin, O. V.; Kovalenko, S. M.; Desenko, S. M.; Van der Eycken, E. V. Tetrahedron Lett. 2010, 51, 2095–2098. doi:10.1016/j.tetlet.2010.02.045
Return to citation in text: [1] -
Jing, X.; Li, Z.; Pan, X.; Wang, Q.; Yan, C.; Zhu, H. Synth. Commun. 2009, 39, 3796–3803. doi:10.1080/00397910902838896
Return to citation in text: [1] -
Cui, X.; Lin, J.-J.; Wang, S.; Li, J.-P.; Xia, X.-S.; Huang, C. Tetrahedron Lett. 2022, 89, 153603. doi:10.1016/j.tetlet.2021.153603
Return to citation in text: [1] -
Svĕtlík, J.; Veizerová, L. Helv. Chim. Acta 2011, 94, 199–205. doi:10.1002/hlca.201000193
Return to citation in text: [1] -
Světlík, J.; Kettmann, V. Tetrahedron Lett. 2011, 52, 1062–1066. doi:10.1016/j.tetlet.2010.12.051
Return to citation in text: [1] -
Tkachenko, V. V.; Muravyova, E. A.; Desenko, S. M.; Shishkin, O. V.; Shishkina, S. V.; Sysoiev, D. O.; Müller, T. J. J.; Chebanov, V. A. Beilstein J. Org. Chem. 2014, 10, 3019–3030. doi:10.3762/bjoc.10.320
Return to citation in text: [1] [2] -
Murlykina, M. V.; Sakhno, Y. I.; Desenko, S. M.; Shishkina, S. V.; Shishkin, O. V.; Sysoiev, D. O.; Kornet, M. N.; Schols, D.; Goeman, J. L.; Van der Eycken, J.; Van der Eycken, E. V.; Chebanov, V. A. Eur. J. Org. Chem. 2015, 4481–4492. doi:10.1002/ejoc.201500469
Return to citation in text: [1] [2] [3] -
Murlykina, M. V.; Sakhno, Y. I.; Desenko, S. M.; Konovalova, I. S.; Shishkin, O. V.; Sysoiev, D. A.; Kornet, M. N.; Chebanov, V. A. Tetrahedron 2013, 69, 9261–9269. doi:10.1016/j.tet.2013.08.055
Return to citation in text: [1] -
Muravyova, E. A.; Desenko, S. M.; Rudenko, R. V.; Shishkina, S. V.; Shishkin, O. V.; Sen’ko, Y. V.; Vashchenko, E. V.; Chebanov, V. A. Tetrahedron 2011, 67, 9389–9400. doi:10.1016/j.tet.2011.09.138
Return to citation in text: [1] -
Sakhno, Y.; Murlykina, M.; Morozova, A.; Kozyrev, A.; Chebanov, V. Fr.-Ukr. J. Chem. 2015, 3, 1–20. doi:10.17721/fujcv3i2p1-20
Return to citation in text: [1] -
Sakhno, Y. I.; Murlykina, M. V.; Zbruyev, O. I.; Kozyryev, A. V.; Shishkina, S. V.; Sysoiev, D.; Musatov, V. I.; Desenko, S. M.; Chebanov, V. A. Beilstein J. Org. Chem. 2020, 16, 281–289. doi:10.3762/bjoc.16.27
Return to citation in text: [1]
| 1. | Graebin, C. S.; Ribeiro, F. V.; Rogério, K. R.; Kümmerle, A. E. Curr. Org. Synth. 2019, 16, 855–899. doi:10.2174/1570179416666190718153703 |
| 2. | John, S. E.; Gulati, S.; Shankaraiah, N. Org. Chem. Front. 2021, 8, 4237–4287. doi:10.1039/d0qo01480j |
| 3. | Graziano, G.; Stefanachi, A.; Contino, M.; Prieto-Díaz, R.; Ligresti, A.; Kumar, P.; Scilimati, A.; Sotelo, E.; Leonetti, F. Int. J. Mol. Sci. 2023, 24, 6581. doi:10.3390/ijms24076581 |
| 4. | Murlykina, M. V.; Morozova, A. D.; Zviagin, I. M.; Sakhno, Y. I.; Desenko, S. M.; Chebanov, V. A. Front. Chem. (Lausanne, Switz.) 2018, 6, 527. doi:10.3389/fchem.2018.00527 |
| 10. | Doğan, İ. S.; Gümüş, M. K.; Gorobets, N. Y.; Reis, R.; Orak, D.; Sipahi, H.; Sari, S.; Chebanov, V. A. Pharm. Chem. J. 2022, 56, 769–776. doi:10.1007/s11094-022-02708-w |
| 11. | Zeng, M.; Xue, Y.; Qin, Y.; Peng, F.; Li, Q.; Zeng, M.-H. Chin. Chem. Lett. 2022, 33, 4891–4895. doi:10.1016/j.cclet.2022.02.075 |
| 12. | Gorobets, N. Y.; Sedash, Y. V.; Ostras, K. S.; Zaremba, O. V.; Shishkina, S. V.; Baumer, V. N.; Shishkin, O. V.; Kovalenko, S. M.; Desenko, S. M.; Van der Eycken, E. V. Tetrahedron Lett. 2010, 51, 2095–2098. doi:10.1016/j.tetlet.2010.02.045 |
| 7. | Damera, T.; Pagadala, R.; Rana, S.; Jonnalagadda, S. B. Catalysts 2023, 13, 1034. doi:10.3390/catal13071034 |
| 8. | Desenko, S. M.; Gorobets, M. Y.; Lipson, V. V.; Sakhno, Y. I.; Chebanov, V. A. Chem. Rec. 2024, 24, e202300244. doi:10.1002/tcr.202300244 |
| 9. | Sakhno, Y. I.; Mykhailenko, M. V.; Kolosov, M. A.; Shvets, E. H.; Musatov, V. I.; Chorna, N. V.; Desenko, S. M.; Chebanov, V. A. Ukr. Bioorg. Acta 2020, 15, 22–26. doi:10.15407/bioorganica2020.02.022 |
| 6. | Chebanov, V. A.; Desenko, S. M.; Lipson, V. V.; Gorobets, N. Y. Multicomponent‐Switched Reactions in Synthesis of Heterocycles. In Multicomponent Reactions towards Heterocycles; Van der Eycken, E.; Sharma, U. K., Eds.; Wiley-VCH: Weinheim, Germany, 2022; pp 287–338. doi:10.1002/9783527832439.ch8 |
| 5. | Scheffelaar, R.; Ruijter, E.; Orru, R. V. A. Top. Heterocycl. Chem. 2010, 25, 95–126. doi:10.1007/7081_2010_44 |
| 4. | Murlykina, M. V.; Morozova, A. D.; Zviagin, I. M.; Sakhno, Y. I.; Desenko, S. M.; Chebanov, V. A. Front. Chem. (Lausanne, Switz.) 2018, 6, 527. doi:10.3389/fchem.2018.00527 |
| 8. | Desenko, S. M.; Gorobets, M. Y.; Lipson, V. V.; Sakhno, Y. I.; Chebanov, V. A. Chem. Rec. 2024, 24, e202300244. doi:10.1002/tcr.202300244 |
| 20. | Muravyova, E. A.; Desenko, S. M.; Rudenko, R. V.; Shishkina, S. V.; Shishkin, O. V.; Sen’ko, Y. V.; Vashchenko, E. V.; Chebanov, V. A. Tetrahedron 2011, 67, 9389–9400. doi:10.1016/j.tet.2011.09.138 |
| 21. | Sakhno, Y.; Murlykina, M.; Morozova, A.; Kozyrev, A.; Chebanov, V. Fr.-Ukr. J. Chem. 2015, 3, 1–20. doi:10.17721/fujcv3i2p1-20 |
| 22. | Sakhno, Y. I.; Murlykina, M. V.; Zbruyev, O. I.; Kozyryev, A. V.; Shishkina, S. V.; Sysoiev, D.; Musatov, V. I.; Desenko, S. M.; Chebanov, V. A. Beilstein J. Org. Chem. 2020, 16, 281–289. doi:10.3762/bjoc.16.27 |
| 8. | Desenko, S. M.; Gorobets, M. Y.; Lipson, V. V.; Sakhno, Y. I.; Chebanov, V. A. Chem. Rec. 2024, 24, e202300244. doi:10.1002/tcr.202300244 |
| 18. | Murlykina, M. V.; Sakhno, Y. I.; Desenko, S. M.; Shishkina, S. V.; Shishkin, O. V.; Sysoiev, D. O.; Kornet, M. N.; Schols, D.; Goeman, J. L.; Van der Eycken, J.; Van der Eycken, E. V.; Chebanov, V. A. Eur. J. Org. Chem. 2015, 4481–4492. doi:10.1002/ejoc.201500469 |
| 19. | Murlykina, M. V.; Sakhno, Y. I.; Desenko, S. M.; Konovalova, I. S.; Shishkin, O. V.; Sysoiev, D. A.; Kornet, M. N.; Chebanov, V. A. Tetrahedron 2013, 69, 9261–9269. doi:10.1016/j.tet.2013.08.055 |
| 17. | Tkachenko, V. V.; Muravyova, E. A.; Desenko, S. M.; Shishkin, O. V.; Shishkina, S. V.; Sysoiev, D. O.; Müller, T. J. J.; Chebanov, V. A. Beilstein J. Org. Chem. 2014, 10, 3019–3030. doi:10.3762/bjoc.10.320 |
| 18. | Murlykina, M. V.; Sakhno, Y. I.; Desenko, S. M.; Shishkina, S. V.; Shishkin, O. V.; Sysoiev, D. O.; Kornet, M. N.; Schols, D.; Goeman, J. L.; Van der Eycken, J.; Van der Eycken, E. V.; Chebanov, V. A. Eur. J. Org. Chem. 2015, 4481–4492. doi:10.1002/ejoc.201500469 |
| 16. | Světlík, J.; Kettmann, V. Tetrahedron Lett. 2011, 52, 1062–1066. doi:10.1016/j.tetlet.2010.12.051 |
| 17. | Tkachenko, V. V.; Muravyova, E. A.; Desenko, S. M.; Shishkin, O. V.; Shishkina, S. V.; Sysoiev, D. O.; Müller, T. J. J.; Chebanov, V. A. Beilstein J. Org. Chem. 2014, 10, 3019–3030. doi:10.3762/bjoc.10.320 |
| 13. | Jing, X.; Li, Z.; Pan, X.; Wang, Q.; Yan, C.; Zhu, H. Synth. Commun. 2009, 39, 3796–3803. doi:10.1080/00397910902838896 |
| 14. | Cui, X.; Lin, J.-J.; Wang, S.; Li, J.-P.; Xia, X.-S.; Huang, C. Tetrahedron Lett. 2022, 89, 153603. doi:10.1016/j.tetlet.2021.153603 |
| 15. | Svĕtlík, J.; Veizerová, L. Helv. Chim. Acta 2011, 94, 199–205. doi:10.1002/hlca.201000193 |
| 18. | Murlykina, M. V.; Sakhno, Y. I.; Desenko, S. M.; Shishkina, S. V.; Shishkin, O. V.; Sysoiev, D. O.; Kornet, M. N.; Schols, D.; Goeman, J. L.; Van der Eycken, J.; Van der Eycken, E. V.; Chebanov, V. A. Eur. J. Org. Chem. 2015, 4481–4492. doi:10.1002/ejoc.201500469 |
© 2025 Sakhno et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.