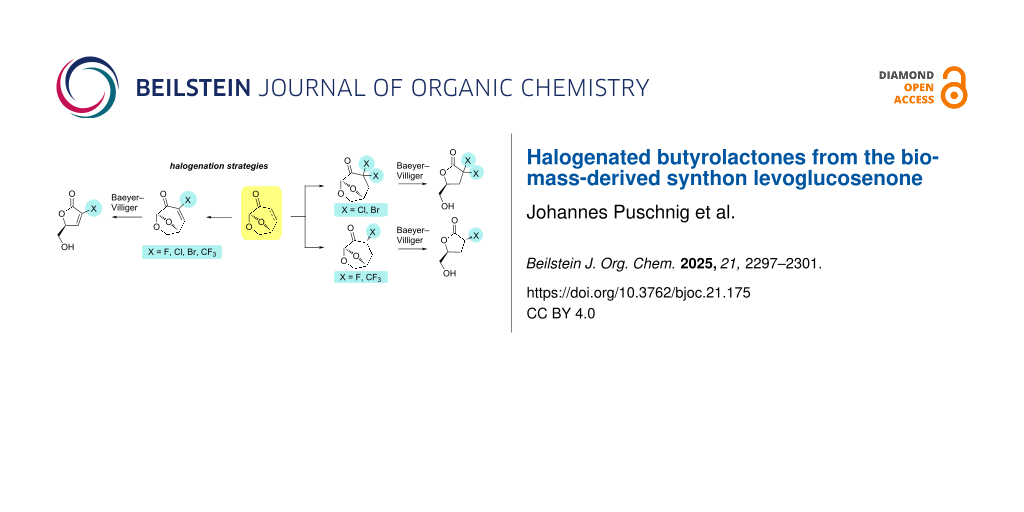
Abstract
Halogenated butyrolactones are found in a variety of bioactive materials and used for the construction of nucleoside analogues. Short procedures for their synthesis have been developed starting with levoglucosenone, which can be obtained in a single step from the pyrolysis of acid-treated cellulose. The processes use inexpensive reagents for the stereoselective C3 functionalization of the bicyclic ring system, with a subsequent Baeyer–Villiger oxidation affording the fluorinated, chlorinated, and brominated dideoxyribonolactones.
Graphical Abstract
Introduction
The γ-butyrolactone ring is a privileged scaffold found in natural products and can be used as a valuable intermediate in synthesis [1,2]. Several nucleoside analogue drugs are prepared using γ-butyrolactones, that when reduced give pentose sugars that can be used as glycosyl donors [3,4]. A number of these clinically used drugs contain fluorine as a hydroxy bioisostere at C2, most notably gemcitabine (1) and sofosbuvir (2). Fluorination at C2 in the nucleoside results in metabolic stability and resistance to hydrolysis as it destabilizes the formation of a C1 oxocarbenium ion [5,6]. Trifluoromethylated γ-butyrolactones also find applications as antiviral agents, for example, lactone 4 which has activity against influenza [7], while chlorinated analogues such as 3 have demonstrated activity against hepatitis C (Figure 1) [8]. Stereoselective methods to access halogenated γ-butyrolactones are therefore valuable, as they enable access to nucleoside analogues which have applications in treating cancer and certain infections.
Figure 1: Halogen-containing butyrolactone-derived bioactives.
Figure 1: Halogen-containing butyrolactone-derived bioactives.
The preparation of 2-halo-2-deoxy-ᴅ-ribose derivatives can be achieved via the modification of the parent sugar [9,10] or chain-elongation strategies from lower homologues. For example, Castro and co-workers have demonstrated the synthesis of a dichlorinated 2-deoxypentose via the addition of dichloro-magnesium enolates to protected ᴅ-glyceraldehyde [11]. In 1988, Hertel et al. from the Lilly laboratories published the first synthesis of the clinically important anticancer agent gemcitabine, using an intermediate γ-butyrolactone constructed using protected ᴅ-glyceraldehyde and ethyl bromodifluoroacetate under Reformatsky conditions [12]. While this reaction is still used in recent patents concerning the synthesis of gemcitabine, the drug’s commercial success has driven interest in alternative procedures for its synthesis [13], as well as the extensive evaluation of other halogenated derivatives [14,15].
In recent years, the chiral biomass derivatives levoglucosenone (LGO, 5) and its reduced form Cyrene® (6) have gained increased attention as platforms for drug discovery [16-19]. The bicyclic ketone 5 is the major product from the pyrolysis of acid-treated cellulose [20], while its reduced form 6, which is sold as a solvent, is an inexpensive commercially available reagent (Scheme 1) [21]. Monohalogenation of LGO giving chloride 7a [22] and bromide 7b [23] is readily achieved in a single step, however, fluorinated 7c, which is a potent inflammasone inhibitor (0.8 ± 0.5 µM), has only been reported in a 12-step procedure starting with glucose in an overall yield of 2% [24]. Enol esters [25], enolates [26], enamines [27,28], and silyl enol ethers [29], some of which can be derived from LGO, have been used in electrophilic fluorination and trifluoromethylation strategies. It was envisaged that halogenation could be combined with the Baeyer–Villiger oxidation which yields the butyrolactones by excision of C5, a reaction which is tolerant to substitution at C3 and can be carried out on a kilogram scale [30]. The present work was focussed on the development of additional halogenation reactions for 5 to give substrates for the Baeyer–Villiger oxidation resulting in halogenated butyrolactones, which is an unexplored chemical space for this biomass derivative.
Scheme 1: Preparation of chlorinated and brominated lactones 8a,b and 11a,b.
Scheme 1: Preparation of chlorinated and brominated lactones 8a,b and 11a,b.
Results and Discussion
The halogenated LGO derivatives 7a and 7b were prepared using literature procedures [22,23]. The reaction of alkenyl halides 7a and 7b with the green oxidant H2O2 gave trace conversion after 3 days; however, the reaction using m-CPBA catalyzed with p-TSA was complete in 24 hours and afforded the halogenated lactones 8a and 8b in 33% and 34% yield, respectively (Scheme 1). We have recently reported the C3 monochlorination and bromination of ketone 6 via enamine 9a [31], and it was envisaged that 9a would be a suitable substrate to achieve the double halogenation using an excess of electrophilic halogen. When enamine 9a was treated with 1.0 mol equivalent of trichloroisocyanouric acid (TCCA), a reagent which can transfer all three chlorine atoms [32], dichlorinated ketone 10a was obtained in 88% yield following acidic workup. Similarly, treatment of enamine 9a with 3.0 equivalents of N-bromosuccinimide and acidic hydrolysis gave dibrominated material 10b in excellent yield. This procedure is attractive due to the ready availability of enamine 9a; however, direct double halogenation of 6 may also be possible using an excess of halogenation agent in DMSO [31]. The Baeyer–Villiger oxidation using H2O2 in MeCN gave the desired chiral lactones 11a and 11b in moderate to good yield following an acidic workup to cleave the intermediate formate ester.
Fluorination of enamine 9a with Selectfluor (SF) resulted only in hydrolysis with conditions adapted from Peng and Shreeves work [28]; and likewise, the base-promoted (KOt-Bu, LHMDS) fluorination of ketone 6 with Selectfluor was unsuccessful. However, when ketone 6 was converted into the silyl enol ether 12 and treated with Selectfluor, fluorinated ketone 13 was formed as the major product after aqueous workup (Scheme 2). When the solution of 13 was concentrated following flash column chromatography, rapid decomposition was observed, which may explain failures with many fluorination attempts. Maintaining some residual solvent allowed for the characterization of compound 13 by NMR spectroscopy. Stereochemical assignment in 13 was based on a large trans-axial coupling constant JH2exo-H3 = 10.6 Hz in the 1H NMR spectrum and interactions between H3endo and H7endo on the oxymethylene bridge in the 2D NOESY NMR spectrum. Given the instability of fluoro-ketone 13, a one-pot procedure was developed in which the reaction mixture containing 13 was subjected to the Baeyer–Villiger oxidation with H2O2 giving the fluorinated and stable lactone 14, which is the expected kinetic product in 22% yield.
Scheme 2: Preparation of fluorinated lactone 14.
Scheme 2: Preparation of fluorinated lactone 14.
Fluorination of α,β-unsaturated ketones is a more challenging transformation than the halogenation reactions presented in Scheme 1 [33]. The previous reports of enamines as suitable substrates led us to prepare and examine enamine 15, which was generated from 5 with 3.0 equivalents of morpholine via an aza-Michael addition and condensation (Scheme 3). It was envisaged that the β-amino group could act as a stable functional group resulting in Baylis–Hillman-like reactivity. An extensive survey of reaction conditions using Selectfluor and N-fluorobenzenesulfonimide (NFSI) as the sources of electrophilic fluorine in the best case gave 15% conversion for NFSI and less than 8% isolated yield of 7c for SF. The major product in these reactions was 5, due to a β-elimination of the amine. In comparison, the reaction of 15 with NBS afforded 7b in an unoptimized yield of 48% after hydrolysis, which demonstrated that halogenation reactions with 15 were viable, but dependent on the characteristics of the electrophile. To avoid the elimination of the amine, β-methyl ether 16 was prepared. The reaction of ketone 16 with Selectfluor promoted by ʟ-proline in MeOH/MeCN, which ensured that the equilibrium between was biased to 16 and not 5, gave full consumption of the starting material in 2 hours and the fluorinated product 7c was obtained in 18% yield. Attempts to combine the methanol addition reaction and the fluorination starting with LGO proceeding through 16 were unsuccessful. Although the yield was low, the synthesis of 7c via this method represents a substantial improvement on previous approaches to this material [24]. The Baeyer–Villiger oxidation of 7c was uneventful and the fluorinated alkene 17 was isolated in 50% yield.
Scheme 3: Fluorination of LGO (5) and conversion to lactone 17.
Scheme 3: Fluorination of LGO (5) and conversion to lactone 17.
The α-trifluoromethylation of ketones and aldehydes can be performed using CF3 donors in copper-catalyzed [34], base- and Lewis acid-mediated reactions [35-37]. The reaction of enamine 9a with Togni’s reagent (18) and subsequent hydrolysis gave the substituted derivative 19 in 35% yield (qNMR) (Scheme 4). The yield was improved using the N-methylpiperazine-derived enamine 9b, which was completely consumed in 6 hours at room temperature. Following acidic hydrolysis of the enamine, a mixture of α-trifluoromethylated ketone diastereomers was obtained, and subsequent epimerization with K2CO3 afforded 19 as a single stereoisomer in 48% yield. The equatorial position of the trifluoromethyl group was established based on NOE interactions between H3endo and H7endo in the oxymethylene bridge. Subsequent Baeyer–Villiger oxidation of 19 gave lactone 20 in moderate yield. Attempts to improve the yield of 19 using base-promoted trifluoromethylation [38] of 6 gave only traces of the desired product.
Scheme 4: Trifluoromethylation of 9a,b and 15 and subsequent Baeyer–Villiger oxidation.
Scheme 4: Trifluoromethylation of 9a,b and 15 and subsequent Baeyer–Villiger oxidation.
Methods for the installation of a CF3-group on enones are limited, although approaches have been applied to quinones, uracils, flavones or arylenones via radical pathways [39-42]. As per the fluorination reactions, we envisaged that a leaving group in the β-position could mask the double bond when incorporating the trifluoromethyl group into 5. When enamine 15 was subjected to the same conditions established for the trifluoromethylation of enamine 9b, only trace amounts of the desired product were detected. It was found that generating the electrophilic CF3 species in situ was crucial for this transformation. By modifying a procedure for the trifluoromethylation of ketene dithioacetals reported by Liu and co-workers using TMSCF3 [43], rapid consumption of enamine 15 was observed. Acidic treatment of the intermediate promoted the hydrolysis/elimination cascade to give enone 21 in 42% yield. Baeyer–Villiger oxidation of this highly activated alkene promoted an epoxidation/Baeyer–Villiger oxidation cascade to yield lactone 22 in 67% yield (dr 2:1). The low diastereoselectivity suggested that the epoxidation happened subsequent to the ring contraction, as epoxidations of 5 are highly stereoselective [44]. Unambiguous assignment of configuration for the diastereomers was not possible on the basis of the selective 1D NOE spectra due to the lack of informative crosspeaks. Limiting the amount of oxidant (H2O2 or peracetic acid) resulted in mixtures, suggesting that the Baeyer–Villiger reaction without epoxidation may be challenging.
Conclusion
In conclusion, we have successfully established halogenation strategies of the biomass derivates 5 and 6, including fluorinations and trifluoromethylation. Baeyer–Villiger oxidations of these materials provide access to chiral halogenated lactones, which could be useful in the enantioselective synthesis of valuable drug precursors. The syntheses feature the use of readily available and cheap starting materials, and we have also demonstrated some of these transformations on a gram scale.
Supporting Information
| Supporting Information File 1: Experimental section, characterization data and copies of spectra. | ||
| Format: PDF | Size: 4.1 MB | Download |
Data Availability Statement
Data generated and analyzed during this study is openly available in Zenodo.org at https://doi.org/10.5281/zenodo.15803415.
References
-
Seitz, M.; Reiser, O. Curr. Opin. Chem. Biol. 2005, 9, 285–292. doi:10.1016/j.cbpa.2005.03.005
Return to citation in text: [1] -
Hur, J.; Jang, J.; Sim, J. Int. J. Mol. Sci. 2021, 22, 2769. doi:10.3390/ijms22052769
Return to citation in text: [1] -
Hoffmann, H. M. R.; Rabe, J. Angew. Chem., Int. Ed. Engl. 1985, 24, 94–110. doi:10.1002/anie.198500941
Return to citation in text: [1] -
Okabe, M.; Sun, R. C.; Tam, S. Y. K.; Todaro, L. J.; Coffen, D. L. J. Org. Chem. 1988, 53, 4780–4786. doi:10.1021/jo00255a021
Return to citation in text: [1] -
Marquez, V. E.; Tseng, C. K. H.; Mitsuya, H.; Aoki, S.; Kelley, J. A.; Ford, H., Jr.; Roth, J. S.; Broder, S.; Johns, D. G.; Driscoll, J. S. J. Med. Chem. 1990, 33, 978–985. doi:10.1021/jm00165a015
Return to citation in text: [1] -
Pankiewicz, K. W. Carbohydr. Res. 2000, 327, 87–105. doi:10.1016/s0008-6215(00)00089-6
Return to citation in text: [1] -
Mizuta, S.; Makau, J. N.; Kitagawa, A.; Kitamura, K.; Otaki, H.; Nishi, K.; Watanabe, K. ChemMedChem 2018, 13, 2390–2399. doi:10.1002/cmdc.201800511
Return to citation in text: [1] -
Pinho, P.; Kalayanov, G.; Westerlind, H.; Rosenquist, Å.; Wähling, H.; Sund, C.; Almeida, M.; Ayesa, S.; Tejbrant, J.; Targett-Adams, P.; Eneroth, A.; Lindqvist, A. Bioorg. Med. Chem. Lett. 2017, 27, 3468–3471. doi:10.1016/j.bmcl.2017.05.075
Return to citation in text: [1] -
Mikhailopulo, I. A.; Sivets, G. G. Synlett 1996, 173–174. doi:10.1055/s-1996-5340
Return to citation in text: [1] -
Larsen, C. H.; Ridgway, B. H.; Shaw, J. T.; Smith, D. M.; Woerpel, K. A. J. Am. Chem. Soc. 2005, 127, 10879–10884. doi:10.1021/ja0524043
Return to citation in text: [1] -
Rague, B.; Chapleur, Y.; Castro, B. J. Chem. Soc., Perkin Trans. 1 1982, 2063–2066. doi:10.1039/p19820002063
Return to citation in text: [1] -
Hertel, L. W.; Kroin, J. S.; Misner, J. W.; Tustin, J. M. J. Org. Chem. 1988, 53, 2406–2409. doi:10.1021/jo00246a002
Return to citation in text: [1] -
Brown, K.; Dixey, M.; Weymouth-Wilson, A.; Linclau, B. Carbohydr. Res. 2014, 387, 59–73. doi:10.1016/j.carres.2014.01.024
Return to citation in text: [1] -
Cini, E.; Barreca, G.; Carcone, L.; Manetti, F.; Rasparini, M.; Taddei, M. Eur. J. Org. Chem. 2018, 2622–2628. doi:10.1002/ejoc.201800158
Return to citation in text: [1] -
Zhou, S.; Mahmoud, S.; Liu, P.; Zhou, L.; Ehteshami, M.; Bassit, L.; Tao, S.; Domaoal, R. A.; Sari, O.; Schutter, C. D.; Amiralaei, S.; Khalil, A.; Ollinger Russell, O.; McBrayer, T.; Whitaker, T.; Abou-Taleb, N.; Amblard, F.; Coats, S. J.; Schinazi, R. F. J. Med. Chem. 2017, 60, 5424–5437. doi:10.1021/acs.jmedchem.7b00067
Return to citation in text: [1] -
Camp, J. E.; Greatrex, B. W. Front. Chem. (Lausanne, Switz.) 2022, 10, 902239. doi:10.3389/fchem.2022.902239
Return to citation in text: [1] -
Comba, M. B.; Tsai, Y.-h.; Sarotti, A. M.; Mangione, M. I.; Suárez, A. G.; Spanevello, R. A. Eur. J. Org. Chem. 2018, 590–604. doi:10.1002/ejoc.201701227
Return to citation in text: [1] -
M. Sarotti, A.; M. Zanardi, M.; A. Spanevello, R. Curr. Org. Synth. 2012, 9, 439–459. doi:10.2174/157017912802651401
Return to citation in text: [1] -
Stanfield, M. K.; Terry, R. S.; Smith, J. A.; Thickett, S. C. Polym. Chem. 2023, 14, 4949–4956. doi:10.1039/d3py01019h
Return to citation in text: [1] -
Halpern, Y.; Riffer, R.; Broido, A. J. Org. Chem. 1973, 38, 204–209. doi:10.1021/jo00942a005
Return to citation in text: [1] -
Marathianos, A.; Liarou, E.; Hancox, E.; Grace, J. L.; Lester, D. W.; Haddleton, D. M. Green Chem. 2020, 22, 5833–5837. doi:10.1039/d0gc02184a
Return to citation in text: [1] -
Sarotti, A. M.; Spanevello, R. A.; Suárez, A. G. Tetrahedron Lett. 2011, 52, 4145–4148. doi:10.1016/j.tetlet.2011.05.143
Return to citation in text: [1] [2] -
Ward, D. D.; Shafizadeh, F. Carbohydr. Res. 1981, 93, 284–287. doi:10.1016/s0008-6215(00)80858-7
Return to citation in text: [1] [2] -
Goto, K.; Ideo, H.; Tsuchida, A.; Hirose, Y.; Maruyama, I.; Noma, S.; Shirai, T.; Amano, J.; Mizuno, M.; Matsuda, A. Bioorg. Med. Chem. 2018, 26, 3763–3772. doi:10.1016/j.bmc.2017.11.041
Return to citation in text: [1] [2] -
Wood, S. H.; Etridge, S.; Kennedy, A. R.; Percy, J. M.; Nelson, D. J. Chem. – Eur. J. 2019, 25, 5574–5585. doi:10.1002/chem.201900029
Return to citation in text: [1] -
Davis, F. A.; Zhou, P.; Murphy, C. K.; Sundarababu, G.; Qi, H.; Han, W.; Przeslawski, R. M.; Chen, B.-C.; Carroll, P. J. J. Org. Chem. 1998, 63, 2273–2280. doi:10.1021/jo972045x
Return to citation in text: [1] -
Timofeeva, D. S.; Ofial, A. R.; Mayr, H. J. Am. Chem. Soc. 2018, 140, 11474–11486. doi:10.1021/jacs.8b07147
Return to citation in text: [1] -
Peng, W.; Shreeve, J. M. J. Org. Chem. 2005, 70, 5760–5763. doi:10.1021/jo0506837
Return to citation in text: [1] [2] -
Fujisawa, H.; Takeuchi, Y. J. Fluorine Chem. 2002, 117, 173–176. doi:10.1016/s0022-1139(02)00185-9
Return to citation in text: [1] -
Bonneau, G.; Peru, A. A. M.; Flourat, A. L.; Allais, F. Green Chem. 2018, 20, 2455–2458. doi:10.1039/c8gc00553b
Return to citation in text: [1] -
Puschnig, J.; Sumby, C. J.; Greatrex, B. W. Eur. J. Org. Chem. 2025, 28, e202500010. doi:10.1002/ejoc.202500010
Return to citation in text: [1] [2] -
Gaspa, S.; Carraro, M.; Pisano, L.; Porcheddu, A.; De Luca, L. Eur. J. Org. Chem. 2019, 3544–3552. doi:10.1002/ejoc.201900449
Return to citation in text: [1] -
Champagne, P. A.; Desroches, J.; Hamel, J.-D.; Vandamme, M.; Paquin, J.-F. Chem. Rev. 2015, 115, 9073–9174. doi:10.1021/cr500706a
Return to citation in text: [1] -
Li, L.; Chen, Q.-Y.; Guo, Y. J. Org. Chem. 2014, 79, 5145–5152. doi:10.1021/jo500713f
Return to citation in text: [1] -
Iseki, K.; Nagai, T.; Kobayashi, Y. Tetrahedron Lett. 1993, 34, 2169–2170. doi:10.1016/s0040-4039(00)60373-8
Return to citation in text: [1] -
Charpentier, J.; Früh, N.; Togni, A. Chem. Rev. 2015, 115, 650–682. doi:10.1021/cr500223h
Return to citation in text: [1] -
Allen, A. E.; MacMillan, D. W. C. J. Am. Chem. Soc. 2010, 132, 4986–4987. doi:10.1021/ja100748y
Return to citation in text: [1] -
Matoušek, V.; Togni, A.; Bizet, V.; Cahard, D. Org. Lett. 2011, 13, 5762–5765. doi:10.1021/ol2023328
Return to citation in text: [1] -
Nagib, D. A.; MacMillan, D. W. C. Nature 2011, 480, 224–228. doi:10.1038/nature10647
Return to citation in text: [1] -
Fang, Z.; Ning, Y.; Mi, P.; Liao, P.; Bi, X. Org. Lett. 2014, 16, 1522–1525. doi:10.1021/ol5004498
Return to citation in text: [1] -
Wang, X.; Ye, Y.; Ji, G.; Xu, Y.; Zhang, S.; Feng, J.; Zhang, Y.; Wang, J. Org. Lett. 2013, 15, 3730–3733. doi:10.1021/ol4016095
Return to citation in text: [1] -
Ilchenko, N. O.; Janson, P. G.; Szabó, K. J. Chem. Commun. 2013, 49, 6614–6616. doi:10.1039/c3cc43357a
Return to citation in text: [1] -
Xu, C.; Liu, J.; Ming, W.; Liu, Y.; Liu, J.; Wang, M.; Liu, Q. Chem. – Eur. J. 2013, 19, 9104–9109. doi:10.1002/chem.201301585
Return to citation in text: [1] -
Ledingham, E. T.; Greatrex, B. W. Tetrahedron 2018, 74, 6107–6115. doi:10.1016/j.tet.2018.08.041
Return to citation in text: [1]
| 24. | Goto, K.; Ideo, H.; Tsuchida, A.; Hirose, Y.; Maruyama, I.; Noma, S.; Shirai, T.; Amano, J.; Mizuno, M.; Matsuda, A. Bioorg. Med. Chem. 2018, 26, 3763–3772. doi:10.1016/j.bmc.2017.11.041 |
| 34. | Li, L.; Chen, Q.-Y.; Guo, Y. J. Org. Chem. 2014, 79, 5145–5152. doi:10.1021/jo500713f |
| 35. | Iseki, K.; Nagai, T.; Kobayashi, Y. Tetrahedron Lett. 1993, 34, 2169–2170. doi:10.1016/s0040-4039(00)60373-8 |
| 36. | Charpentier, J.; Früh, N.; Togni, A. Chem. Rev. 2015, 115, 650–682. doi:10.1021/cr500223h |
| 37. | Allen, A. E.; MacMillan, D. W. C. J. Am. Chem. Soc. 2010, 132, 4986–4987. doi:10.1021/ja100748y |
| 1. | Seitz, M.; Reiser, O. Curr. Opin. Chem. Biol. 2005, 9, 285–292. doi:10.1016/j.cbpa.2005.03.005 |
| 2. | Hur, J.; Jang, J.; Sim, J. Int. J. Mol. Sci. 2021, 22, 2769. doi:10.3390/ijms22052769 |
| 8. | Pinho, P.; Kalayanov, G.; Westerlind, H.; Rosenquist, Å.; Wähling, H.; Sund, C.; Almeida, M.; Ayesa, S.; Tejbrant, J.; Targett-Adams, P.; Eneroth, A.; Lindqvist, A. Bioorg. Med. Chem. Lett. 2017, 27, 3468–3471. doi:10.1016/j.bmcl.2017.05.075 |
| 23. | Ward, D. D.; Shafizadeh, F. Carbohydr. Res. 1981, 93, 284–287. doi:10.1016/s0008-6215(00)80858-7 |
| 7. | Mizuta, S.; Makau, J. N.; Kitagawa, A.; Kitamura, K.; Otaki, H.; Nishi, K.; Watanabe, K. ChemMedChem 2018, 13, 2390–2399. doi:10.1002/cmdc.201800511 |
| 24. | Goto, K.; Ideo, H.; Tsuchida, A.; Hirose, Y.; Maruyama, I.; Noma, S.; Shirai, T.; Amano, J.; Mizuno, M.; Matsuda, A. Bioorg. Med. Chem. 2018, 26, 3763–3772. doi:10.1016/j.bmc.2017.11.041 |
| 5. | Marquez, V. E.; Tseng, C. K. H.; Mitsuya, H.; Aoki, S.; Kelley, J. A.; Ford, H., Jr.; Roth, J. S.; Broder, S.; Johns, D. G.; Driscoll, J. S. J. Med. Chem. 1990, 33, 978–985. doi:10.1021/jm00165a015 |
| 6. | Pankiewicz, K. W. Carbohydr. Res. 2000, 327, 87–105. doi:10.1016/s0008-6215(00)00089-6 |
| 21. | Marathianos, A.; Liarou, E.; Hancox, E.; Grace, J. L.; Lester, D. W.; Haddleton, D. M. Green Chem. 2020, 22, 5833–5837. doi:10.1039/d0gc02184a |
| 3. | Hoffmann, H. M. R.; Rabe, J. Angew. Chem., Int. Ed. Engl. 1985, 24, 94–110. doi:10.1002/anie.198500941 |
| 4. | Okabe, M.; Sun, R. C.; Tam, S. Y. K.; Todaro, L. J.; Coffen, D. L. J. Org. Chem. 1988, 53, 4780–4786. doi:10.1021/jo00255a021 |
| 22. | Sarotti, A. M.; Spanevello, R. A.; Suárez, A. G. Tetrahedron Lett. 2011, 52, 4145–4148. doi:10.1016/j.tetlet.2011.05.143 |
| 13. | Brown, K.; Dixey, M.; Weymouth-Wilson, A.; Linclau, B. Carbohydr. Res. 2014, 387, 59–73. doi:10.1016/j.carres.2014.01.024 |
| 16. | Camp, J. E.; Greatrex, B. W. Front. Chem. (Lausanne, Switz.) 2022, 10, 902239. doi:10.3389/fchem.2022.902239 |
| 17. | Comba, M. B.; Tsai, Y.-h.; Sarotti, A. M.; Mangione, M. I.; Suárez, A. G.; Spanevello, R. A. Eur. J. Org. Chem. 2018, 590–604. doi:10.1002/ejoc.201701227 |
| 18. | M. Sarotti, A.; M. Zanardi, M.; A. Spanevello, R. Curr. Org. Synth. 2012, 9, 439–459. doi:10.2174/157017912802651401 |
| 19. | Stanfield, M. K.; Terry, R. S.; Smith, J. A.; Thickett, S. C. Polym. Chem. 2023, 14, 4949–4956. doi:10.1039/d3py01019h |
| 43. | Xu, C.; Liu, J.; Ming, W.; Liu, Y.; Liu, J.; Wang, M.; Liu, Q. Chem. – Eur. J. 2013, 19, 9104–9109. doi:10.1002/chem.201301585 |
| 12. | Hertel, L. W.; Kroin, J. S.; Misner, J. W.; Tustin, J. M. J. Org. Chem. 1988, 53, 2406–2409. doi:10.1021/jo00246a002 |
| 20. | Halpern, Y.; Riffer, R.; Broido, A. J. Org. Chem. 1973, 38, 204–209. doi:10.1021/jo00942a005 |
| 44. | Ledingham, E. T.; Greatrex, B. W. Tetrahedron 2018, 74, 6107–6115. doi:10.1016/j.tet.2018.08.041 |
| 11. | Rague, B.; Chapleur, Y.; Castro, B. J. Chem. Soc., Perkin Trans. 1 1982, 2063–2066. doi:10.1039/p19820002063 |
| 38. | Matoušek, V.; Togni, A.; Bizet, V.; Cahard, D. Org. Lett. 2011, 13, 5762–5765. doi:10.1021/ol2023328 |
| 9. | Mikhailopulo, I. A.; Sivets, G. G. Synlett 1996, 173–174. doi:10.1055/s-1996-5340 |
| 10. | Larsen, C. H.; Ridgway, B. H.; Shaw, J. T.; Smith, D. M.; Woerpel, K. A. J. Am. Chem. Soc. 2005, 127, 10879–10884. doi:10.1021/ja0524043 |
| 14. | Cini, E.; Barreca, G.; Carcone, L.; Manetti, F.; Rasparini, M.; Taddei, M. Eur. J. Org. Chem. 2018, 2622–2628. doi:10.1002/ejoc.201800158 |
| 15. | Zhou, S.; Mahmoud, S.; Liu, P.; Zhou, L.; Ehteshami, M.; Bassit, L.; Tao, S.; Domaoal, R. A.; Sari, O.; Schutter, C. D.; Amiralaei, S.; Khalil, A.; Ollinger Russell, O.; McBrayer, T.; Whitaker, T.; Abou-Taleb, N.; Amblard, F.; Coats, S. J.; Schinazi, R. F. J. Med. Chem. 2017, 60, 5424–5437. doi:10.1021/acs.jmedchem.7b00067 |
| 39. | Nagib, D. A.; MacMillan, D. W. C. Nature 2011, 480, 224–228. doi:10.1038/nature10647 |
| 40. | Fang, Z.; Ning, Y.; Mi, P.; Liao, P.; Bi, X. Org. Lett. 2014, 16, 1522–1525. doi:10.1021/ol5004498 |
| 41. | Wang, X.; Ye, Y.; Ji, G.; Xu, Y.; Zhang, S.; Feng, J.; Zhang, Y.; Wang, J. Org. Lett. 2013, 15, 3730–3733. doi:10.1021/ol4016095 |
| 42. | Ilchenko, N. O.; Janson, P. G.; Szabó, K. J. Chem. Commun. 2013, 49, 6614–6616. doi:10.1039/c3cc43357a |
| 27. | Timofeeva, D. S.; Ofial, A. R.; Mayr, H. J. Am. Chem. Soc. 2018, 140, 11474–11486. doi:10.1021/jacs.8b07147 |
| 28. | Peng, W.; Shreeve, J. M. J. Org. Chem. 2005, 70, 5760–5763. doi:10.1021/jo0506837 |
| 25. | Wood, S. H.; Etridge, S.; Kennedy, A. R.; Percy, J. M.; Nelson, D. J. Chem. – Eur. J. 2019, 25, 5574–5585. doi:10.1002/chem.201900029 |
| 26. | Davis, F. A.; Zhou, P.; Murphy, C. K.; Sundarababu, G.; Qi, H.; Han, W.; Przeslawski, R. M.; Chen, B.-C.; Carroll, P. J. J. Org. Chem. 1998, 63, 2273–2280. doi:10.1021/jo972045x |
| 28. | Peng, W.; Shreeve, J. M. J. Org. Chem. 2005, 70, 5760–5763. doi:10.1021/jo0506837 |
| 33. | Champagne, P. A.; Desroches, J.; Hamel, J.-D.; Vandamme, M.; Paquin, J.-F. Chem. Rev. 2015, 115, 9073–9174. doi:10.1021/cr500706a |
| 32. | Gaspa, S.; Carraro, M.; Pisano, L.; Porcheddu, A.; De Luca, L. Eur. J. Org. Chem. 2019, 3544–3552. doi:10.1002/ejoc.201900449 |
| 31. | Puschnig, J.; Sumby, C. J.; Greatrex, B. W. Eur. J. Org. Chem. 2025, 28, e202500010. doi:10.1002/ejoc.202500010 |
| 22. | Sarotti, A. M.; Spanevello, R. A.; Suárez, A. G. Tetrahedron Lett. 2011, 52, 4145–4148. doi:10.1016/j.tetlet.2011.05.143 |
| 23. | Ward, D. D.; Shafizadeh, F. Carbohydr. Res. 1981, 93, 284–287. doi:10.1016/s0008-6215(00)80858-7 |
| 31. | Puschnig, J.; Sumby, C. J.; Greatrex, B. W. Eur. J. Org. Chem. 2025, 28, e202500010. doi:10.1002/ejoc.202500010 |
| 29. | Fujisawa, H.; Takeuchi, Y. J. Fluorine Chem. 2002, 117, 173–176. doi:10.1016/s0022-1139(02)00185-9 |
| 30. | Bonneau, G.; Peru, A. A. M.; Flourat, A. L.; Allais, F. Green Chem. 2018, 20, 2455–2458. doi:10.1039/c8gc00553b |
© 2025 Puschnig et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.