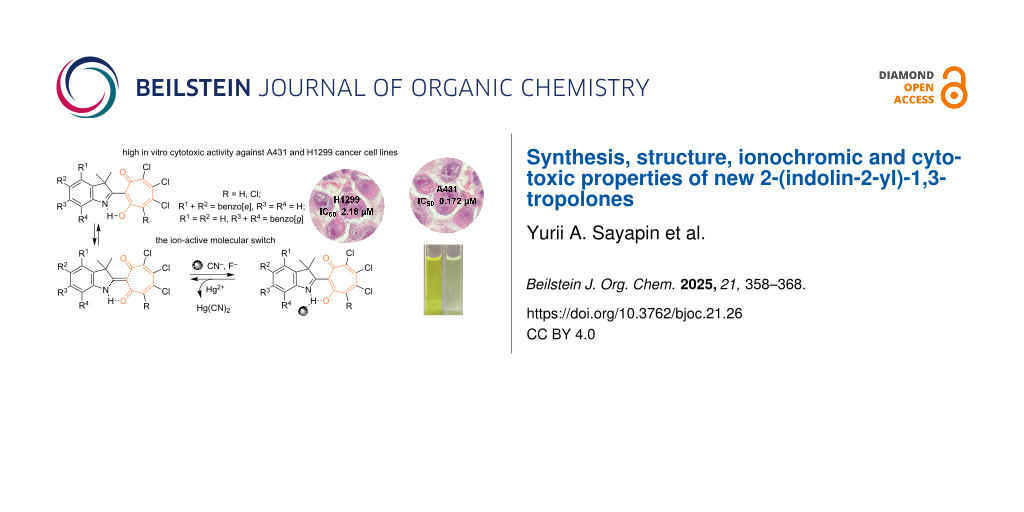
Abstract
The acid-catalyzed reaction of benzo[e(g)] derivatives of 2,3,3-trimethylindolenines with o-chloranil leads to new 2-(benzo[e(g)]indolin-2-yl)-5,6,7-trichloro-1,3-tropolones and 2-(benzo[e(g)]indolin-2-yl)-4,5,6,7-tetrachloro-1,3-tropolones. Based on the results of PBE0/6-311+G(d,p) calculations, the structural and energetic characteristics of the tautomeric forms of the obtained 1,3-tropolones were determined. The structure of 2-(3,3-dimethyl-3H-benzo[g]indolin-2-yl)-5,6,7-trichloro-1,3-tropolone was determined by X-ray diffraction analysis. The compounds obtained are capable of switching emission at 420–440 nm and 476–530 nm upon successive exposure to CN− and Hg2+ ions in an acetonitrile solution. 2-(1,1-Dimethyl-1H-benzo[e]indolin-2-yl)-5,6,7-trichloro-1,3-tropolone exhibited high in vitro cytotoxic activity against A431 skin cancer and H1299 lung cancer cell lines.
Graphical Abstract
Introduction
1,2-Benzoquinones represent unique building blocks for various classes of heterocyclic systems. Their structure depends on the reactivity of the initial heterocycles and 1,2-benzoquinones, as well as on the reaction conditions. In the series of 2-methylquinolines [1], 2-methylquinoxalines [2], 2-methylquinazolinones [3], 2-methylbenzoxazinones [4], and 2-methylbenzoxa(thia)zoles [5] the interaction with sterically hindered 1,2-benzoquinones and 3,4,5,6-tetrachloro-1,2-benzoquinone proceeds with the expansion of the o-quinone ring and results in 2-hetaryl-substituted 1,3-tropolones 1 (Scheme 1), which exhibit antibacterial [4] and cytotoxic activity [6,7].
Scheme 1: Synthesis of 2-hetaryl-substituted 1,3-tropolones 1.
Scheme 1: Synthesis of 2-hetaryl-substituted 1,3-tropolones 1.
Existing approaches to the synthesis of 1,3-tropolone derivatives have a number of drawbacks, namely, limited synthesis methodology and low yields of the target compounds [8-15]. Over the past decade, only a few reports have been published [16,17].
Indole derivatives, including those with conjugated aromatic and alicyclic rings, are of considerable interest because of their diverse biological activities (antibacterial, antimicrobial, anticancer, antidiabetic, etc.) [18-22]. For this reason, the synthetic goal of this work is to obtain heterocyclic indole compounds conjugated with a 1,3-tropolone moiety.
The variability of the products of acid-catalyzed reactions in the series of 2,3,3-trimethylindolenine with 1,2-benzoquinone derivatives depends on the nature of the substituents in the 1,2-benzoquinone. Thus, the interaction of 2,3,3-trimethylindolenine with 3,5-di(tert-butyl)-1,2-benzoquinone leads to the formation of indolo[1,2-a]indoline derivatives [23], while the presence of a nitro group in 4,6-di(tert-butyl)-3-nitro-1,2-benzoquinone the reaction with 2,3,3-trimethylindolenine leads to an o-quinone ring contraction and the formation of 2-azabicyclic products and pyridino[1,2-a]benzo[e]indol-10,11-diones [24]. o-Chloranil was found to be the most efficient 1,2-benzoquinone, which engages in o-quinone ring-expansion reactions with 2,3,3-trimethylindolenines to form hard-to-reach polychlorinated derivatives of 2-(indolin-2-yl)-1,3-tropolones [5]. Unlike the cross-aldol reaction of o-chloranil with methyl ketones [13-15], which is always accompanied by the removal of one of the chlorine atoms from the seven-membered ring, the acid-catalyzed reaction between methylene-active heterocyclic compounds and o-chloranil depends on the reaction conditions and can proceed with or without the inclusion of a dehydrochlorination stage and leads to 5,6,7-trichloro- or 4,5,6,7-tetrachlorotropones, respectively [5,25].
The present work reports the synthesis of new 2-(indolin-2-yl)-1,3-tropolones by the reaction of o-chloranil with benzoannelated derivatives of 2,3,3-trimethylindolenine, a comprehensive evaluation of the structure of the compounds obtained using quantum chemical methods, X-ray diffraction analysis, two-dimensional correlation NMR spectroscopy, as well as investigation of their ionochromic properties towards CN− and Hg2+ ions and evaluation of in vitro cytotoxic activity against A431 skin cancer and H1299 lung cancer cell lines.
Results and Discussion
We found that boiling of equimolar amounts of benzannelated 2,3,3-trimethylindolenines 2a,b and o-chloranil (3) (method A) in dioxane leads to the formation of trichlorosubstituted 1,3-tropolones 7a,b as the main reaction products (Scheme 2). Heating 2a,b in acetic acid with a two-fold excess of o-chloranil (3) (method B) is accompanied by the formation of tetrachlorosubstituted 1,3-tropolones 8a,b (Scheme 2 and Supporting Information File 1).
Scheme 2: Synthesis of 1,3-tropolones 7a,b and 8a,b. Reagents and conditions: method A: dioxane, reflux; method B: AcOH, 40–50 °C.
Scheme 2: Synthesis of 1,3-tropolones 7a,b and 8a,b. Reagents and conditions: method A: dioxane, reflux; meth...
As shown in Scheme 2, in the initial step, the aldol condensation of 2,3,3-trimethylindolenine 2 with o-chloranil (3) leads to the intermediate compounds, 6-(2-hetarylmethylene)-6-hydroxy-2,4-cyclohexadien-1-ones 4. Such intermediates were isolated preparatively and structurally characterized in the reactions of 2-methylquinolines with 3,5-di-(tert-butyl)-1,2-benzoquinone [1] and benzophenones with o-chloranil [15]. The norcaradiene derivatives 5, formed in the next step by the intramolecular cyclization reaction of 4, undergo thermal isomerization into 2,3-dihydrotropolones 6. The formation of 2-(indolin-2-yl)-5,6,7-trichloro-1,3-tropolones 7a,b is accompanied by dehydrochlorination of 6 upon boiling in dioxane according to method A. One of the conditions for carrying out the reaction according to method B is the use of a two-fold excess of quinone 3. Oxidation of dihydrotropolones 6 with an excess of quinone 3 leads to the formation of 2-(indolin-2-yl)-4,5,6,7-tetrachloro-1,3-tropolones 8a,b as final products. The detailed reaction mechanism in acetic acid solution was studied by the PBE0/6-311+G(d,p) method on the example of the interaction of 2-methylquinolines and 2-methylbenzazoles with 1,2-benzoquinone and o-chloroanil, respectively [1,25].
The structures of compounds 7 and 8 obtained by methods A and B were confirmed by 1H NMR, IR spectroscopy and mass spectrometry (Supporting Information File 2). A distinctive feature of the 1H NMR spectra of 7 in CDCl3 is the signal of the tropolone ring proton, which appears at 6.9 ppm. A characteristic specificity of the 1H NMR spectra of compounds 7 and 8 is the presence of signals of hydroxy group protons forming a strong hydrogen bond with the indoline nitrogen atom, which closes the six-membered chelate cycle. These signals are observed in the weak field at 15.2–15.8 ppm for 7a,b and 14.3–14.8 ppm for 8a,b, respectively, as a broadened singlet peak.
A dynamic equilibrium of tautomeric forms 7, 8 (OH)–7, 8 (NH) exists in solution, which can be detected by the broadening of the hydroxy group proton signal in the 1H NMR spectrum (Scheme 2). The structures of tautomeric forms of 7a, 7b, 8a, 8b and their energy characteristics were calculated by PBE0/6-311+G(d,p) method in the gas phase and polar solvent (DMSO) (Table 1, Figure 1 and Supporting Information File 3). According to the obtained data, the (NH) isomers of 7 and 8 are thermodynamically more stable than the corresponding (OH) forms. Increasing the polarity of the medium additionally enhances their stabilization. The introduction of an additional acceptor substituent to the tropolone moiety has a similar effect. In addition, compared to compounds 7a,b, compounds 8a,b show a marked increase in the intramolecular hydrogen bond length NH···O of the (NH) isomers and a decrease in the magnitude of hydrogen bond N···HO of the (OH) forms (Figure 1). On the other hand, the position of the benzoannelated fragment does not significantly affect the structural and energetic parameters of the studied compounds.
Table 1: Total energies with zero-point energy correction (Etot + ZPE, a.u.) and relative energies (ΔE, kcal/mol) of (NH) and (OH) isomers of compounds 7 and 8 calculated by the PBE0/6-311+G(d,p) method in the gas phase (gas) and DMSO solution (sol).
| compound | Etot + ZPE(gas) | ΔEgas | Etot + ZPE(sol) | ΔEsol |
| 7a (NH) | −2392.806710 | 0 | −2392.818223 | 0 |
| 7a (OH) | −2392.803386 | 2.1 | −2392.812918 | 3.3 |
| 7b (NH) | −2392.809986 | 0 | −2392.821048 | 0 |
| 7b (OH) | −2392.806819 | 2.0 | −2392.816317 | 3.0 |
| 8a (NH) | −2852.269500 | 0 | −2852.281433 | 0 |
| 8a (OH) | −2852.263504 | 3.8 | – | – |
| 8b (NH) | −2852.272654 | 0 | −2852.284199 | 0 |
| 8b (OH) | −2852.266797 | 3.7 | – | – |
![[1860-5397-21-26-1]](/bjoc/content/figures/1860-5397-21-26-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Structural characteristics of (NH) and (OH) tautomeric forms of compounds 7 and 8 in the gas phase according to PBE0/6-311+G(d,p) calculations. Bond lengths are given in Ångstrom.
Figure 1: Structural characteristics of (NH) and (OH) tautomeric forms of compounds 7 and 8 in the gas phase ...
The difference in the energy characteristics of the (NH) and (OH) tautomeric forms of compounds 7a, 7b, 8a, and 8b in the polar solvent and the gas phase is small (2.0–3.8 kcal/mol), and probably the nature of the solvent can influence the shift of the equilibrium towards one of the tautomeric forms.
To reveal the predominance of either the (NH) or (OH) tautomeric form of compounds 7a, 7b, 8a, and 8b in solution depending on the nature of the solvent, we carried out a detailed study using compound 7a. A complete signal assignment of 1H and 13C NMR spectra was carried out, based on characteristic values of chemical shifts and cross-peak analysis in two-dimensional spectra of 1H,1H COSY correlations, as well as 1H,13C correlations HSQC, HMBC, and 1H,15N HMBC spectra in DMSO-d6 and CDCl3 (Supporting Information File 2).
In the 1H NMR spectrum of 7a in DMSO-d6, the signal of the weak field proton is shifted to a stronger field of 14.23 ppm and appears as a narrow singlet (Supporting Information File 2, Figure S17). Analysis of two-dimensional correlation spectra of heteronuclear NMR spectroscopy shows that compound 7a in DMSO-d6 solution exists in the (NH) tautomeric form. Thus, in the 1H,15N HMBC spectrum of 7a there are cross peaks of the indolenine nitrogen atom at 162.2 ppm with a weak field proton at δH 14.23 ppm, as well as aromatic protons H(4') and H(5') with δH 7.94 ppm and 7.97 ppm, respectively (Supporting Information File 2, Figure S19). In the two-dimensional 1H,13C HMBC spectrum of 7a, a correlation of the NH proton with the bridging carbon atoms C(3a'), C(9b'), and the quaternary carbon atom C(1') of the indolenine fragment is observed with δH 136.6 ppm, 134.0 ppm, and 53.6 ppm, respectively (Supporting Information File 2, Figure S20). The most important HMBC correlations for the analysis of structure 7a are schematically depicted in Figure 2.
Figure 2: Scheme of HMBC correlations of compound 7a in DMSO-d6.
Figure 2: Scheme of HMBC correlations of compound 7a in DMSO-d6.
At the same time, in the 1H,15N HMBC spectra of 7a in CDCl3, there are no cross-peaks of the proton in the region of δH 15.15 ppm with the nitrogen atom of the indolenine fragment at 162.7 ppm, and in the 1H,13C HMBC spectrum, no correlations of the down-field proton with the carbon atoms of the indolenine fragment are observed (Supporting Information File 2, Figures S21 and S22). Thus, in CDCl3, compound 7a is predominantly in the (OH) tautomeric form.
The structure of 2-(3,3-dimethyl-3H-benzo[g]indolin-2-yl)-5,6,7-trichloro-1,3-tropolone (7b) was established by X-ray diffraction analysis (Figure 3). The main distances and angles are summarized in Supporting Information File 3.
![[1860-5397-21-26-3]](/bjoc/content/figures/1860-5397-21-26-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Molecular structure of 2-(3,3-dimethyl-3H-benzo[g]indolin-2-yl)-5,6,7-trichloro-1,3-tropolone (7b).
Figure 3: Molecular structure of 2-(3,3-dimethyl-3H-benzo[g]indolin-2-yl)-5,6,7-trichloro-1,3-tropolone (7b).
To compare the structural characteristics, we combined the molecule 7b (solid lines) and the previously obtained 2-(3,3-dimethylindolin-2-yl)-5,6,7-trichloro-1,3-tropolone [5] (dashed lines) at the positions of N(1), C(2) and C(9) atoms (hydrogen atoms removed) (Figure 4).
![[1860-5397-21-26-4]](/bjoc/content/figures/1860-5397-21-26-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Result of matching structures of 7b (solid lines) and 2-(3,3-dimethylindolin-2-yl)-5,6,7-trichloro-1,3-tropolone (dashed lines) at the positions of N(1), C(2), and C(9) atoms.
Figure 4: Result of matching structures of 7b (solid lines) and 2-(3,3-dimethylindolin-2-yl)-5,6,7-trichloro-...
While maintaining the general configuration of the molecular framework, there are minor differences in the details of their structures, manifested in the observed distances between the atoms C12)···C(13A) = 0.31 Å, Cl(2)···Cl(2A) = 0.76 Å and in the geometric parameters of the tropolone rings. The angle between the planes C(2)C(3)C(6)C(7) and C(1)C(2)C(7) is equal to 42.9° (42.1°) (here and below, when comparing geometric values, similar values are given in parentheses for the previously obtained 2-(indolin-2-yl)-1,3-tropolone)). The angle between the planes C(2)C(3)C(6)C(7) and (3)C(4)C(5)C(6) is equal to 27.6° (34°). In compound 7b, as in 2-(indolin-2-yl)-1,3-tropolone, an intramolecular hydrogen bond N(1)–H(1)···O(2) was realized with parameters: distances N–H = 0.86 (0.84) Å, H···O = 1.79 (1.83) Å, and N···O = 2.513 (2.518) Å, angle N–H–O 140.4° (138.0°).
The electronic absorption spectra of compounds 7a,b and 8a,b in acetonitrile have long wavelength bands in the region of 425–432 nm (Table 2). They exhibit dual-maxima fluorescence at 476–530 nm with normal and large Stokes shift values (Table 2, Figure 5).
Table 2: Absorption and fluorescence spectral data of compounds 7a,b and 8a,b in CH3CN.a
| compound |
absorption, λmax, nm
(ε, L mol−1 cm−1) |
fluorescence, λmax, nm
(Ifl, rel. units) |
Stokes shift,
(Δνfl, cm−1) |
| 7a | 276 (17200), 300 (18400), 427 (20400) | 485 (260), 520 (180) | 2800, 4200 |
| 7b | 292 (14000), 432 (19100) | 495 (220), 523 (190) | 2950, 4050 |
| 8a | 300 (14800), 310 (15200), 418 (20000) | 476 (420), 520 (190) | 2900, 4700 |
| 8b | 300 (14400), 425 (21900) | 491(480), 530 (250) | 3150, 4650 |
aIfl – the relative fluorescence intensity; c 2.5 × 10−5 mol L−1, λex 417 nm, PMT voltage 940 V.
![[1860-5397-21-26-5]](/bjoc/content/figures/1860-5397-21-26-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Absorption and emission spectra of compound 8b in acetonitrile before (1,1’) (c 2.5 × 10−5 mol L–1) and after the addition of CN− (2,2’) and F− (3,3’) ions (c 5.0 × 10−5 mol L−1).
Figure 5: Absorption and emission spectra of compound 8b in acetonitrile before (1,1’) (c 2.5 × 10−5 mol L–1)...
This is consistent with the above conclusion about the existence of tautomeric equilibrium 7,8 (OH)–7,8 (NH) in solutions based on NMR data and DFT quantum chemical calculations (Scheme 2). The emission with a larger Stokes shift appears to correspond to the 7,8 (OH) form and is caused by the excited-state intramolecular proton transfer (ESIPT) process due to intramolecular O→N proton migration in the singlet excited state [26,27].
The ionochromic sensitivity of compounds 7a,b and 8a,b to anions was investigated in acetonitrile upon addition of tetra-n-butylammonium salts (TBAX: F, Cl, Br, I, CN). Exclusively cyanide and fluoride anions lead to a naked-eye effect due to a change of the solution’s colour from yellow-orange to pale yellow (Figure 5). At the same time, a new fluorescence band appears at 420–440 nm. The Stokes shifts of fluorescence decrease to normal values of 1800–2000 cm−1, indicating a complete inhibition of the ESIPT process. These spectral transformations are usually caused by the formation of a strong N–H(O–H)···CN− (F−) hydrogen bond up to deprotonation [28]. Using the principle of “relay recognition” [29], we investigated the sensitivity of in situ obtained complexes 9 and 10 with CN− to different cations. It appeared that the addition of an equivalent amount of Hg(ClO4)2 to an acetonitrile solution selectively and completely restores the initial absorption and fluorescence spectra (Scheme 3).
Scheme 3: Possible binding mode of 7 and 8 with CN− and F−.
Scheme 3: Possible binding mode of 7 and 8 with CN− and F−.
Thus, the obtained compounds represent molecular switches of optical and fluorescent properties under sequential addition of CN− and Hg2+ ions and the transformation cycle presented in Scheme 3 can be repeated at least 5–6 times.
The resulting compounds 7b and 8a,b have extremely low water solubility, which makes it impossible to prepare concentrations sufficient for studying cellular cytotoxicity. In this regard, 1,3-tropolone 7a, which has an acceptable level of solubility in water, was used for biological testing. The in vitro cytotoxic activity was investigated with A431 skin cancer and H1299 lung cancer cell lines. A standard MTT assay was used to determine the anticancer activity of compound 7a against these cultures (Figure 6). The viability test is an important method to determine which new compounds are able to target cancer cells. The results of this test (IC50 value ± 95% confidence interval) allow us to evaluate the possibility of carrying out subsequent stages of research and selecting promising compounds for the further development of anticancer drugs [30].
![[1860-5397-21-26-6]](/bjoc/content/figures/1860-5397-21-26-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: Dose–response curves for H1299 and A431 cells treated with compound 7a for 24 h. *Significant difference compared to control samples, p < 0.05.
Figure 6: Dose–response curves for H1299 and A431 cells treated with compound 7a for 24 h. *Significant diffe...
The study showed that the IC50 inhibitory concentration of 2-(1,1-dimethyl-1H-benzo[e]indolin-2-yl)-5,6,7-trichloro-1,3-tropolone (7a) against the A431 skin cancer cell culture, at which the number of live cells was reduced by 50%, was 0.172 ± 0.029 μM. For the H1299 culture, this value was 2.18 ± 0.7 μM. In comparison, the IC50 value of the standard anticancer drug cisplatin at 24 hour incubation is 137 ± 12 μM for the A431 culture [31] and 34.9 μM for the H1299 culture [32], which is significantly higher than the values obtained for compound 7a.
Conclusion
In summary, new 2-(benzo[e(g)]indolin-2-yl)-5,6,7-trichloro-1,3-tropolones 7a,b and 2-(benzo[e(g)]indolin-2-yl)-4,5,6,7-tetrachloro-1,3-tropolones 8a,b were obtained by acid-catalyzed reactions of benzo[e(g)] derivatives of 2,3,3-trimethylindolenines with o-chloranil. The energetic characteristics of the tautomeric forms of 1,3-tropolones 7 and 8 were determined by PBE0/6-3-311+G(d,p) calculations, and the structure of 2-(3,3-dimethyl-3H-benzo[g]indolin-2-yl)-5,6,7-trichloro-1,3-tropolone (7b) was established by X-ray diffraction analysis. Using two-dimensional correlation NMR spectroscopy methods, it was shown that the nature of the solvent significantly affects the equilibrium of the tautomeric forms of the 1,3-tropolones. The obtained compounds represent molecular switches of optical and fluorescent properties under sequential addition of CN− and Hg2+ ions. 2-(1,1-Dimethyl-1H-benzo[e]indolin-2-yl)-5,6,7-trichloro-1,3-tropolone (7a) was found to exhibit high in vitro cytotoxic activity against A431 skin cancer and H1299 lung cancer cell lines. In addition, the IC50 values of compound 7a are significantly lower than the IC50 values of cisplastin, which is widely used in the therapy of tumors, including lung cancer.
Experimental
General
1Н, 13С and 15N NMR spectra were obtained on the integrated analytical LC–SPE–NMR–MS system AVANCE-600 (Bruker) (600 MHz, 1Н; 150 MHz, 13С; 60 MHz, 15N) at 20 °C in CDCl3 and DMSO-d6. The signals were referenced to the signals of residual proton signals of corresponding deutero-solvents. IR spectra were recorded on a Varian Excalibur 3100 FTIR instrument using the attenuated total internal reflection technique. Mass spectra were obtained on a Finnigan Mat Incos 50 spectrometer. Electronic absorption spectra were obtained on a Varian Cary 100 spectrophotometer. Emission spectra were recorded on a Varian Cary Eclipse spectrofluorimeter. Acetonitrile of spectroscopic grade (Aldrich), previously purified by distillation, and tetra-n-butylammonium salts (TBAX: F, Cl, Br, I, CN) (Aldrich) were used to prepare the solutions. Spectral fluorescent experiments were performed using quartz cells (lcuvette 1.0 cm, volume V 2 mL). Stock solutions of compounds 7 or 8 (c 5.0 × 10−5 mol L−1) and tetrabutylammonium salts (c 1.0 × 10−4 mol L−1) in acetonitrile were used. The corresponding solution (1 mL) and the tetra-n-butylammonium salt (1 mL) solution were mixed directly in the cell and thoroughly stirred. Hence, the working concentrations of compounds 7 or 8 and anions was 2.5 × 10−5 mol L−1 and 5.0 × 10−5 mol L−1, respectively. All spectral experiments were performed at room temperature (23 °C). Chromatography was carried out on columns filled with Al2O3 of II–III degree of activity according to Brockmann. Melting points were determined on a Fisher-Johns melting point apparatus. 1,1,2-Trimethyl-1H-benzo[e]indolenine (2a, 98%, Alfa Aesar), 2,3,3-trimethyl-3H-benzo[g]indolenine (2b, 98%, Alfa Aesar) and tetrachloro-o-benzoquinone (o-chloranil, 3) (97%, ACROS organics) were used as the starting reagents. NMR and IR spectra were recorded on the equipment of the Center for Collective Usage “Molecular Spectroscopy” of Southern Federal University.
X-ray diffraction study
The unit cell parameters and reflection intensities for compound 7b (a three-dimensional set) were measured on an Xcalibur EOS autodiffractometer (Mo Kα irradiation, graphite monochromator, 150 K). Orange monoclinic crystals, chemical formula C21H14NO2Cl3, М = 418.68, a = 11.4480(3), b = 7.4883(2), с = 21.7720(6) Å, β = 102.590(3)°, V = 1821.55(9) Å3, Z = 4, ρcalc = 1.527 g cm−3, μ(Мо Кα) = 0.520 mm−1, P21/c space group. Intensities of 15441 reflections were measured in the reciprocal space (2θ ≤ 64.14°) using the ω/2θ-scanning method from a single crystal with dimensions of 0.31 × 0.25 × 0.20 mm. An empirical accounting of absorption was carried out using the Multiscan procedure. After exclusion of systematically cancelled reflections and averaging intensities of equivalent reflections, the working array of measured F2(hkl) and σ(F2) reflections contained 6352 independent reflections, 5089 of which with F2 > 4σ(F2). The structure was solved with the direct method and was refined by the full-matrix least-squares procedure (LSP) with respect to F2 in anisotropic approximation for non-hydrogen atoms (hydrogen atoms isotropic) using the SHELXTL program. In the crystal structure, most of the H atoms are localized in the Fourier synthesis of the difference electron density, then the coordinates and isotropic thermal parameters of all H atoms were calculated by the LSP using the “rider” model [33]. The absolute shifts of all 247 varied structure parameters were less than 0.001 σ, the final value of the factor R1 = 0.0385. The CIF file containing atomic coordinates, full tables of bond lengths, bond angles, and thermal parameters of compound 7b have been deposited at the Cambridge Crystallographic Data Centre (CCDC 2040907) and can be obtained upon request on the website: https://www.ccdc.cam.ac.uk/data_request/cif.
Computational methods
Quantum chemical calculations were performed using the Gaussian 09 software package [34] with hybrid functional PBE0 [35,36] and 6-311+G (d,p) basis set. Solvent effects were modeled by using polarizable continuum model (PCM) [37] within the integral equation formalism (IEFPCM) [38].
Biological experiments
The MTT colorimetric test for cell viability assessment is based on the reduction by NADPH-H-dependent cellular oxidoreductase enzymes of the tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, which has yellow color, into violet-blue formazan, with absorption maximum in the range of 540–560 nm. The optical density of the solution in this wavelength range is an indirect indicator of the number of live cells in the culture. The cytotoxic activity of the test substance was determined by the decrease in optical density of experimental samples compared to control samples [39].
A431 and H1299 cells were seeded into 96-well plates at 15000/well in DMEM medium supplemented with 10% EFV and cultured under standard conditions at 5.0% CO2 and 37 °C. The next day, the medium was replaced and test compound 7a was added in a series of two-fold dilutions from 0.12 μM to 120 μM. In the control wells, the medium was replaced without adding the compound 7a. The cells were then incubated under the same standard conditions for 24 h, after which the medium was replaced and 10% MTT solution in DMEM medium supplemented with 10% EFV was added and incubated for another 2 h under CO2 incubator conditions. At the end of cultivation, the medium with MTT was completely removed from the wells, and the formed formazan crystals were dissolved in DMSO and the optical density of the resulting solution was measured at 540 nm. The level of cytotoxic activity was determined by the change in optical density at 540 nm in wells treated with the compound 7a or cisplatin compared to control wells. The experiment was carried out in three biological replicates with 6 technical replicates in each. Results were analyzed using one-way ANOVA followed by Tukey’s post hoc test of significance. IC50 values were determined in the RStudio development environment using the DRC software package [40].
Supporting Information
| Supporting Information File 1: Experimental procedures and characterization data for all novel compounds 7a, 7b, 8a and 8b. | ||
| Format: PDF | Size: 242.1 KB | Download |
| Supporting Information File 2: 1H, 13C NMR, IR and HRMS spectra of all novel compounds. | ||
| Format: PDF | Size: 2.6 MB | Download |
| Supporting Information File 3: X-ray analysis data of 7b and DFT quantum chemical calculations for 7a, 7b, 8a and 8b. | ||
| Format: PDF | Size: 641.6 KB | Download |
| Supporting Information File 4: Crystallographic information file for compound 7b. | ||
| Format: CIF | Size: 17.1 KB | Download |
Acknowledgements
XRD studies were performed in accordance with the State Task, State registration № 124013100858-3 (V. V. Tkachev, G. V. Shilov, S. M. Aldoshin). Y. A. Sayapin and A. D. Dubonosov worked in the scope of the State Task to the Southern Scientific Centre of the Russian Academy of Sciences for 2025, State registration № 125012000461-8. Biological studies were performed in the scope of the State Task, State registration № 124022100044-2 (A. S. Goncharova, S. Y. Filippova, N. S. Kuznetsova, A. Y. Maksimov).
Funding
The research was financially supported by the Ministry of Science and Higher Education of the Russian Federation in the scope of the State Task in the Field of Science (No. FENW-2023-0017). Y. A. Boumber was supported by the start-up funds from UAB and by the UAB O’Neal NCI Cancer Center Support Core Grant 530CA013148.
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Minkin, V. I.; Aldoshin, S. M.; Komissarov, V. N.; Dorogan, I. V.; Sayapin, Y. A.; Tkachev, V. V.; Starikov, A. G. Russ. Chem. Bull. 2006, 55, 2032–2055. doi:10.1007/s11172-006-0547-x
Return to citation in text: [1] [2] [3] -
Sayapin, Y. A.; Komissarov, V. N.; Bang, D. N.; Dorogan, I. V.; Minkin, V. I.; Tkachev, V. V.; Shilov, G. V.; Aldoshin, S. M.; Charushin, V. N. Mendeleev Commun. 2008, 18, 180–182. doi:10.1016/j.mencom.2008.07.002
Return to citation in text: [1] -
Sayapin, Y. A.; Gusakov, E. A.; Kolodina, A. A.; Komissarov, V. N.; Dorogan, I. V.; Tkachev, V. V.; Shilov, G. V.; Nosova, E. V.; Aldoshin, S. M.; Charushin, V. N.; Minkin, V. I. Russ. Chem. Bull. 2014, 63, 1364–1372. doi:10.1007/s11172-014-0604-9
Return to citation in text: [1] -
Sayapin, Y. A.; Gusakov, E. A.; Dorogan, I. V.; Tupaeva, I. O.; Teimurazov, M. G.; Fursova, N. K.; Ovchinnikov, K. V.; Minkin, V. I. Russ. J. Bioorg. Chem. 2016, 42, 224–228. doi:10.1134/s1068162016020114
Return to citation in text: [1] [2] -
Sayapin, Y. A.; Tupaeva, I. O.; Kolodina, A. A.; Gusakov, E. A.; Komissarov, V. N.; Dorogan, I. V.; Makarova, N. I.; Metelitsa, A. V.; Tkachev, V. V.; Aldoshin, S. M.; Minkin, V. I. Beilstein J. Org. Chem. 2015, 11, 2179–2188. doi:10.3762/bjoc.11.236
Return to citation in text: [1] [2] [3] [4] -
Gusakov, E. A.; Topchu, I. A.; Mazitova, A. M.; Dorogan, I. V.; Bulatov, E. R.; Serebriiskii, I. G.; Abramova, Z. I.; Tupaeva, I. O.; Demidov, O. P.; Toan, D. N.; Lam, T. D.; Bang, D. N.; Boumber, Y. A.; Sayapin, Y. A.; Minkin, V. I. RSC Adv. 2021, 11, 4555–4571. doi:10.1039/d0ra10610k
Return to citation in text: [1] -
Kit, O. I.; Minkin, V. I.; Lukbanova, E. A.; Sayapin, Y. A.; Gusakov, E. A.; Sitkovskaya, A. O.; Filippova, S. Y.; Komarova, E. F.; Volkova, A. V.; Khodakova, D. V.; Mindar, M. V.; Lazutin, Y. N.; Engibaryan, M. A.; Kolesnikov, V. E. Bull. Sib. Med. 2022, 21, 60–66. doi:10.20538/1682-0363-2022-2-60-66
Return to citation in text: [1] -
Johns, R. B.; Johnson, A. W. Chem. Ind. 1954, 192–193.
Return to citation in text: [1] -
Chapman, O. L.; Fitton, P. J. Am. Chem. Soc. 1963, 85, 41–47. doi:10.1021/ja00884a008
Return to citation in text: [1] -
Becker, A. M.; Rickards, R. W. Org. Prep. Proced. Int. 1983, 15, 239–242. doi:10.1080/00304948309356648
Return to citation in text: [1] -
Rahman, M. M.; Matano, Y.; Suzuki, H. J. Chem. Soc., Perkin Trans. 1 1999, 1533–1542. doi:10.1039/a900751b
Return to citation in text: [1] -
Zinser, H.; Henkel, S.; Föhlisch, B. Eur. J. Org. Chem. 2004, 1344–1356. doi:10.1002/ejoc.200300600
Return to citation in text: [1] -
Schenck, G. O.; Brähler, B.; Cziesla, M. Angew. Chem. 1956, 68, 247–248. doi:10.1002/ange.19560680707
Return to citation in text: [1] [2] -
Kogler, H.; Fehlhaber, H.-W.; Leube, K.; Dürckheimer, W. Chem. Ber. 1989, 122, 2205–2206. doi:10.1002/cber.19891221123
Return to citation in text: [1] [2] -
Li, H.; Li, W.; Li, Z. Chem. Commun. 2009, 3264–3266. doi:10.1039/b903515j
Return to citation in text: [1] [2] [3] -
Mondal, A.; Hazra, R.; Grover, J.; Raghu, M.; Ramasastry, S. S. V. ACS Catal. 2018, 8, 2748–2753. doi:10.1021/acscatal.8b00397
Return to citation in text: [1] -
Chien, P.-C.; Chen, Y.-R.; Chen, Y.-J.; Chang, C.-F.; Marri, G.; Lin, W. Adv. Synth. Catal. 2024, 366, 420–425. doi:10.1002/adsc.202301344
Return to citation in text: [1] -
Humphrey, G. R.; Kuethe, J. T. Chem. Rev. 2006, 106, 2875–2911. doi:10.1021/cr0505270
Return to citation in text: [1] -
Shiri, M. Chem. Rev. 2012, 112, 3508–3549. doi:10.1021/cr2003954
Return to citation in text: [1] -
Lygin, A. V.; de Meijere, A. Angew. Chem., Int. Ed. 2010, 49, 9094–9124. doi:10.1002/anie.201000723
Return to citation in text: [1] -
Zhuo, C.-X.; Zhang, W.; You, S.-L. Angew. Chem., Int. Ed. 2012, 51, 12662–12686. doi:10.1002/anie.201204822
Return to citation in text: [1] -
Bandini, M.; Eichholzer, A. Angew. Chem., Int. Ed. 2009, 48, 9608–9644. doi:10.1002/anie.200901843
Return to citation in text: [1] -
Sayapin, Y. A.; Tupaeva, I. O.; Gusakov, E. A.; Shilov, G. V.; Tkachev, V. V.; Aldoshin, S. M.; Minkin, V. I. Dokl. Chem. 2015, 460, 33–36. doi:10.1134/s0012500815020019
Return to citation in text: [1] -
Sayapin, Y. A.; Dorogan, I. V.; Gusakov, E. A.; Bang, D. N.; Tkachev, V. V.; Tupaeva, I. O.; Tran, D. L.; Nguyen, T. V.; Duong, T. N.; Dinh, H. V.; Krasnikova, T. A.; Aldoshin, S. M.; Minkin, V. I. ACS Omega 2021, 6, 18226–18234. doi:10.1021/acsomega.1c02033
Return to citation in text: [1] -
Sayapin, Y. A.; Duong, B. N.; Komissarov, V. N.; Dorogan, I. V.; Makarova, N. I.; Bondareva, I. O.; Tkachev, V. V.; Shilov, G. V.; Aldoshin, S. M.; Minkin, V. I. Tetrahedron 2010, 66, 8763–8771. doi:10.1016/j.tet.2010.08.077
Return to citation in text: [1] [2] -
Joshi, H. C.; Antonov, L. Molecules 2021, 26, 1475. doi:10.3390/molecules26051475
Return to citation in text: [1] -
Kwon, J. E.; Park, S. Y. Adv. Mater. (Weinheim, Ger.) 2011, 23, 3615–3642. doi:10.1002/adma.201102046
Return to citation in text: [1] -
Kaur, N.; Kaur, G.; Fegade, U. A.; Singh, A.; Sahoo, S. K.; Kuwar, A. S.; Singh, N. TrAC, Trends Anal. Chem. 2017, 95, 86–109. doi:10.1016/j.trac.2017.08.003
Return to citation in text: [1] -
Suganya, S.; Naha, S.; Velmathi, S. ChemistrySelect 2018, 3, 7231–7268. doi:10.1002/slct.201801222
Return to citation in text: [1] -
Bai, L.; Gao, C.; Liu, Q.; Yu, C.; Zhang, Z.; Cai, L.; Yang, B.; Qian, Y.; Yang, J.; Liao, X. Eur. J. Med. Chem. 2017, 140, 349–382. doi:10.1016/j.ejmech.2017.09.034
Return to citation in text: [1] -
Bannon, J. H.; Fichtner, I.; O'Neill, A.; Pampillón, C.; Sweeney, N. J.; Strohfeldt, K.; Watson, R. W.; Tacke, M.; Mc Gee, M. M. Br. J. Cancer 2007, 97, 1234–1241. doi:10.1038/sj.bjc.6604021
Return to citation in text: [1] -
Tang, Z.; Du, W.; Xu, F.; Sun, X.; Chen, W.; Cui, J.; Tang, W.; Yang, F.; Teng, F.; Lin, J.; Liu, B.; Dong, J. Int. J. Biol. Sci. 2022, 18, 2060–2074. doi:10.7150/ijbs.66630
Return to citation in text: [1] -
SHELXTL; Structure Determination Software Suite, Version 6.14; Bruker AXS: Madison, Wisconsin, USA, 2000.
Return to citation in text: [1] -
Gaussian 09, Revision A.01; Gaussian, Inc.: Wallingford, CT, 2009.
Return to citation in text: [1] -
Perdew, J. P.; Burke, K.; Ernzerhof, M. Phys. Rev. Lett. 1996, 77, 3865–3868. doi:10.1103/physrevlett.77.3865
Return to citation in text: [1] -
Adamo, C.; Barone, V. J. Chem. Phys. 1999, 110, 6158–6170. doi:10.1063/1.478522
Return to citation in text: [1] -
Barone, V.; Cossi, M.; Tomasi, J. J. Chem. Phys. 1997, 107, 3210–3221. doi:10.1063/1.474671
Return to citation in text: [1] -
Cancès, E.; Mennucci, B.; Tomasi, J. J. Chem. Phys. 1997, 107, 3032–3041. doi:10.1063/1.474659
Return to citation in text: [1] -
Mosmann, T. J. Immunol. Methods 1983, 65, 55–63. doi:10.1016/0022-1759(83)90303-4
Return to citation in text: [1] -
Ritz, C.; Baty, F.; Streibig, J. C.; Gerhard, D. PLoS One 2015, 10, e0146021. doi:10.1371/journal.pone.0146021
Return to citation in text: [1]
| 35. | Perdew, J. P.; Burke, K.; Ernzerhof, M. Phys. Rev. Lett. 1996, 77, 3865–3868. doi:10.1103/physrevlett.77.3865 |
| 36. | Adamo, C.; Barone, V. J. Chem. Phys. 1999, 110, 6158–6170. doi:10.1063/1.478522 |
| 37. | Barone, V.; Cossi, M.; Tomasi, J. J. Chem. Phys. 1997, 107, 3210–3221. doi:10.1063/1.474671 |
| 38. | Cancès, E.; Mennucci, B.; Tomasi, J. J. Chem. Phys. 1997, 107, 3032–3041. doi:10.1063/1.474659 |
| 1. | Minkin, V. I.; Aldoshin, S. M.; Komissarov, V. N.; Dorogan, I. V.; Sayapin, Y. A.; Tkachev, V. V.; Starikov, A. G. Russ. Chem. Bull. 2006, 55, 2032–2055. doi:10.1007/s11172-006-0547-x |
| 5. | Sayapin, Y. A.; Tupaeva, I. O.; Kolodina, A. A.; Gusakov, E. A.; Komissarov, V. N.; Dorogan, I. V.; Makarova, N. I.; Metelitsa, A. V.; Tkachev, V. V.; Aldoshin, S. M.; Minkin, V. I. Beilstein J. Org. Chem. 2015, 11, 2179–2188. doi:10.3762/bjoc.11.236 |
| 5. | Sayapin, Y. A.; Tupaeva, I. O.; Kolodina, A. A.; Gusakov, E. A.; Komissarov, V. N.; Dorogan, I. V.; Makarova, N. I.; Metelitsa, A. V.; Tkachev, V. V.; Aldoshin, S. M.; Minkin, V. I. Beilstein J. Org. Chem. 2015, 11, 2179–2188. doi:10.3762/bjoc.11.236 |
| 25. | Sayapin, Y. A.; Duong, B. N.; Komissarov, V. N.; Dorogan, I. V.; Makarova, N. I.; Bondareva, I. O.; Tkachev, V. V.; Shilov, G. V.; Aldoshin, S. M.; Minkin, V. I. Tetrahedron 2010, 66, 8763–8771. doi:10.1016/j.tet.2010.08.077 |
| 4. | Sayapin, Y. A.; Gusakov, E. A.; Dorogan, I. V.; Tupaeva, I. O.; Teimurazov, M. G.; Fursova, N. K.; Ovchinnikov, K. V.; Minkin, V. I. Russ. J. Bioorg. Chem. 2016, 42, 224–228. doi:10.1134/s1068162016020114 |
| 1. | Minkin, V. I.; Aldoshin, S. M.; Komissarov, V. N.; Dorogan, I. V.; Sayapin, Y. A.; Tkachev, V. V.; Starikov, A. G. Russ. Chem. Bull. 2006, 55, 2032–2055. doi:10.1007/s11172-006-0547-x |
| 3. | Sayapin, Y. A.; Gusakov, E. A.; Kolodina, A. A.; Komissarov, V. N.; Dorogan, I. V.; Tkachev, V. V.; Shilov, G. V.; Nosova, E. V.; Aldoshin, S. M.; Charushin, V. N.; Minkin, V. I. Russ. Chem. Bull. 2014, 63, 1364–1372. doi:10.1007/s11172-014-0604-9 |
| 5. | Sayapin, Y. A.; Tupaeva, I. O.; Kolodina, A. A.; Gusakov, E. A.; Komissarov, V. N.; Dorogan, I. V.; Makarova, N. I.; Metelitsa, A. V.; Tkachev, V. V.; Aldoshin, S. M.; Minkin, V. I. Beilstein J. Org. Chem. 2015, 11, 2179–2188. doi:10.3762/bjoc.11.236 |
| 2. | Sayapin, Y. A.; Komissarov, V. N.; Bang, D. N.; Dorogan, I. V.; Minkin, V. I.; Tkachev, V. V.; Shilov, G. V.; Aldoshin, S. M.; Charushin, V. N. Mendeleev Commun. 2008, 18, 180–182. doi:10.1016/j.mencom.2008.07.002 |
| 13. | Schenck, G. O.; Brähler, B.; Cziesla, M. Angew. Chem. 1956, 68, 247–248. doi:10.1002/ange.19560680707 |
| 14. | Kogler, H.; Fehlhaber, H.-W.; Leube, K.; Dürckheimer, W. Chem. Ber. 1989, 122, 2205–2206. doi:10.1002/cber.19891221123 |
| 15. | Li, H.; Li, W.; Li, Z. Chem. Commun. 2009, 3264–3266. doi:10.1039/b903515j |
| 16. | Mondal, A.; Hazra, R.; Grover, J.; Raghu, M.; Ramasastry, S. S. V. ACS Catal. 2018, 8, 2748–2753. doi:10.1021/acscatal.8b00397 |
| 17. | Chien, P.-C.; Chen, Y.-R.; Chen, Y.-J.; Chang, C.-F.; Marri, G.; Lin, W. Adv. Synth. Catal. 2024, 366, 420–425. doi:10.1002/adsc.202301344 |
| 23. | Sayapin, Y. A.; Tupaeva, I. O.; Gusakov, E. A.; Shilov, G. V.; Tkachev, V. V.; Aldoshin, S. M.; Minkin, V. I. Dokl. Chem. 2015, 460, 33–36. doi:10.1134/s0012500815020019 |
| 8. | Johns, R. B.; Johnson, A. W. Chem. Ind. 1954, 192–193. |
| 9. | Chapman, O. L.; Fitton, P. J. Am. Chem. Soc. 1963, 85, 41–47. doi:10.1021/ja00884a008 |
| 10. | Becker, A. M.; Rickards, R. W. Org. Prep. Proced. Int. 1983, 15, 239–242. doi:10.1080/00304948309356648 |
| 11. | Rahman, M. M.; Matano, Y.; Suzuki, H. J. Chem. Soc., Perkin Trans. 1 1999, 1533–1542. doi:10.1039/a900751b |
| 12. | Zinser, H.; Henkel, S.; Föhlisch, B. Eur. J. Org. Chem. 2004, 1344–1356. doi:10.1002/ejoc.200300600 |
| 13. | Schenck, G. O.; Brähler, B.; Cziesla, M. Angew. Chem. 1956, 68, 247–248. doi:10.1002/ange.19560680707 |
| 14. | Kogler, H.; Fehlhaber, H.-W.; Leube, K.; Dürckheimer, W. Chem. Ber. 1989, 122, 2205–2206. doi:10.1002/cber.19891221123 |
| 15. | Li, H.; Li, W.; Li, Z. Chem. Commun. 2009, 3264–3266. doi:10.1039/b903515j |
| 24. | Sayapin, Y. A.; Dorogan, I. V.; Gusakov, E. A.; Bang, D. N.; Tkachev, V. V.; Tupaeva, I. O.; Tran, D. L.; Nguyen, T. V.; Duong, T. N.; Dinh, H. V.; Krasnikova, T. A.; Aldoshin, S. M.; Minkin, V. I. ACS Omega 2021, 6, 18226–18234. doi:10.1021/acsomega.1c02033 |
| 6. | Gusakov, E. A.; Topchu, I. A.; Mazitova, A. M.; Dorogan, I. V.; Bulatov, E. R.; Serebriiskii, I. G.; Abramova, Z. I.; Tupaeva, I. O.; Demidov, O. P.; Toan, D. N.; Lam, T. D.; Bang, D. N.; Boumber, Y. A.; Sayapin, Y. A.; Minkin, V. I. RSC Adv. 2021, 11, 4555–4571. doi:10.1039/d0ra10610k |
| 7. | Kit, O. I.; Minkin, V. I.; Lukbanova, E. A.; Sayapin, Y. A.; Gusakov, E. A.; Sitkovskaya, A. O.; Filippova, S. Y.; Komarova, E. F.; Volkova, A. V.; Khodakova, D. V.; Mindar, M. V.; Lazutin, Y. N.; Engibaryan, M. A.; Kolesnikov, V. E. Bull. Sib. Med. 2022, 21, 60–66. doi:10.20538/1682-0363-2022-2-60-66 |
| 39. | Mosmann, T. J. Immunol. Methods 1983, 65, 55–63. doi:10.1016/0022-1759(83)90303-4 |
| 4. | Sayapin, Y. A.; Gusakov, E. A.; Dorogan, I. V.; Tupaeva, I. O.; Teimurazov, M. G.; Fursova, N. K.; Ovchinnikov, K. V.; Minkin, V. I. Russ. J. Bioorg. Chem. 2016, 42, 224–228. doi:10.1134/s1068162016020114 |
| 18. | Humphrey, G. R.; Kuethe, J. T. Chem. Rev. 2006, 106, 2875–2911. doi:10.1021/cr0505270 |
| 19. | Shiri, M. Chem. Rev. 2012, 112, 3508–3549. doi:10.1021/cr2003954 |
| 20. | Lygin, A. V.; de Meijere, A. Angew. Chem., Int. Ed. 2010, 49, 9094–9124. doi:10.1002/anie.201000723 |
| 21. | Zhuo, C.-X.; Zhang, W.; You, S.-L. Angew. Chem., Int. Ed. 2012, 51, 12662–12686. doi:10.1002/anie.201204822 |
| 22. | Bandini, M.; Eichholzer, A. Angew. Chem., Int. Ed. 2009, 48, 9608–9644. doi:10.1002/anie.200901843 |
| 40. | Ritz, C.; Baty, F.; Streibig, J. C.; Gerhard, D. PLoS One 2015, 10, e0146021. doi:10.1371/journal.pone.0146021 |
| 5. | Sayapin, Y. A.; Tupaeva, I. O.; Kolodina, A. A.; Gusakov, E. A.; Komissarov, V. N.; Dorogan, I. V.; Makarova, N. I.; Metelitsa, A. V.; Tkachev, V. V.; Aldoshin, S. M.; Minkin, V. I. Beilstein J. Org. Chem. 2015, 11, 2179–2188. doi:10.3762/bjoc.11.236 |
| 1. | Minkin, V. I.; Aldoshin, S. M.; Komissarov, V. N.; Dorogan, I. V.; Sayapin, Y. A.; Tkachev, V. V.; Starikov, A. G. Russ. Chem. Bull. 2006, 55, 2032–2055. doi:10.1007/s11172-006-0547-x |
| 25. | Sayapin, Y. A.; Duong, B. N.; Komissarov, V. N.; Dorogan, I. V.; Makarova, N. I.; Bondareva, I. O.; Tkachev, V. V.; Shilov, G. V.; Aldoshin, S. M.; Minkin, V. I. Tetrahedron 2010, 66, 8763–8771. doi:10.1016/j.tet.2010.08.077 |
| 33. | SHELXTL; Structure Determination Software Suite, Version 6.14; Bruker AXS: Madison, Wisconsin, USA, 2000. |
| 31. | Bannon, J. H.; Fichtner, I.; O'Neill, A.; Pampillón, C.; Sweeney, N. J.; Strohfeldt, K.; Watson, R. W.; Tacke, M.; Mc Gee, M. M. Br. J. Cancer 2007, 97, 1234–1241. doi:10.1038/sj.bjc.6604021 |
| 32. | Tang, Z.; Du, W.; Xu, F.; Sun, X.; Chen, W.; Cui, J.; Tang, W.; Yang, F.; Teng, F.; Lin, J.; Liu, B.; Dong, J. Int. J. Biol. Sci. 2022, 18, 2060–2074. doi:10.7150/ijbs.66630 |
| 29. | Suganya, S.; Naha, S.; Velmathi, S. ChemistrySelect 2018, 3, 7231–7268. doi:10.1002/slct.201801222 |
| 30. | Bai, L.; Gao, C.; Liu, Q.; Yu, C.; Zhang, Z.; Cai, L.; Yang, B.; Qian, Y.; Yang, J.; Liao, X. Eur. J. Med. Chem. 2017, 140, 349–382. doi:10.1016/j.ejmech.2017.09.034 |
| 26. | Joshi, H. C.; Antonov, L. Molecules 2021, 26, 1475. doi:10.3390/molecules26051475 |
| 27. | Kwon, J. E.; Park, S. Y. Adv. Mater. (Weinheim, Ger.) 2011, 23, 3615–3642. doi:10.1002/adma.201102046 |
| 28. | Kaur, N.; Kaur, G.; Fegade, U. A.; Singh, A.; Sahoo, S. K.; Kuwar, A. S.; Singh, N. TrAC, Trends Anal. Chem. 2017, 95, 86–109. doi:10.1016/j.trac.2017.08.003 |
© 2025 Sayapin et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.