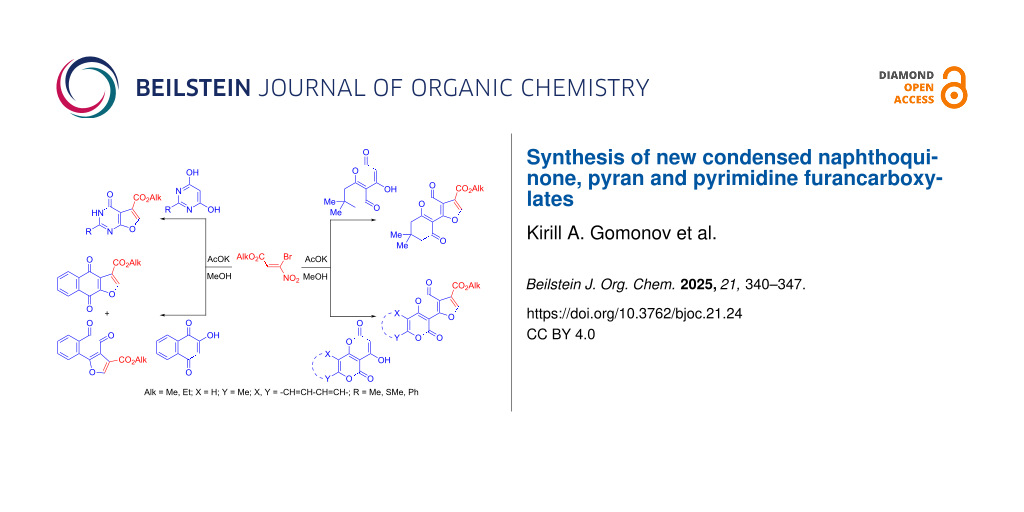
Abstract
New representatives of dioxodihydronaphtho[2,3-b]furan-, furo[3,2-c][1]benzopyran-, furo[2,3-d]pyrano[4,3-b]pyran-, furo[2',3':4,5]pyrano[3,2-c]chromene-, and furo[2,3-d]pyrimidine carboxylates were obtained from the reactions of alkyl 3-bromo-3-nitroacrylates with representatives of carbo- and heterocyclic CH-acids under simple conditions, without the use of organocatalysts. The structures of the synthesized compounds were proven by a set of physicochemical methods, including X-ray diffraction analysis.
Graphical Abstract
Introduction
The combination of the furan ring with various carbo- and heterocyclic structures leads to the production of new types of heterocyclic compounds with practical useful properties [1-6]. Representatives of the naphthofuran series exhibit anticancer [7] and antiinfectious activity [8]. Anticancer properties have also been found in representatives of the furopyran, furocoumarin [9,10], and furopyrimidine series [11,12].
It is known that the main approaches to the synthesis of naphtho[2,3-b]furan-4,9-diones are reactions of hydroxynaphthalene-1,4-dione with ethane-1,2-diol [13], chloroacetaldehyde [14], ethenyl methyl sulfone [15], or ethenyl acetate [16] (Scheme 1). In turn, naphtho[2,3-b]furan-4,9-diones containing an aromatic substituent in the third position can be obtained as a result of the interaction of gem-bromonitroalkenes with 2-hydroxynaphthalene-1,4-dione [17-19] (Scheme 1).
Scheme 1: Approaches to the synthesis of naphtho[2,3-b]furan-4,9-diones.
Scheme 1: Approaches to the synthesis of naphtho[2,3-b]furan-4,9-diones.
Furo[2,3-d]pyrimidin-4(3H)-ones are obtained on the basis of furoamines [20,21], as well as by the interaction of pyrimidinols with α-halocarbonyl compounds [22], nitroalkenes [23] or gem-chloronitroalkenes [21,24,25] (Scheme 2).
Scheme 2: Approaches to the synthesis of furo[2,3-d]pyrimidin-4(3H)-ones.
Scheme 2: Approaches to the synthesis of furo[2,3-d]pyrimidin-4(3H)-ones.
At the same time, the construction of furan-containing pyranopyrans and pyranochromenes in the literature is represented only by the reactions of 4-hydroxy-7-methyl-2H,5H-pyrano[4,3-b]pyran-2,5-dione and 4-hydroxy-2H,5H-pyrano[3,2-c]chromene-2,5-dione with ethynylbenzene [26] (Scheme 3).
Scheme 3: Approaches to the synthesis of furan-containing pyranopyrans and pyranochromenes.
Scheme 3: Approaches to the synthesis of furan-containing pyranopyrans and pyranochromenes.
The possibility of the formation of substituted furan structures is shown by the example of the reaction of nitroalkenes with aliphatic CH acids [27]. In addition, we have previously demonstrated the effective use of alkyl 3-bromo-3-nitroacrylates in the preparation of condensed furancarboxylates using potassium acetate as a catalyst [28-30].
The present study is aimed at developing methods for the synthesis of a wide range of condensed furancarboxylates based on the interaction of alkyl 3-bromo-3-nitroacrylates [31] with carbo- and heterocyclic CH-acids of the naphthoquinone, pyran, and pyrimidine series.
Results and Discussion
We have proposed a synthesis of dihydronaphthofuran-3-carboxylates based on the interaction of alkyl 3-bromo-3-nitroacrylates 1a,b with 2-hydroxynaphthalene-1,4-dione (2a). The reaction proceeds successfully in a methanol solution in the presence of AcOK for 3 h at room temperature, resulting in the formation of a mixture of alkyl 4,9-dioxo-4,9-dihydronaphtho[2,3-b]furan-3-carboxylate 3a,b and alkyl 4,5-dioxo-4,5-dihydronaphtho[1,2-b]furan-3-carboxylate 4a,b with a total yield of up to 73% (ratio 3a/4a = 2:1; 3b/4b = 2:1, according to 1H NMR spectroscopy) (Scheme 4). Using preparative chromatography, the mixture was separated into individual substances.
Scheme 4: Reaction of alkyl 3-bromo-3-nitroacrylates 1a,b with 2-hydroxynaphthalene-1,4-dione (2a).
Scheme 4: Reaction of alkyl 3-bromo-3-nitroacrylates 1a,b with 2-hydroxynaphthalene-1,4-dione (2a).
In turn, alkyl 7,7-dimethyl-4,9-dioxo-6,7,8,9-tetrahydro-4H-furo[3,2-c]chromene-3-carboxylates 5a,b with a yield of 84–85% were obtained under the same conditions as a result of the interaction of bromonitroacrylates 1a,b with 4-hydroxy-7,7-dimethyl-7,8-dihydro-2H-chromene-2,5(6H)-dione (2b) (ratio acrylate/CH-acid/AcOK = 1:1:1.5, room temperature, 1 h) (Scheme 5).
Scheme 5: Reaction of alkyl 3-bromo-3-nitroacrylates 1a,b with 4-hydroxy-7,7-dimethyl-7,8-dihydro-2H-1-benzopyran-2,5(6H)-dione (2b).
Scheme 5: Reaction of alkyl 3-bromo-3-nitroacrylates 1a,b with 4-hydroxy-7,7-dimethyl-7,8-dihydro-2H-1-benzop...
Polycyclic furan-3-carboxylates – alkyl 7-methyl-4,9-dioxo-4H,9H-furo[2,3-d]pyran[4,3-b]pyran-3-carboxylates 6a,b or alkyl 4,11-dioxo-4H,11H-furo[2',3':4,5]pyran[3,2-c]chromene-1-carboxylates 6c,d were obtained by reacting bromonitroacrylates 1a,b with 4-hydroxy-7-methyl-2H,5H-pyrano[4,3-b]pyran-2,5-dione (2c) or 4-hydroxy-2H,5H-pyrano[3,2-c][1]benzopyran-2,5-dione (2d) in yields up to 51% (Scheme 6).
Scheme 6: Reaction of alkyl 3-bromo-3-nitroacrylates 1a,b with 4-hydroxy-7-methyl-2H,5H-pyrano[4,3-b]pyran-2,5-dione (2c) and 4-hydroxy-2H,5H-pyrano[3,2-c][1]benzopyran-2,5-dione (2d).
Scheme 6: Reaction of alkyl 3-bromo-3-nitroacrylates 1a,b with 4-hydroxy-7-methyl-2H,5H-pyrano[4,3-b]pyran-2,...
Furopyrimidines 7a–f were obtained by reacting bromonitroacrylates 1a,b with representatives of substituted pyrimidines 2e–g. The reactions proceed successfully by refluxing in a water–alcohol (in the case of 2e) or alcohol (in the case of 2f,g) solution for 1–3 h (ratio of acrylate/CH-acid/AcOK = 1:1:1.5) with a yield of 43–64% (Scheme 7).
Scheme 7: Reaction of alkyl 3-bromo-3-nitroacrylates 1a,b with pyrimidine-4,6-diols 2e–g.
Scheme 7: Reaction of alkyl 3-bromo-3-nitroacrylates 1a,b with pyrimidine-4,6-diols 2e–g.
The structures of the obtained condensed furans 3–7 were characterized by a set of physicochemical methods, including X-ray diffraction analysis.
Analysis of the IR spectra of compounds 5a–6d containing an alkoxyfuropyran fragment shows that in the case of methyl esters 5a, 6a, and 6c the absorption band of the alkoxycarbonyl function is shifted to a lower frequency region (1714–1721 cm−1), while ethyl esters 5b, 6b, and 6d are characterized by higher frequency values (1725–1731 cm−1). This observation may be due to the position of the ester fragment relative to the heterocyclic system. The analysis of the results of X-ray diffraction analysis shows that the torsion angle C(10)–C(3)–C(16)–O(17) of methyl esters 5a and 6c is −0.8(2), and 5(2)°, and of ethyl esters 5b and 6b is −15.6(2) and −11.5(2)°, respectively, indicating that in the case of methyl esters, the alkoxycarbonyl fragment is less out of plane, causing a greater conjugation with the furan fragment.
The possibility of exhibiting fluorescent properties was investigated for tetracyclic furan-3-carboxylates 6c,d. It was shown that the increased number of conjugated bonds in their molecules leads to a decrease in the energy of the singlet–singlet π→π* transition and the possibility of exhibiting luminescence of the molecules under study. According to the luminescence excitation spectral data, they luminesce in DMSO solution when irradiated with light at wavelengths of 352 and 283 nm.
Compounds 7a–f are capable of existing as lactim–lactam tautomers due to the presence of an amide fragment in their structure (Scheme 7). At the same time, the 1H and 13C NMR spectra of compounds 7a–f indicate their individuality. The presence of a broadened signal in the 1H NMR spectra (DMSO-d6) in the region of 12.49–12.84 ppm and a C4 signal in the 13C NMR spectra (DMSO-d6) in the region of 158.1–159.0 ppm does not allow us to unambiguously determine the tautomeric form of compounds 7a–f in solution. In turn, in the IR spectra (KBr) of compounds 7a–f, absorption bands of the ester fragment in the region of 1714–1750 cm−1 and absorption bands of the carbonyl group of the amide fragment in the region of 1674–1688 cm−1 are observed, which suggests the existence of these compounds in the solid phase in the lactam form. The study of compound 7a by X-ray diffraction analysis convincingly confirms its existence in crystalline form in the lactam form. Thus, we have developed a method for synthesizing a wide range of condensed furancarboxylates. The structures of the compounds obtained were proven by physicochemical methods, as well as by X-ray diffraction analysis. The obtained compounds, due to the combination of different heterocyclic structures in their molecules, may be interesting as potential biologically active substances. In turn, the presence of active electrophilic centers in their structures expands the possibilities of further transformation and obtaining new compounds on their basis.
Experimental
General information
IR spectra were recorded on a Shimadzu IRPrestige-21 FT-IR spectrometer for samples in KBr pellets over 400–4000 cm−1 range. The electronic absorption spectra were recorded on a Shimadzu UV-2401 PC spectrometer for samples in DMSO solutions in fused quartz cuvettes (optical path length 1.01 mm). Luminescence excitation spectra and luminescence spectra were recorded on a Shimadzu SF-6000 spectrofluorimeter in a 1 cm thick quartz cuvette at room temperature in a DMSO solution (c = 10−5 mol/L). 1Н, 13С{1H}, 1H-13C HMQC, and 1H-13C HMBC NMR spectra were registered on a Jeol ECX–400A instrument at 400 (for 1H nuclei) and 100 MHz (for 13С nuclei) in CDCl3 (δH 7.26, δC 77.16 ppm) and DMSO-d6 (δH 2.47, δC 40.0 ppm) using residual signals of the nondeuterated solvent as the references. Elemental analysis was performed on a EuroVector EA3000 (dual mode) elemental analyzer. Melting points were determined on a PTP-M melting point apparatus. The reaction completion and purity of the obtained compounds were controlled by TLC on Silufol UV-254 plates with 3:1 hexane/EtOAc mobile phase and visualized under UV light (λ 254 nm).
X-ray structure determination
X-ray diffraction studies of single crystals of compounds 5a, 5b, 6b, 6c, and 7a were carried out on a Bruker D8 QUEST diffractometer. The cell parameters and experimental data were obtained at 100 K (graphite monochromator, λMo Kα = 0.71073 Å, ω and φ scanning in 0.5° steps) at the distributed spectral-analytical center of shared facilities for study of structure, composition and properties of substances and materials of FRC Kazan scientific center of Russian academy of sciences. Crystals of compounds suitable for X-ray structural analysis were obtained by crystallization from MeOH (5a), EtOH (5b), or DMFA (6b, 6c, and 7a). Single crystals of a suitable size were glued to the top of a glass fiber in a random orientation. The preliminary unit cell parameters were determined using three runs at different ω angle positions with 12 frames per run (φ-scan technique). Data collection and indexing, determination and refinement of unit cell parameters were carried out using the APEX2 software package [32]. Absorption correction was carried out according to the SADABS program [33]. The structures were solved by the direct method according to the SHELXT-2014/5 program [34] and refined by the full-matrix least-square on F2 according to the SHELXL-2018/3 program [35]. All calculations were performed using the WingX-2020.1 software package [36]. Structure 6c was refined using OLEX2-1.5 software package [37]. Non-hydrogen atoms were refined in anisotropic approximation. The hydrogen atoms were placed in calculated positions and refined according to the rider model. The figures were performed in the Mercury 2020.3 program [38], the analysis of intermolecular contacts was performed according to the PLATON program [39].
The crystal of 5b (Z’ = 3), contains 3 independent molecules, the crystals of 6c and 7a (Z’ = 2) contain 2 independent molecules, with all independent molecules having similar conformation and equivalent geometric parameters within the experimental error limits.
The crystallographic data of the structure are deposited in the Cambridge Crystal Structure Data Bank (CCDC 5a: 2403284; CCDC 5b: 2403285; CCDC 6b: 2403316; CCDC 6c: 2403286; CCDC 7a: 2403287). Statistics on the collection of X-ray diffraction data and refinement of the structure are shown in Table S1 in Supporting Information File 1.
Supporting Information
| Supporting Information File 1: General synthetic procedures, characterization data and copies of IR spectra, 1H, 13C spectra of all synthesized compounds, as well as 1H-13C HMQC, 1H-13C HMBC spectra and the crystallographic data for compounds 5a, 5b, 6b, 6c, and 7a. | ||
| Format: PDF | Size: 7.4 MB | Download |
Acknowledgements
The physicochemical studies were performed on the equipment of Center for Collective Use ''Physical and chemical methods for the study of nitro compounds, coordination, biologically active substances and nanostructured materials'' at the Interdisciplinary Resource Center for Collective Use ''Modern physical and chemical methods for the formation and study of materials for the needs of industry, science, and education'' of the Herzen State Pedagogical University of Russia.
Funding
The X-ray diffraction study was financially supported within the framework of the state assignment for the Federal Research Center “Kazan Scientific Center of the Russian Academy of Sciences”. The research was supported by an internal grant of the Herzen State Pedagogical University of Russia (project № 3VG).
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Chen, C.; Tang, Z.-B.; Liu, Z. Chin. Chem. Lett. 2023, 34, 108396. doi:10.1016/j.cclet.2023.108396
Return to citation in text: [1] -
Santana, L.; Uriarte, E.; Roleira, F.; Milhazes, N.; Borges, F. Curr. Med. Chem. 2004, 11, 3239–3261. doi:10.2174/0929867043363721
Return to citation in text: [1] -
Traven, V. F. Molecules 2004, 9, 50–66. doi:10.3390/90300050
Return to citation in text: [1] -
Singh, N.; Rajotiya, K.; Lamba, N.; Singh, H. L.; Ameta, K. L.; Singh, S. Curr. Org. Chem. 2022, 26, 324–341. doi:10.2174/1385272826666220126155703
Return to citation in text: [1] -
Uchuskin, M. G.; Shcherbinin, V. A.; Butin, A. V. Chem. Heterocycl. Compd. 2014, 50, 619–633. doi:10.1007/s10593-014-1515-2
Return to citation in text: [1] -
Li, C.; Yin, K.; Zhou, X.; Zhang, F.; Shen, Z. Tetrahedron 2023, 149, 133717. doi:10.1016/j.tet.2023.133717
Return to citation in text: [1] -
Kongkathip, N.; Kongkathip, B.; Siripong, P.; Sangma, C.; Luangkamin, S.; Niyomdecha, M.; Pattanapa, S.; Piyaviriyagul, S.; Kongsaeree, P. Bioorg. Med. Chem. 2003, 11, 3179–3191. doi:10.1016/s0968-0896(03)00226-8
Return to citation in text: [1] -
Koumpoura, C. L.; Nguyen, M.; Bijani, C.; Vendier, L.; Salina, E. G.; Buroni, S.; Degiacomi, G.; Cojean, S.; Loiseau, P. M.; Benoit-Vical, F.; García-Sosa, A. T.; Robert, A.; Baltas, M. ACS Omega 2022, 7, 35635–35655. doi:10.1021/acsomega.2c03421
Return to citation in text: [1] -
Dong, Y.; Shi, Q.; Nakagawa-Goto, K.; Wu, P.-C.; Morris-Natschke, S. L.; Brossi, A.; Bastow, K. F.; Lang, J.-Y.; Hung, M.-C.; Lee, K.-H. Bioorg. Med. Chem. 2010, 18, 803–808. doi:10.1016/j.bmc.2009.11.049
Return to citation in text: [1] -
Patel, J. Adv. Appl. Sci. Res. 2021, 12 (2), 13.
Return to citation in text: [1] -
Song, B.; Nie, L.; Bozorov, K.; Kuryazov, R.; Zhao, J.; Aisa, H. A. Mol. Diversity 2023, 27, 1767–1783. doi:10.1007/s11030-022-10529-y
Return to citation in text: [1] -
Tang, X.; Zheng, A.; Wu, F.; Liao, C.; Hu, Y.; Luo, C. Synth. Commun. 2022, 52, 994–1003. doi:10.1080/00397911.2022.2060117
Return to citation in text: [1] -
Maity, S.; Gupta, S. K.; Panda, N. Asian J. Org. Chem. 2021, 10, 3355–3363. doi:10.1002/ajoc.202100580
Return to citation in text: [1] -
Tseng, C.-H.; Lin, C.-S.; Shih, P.-K.; Tsao, L.-T.; Wang, J.-P.; Cheng, C.-M.; Tzeng, C.-C.; Chen, Y.-L. Bioorg. Med. Chem. 2009, 17, 6773–6779. doi:10.1016/j.bmc.2009.07.054
Return to citation in text: [1] -
Karnsomwan, W.; Netcharoensirisuk, P.; Rungrotmongkol, T.; De-Eknamkul, W.; Chamni, S. Chem. Pharm. Bull. 2017, 65, 253–260. doi:10.1248/cpb.c16-00727
Return to citation in text: [1] -
Acuña, J.; Piermattey, J.; Caro, D.; Bannwitz, S.; Barrios, L.; López, J.; Ocampo, Y.; Vivas-Reyes, R.; Aristizábal, F.; Gaitán, R.; Müller, K.; Franco, L. Molecules 2018, 23, 186. doi:10.3390/molecules23010186
Return to citation in text: [1] -
Wood, J. M.; Satam, N. S.; Almeida, R. G.; Cristani, V. S.; de Lima, D. P.; Dantas-Pereira, L.; Salomão, K.; Menna-Barreto, R. F. S.; Namboothiri, I. N. N.; Bower, J. F.; da Silva Júnior, E. N. Bioorg. Med. Chem. 2020, 28, 115565. doi:10.1016/j.bmc.2020.115565
Return to citation in text: [1] -
Baiju, T. V.; Almeida, R. G.; Sivanandan, S. T.; de Simone, C. A.; Brito, L. M.; Cavalcanti, B. C.; Pessoa, C.; Namboothiri, I. N. N.; da Silva Júnior, E. N. Eur. J. Med. Chem. 2018, 151, 686–704. doi:10.1016/j.ejmech.2018.03.079
Return to citation in text: [1] -
Zhang, R.; Xu, D.; Xie, J. Chin. J. Chem. 2012, 30, 1690–1694. doi:10.1002/cjoc.201200499
Return to citation in text: [1] -
Martin‐Kohler, A.; Widmer, J.; Bold, G.; Meyer, T.; Séquin, U.; Traxler, P. Helv. Chim. Acta 2004, 87, 956–975. doi:10.1002/hlca.200490089
Return to citation in text: [1] -
DiMauro, E. F.; Newcomb, J.; Nunes, J. J.; Bemis, J. E.; Boucher, C.; Buchanan, J. L.; Buckner, W. H.; Cheng, A.; Faust, T.; Hsieh, F.; Huang, X.; Lee, J. H.; Marshall, T. L.; Martin, M. W.; McGowan, D. C.; Schneider, S.; Turci, S. M.; White, R. D.; Zhu, X. Bioorg. Med. Chem. Lett. 2007, 17, 2305–2309. doi:10.1016/j.bmcl.2007.01.057
Return to citation in text: [1] [2] -
Secrist, J. A., III; Liu, P. S. J. Org. Chem. 1978, 43, 3937–3941. doi:10.1021/jo00414a029
Return to citation in text: [1] -
Li, C.; Zhang, F. Tetrahedron Lett. 2017, 58, 1572–1575. doi:10.1016/j.tetlet.2017.03.019
Return to citation in text: [1] -
Bata, N.; Chaikuad, A.; Bakas, N. A.; Limpert, A. S.; Lambert, L. J.; Sheffler, D. J.; Berger, L. M.; Liu, G.; Yuan, C.; Wang, L.; Peng, Y.; Dong, J.; Celeridad, M.; Layng, F.; Knapp, S.; Cosford, N. D. P. J. Med. Chem. 2022, 65, 1352–1369. doi:10.1021/acs.jmedchem.1c00804
Return to citation in text: [1] -
Dauzonne, D.; Adam-Launay, A. Tetrahedron 1992, 48, 3069–3080. doi:10.1016/s0040-4020(01)92249-5
Return to citation in text: [1] -
Kobayashi, K.; Nagase, K.; Morikawa, O.; Konishi, H. Heterocycles 2003, 60, 939–946. doi:10.3987/com-02-9690
Return to citation in text: [1] -
Iwai, K.; Kamidate, R.; Wada, K.; Asahara, H.; Nishiwaki, N. Beilstein J. Org. Chem. 2023, 19, 892–900. doi:10.3762/bjoc.19.67
Return to citation in text: [1] -
Pelipko, V. V.; Baichurin, R. I.; Lyssenko, K. A.; Dotsenko, V. V.; Makarenko, S. V. Mendeleev Commun. 2022, 32, 454–456. doi:10.1016/j.mencom.2022.07.009
Return to citation in text: [1] -
Pelipko, V. V.; Baichurin, R. I.; Kondrashov, E. V.; Makarenko, S. V. Russ. J. Gen. Chem. 2021, 91, 167–172. doi:10.1134/s1070363221020031
Return to citation in text: [1] -
Pekki, A. I.; Makarenko, S. V.; Altukhov, K. V.; Berestovitskaya, V. M. Russ. J. Gen. Chem. 2010, 80, 1048–1049. doi:10.1134/s1070363210050361
Return to citation in text: [1] -
Kuritsyna, M. A.; Pelipko, V. V.; Kataeva, O. N.; Baichurin, R. I.; Sadikov, K. D.; Smirnov, A. S.; Makarenko, S. V. Russ. Chem. Bull. 2021, 70, 1605–1612. doi:10.1007/s11172-021-3257-5
Return to citation in text: [1] -
APEX2, version 2.1; SAINTPlus, version 7.31A, Data Reduction and Correction Program; Bruker AXS Inc.: Madison, WI-53719, USA, 2006.
Return to citation in text: [1] -
SADABS, Madison, USA; Bruker AXS Inc., 1997.
Return to citation in text: [1] -
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3–8. doi:10.1107/s2053273314026370
Return to citation in text: [1] -
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8. doi:10.1107/s2053229614024218
Return to citation in text: [1] -
Farrugia, L. J. J. Appl. Crystallogr. 2012, 45, 849–854. doi:10.1107/s0021889812029111
Return to citation in text: [1] -
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339–341. doi:10.1107/s0021889808042726
Return to citation in text: [1] -
Macrae, C. F.; Sovago, I.; Cottrell, S. J.; Galek, P. T. A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G. P.; Stevens, J. S.; Towler, M.; Wood, P. A. J. Appl. Crystallogr. 2020, 53, 226–235. doi:10.1107/s1600576719014092
Return to citation in text: [1] -
Spek, A. L. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2009, 65, 148–155. doi:10.1107/s090744490804362x
Return to citation in text: [1]
| 1. | Chen, C.; Tang, Z.-B.; Liu, Z. Chin. Chem. Lett. 2023, 34, 108396. doi:10.1016/j.cclet.2023.108396 |
| 2. | Santana, L.; Uriarte, E.; Roleira, F.; Milhazes, N.; Borges, F. Curr. Med. Chem. 2004, 11, 3239–3261. doi:10.2174/0929867043363721 |
| 3. | Traven, V. F. Molecules 2004, 9, 50–66. doi:10.3390/90300050 |
| 4. | Singh, N.; Rajotiya, K.; Lamba, N.; Singh, H. L.; Ameta, K. L.; Singh, S. Curr. Org. Chem. 2022, 26, 324–341. doi:10.2174/1385272826666220126155703 |
| 5. | Uchuskin, M. G.; Shcherbinin, V. A.; Butin, A. V. Chem. Heterocycl. Compd. 2014, 50, 619–633. doi:10.1007/s10593-014-1515-2 |
| 6. | Li, C.; Yin, K.; Zhou, X.; Zhang, F.; Shen, Z. Tetrahedron 2023, 149, 133717. doi:10.1016/j.tet.2023.133717 |
| 11. | Song, B.; Nie, L.; Bozorov, K.; Kuryazov, R.; Zhao, J.; Aisa, H. A. Mol. Diversity 2023, 27, 1767–1783. doi:10.1007/s11030-022-10529-y |
| 12. | Tang, X.; Zheng, A.; Wu, F.; Liao, C.; Hu, Y.; Luo, C. Synth. Commun. 2022, 52, 994–1003. doi:10.1080/00397911.2022.2060117 |
| 26. | Kobayashi, K.; Nagase, K.; Morikawa, O.; Konishi, H. Heterocycles 2003, 60, 939–946. doi:10.3987/com-02-9690 |
| 9. | Dong, Y.; Shi, Q.; Nakagawa-Goto, K.; Wu, P.-C.; Morris-Natschke, S. L.; Brossi, A.; Bastow, K. F.; Lang, J.-Y.; Hung, M.-C.; Lee, K.-H. Bioorg. Med. Chem. 2010, 18, 803–808. doi:10.1016/j.bmc.2009.11.049 |
| 10. | Patel, J. Adv. Appl. Sci. Res. 2021, 12 (2), 13. |
| 27. | Iwai, K.; Kamidate, R.; Wada, K.; Asahara, H.; Nishiwaki, N. Beilstein J. Org. Chem. 2023, 19, 892–900. doi:10.3762/bjoc.19.67 |
| 8. | Koumpoura, C. L.; Nguyen, M.; Bijani, C.; Vendier, L.; Salina, E. G.; Buroni, S.; Degiacomi, G.; Cojean, S.; Loiseau, P. M.; Benoit-Vical, F.; García-Sosa, A. T.; Robert, A.; Baltas, M. ACS Omega 2022, 7, 35635–35655. doi:10.1021/acsomega.2c03421 |
| 23. | Li, C.; Zhang, F. Tetrahedron Lett. 2017, 58, 1572–1575. doi:10.1016/j.tetlet.2017.03.019 |
| 7. | Kongkathip, N.; Kongkathip, B.; Siripong, P.; Sangma, C.; Luangkamin, S.; Niyomdecha, M.; Pattanapa, S.; Piyaviriyagul, S.; Kongsaeree, P. Bioorg. Med. Chem. 2003, 11, 3179–3191. doi:10.1016/s0968-0896(03)00226-8 |
| 21. | DiMauro, E. F.; Newcomb, J.; Nunes, J. J.; Bemis, J. E.; Boucher, C.; Buchanan, J. L.; Buckner, W. H.; Cheng, A.; Faust, T.; Hsieh, F.; Huang, X.; Lee, J. H.; Marshall, T. L.; Martin, M. W.; McGowan, D. C.; Schneider, S.; Turci, S. M.; White, R. D.; Zhu, X. Bioorg. Med. Chem. Lett. 2007, 17, 2305–2309. doi:10.1016/j.bmcl.2007.01.057 |
| 24. | Bata, N.; Chaikuad, A.; Bakas, N. A.; Limpert, A. S.; Lambert, L. J.; Sheffler, D. J.; Berger, L. M.; Liu, G.; Yuan, C.; Wang, L.; Peng, Y.; Dong, J.; Celeridad, M.; Layng, F.; Knapp, S.; Cosford, N. D. P. J. Med. Chem. 2022, 65, 1352–1369. doi:10.1021/acs.jmedchem.1c00804 |
| 25. | Dauzonne, D.; Adam-Launay, A. Tetrahedron 1992, 48, 3069–3080. doi:10.1016/s0040-4020(01)92249-5 |
| 16. | Acuña, J.; Piermattey, J.; Caro, D.; Bannwitz, S.; Barrios, L.; López, J.; Ocampo, Y.; Vivas-Reyes, R.; Aristizábal, F.; Gaitán, R.; Müller, K.; Franco, L. Molecules 2018, 23, 186. doi:10.3390/molecules23010186 |
| 20. | Martin‐Kohler, A.; Widmer, J.; Bold, G.; Meyer, T.; Séquin, U.; Traxler, P. Helv. Chim. Acta 2004, 87, 956–975. doi:10.1002/hlca.200490089 |
| 21. | DiMauro, E. F.; Newcomb, J.; Nunes, J. J.; Bemis, J. E.; Boucher, C.; Buchanan, J. L.; Buckner, W. H.; Cheng, A.; Faust, T.; Hsieh, F.; Huang, X.; Lee, J. H.; Marshall, T. L.; Martin, M. W.; McGowan, D. C.; Schneider, S.; Turci, S. M.; White, R. D.; Zhu, X. Bioorg. Med. Chem. Lett. 2007, 17, 2305–2309. doi:10.1016/j.bmcl.2007.01.057 |
| 15. | Karnsomwan, W.; Netcharoensirisuk, P.; Rungrotmongkol, T.; De-Eknamkul, W.; Chamni, S. Chem. Pharm. Bull. 2017, 65, 253–260. doi:10.1248/cpb.c16-00727 |
| 22. | Secrist, J. A., III; Liu, P. S. J. Org. Chem. 1978, 43, 3937–3941. doi:10.1021/jo00414a029 |
| 14. | Tseng, C.-H.; Lin, C.-S.; Shih, P.-K.; Tsao, L.-T.; Wang, J.-P.; Cheng, C.-M.; Tzeng, C.-C.; Chen, Y.-L. Bioorg. Med. Chem. 2009, 17, 6773–6779. doi:10.1016/j.bmc.2009.07.054 |
| 13. | Maity, S.; Gupta, S. K.; Panda, N. Asian J. Org. Chem. 2021, 10, 3355–3363. doi:10.1002/ajoc.202100580 |
| 17. | Wood, J. M.; Satam, N. S.; Almeida, R. G.; Cristani, V. S.; de Lima, D. P.; Dantas-Pereira, L.; Salomão, K.; Menna-Barreto, R. F. S.; Namboothiri, I. N. N.; Bower, J. F.; da Silva Júnior, E. N. Bioorg. Med. Chem. 2020, 28, 115565. doi:10.1016/j.bmc.2020.115565 |
| 18. | Baiju, T. V.; Almeida, R. G.; Sivanandan, S. T.; de Simone, C. A.; Brito, L. M.; Cavalcanti, B. C.; Pessoa, C.; Namboothiri, I. N. N.; da Silva Júnior, E. N. Eur. J. Med. Chem. 2018, 151, 686–704. doi:10.1016/j.ejmech.2018.03.079 |
| 19. | Zhang, R.; Xu, D.; Xie, J. Chin. J. Chem. 2012, 30, 1690–1694. doi:10.1002/cjoc.201200499 |
| 32. | APEX2, version 2.1; SAINTPlus, version 7.31A, Data Reduction and Correction Program; Bruker AXS Inc.: Madison, WI-53719, USA, 2006. |
| 28. | Pelipko, V. V.; Baichurin, R. I.; Lyssenko, K. A.; Dotsenko, V. V.; Makarenko, S. V. Mendeleev Commun. 2022, 32, 454–456. doi:10.1016/j.mencom.2022.07.009 |
| 29. | Pelipko, V. V.; Baichurin, R. I.; Kondrashov, E. V.; Makarenko, S. V. Russ. J. Gen. Chem. 2021, 91, 167–172. doi:10.1134/s1070363221020031 |
| 30. | Pekki, A. I.; Makarenko, S. V.; Altukhov, K. V.; Berestovitskaya, V. M. Russ. J. Gen. Chem. 2010, 80, 1048–1049. doi:10.1134/s1070363210050361 |
| 31. | Kuritsyna, M. A.; Pelipko, V. V.; Kataeva, O. N.; Baichurin, R. I.; Sadikov, K. D.; Smirnov, A. S.; Makarenko, S. V. Russ. Chem. Bull. 2021, 70, 1605–1612. doi:10.1007/s11172-021-3257-5 |
| 39. | Spek, A. L. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2009, 65, 148–155. doi:10.1107/s090744490804362x |
| 37. | Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339–341. doi:10.1107/s0021889808042726 |
| 38. | Macrae, C. F.; Sovago, I.; Cottrell, S. J.; Galek, P. T. A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G. P.; Stevens, J. S.; Towler, M.; Wood, P. A. J. Appl. Crystallogr. 2020, 53, 226–235. doi:10.1107/s1600576719014092 |
| 35. | Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8. doi:10.1107/s2053229614024218 |
| 36. | Farrugia, L. J. J. Appl. Crystallogr. 2012, 45, 849–854. doi:10.1107/s0021889812029111 |
| 34. | Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3–8. doi:10.1107/s2053273314026370 |
© 2025 Gomonov et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.