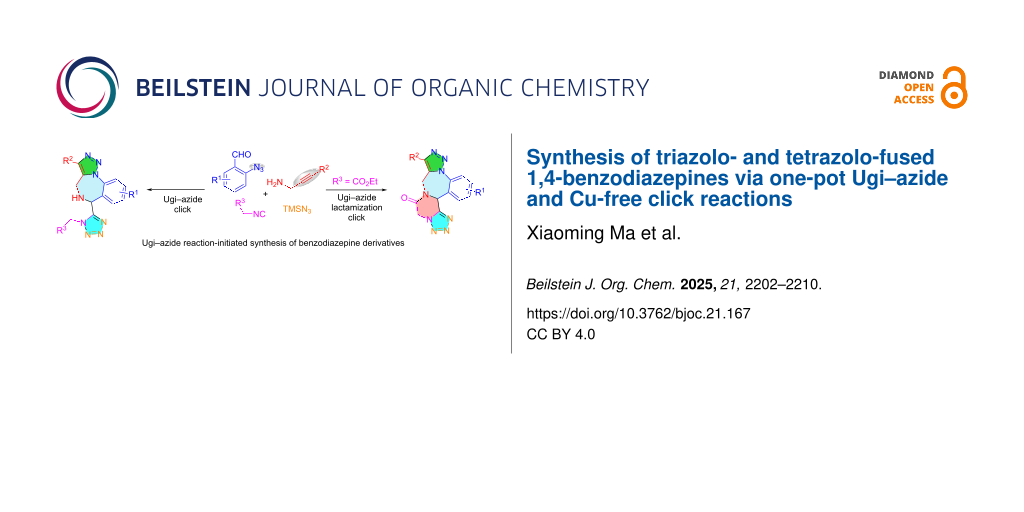
Abstract
A one-pot Ugi–azide reaction followed by intramolecular Cu-free azide–alkyne cycloaddition generates a polycyclic scaffold 7 bearing polycyclic triazole, tetrazole, and benzodiazepine rings. This method could be extended for obtaining a more complicated scaffold 8 containing a piperazinone ring.
Graphical Abstract
Introduction
Triazole, tetrazole, and benzodiazepine are privileged heterocyclic rings commonly found in drug molecules and functional materials [1-5]. For example, triazole-fused 1,4-benzodiazepins are protease inhibitors [6] and part of drug molecules such as alprazolam [7], estazolam [8], and triazolam [9] (Figure 1). Tetrazole-containing functional materials have been developed as photographic sensitizers, diagnostic contrast agents, and high-energy propellants [10-18]. Tetrazole is also found in several bioactive compounds [19-24], such as tasosartan, alfentanil, and cefmenoxime for the treatment of hypertension, anesthesia, and bacterial infections [4].
Figure 1: Some bioactive molecules bearing triazole, tetrazole, and 1,4-benzodiazepin rings.
Figure 1: Some bioactive molecules bearing triazole, tetrazole, and 1,4-benzodiazepin rings.
Among the reported methods for the synthesis of tetrazoles [25-28], the Ugi–azide reaction is a good approach for constructing 1,5-disubstituted-tetrazoles (1,5-DS-T) [29-31]. This scaffold can be subsequently linked to 1,2,3-triazole [32], 4H-chromen-4-one [33], pyrrolo[3,4-b]indolizine [34], and other heterocyclic scaffolds to obtain biologically interesting compounds (Scheme 1) [35-41].
Scheme 1: Ugi–azide reaction for the synthesis of 1,5-DS-T-containing heterocycles.
Scheme 1: Ugi–azide reaction for the synthesis of 1,5-DS-T-containing heterocycles.
The development of methods for the synthesis of triazole, tetrazole, piperazinone, and 1,4-benzodiazepine motifs are attractive from both synthetic and medicinal chemistry considerations [42-45]. We herein propose a one-pot synthesis involving an Ugi–azide 4-component (4-CR) reaction followed by lactamization and azide–alkyne cycloaddition for assembling triazole-fused and tetrazole-tethered 1,4-benzodiazepines 7 and triazole-, tetrazole-, and piperazinone-fused 1,4-benzodiazepines 8 (Scheme 2). Functional groups including ester, azido, and alkynyl present in the starting materials are responsible for the post-Ugi transformations.
Scheme 2: Proposed Ugi–azide-initiated synthesis of polyheterocyclic scaffolds 7 and 8.
Scheme 2: Proposed Ugi–azide-initiated synthesis of polyheterocyclic scaffolds 7 and 8.
Results and Discussion
We first attempted the Ugi–azide 4-CR at a 0.2 mmol scale with equal molar amounts of 2-azidobenzaldehyde (1a), propargylamine (2a), tert-butyl isocyanide (3a), and TMSN3 (4) in 2 mL of MeOH. After heating the reaction mixture at 40 °C for 12 h, the solvent was removed and changed to 2 mL of MeCN followed by heating at 130 °C for 2 h (Scheme 3A). However, no desired product was detected in the reaction mixture. The functional groups in four starting materials may not be compatible under the 4-CR conditions, in which propargylamine may undergo competitive reaction with 2-azidobenzaldehyde (1a) and TMSN3 (4). A literature search revealed that Shaabani’s group reported a reaction of 2-azidobenzaldehyde (1a) and propargylamine (2a) to obtain triazolobenzodiazepine which in turn can serve as a cyclic imine for a modified Joullié–Ugi 3-CR with isocyanide and trimethylsilyl azide (TMSN₃) in the synthesis of tetrazole-tethered triazolobenzodiazepines (Scheme 3B). We envisioned that the reaction conditions may need to be modified to introduce reactants in a sequential manner. Thus, we changed the reaction conditions by first reacting 0.2 mmol each of 1a and 2a in 2 mL MeOH at 40 °C for 40 min to form the Schiff base Int-I followed by the addition of 0.2 mmol each of 3a and TMSN3 (4) to obtain the desired 1,5-DS-T 5a as Ugi–azide adduct. After evaporation of the solvent MeOH, the residue was redissolved in 2 mL of MeCN and heated at 130 °C for 2 h in a sealed vial to give product 7a in 90% yield after purification via Cu-free intramolecular click reaction (Scheme 3C) [46]. This Cu-free intramolecular cyclization provides key practical advantages over traditional copper-catalyzed azide–alkyne cycloaddition (CuAAC) reactions, including operational simplicity and the absence of metal contaminants, which is crucial for pharmaceutical applications.
Scheme 3: 4-CR vs stepwise Ugi–azide reactions for the synthesis of 7a.
Scheme 3: 4-CR vs stepwise Ugi–azide reactions for the synthesis of 7a.
After having identified suitable reaction conditions of the Ugi–azide and click reactions for the synthesis of triazole-fused and tetrazole-tethered benzodiazepine 7a, we synthesized additional analogs by conducting the reaction of 2-azidobenzaldehyde (1a) with ten different isocyanides 3, two 2-yn-1-amines (propargylamine (2a), 3-phenylprop-2-yn-1-amine (2b)), and TMSN3 to give products 7a–k in 36–90% yields (Scheme 4).
Scheme 4: Synthesis of benzodiazepines 7a–k. Reaction conditions: 1) 0.2 mmol each of 2-azidobenzaldehyde (1a) and 2-yn-1-amines 2 in MeOH (2 mL), 40 °C for 40 min; then add 0.2 mmol each of isocyanides 3 and TMSN3 (4), 40 °C for 12 h; 2) change solvent to MeCN (2 mL), 130 °C for 2 h.
Scheme 4: Synthesis of benzodiazepines 7a–k. Reaction conditions: 1) 0.2 mmol each of 2-azidobenzaldehyde (1a...
As shown in Scheme 2, we also propose the synthesis of triazole-, tetrazole-, and piperazinone-fused 1,4-benzodiazepines 8. For the synthesis of this unique polycyclic scaffold, 2-isocyanoacetate 9 played a critical role in the formation of the piperazinone ring. Thus, the reaction of 0.2 mmol each of 1a and 2a led to the formation of Int-I which then reacted with 0.2 mmol each of 9 and 4 to form 1,5-DS-T 5b which consequently underwent lactamization to form 6a followed by an intramolecular click reaction to afford highly condensed polycyclic product 8a in 92% isolated yield (Scheme 5).
Scheme 5: Synthesis of polycyclic compound 8a.
Scheme 5: Synthesis of polycyclic compound 8a.
Next, the reaction scope was explored by reacting four different 2-azidobenzaldehydes 1 and five different 2-yn-1-amines 2 with 2-isocyanoacetate (9) and TMSN3 (4) to give products 8a–h (Scheme 6). The reaction of five different 2-yn-1-amines 2 yielded products 8a–e in 77–92% yields, which demonstrates a good tolerance of substituents R2 on 2-yn-1-amines 2. The reaction of 6-azidobenzo[d][1,3]dioxol-5-carbaldehyde (1b) with propargylamine (2a), 2-isocyanoacetate (9), and TMSN3 (4) gave product 8f in 77% yield. Azidobenzaldehyde bearing a Cl group gave product 8g in 79% yield. However, the reaction of 2-azido-5-bromobenzaldehyde (1d) gave only a trace amount of product 8h. Instead, compound 8h', an intermediate without lactamization, was isolated in 59% yield. It is likely that the bromo group on the phenyl ring interfered with the lactamization process.
Scheme 6: Synthesis of product analogs 8. Reaction conditions: 1) 0.2 mmol each of 2-azidobenzaldehyde (1a) and 2-yn-1-amines 2 in MeOH (2 mL), 40 °C for 40 min; then addition of 0.2 mmol each of 2-isocyanoacetate (9) and TMSN3 (4), 40 °C for 12 h; 2) changing solvent to MeCN (2 mL), 130 °C for 2 h.
Scheme 6: Synthesis of product analogs 8. Reaction conditions: 1) 0.2 mmol each of 2-azidobenzaldehyde (1a) a...
Two control reactions were conducted to study the reaction process. The reaction of 1a, 2a, 3b, and 4 at 40 °C afforded the Ugi–azide product 10 in 85% yield without formation of a triazole ring, which indicates that the intramolecular click reaction needs a higher temperature (Scheme 7A). The reaction involving the lactamization step was carried out using 2-isocyanoacetate (9) which gave the tetrazole-fused piperazinone 6a in 93% yield, which also indicates that at this reaction temperature, lactamization could take place prior to the click reaction (Scheme 7B).
Scheme 7: Control reactions to trap the Ugi–azide adduct.
Scheme 7: Control reactions to trap the Ugi–azide adduct.
Compounds 6a and 8a have same molecular weights, but their 1H NMR spectra are different (Figure 2). The most evident differences are the disappearance of the signal for the alkyne H at 2.36 ppm in the spectrum of 6a and the appearance of a peak at 8.14 ppm for the proton on the triazole ring of 8a. The aromatic protons in 8a show two distinct doublets and two triplets which are slightly downfield-shifted as compared to the aromatic protons in compound 6a. This observation reflects a rigid conformation of aromatic Hs after forming the 7-membered 1,4-diazepine ring in 8a. In addition, the 1H-1H COSY and HSQC analysis of compound 8a were conducted and the spectra are provided in Supporting Information File 1.
![[1860-5397-21-167-2]](/bjoc/content/figures/1860-5397-21-167-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: 1H NMR spectra of compounds 6a (red) and 8a (blue).
Figure 2: 1H NMR spectra of compounds 6a (red) and 8a (blue).
To evaluate the scalability of this protocol, we performed the synthesis of diazepine 8a on a gram scale with 10 mmol of 1a, which led to the formation of product 8a in 91% yield (Scheme 8).
Scheme 8: Gram-scale one-pot synthesis of 8a.
Scheme 8: Gram-scale one-pot synthesis of 8a.
Conclusion
We have developed a new synthetic method to access triazole-fused and tetrazole-tethered benzodiazepines 7 via Ugi–azide/intramolecular click reaction under Cu-catalyst-free conditions. The 4-CR Ugi–azide reaction was modified to be a one-pot two-step reaction process to address functional group compatibility issues. By using 2-isocyanoacetate, the Ugi–azide adducts could undergo lactamization and lead to the formation of highly condensed 1,4-benzodiazepines 8 fused with triazole, tetrazole, and piperazinone rings. This is a new example of combining MCR and post-condensation modification as a one-pot synthesis to access novel heterocyclic scaffolds in a highly efficient manner. It was found that brominated azides do not undergo lactamization under the current conditions which presents an opportunity for further optimization of reaction conditions to expand the scope.
Experimental
General procedure for synthesis of analogs 7 and 8
A solution of 2-azidobenzaldehyde 1 (0.2 mmol, 1 equiv) and propargylamine 2 (0.2 mmol, 1 equiv) in MeOH (2 mL) was heated at 40 °C for 40 min in a metal bath, followed by the addition of isocyanides 3 (0.2 mmol, 1 equiv) and TMSN3 (4, 0.2 mmol, 1 equiv) and further stirred at 40 °C for 12 h. Then, the reaction mixture was evaporated to remove MeOH solvent and the residue was redissolved in MeCN (2 mL) and heated at 130 °C for 2 h in a sealed vial. After the reaction had reached completion as monitored by TLC, the reaction mixture was concentrated in vacuo. Column chromatography on silica gel afforded products 7a–k in 36–90% yields, 8a–g in 77–92% yields.
Note: When 2-yn-1-amine hydrochlorides 2b–e were employed in this one-pot reaction, Et3N (0.3 mol, 1.5 equiv) was added into the vessel at the initial stage.
Supporting Information
| Supporting Information File 1: Experimental section, characterization data and copies of spectra. | ||
| Format: PDF | Size: 4.4 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Keri, R. S.; Patil, S. A.; Budagumpi, S.; Nagaraja, B. M. Chem. Biol. Drug Des. 2015, 86, 410–423. doi:10.1111/cbdd.12527
Return to citation in text: [1] -
Soyka, M. N. Engl. J. Med. 2017, 376, 1147–1157. doi:10.1056/nejmra1611832
Return to citation in text: [1] -
Sigel, E.; Ernst, M. Trends Pharmacol. Sci. 2018, 39, 659–671. doi:10.1016/j.tips.2018.03.006
Return to citation in text: [1] -
Neochoritis, C. G.; Zhao, T.; Dömling, A. Chem. Rev. 2019, 119, 1970–2042. doi:10.1021/acs.chemrev.8b00564
Return to citation in text: [1] [2] -
Dhiman, N.; Kaur, K.; Jaitak, V. Bioorg. Med. Chem. 2020, 28, 115599. doi:10.1016/j.bmc.2020.115599
Return to citation in text: [1] -
Mohapatra, D. K.; Maity, P. K.; Shabab, M.; Khan, M. I. Bioorg. Med. Chem. Lett. 2009, 19, 5241–5245. doi:10.1016/j.bmcl.2009.06.107
Return to citation in text: [1] -
Ye, Z.; Ding, M.; Wu, Y.; Li, Y.; Hua, W.; Zhang, F. Green Chem. 2018, 20, 1732–1737. doi:10.1039/c7gc03739b
Return to citation in text: [1] -
Massah, A. R.; Gharaghani, S.; Lordejani, H. A.; Asakere, N. Med. Chem. Res. 2016, 25, 1538–1550. doi:10.1007/s00044-016-1585-z
Return to citation in text: [1] -
Fustero, S.; González, J.; Del Pozo, C. Molecules 2006, 11, 583–588. doi:10.3390/11080583
Return to citation in text: [1] -
Wei, C.-X.; Bian, M.; Gong, G.-H. Molecules 2015, 20, 5528–5553. doi:10.3390/molecules20045528
Return to citation in text: [1] -
Frija, L. M. T.; Ismael, A.; Cristiano, M. L. S. Molecules 2010, 15, 3757–3774. doi:10.3390/molecules15053757
Return to citation in text: [1] -
Myznikov, L. V.; Hrabalek, A.; Koldobskii, G. I. Chem. Heterocycl. Compd. (N. Y., NY, U. S.) 2007, 43, 1–9. doi:10.1007/s10593-007-0001-5
Return to citation in text: [1] -
Lv, F.; Liu, Y.; Zou, J.; Zhang, D.; Yao, Z. Dyes Pigm. 2006, 68, 211–216. doi:10.1016/j.dyepig.2004.07.017
Return to citation in text: [1] -
Song, W.; Wang, Y.; Qu, J.; Madden, M. M.; Lin, Q. Angew. Chem., Int. Ed. 2008, 47, 2832–2835. doi:10.1002/anie.200705805
Return to citation in text: [1] -
Shmatova, O. I.; Nenajdenko, V. G. J. Org. Chem. 2013, 78, 9214–9222. doi:10.1021/jo401428q
Return to citation in text: [1] -
Dippold, A. A.; Izsák, D.; Klapötke, T. M.; Pflüger, C. Chem. – Eur. J. 2016, 22, 1768–1778. doi:10.1002/chem.201504624
Return to citation in text: [1] -
Fischer, D.; Klapötke, T. M.; Stierstorfer, J. Angew. Chem., Int. Ed. 2015, 54, 10299–10302. doi:10.1002/anie.201502919
Return to citation in text: [1] -
Nasrollahzadeh, M.; Nezafat, Z.; Bidgoli, N. S. S.; Shafiei, N. Mol. Catal. 2021, 513, 111788. doi:10.1016/j.mcat.2021.111788
Return to citation in text: [1] -
Muraglia, E.; Kinzel, O. D.; Laufer, R.; Miller, M. D.; Moyer, G.; Munshi, V.; Orvieto, F.; Palumbi, M. C.; Pescatore, G.; Rowley, M.; Williams, P. D.; Summa, V. Bioorg. Med. Chem. Lett. 2006, 16, 2748–2752. doi:10.1016/j.bmcl.2006.02.024
Return to citation in text: [1] -
Kambe, T.; Correia, B. E.; Niphakis, M. J.; Cravatt, B. F. J. Am. Chem. Soc. 2014, 136, 10777–10782. doi:10.1021/ja505517t
Return to citation in text: [1] -
Chauhan, K.; Singh, P.; Kumar, V.; Shukla, P. K.; Siddiqi, M. I.; Chauhan, P. M. S. Eur. J. Med. Chem. 2014, 78, 442–454. doi:10.1016/j.ejmech.2014.03.069
Return to citation in text: [1] -
Yuan, Y.; Li, M.; Apostolopoulos, V.; Matsoukas, J.; Wolf, W. M.; Blaskovich, M. A. T.; Bojarska, J.; Ziora, Z. M. Eur. J. Med. Chem. 2024, 279, 116870. doi:10.1016/j.ejmech.2024.116870
Return to citation in text: [1] -
Chowdhury, M. G.; Kapoor, S.; Muthukumar, V.; Chatterjee, D. R.; Shard, A. Bioorg. Chem. 2025, 154, 108029. doi:10.1016/j.bioorg.2024.108029
Return to citation in text: [1] -
Li, G.; Jiang, B.; Wang, J.-Y.; Hao, W.-J. Synlett 2023, 34, 243–248. doi:10.1055/a-1971-6187
Return to citation in text: [1] -
Savych, O.; Kuchkovska, Y. O.; Bogolyubsky, A. V.; Konovets, A. I.; Gubina, K. E.; Pipko, S. E.; Zhemera, A. V.; Grishchenko, A. V.; Khomenko, D. N.; Brovarets, V. S.; Doroschuk, R.; Moroz, Y. S.; Grygorenko, O. O. ACS Comb. Sci. 2019, 21, 635–642. doi:10.1021/acscombsci.9b00120
Return to citation in text: [1] -
Soni, A.; Kumar, P.; Tomar, V.; Joshi, R. K.; Nemiwal, M. Synth. Commun. 2023, 53, 1469–1505. doi:10.1080/00397911.2023.2226271
Return to citation in text: [1] -
Moraes, A. M.; da Silva, T. L.; de Mattos, M. C. S. Curr. Org. Chem. 2023, 27, 1319–1328. doi:10.2174/0113852728263929230919074722
Return to citation in text: [1] -
Pharande, S. G.; Rentería-Gómez, M. A.; Gámez-Montaño, R. New J. Chem. 2018, 42, 11294–11298. doi:10.1039/c8nj02312c
Return to citation in text: [1] -
Medda, F.; Martinez-Ariza, G.; Hulme, C. Tetrahedron Lett. 2015, 56, 5295–5298. doi:10.1016/j.tetlet.2015.07.083
Return to citation in text: [1] -
Haldar, S.; Saha, S.; Mandal, S.; Jana, C. K. Green Chem. 2018, 20, 3463–3467. doi:10.1039/c8gc01544a
Return to citation in text: [1] -
Mohammadkhani, L.; Heravi, M. M. Mol. Diversity 2020, 24, 841–853. doi:10.1007/s11030-019-09972-1
Return to citation in text: [1] -
Aguilar-Morales, C. M.; Araujo-Huitrado, J. G.; López-Hernández, Y.; Contreras-Celedón, C.; Islas-Jácome, A.; Granados-López, A. J.; Solorio-Alvarado, C. R.; López, J. A.; Chacón-García, L.; Cortés-García, C. J. Molecules 2021, 26, 6104. doi:10.3390/molecules26206104
Return to citation in text: [1] -
Cano, P. A.; Islas-Jácome, A.; González-Marrero, J.; Yépez-Mulia, L.; Calzada, F.; Gámez-Montaño, R. Bioorg. Med. Chem. 2014, 22, 1370–1376. doi:10.1016/j.bmc.2013.12.069
Return to citation in text: [1] -
Niño‐Pantoja, I.; Gallardo‐Alfonzo, A.; Solis‐Santos, M.; Ordoñez, M.; Contreras‐Celedón, C.; Islas‐Jácome, A.; Chacón‐García, L.; Cortés‐García, C. J. Eur. J. Org. Chem. 2022, e202200230. doi:10.1002/ejoc.202200230
Return to citation in text: [1] -
Zarganes‐Tzitzikas, T.; Patil, P.; Khoury, K.; Herdtweck, E.; Dömling, A. Eur. J. Org. Chem. 2015, 51–55. doi:10.1002/ejoc.201403401
Return to citation in text: [1] -
Saha, D.; Kharbanda, A.; Essien, N.; Zhang, L.; Cooper, R.; Basak, D.; Kendrick, S.; Frett, B.; Li, H.-y. Org. Chem. Front. 2019, 6, 2234–2239. doi:10.1039/c9qo00389d
Return to citation in text: [1] -
Lohmann, N.; Milovanović, V.; Piekarski, D. G.; García Mancheño, O. Org. Biomol. Chem. 2022, 20, 2896–2908. doi:10.1039/d2ob00101b
Return to citation in text: [1] -
Sun, B.-B.; Liu, K.; Gao, Q.; Fang, W.; Lu, S.; Wang, C.-R.; Yao, C.-Z.; Cao, H.-Q.; Yu, J. Nat. Commun. 2022, 13, 7065. doi:10.1038/s41467-022-34887-1
Return to citation in text: [1] -
Yang, M.-L.; Zhao, L.; Chen, H.-R.; Ding, M.-W. J. Org. Chem. 2023, 88, 1898–1906. doi:10.1021/acs.joc.2c02621
Return to citation in text: [1] -
Niu, J.; Wang, Y.; Yan, S.; Zhang, Y.; Ma, X.; Zhang, Q.; Zhang, W. Beilstein J. Org. Chem. 2024, 20, 912–920. doi:10.3762/bjoc.20.81
Return to citation in text: [1] -
Zuo, H.-D.; Chen, X.; Zhang, Y.; Liu, J.-W.; Yan, S.-H.; Li, G.; Wang, J.-Y. Org. Lett. 2024, 26, 3828–3833. doi:10.1021/acs.orglett.4c00981
Return to citation in text: [1] -
Donald, J. R.; Martin, S. F. Org. Lett. 2011, 13, 852–855. doi:10.1021/ol1028404
Return to citation in text: [1] -
Nazeri, M. T.; Farhid, H.; Mohammadian, R.; Shaabani, A. ACS Comb. Sci. 2020, 22, 361–400. doi:10.1021/acscombsci.0c00046
Return to citation in text: [1] -
Aguilar-Morales, C. M.; de Loera, D.; Contreras-Celedón, C.; Cortés-García, C. J.; Chacón-García, L. Synth. Commun. 2019, 49, 2086–2095. doi:10.1080/00397911.2019.1616301
Return to citation in text: [1] -
Nazeri, M. T.; Ghasemi, M.; Ahmadi, M.; Shaabani, A.; Notash, B. J. Org. Chem. 2023, 88, 13504–13519. doi:10.1021/acs.joc.3c01013
Return to citation in text: [1] -
Ma, X.; Zhang, X.; Qiu, W.; Zhang, W.; Wan, B.; Evans, J.; Zhang, W. Molecules 2019, 24, 601. doi:10.3390/molecules24030601
Return to citation in text: [1]
| 1. | Keri, R. S.; Patil, S. A.; Budagumpi, S.; Nagaraja, B. M. Chem. Biol. Drug Des. 2015, 86, 410–423. doi:10.1111/cbdd.12527 |
| 2. | Soyka, M. N. Engl. J. Med. 2017, 376, 1147–1157. doi:10.1056/nejmra1611832 |
| 3. | Sigel, E.; Ernst, M. Trends Pharmacol. Sci. 2018, 39, 659–671. doi:10.1016/j.tips.2018.03.006 |
| 4. | Neochoritis, C. G.; Zhao, T.; Dömling, A. Chem. Rev. 2019, 119, 1970–2042. doi:10.1021/acs.chemrev.8b00564 |
| 5. | Dhiman, N.; Kaur, K.; Jaitak, V. Bioorg. Med. Chem. 2020, 28, 115599. doi:10.1016/j.bmc.2020.115599 |
| 9. | Fustero, S.; González, J.; Del Pozo, C. Molecules 2006, 11, 583–588. doi:10.3390/11080583 |
| 42. | Donald, J. R.; Martin, S. F. Org. Lett. 2011, 13, 852–855. doi:10.1021/ol1028404 |
| 43. | Nazeri, M. T.; Farhid, H.; Mohammadian, R.; Shaabani, A. ACS Comb. Sci. 2020, 22, 361–400. doi:10.1021/acscombsci.0c00046 |
| 44. | Aguilar-Morales, C. M.; de Loera, D.; Contreras-Celedón, C.; Cortés-García, C. J.; Chacón-García, L. Synth. Commun. 2019, 49, 2086–2095. doi:10.1080/00397911.2019.1616301 |
| 45. | Nazeri, M. T.; Ghasemi, M.; Ahmadi, M.; Shaabani, A.; Notash, B. J. Org. Chem. 2023, 88, 13504–13519. doi:10.1021/acs.joc.3c01013 |
| 8. | Massah, A. R.; Gharaghani, S.; Lordejani, H. A.; Asakere, N. Med. Chem. Res. 2016, 25, 1538–1550. doi:10.1007/s00044-016-1585-z |
| 46. | Ma, X.; Zhang, X.; Qiu, W.; Zhang, W.; Wan, B.; Evans, J.; Zhang, W. Molecules 2019, 24, 601. doi:10.3390/molecules24030601 |
| 7. | Ye, Z.; Ding, M.; Wu, Y.; Li, Y.; Hua, W.; Zhang, F. Green Chem. 2018, 20, 1732–1737. doi:10.1039/c7gc03739b |
| 34. | Niño‐Pantoja, I.; Gallardo‐Alfonzo, A.; Solis‐Santos, M.; Ordoñez, M.; Contreras‐Celedón, C.; Islas‐Jácome, A.; Chacón‐García, L.; Cortés‐García, C. J. Eur. J. Org. Chem. 2022, e202200230. doi:10.1002/ejoc.202200230 |
| 6. | Mohapatra, D. K.; Maity, P. K.; Shabab, M.; Khan, M. I. Bioorg. Med. Chem. Lett. 2009, 19, 5241–5245. doi:10.1016/j.bmcl.2009.06.107 |
| 35. | Zarganes‐Tzitzikas, T.; Patil, P.; Khoury, K.; Herdtweck, E.; Dömling, A. Eur. J. Org. Chem. 2015, 51–55. doi:10.1002/ejoc.201403401 |
| 36. | Saha, D.; Kharbanda, A.; Essien, N.; Zhang, L.; Cooper, R.; Basak, D.; Kendrick, S.; Frett, B.; Li, H.-y. Org. Chem. Front. 2019, 6, 2234–2239. doi:10.1039/c9qo00389d |
| 37. | Lohmann, N.; Milovanović, V.; Piekarski, D. G.; García Mancheño, O. Org. Biomol. Chem. 2022, 20, 2896–2908. doi:10.1039/d2ob00101b |
| 38. | Sun, B.-B.; Liu, K.; Gao, Q.; Fang, W.; Lu, S.; Wang, C.-R.; Yao, C.-Z.; Cao, H.-Q.; Yu, J. Nat. Commun. 2022, 13, 7065. doi:10.1038/s41467-022-34887-1 |
| 39. | Yang, M.-L.; Zhao, L.; Chen, H.-R.; Ding, M.-W. J. Org. Chem. 2023, 88, 1898–1906. doi:10.1021/acs.joc.2c02621 |
| 40. | Niu, J.; Wang, Y.; Yan, S.; Zhang, Y.; Ma, X.; Zhang, Q.; Zhang, W. Beilstein J. Org. Chem. 2024, 20, 912–920. doi:10.3762/bjoc.20.81 |
| 41. | Zuo, H.-D.; Chen, X.; Zhang, Y.; Liu, J.-W.; Yan, S.-H.; Li, G.; Wang, J.-Y. Org. Lett. 2024, 26, 3828–3833. doi:10.1021/acs.orglett.4c00981 |
| 25. | Savych, O.; Kuchkovska, Y. O.; Bogolyubsky, A. V.; Konovets, A. I.; Gubina, K. E.; Pipko, S. E.; Zhemera, A. V.; Grishchenko, A. V.; Khomenko, D. N.; Brovarets, V. S.; Doroschuk, R.; Moroz, Y. S.; Grygorenko, O. O. ACS Comb. Sci. 2019, 21, 635–642. doi:10.1021/acscombsci.9b00120 |
| 26. | Soni, A.; Kumar, P.; Tomar, V.; Joshi, R. K.; Nemiwal, M. Synth. Commun. 2023, 53, 1469–1505. doi:10.1080/00397911.2023.2226271 |
| 27. | Moraes, A. M.; da Silva, T. L.; de Mattos, M. C. S. Curr. Org. Chem. 2023, 27, 1319–1328. doi:10.2174/0113852728263929230919074722 |
| 28. | Pharande, S. G.; Rentería-Gómez, M. A.; Gámez-Montaño, R. New J. Chem. 2018, 42, 11294–11298. doi:10.1039/c8nj02312c |
| 32. | Aguilar-Morales, C. M.; Araujo-Huitrado, J. G.; López-Hernández, Y.; Contreras-Celedón, C.; Islas-Jácome, A.; Granados-López, A. J.; Solorio-Alvarado, C. R.; López, J. A.; Chacón-García, L.; Cortés-García, C. J. Molecules 2021, 26, 6104. doi:10.3390/molecules26206104 |
| 4. | Neochoritis, C. G.; Zhao, T.; Dömling, A. Chem. Rev. 2019, 119, 1970–2042. doi:10.1021/acs.chemrev.8b00564 |
| 33. | Cano, P. A.; Islas-Jácome, A.; González-Marrero, J.; Yépez-Mulia, L.; Calzada, F.; Gámez-Montaño, R. Bioorg. Med. Chem. 2014, 22, 1370–1376. doi:10.1016/j.bmc.2013.12.069 |
| 19. | Muraglia, E.; Kinzel, O. D.; Laufer, R.; Miller, M. D.; Moyer, G.; Munshi, V.; Orvieto, F.; Palumbi, M. C.; Pescatore, G.; Rowley, M.; Williams, P. D.; Summa, V. Bioorg. Med. Chem. Lett. 2006, 16, 2748–2752. doi:10.1016/j.bmcl.2006.02.024 |
| 20. | Kambe, T.; Correia, B. E.; Niphakis, M. J.; Cravatt, B. F. J. Am. Chem. Soc. 2014, 136, 10777–10782. doi:10.1021/ja505517t |
| 21. | Chauhan, K.; Singh, P.; Kumar, V.; Shukla, P. K.; Siddiqi, M. I.; Chauhan, P. M. S. Eur. J. Med. Chem. 2014, 78, 442–454. doi:10.1016/j.ejmech.2014.03.069 |
| 22. | Yuan, Y.; Li, M.; Apostolopoulos, V.; Matsoukas, J.; Wolf, W. M.; Blaskovich, M. A. T.; Bojarska, J.; Ziora, Z. M. Eur. J. Med. Chem. 2024, 279, 116870. doi:10.1016/j.ejmech.2024.116870 |
| 23. | Chowdhury, M. G.; Kapoor, S.; Muthukumar, V.; Chatterjee, D. R.; Shard, A. Bioorg. Chem. 2025, 154, 108029. doi:10.1016/j.bioorg.2024.108029 |
| 24. | Li, G.; Jiang, B.; Wang, J.-Y.; Hao, W.-J. Synlett 2023, 34, 243–248. doi:10.1055/a-1971-6187 |
| 10. | Wei, C.-X.; Bian, M.; Gong, G.-H. Molecules 2015, 20, 5528–5553. doi:10.3390/molecules20045528 |
| 11. | Frija, L. M. T.; Ismael, A.; Cristiano, M. L. S. Molecules 2010, 15, 3757–3774. doi:10.3390/molecules15053757 |
| 12. | Myznikov, L. V.; Hrabalek, A.; Koldobskii, G. I. Chem. Heterocycl. Compd. (N. Y., NY, U. S.) 2007, 43, 1–9. doi:10.1007/s10593-007-0001-5 |
| 13. | Lv, F.; Liu, Y.; Zou, J.; Zhang, D.; Yao, Z. Dyes Pigm. 2006, 68, 211–216. doi:10.1016/j.dyepig.2004.07.017 |
| 14. | Song, W.; Wang, Y.; Qu, J.; Madden, M. M.; Lin, Q. Angew. Chem., Int. Ed. 2008, 47, 2832–2835. doi:10.1002/anie.200705805 |
| 15. | Shmatova, O. I.; Nenajdenko, V. G. J. Org. Chem. 2013, 78, 9214–9222. doi:10.1021/jo401428q |
| 16. | Dippold, A. A.; Izsák, D.; Klapötke, T. M.; Pflüger, C. Chem. – Eur. J. 2016, 22, 1768–1778. doi:10.1002/chem.201504624 |
| 17. | Fischer, D.; Klapötke, T. M.; Stierstorfer, J. Angew. Chem., Int. Ed. 2015, 54, 10299–10302. doi:10.1002/anie.201502919 |
| 18. | Nasrollahzadeh, M.; Nezafat, Z.; Bidgoli, N. S. S.; Shafiei, N. Mol. Catal. 2021, 513, 111788. doi:10.1016/j.mcat.2021.111788 |
| 29. | Medda, F.; Martinez-Ariza, G.; Hulme, C. Tetrahedron Lett. 2015, 56, 5295–5298. doi:10.1016/j.tetlet.2015.07.083 |
| 30. | Haldar, S.; Saha, S.; Mandal, S.; Jana, C. K. Green Chem. 2018, 20, 3463–3467. doi:10.1039/c8gc01544a |
| 31. | Mohammadkhani, L.; Heravi, M. M. Mol. Diversity 2020, 24, 841–853. doi:10.1007/s11030-019-09972-1 |
© 2025 Ma et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.