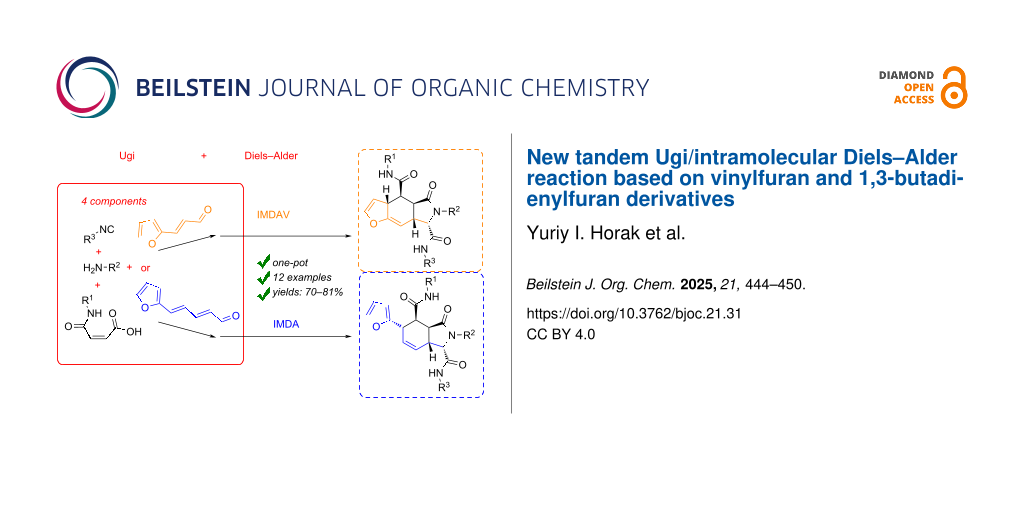
Abstract
A new tandem sequence involving the Ugi reaction and Diels–Alder [4 + 2] cycloaddition based on vinylfuran and 1,3-butadienylfuran derivatives was designed and studied. It was found that in the case of 3-(furan-2-yl)acrylaldehyde, a one-pot Ugi reaction and intramolecular Diels–Alder vinylarene (IMDAV) reaction leads to the formation of the insufficiently studied furo[2,3-f]isoindole derivatives. Ugi adducts formed from (E)-3-(furan-2-yl)acrylaldehyde, maleic acid monoanilide, isonitrile, and an amine spontaneously underwent the IMDAV reaction with a high level of stereoselectivity, leading to single pairs of enantiomers of 4,4a,5,6,7,7a-hexahydro-3aH-furo[2,3-f]isoindole core in excellent yields. Under the same conditions, the (2E,4E)-5-(furan-2-yl)penta-2,4-dienal gives an Ugi adduct that undergoes the IMDA reaction without involving the furan core. The cycloaddition leads to the formation of 2,3,3a,4,5,7a-hexahydro-1H-isoindoles in high yields. The studied tandem Ugi and intramolecular Diels–Alder reactions allow high substituent variation in the named isoindoles.
Graphical Abstract
Introduction
Energy-saving and environmentally friendly synthetic strategies, especially one-pot multicomponent and tandem reactions, are key to modern organic and medicinal chemistry [1-7] and have proven to be successful in generating diverse heterocyclic scaffolds, as highlighted in a recent book [8]. Multicomponent reactions, for instance, have contributed to high-throughput screening for drug discovery, facilitating the identification of new therapeutic compounds. [9,10]. A notable example is ivosidenib, approved in 2018, which was synthesized using the Ugi reaction to target mutated IDH1 in acute myeloid leukemia [11-13]. Advancements in one-pot syntheses, like combining the Ugi reaction with other methods [14-18], for example, the Huisgen cycloaddition, have led to the creation of unique [1,2,3]triazolo[1,5-a]pyrazine derivatives [19]. Tandem reactions are particularly valued for their atom and step-economy, making them promising for future commercial applications.
Partly hydrogenated isoindoles and their derivatives exhibit broad biological activities and are considered privileged motifs in medicinal chemistry [20,21]. These compounds, when condensed with aromatics or heterocycles, form heterolignans, which are synthetic derivatives of naturally occurring lignans [22-25]. This has gained significant attention in drug discovery [26].
Several synthetic routes, such as tandem Pummerer/Diels–Alder or Wittig/Diels–Alder approaches, have been developed for heterolignan construction [27,28]. For instance, the intramolecular Diels–Alder reactions of vinylarenes (IMDAV) strategy [29,30] has been used to synthesize annulated isoindoles, including thieno[2,3-f]isoindoles [31,32] and furo[2,3-f]isoindoles [33,34], from thienyl- or furylallylamines and unsaturated acid derivatives. Also, benzoisoindoles have been synthesized via the pseudo-four-component reaction of 3-phenylallylamines with maleic anhydride [35]. Finally, furfural was utilized in the Ugi reactions with unsaturated acids leading to the intramolecular Diels–Alder furan (IMDAF) reaction (Figure 1) [36].
Figure 1: State of the art of Ugi/Diels–Alder reaction based on furan.
Figure 1: State of the art of Ugi/Diels–Alder reaction based on furan.
In the present work, the new tandem sequence involving an Ugi reaction and Diels–Alder [4 + 2] cycloaddition based on vinylfuran, 1,3-butadienylfuran derivatives was designed and studied.
Results and Discussion
To carry out an intramolecular Diels–Alder reaction, we tested (E)-3-(furan-2-yl)acrylaldehyde (1a) in the Ugi reaction with a maleic acid to form adducts containing both, diene and dienophilic fragments. It was found that aldehyde 1a reacted with amines 2, isonitriles 3, and maleic acid monoanilide (4a) to form an adduct that spontaneously underwent a [4 + 2] cycloaddition giving furoisoindoles 5a–h under the Ugi reaction conditions (Scheme 1). A furan double bond and an exocyclic double bond, forming the diene, enter the intramolecular cyclization, and a residue formed by the monoanilide of maleic acid acts as a dienophile. Notably, non-cyclized Ugi adducts A1 were not found in the products. The products were identified via NMR as 4,4a,5,6,7,7a-hexahydro-3aH-furo[2,3-f]isoindoles.
Scheme 1: Preparation of 4,4a,5,6,7,7a-hexahydro-3aH-furo[2,3-f]isoindoles via Ugi/IMDAV tandem reaction.
Scheme 1: Preparation of 4,4a,5,6,7,7a-hexahydro-3aH-furo[2,3-f]isoindoles via Ugi/IMDAV tandem reaction.
The structures of compounds 5 were confirmed by NMR spectroscopy data (for details and discussion see Supporting Information File 1). The NMR data for the obtained furoindole skeleton signals and spin-coupling constants agree with our previous results on tandem acylation/Diels–Alder reactions [31-33] and lead us to conclude that the final Diels–Alder reaction products are formed as the exo-adducts. Moreover, since we do not observe other stereoisomers we speculate that the Ugi and Diels–Alder reactions occur under a coordinated mechanism.
Noteworthy, the primary kinetic product of the cycloaddition reaction 5 is not transformed into the thermodynamic product 6 via a H-shift at the last stage. The expected aromatization with the formation of a furan ring, as happens in similar reactions, does not occur (Scheme 2).
Scheme 2: Kinetic product 5 does not transform into thermodynamic product 6.
Scheme 2: Kinetic product 5 does not transform into thermodynamic product 6.
However, if maleic acid was used instead of its monoanilide, then an aromatic furan product was obtained (Scheme 3). Most likely, the presence of a carboxyl group near the hydrogen in the 3-position of the furan ring contributes to its shift and isomerization to a more thermodynamically beneficial aromatic product.
Since we previously studied the reaction of 3-(thien-2-yl)allylamines with maleic anhydride [30], followed by a domino sequence involving successive acylation/[4 + 2] cycloaddition steps, that leads to the formation of the thieno[2,3-f]isoindole core, it was interesting to investigate the possibility of obtaining related compounds in the Ugi/IMDAV reaction. It was found that the reaction of (E)-3-(thiophen-2-yl)acrylaldehyde proceeds similarly to furylacrylaldehyde and with a high level of stereoselectivity, forms a single pair of enantiomers of 4,4a,5,6,7,7a-hexahydro-3aH-thieno[2,3-f]isoindoles in excellent yield (Scheme 4). The H-shift does not take place at the last stage, which correlates with the results obtained earlier. This shows the possibility of replacing the furan ring with a thiophene one, which significantly expands the possibilities of this approach for achieving molecular diversity of derivatives for creating libraries of compounds for bioscreening.
Finally, in such a tandem Ugi/IMDA reaction, furyl-2-pentadienal was studied. It was found, that the Ugi adduct formed from (2E,4E)-5-(furan-2-yl)penta-2,4-dienal (8) underwent the IMDA reaction, without involving the furan core in the cycloaddition, thus leading to the 2,3,3a,4,5,7a-hexahydro-1H-isoindole core 9. In the IMDA stage, the exocyclic diene system is involved (Scheme 5). The yields of the products listed below for the Ugi/Diels–Alder tandem reactions were 73% (9a) and 70% (9b). The structures of these compounds were confirmed by NMR spectroscopy (see Supporting Information File 1).
Conclusion
In summary, we showed the practical usage of 3-(furan-2-yl)acrylaldehyde in the four-component Ugi reaction with the prospect of further intramolecular [4 + 2] cycloaddition of the Ugi reaction product. We managed to carry out these two tandem reactions in one-pot, and, thus, proposed a new variant of the Ugi/Diels–Alder tandem reaction, which is highly variable and promising for implementation in combinatorial synthesis.
Supporting Information
| Supporting Information File 1: Experimental procedures, compound characterizations, and NMR spectra. | ||
| Format: PDF | Size: 4.4 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Herrera, R. P.; Marqués‐López, E., Eds. Multicomponent Reactions: Concepts and Applications for Design and Synthesis; Wiley-VCH: Weinheim, Germany, 2015. doi:10.1002/9781118863992
Return to citation in text: [1] -
Zhu, J.; Bienaymé, H., Eds. Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005. doi:10.1002/3527605118
Return to citation in text: [1] -
Langer, P. Eur. J. Org. Chem. 2024, 27, e202400153. doi:10.1002/ejoc.202400153
Return to citation in text: [1] -
Müller, T. J. J.; D'Souza, D. M. Pure Appl. Chem. 2008, 80, 609–620. doi:10.1351/pac200880030609
Return to citation in text: [1] -
Gers, C. F.; Rosellen, J.; Merkul, E.; Müller, T. J. J. Beilstein J. Org. Chem. 2011, 7, 1173–1181. doi:10.3762/bjoc.7.136
Return to citation in text: [1] -
John, S. E.; Gulati, S.; Shankaraiah, N. Org. Chem. Front. 2021, 8, 4237–4287. doi:10.1039/d0qo01480j
Return to citation in text: [1] -
Levi, L.; Müller, T. J. J. Chem. Soc. Rev. 2016, 45, 2825–2846. doi:10.1039/c5cs00805k
Return to citation in text: [1] -
Ameta, K. L.; Dandia, A., Eds. Multicomponent Reactions: Synthesis of Bioactive Heterocycles; CRC Press: Boca Raton, FL, USA, 2017. doi:10.1201/9781315369754
Return to citation in text: [1] -
Graebin, C. S.; Ribeiro, F. V.; Rogério, K. R.; Kümmerle, A. E. Curr. Org. Synth. 2019, 16, 855–899. doi:10.2174/1570179416666190718153703
Return to citation in text: [1] -
Fouad, M. A.; Abdel-Hamid, H.; Ayoup, M. S. RSC Adv. 2020, 10, 42644–42681. doi:10.1039/d0ra07501a
Return to citation in text: [1] -
Dömling, A.; Ugi, I. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. doi:10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u
Return to citation in text: [1] -
Mason, K. M.; Meyers, M. S.; Fox, A. M.; Luesse, S. B. Beilstein J. Org. Chem. 2016, 12, 2032–2037. doi:10.3762/bjoc.12.191
Return to citation in text: [1] -
Rocha, R. O.; Rodrigues, M. O.; Neto, B. A. D. ACS Omega 2020, 5, 972–979. doi:10.1021/acsomega.9b03684
Return to citation in text: [1] -
Rudick, J. G.; Shaabani, S.; Dömling, A. Front. Chem. (Lausanne, Switz.) 2020, 7, 918. doi:10.3389/fchem.2019.00918
Return to citation in text: [1] -
Nazeri, M. T.; Farhid, H.; Mohammadian, R.; Shaabani, A. ACS Comb. Sci. 2020, 22, 361–400. doi:10.1021/acscombsci.0c00046
Return to citation in text: [1] -
Ojeda, G. M.; Ranjan, P.; Fedoseev, P.; Amable, L.; Sharma, U. K.; Rivera, D. G.; Van der Eycken, E. V. Beilstein J. Org. Chem. 2019, 15, 2447–2457. doi:10.3762/bjoc.15.237
Return to citation in text: [1] -
Niu, J.; Wang, Y.; Yan, S.; Zhang, Y.; Ma, X.; Zhang, Q.; Zhang, W. Beilstein J. Org. Chem. 2024, 20, 912–920. doi:10.3762/bjoc.20.81
Return to citation in text: [1] -
Ilyin, A.; Kysil, V.; Krasavin, M.; Kurashvili, I.; Ivachtchenko, A. V. J. Org. Chem. 2006, 71, 9544–9547. doi:10.1021/jo061825f
Return to citation in text: [1] -
Pokhodylo, N. T.; Тupychak, M. A.; Goreshnik, E. A.; Obushak, M. D. Synthesis 2023, 55, 977–988. doi:10.1055/s-0042-1751382
Return to citation in text: [1] -
Pemberton, N.; Mogemark, M.; Arlbrandt, S.; Bold, P.; Cox, R. J.; Gardelli, C.; Holden, N. S.; Karabelas, K.; Karlsson, J.; Lever, S.; Li, X.; Lindmark, H.; Norberg, M.; Perry, M. W. D.; Petersen, J.; Blomqvist, S. R.; Thomas, M.; Tyrchan, C.; Eriksson, A. W.; Zlatoidsky, P.; Öster, L. J. Med. Chem. 2018, 61, 5435–5441. doi:10.1021/acs.jmedchem.8b00447
Return to citation in text: [1] -
Mariaule, G.; De Cesco, S.; Airaghi, F.; Kurian, J.; Schiavini, P.; Rocheleau, S.; Huskić, I.; Auclair, K.; Mittermaier, A.; Moitessier, N. J. Med. Chem. 2016, 59, 4221–4234. doi:10.1021/acs.jmedchem.5b01296
Return to citation in text: [1] -
Mishra, R.; Sachan, N.; Kumar, N.; Mishra, I.; Chand, P. J. Heterocycl. Chem. 2018, 55, 2019–2034. doi:10.1002/jhet.3249
Return to citation in text: [1] -
Tilve, S. G.; Torney, P. S.; Patre, R. E.; Kamat, D. P.; Srinivasan, B. R.; Zubkov, F. I. Tetrahedron Lett. 2016, 57, 2266–2268. doi:10.1016/j.tetlet.2016.04.038
Return to citation in text: [1] -
Huang, L.; Ye, L.; Li, X.-H.; Li, Z.-L.; Lin, J.-S.; Liu, X.-Y. Org. Lett. 2016, 18, 5284–5287. doi:10.1021/acs.orglett.6b02599
Return to citation in text: [1] -
Li, J.; Zhang, X.; Renata, H. Angew. Chem., Int. Ed. 2019, 58, 11657–11660. doi:10.1002/anie.201904102
Return to citation in text: [1] -
Rifai, Y.; Tani, H. B.; Nur, M.; Aswad, M.; Lallo, S.; Wahyudin, E. Arch. Pharm. (Weinheim, Ger.) 2016, 349, 848–852. doi:10.1002/ardp.201600069
Return to citation in text: [1] -
Sarkar, T. K.; Panda, N.; Basak, S. J. Org. Chem. 2003, 68, 6919–6927. doi:10.1021/jo0344081
Return to citation in text: [1] -
Huang, J.; Du, X.; Van Hecke, K.; Van der Eycken, E. V.; Pereshivko, O. P.; Peshkov, V. A. Eur. J. Org. Chem. 2017, 4379–4388. doi:10.1002/ejoc.201700747
Return to citation in text: [1] -
Krishna, G.; Grudinin, D. G.; Nikitina, E. V.; Zubkov, F. I. Synthesis 2022, 54, 797–863. doi:10.1055/s-0040-1705983
Return to citation in text: [1] -
Yakovleva, E. D.; Shelukho, E. R.; Nadirova, M. A.; Erokhin, P. P.; Simakova, D. N.; Khrustalev, V. N.; Grigoriev, M. S.; Novikov, A. P.; Romanycheva, A. A.; Shetnev, A. A.; Bychkova, O. P.; Trenin, A. S.; Zubkov, F. I.; Zaytsev, V. P. Org. Biomol. Chem. 2024, 22, 2643–2653. doi:10.1039/d3ob01933k
Return to citation in text: [1] [2] -
Nadirova, M. A.; Laba, Y.-O. V.; Zaytsev, V. P.; Sokolova, J. S.; Pokazeev, K. M.; Anokhina, V. A.; Khrustalev, V. N.; Horak, Y. I.; Lytvyn, R. Z.; Siczek, M.; Kinzhybalo, V.; Zubavichus, Y. V.; Kuznetsov, M. L.; Obushak, M. D.; Zubkov, F. I. Synthesis 2020, 52, 2196–2223. doi:10.1055/s-0039-1690833
Return to citation in text: [1] [2] -
Horak, Y. I.; Lytvyn, R. Z.; Laba, Y.-O. V.; Homza, Y. V.; Zaytsev, V. P.; Nadirova, M. A.; Nikanorova, T. V.; Zubkov, F. I.; Varlamov, A. V.; Obushak, M. D. Tetrahedron Lett. 2017, 58, 4103–4106. doi:10.1016/j.tetlet.2017.09.038
Return to citation in text: [1] [2] -
Horak, Y. I.; Lytvyn, R. Z.; Homza, Y. V.; Zaytsev, V. P.; Mertsalov, D. F.; Babkina, M. N.; Nikitina, E. V.; Lis, T.; Kinzhybalo, V.; Matiychuk, V. S.; Zubkov, F. I.; Varlamov, A. V.; Obushak, M. D. Tetrahedron Lett. 2015, 56, 4499–4501. doi:10.1016/j.tetlet.2015.05.115
Return to citation in text: [1] [2] -
Zubkov, F. I.; Zaytsev, V. P.; Mertsalov, D. F.; Nikitina, E. V.; Horak, Y. I.; Lytvyn, R. Z.; Homza, Y. V.; Obushak, M. D.; Dorovatovskii, P. V.; Khrustalev, V. N.; Varlamov, A. V. Tetrahedron 2016, 72, 2239–2253. doi:10.1016/j.tet.2016.03.023
Return to citation in text: [1] -
Voronov, A. A.; Alekseeva, K. A.; Ryzhkova, E. A.; Zarubaev, V. V.; Galochkina, A. V.; Zaytsev, V. P.; Majik, M. S.; Tilve, S. G.; Gurbanov, A. V.; Zubkov, F. I. Tetrahedron Lett. 2018, 59, 1108–1111. doi:10.1016/j.tetlet.2018.02.015
Return to citation in text: [1] -
Paulvannan, K. Tetrahedron Lett. 1999, 40, 1851–1854. doi:10.1016/s0040-4039(99)00072-6
Return to citation in text: [1]
| 30. | Yakovleva, E. D.; Shelukho, E. R.; Nadirova, M. A.; Erokhin, P. P.; Simakova, D. N.; Khrustalev, V. N.; Grigoriev, M. S.; Novikov, A. P.; Romanycheva, A. A.; Shetnev, A. A.; Bychkova, O. P.; Trenin, A. S.; Zubkov, F. I.; Zaytsev, V. P. Org. Biomol. Chem. 2024, 22, 2643–2653. doi:10.1039/d3ob01933k |
| 1. | Herrera, R. P.; Marqués‐López, E., Eds. Multicomponent Reactions: Concepts and Applications for Design and Synthesis; Wiley-VCH: Weinheim, Germany, 2015. doi:10.1002/9781118863992 |
| 2. | Zhu, J.; Bienaymé, H., Eds. Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005. doi:10.1002/3527605118 |
| 3. | Langer, P. Eur. J. Org. Chem. 2024, 27, e202400153. doi:10.1002/ejoc.202400153 |
| 4. | Müller, T. J. J.; D'Souza, D. M. Pure Appl. Chem. 2008, 80, 609–620. doi:10.1351/pac200880030609 |
| 5. | Gers, C. F.; Rosellen, J.; Merkul, E.; Müller, T. J. J. Beilstein J. Org. Chem. 2011, 7, 1173–1181. doi:10.3762/bjoc.7.136 |
| 6. | John, S. E.; Gulati, S.; Shankaraiah, N. Org. Chem. Front. 2021, 8, 4237–4287. doi:10.1039/d0qo01480j |
| 7. | Levi, L.; Müller, T. J. J. Chem. Soc. Rev. 2016, 45, 2825–2846. doi:10.1039/c5cs00805k |
| 14. | Rudick, J. G.; Shaabani, S.; Dömling, A. Front. Chem. (Lausanne, Switz.) 2020, 7, 918. doi:10.3389/fchem.2019.00918 |
| 15. | Nazeri, M. T.; Farhid, H.; Mohammadian, R.; Shaabani, A. ACS Comb. Sci. 2020, 22, 361–400. doi:10.1021/acscombsci.0c00046 |
| 16. | Ojeda, G. M.; Ranjan, P.; Fedoseev, P.; Amable, L.; Sharma, U. K.; Rivera, D. G.; Van der Eycken, E. V. Beilstein J. Org. Chem. 2019, 15, 2447–2457. doi:10.3762/bjoc.15.237 |
| 17. | Niu, J.; Wang, Y.; Yan, S.; Zhang, Y.; Ma, X.; Zhang, Q.; Zhang, W. Beilstein J. Org. Chem. 2024, 20, 912–920. doi:10.3762/bjoc.20.81 |
| 18. | Ilyin, A.; Kysil, V.; Krasavin, M.; Kurashvili, I.; Ivachtchenko, A. V. J. Org. Chem. 2006, 71, 9544–9547. doi:10.1021/jo061825f |
| 36. | Paulvannan, K. Tetrahedron Lett. 1999, 40, 1851–1854. doi:10.1016/s0040-4039(99)00072-6 |
| 11. | Dömling, A.; Ugi, I. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. doi:10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u |
| 12. | Mason, K. M.; Meyers, M. S.; Fox, A. M.; Luesse, S. B. Beilstein J. Org. Chem. 2016, 12, 2032–2037. doi:10.3762/bjoc.12.191 |
| 13. | Rocha, R. O.; Rodrigues, M. O.; Neto, B. A. D. ACS Omega 2020, 5, 972–979. doi:10.1021/acsomega.9b03684 |
| 31. | Nadirova, M. A.; Laba, Y.-O. V.; Zaytsev, V. P.; Sokolova, J. S.; Pokazeev, K. M.; Anokhina, V. A.; Khrustalev, V. N.; Horak, Y. I.; Lytvyn, R. Z.; Siczek, M.; Kinzhybalo, V.; Zubavichus, Y. V.; Kuznetsov, M. L.; Obushak, M. D.; Zubkov, F. I. Synthesis 2020, 52, 2196–2223. doi:10.1055/s-0039-1690833 |
| 32. | Horak, Y. I.; Lytvyn, R. Z.; Laba, Y.-O. V.; Homza, Y. V.; Zaytsev, V. P.; Nadirova, M. A.; Nikanorova, T. V.; Zubkov, F. I.; Varlamov, A. V.; Obushak, M. D. Tetrahedron Lett. 2017, 58, 4103–4106. doi:10.1016/j.tetlet.2017.09.038 |
| 33. | Horak, Y. I.; Lytvyn, R. Z.; Homza, Y. V.; Zaytsev, V. P.; Mertsalov, D. F.; Babkina, M. N.; Nikitina, E. V.; Lis, T.; Kinzhybalo, V.; Matiychuk, V. S.; Zubkov, F. I.; Varlamov, A. V.; Obushak, M. D. Tetrahedron Lett. 2015, 56, 4499–4501. doi:10.1016/j.tetlet.2015.05.115 |
| 9. | Graebin, C. S.; Ribeiro, F. V.; Rogério, K. R.; Kümmerle, A. E. Curr. Org. Synth. 2019, 16, 855–899. doi:10.2174/1570179416666190718153703 |
| 10. | Fouad, M. A.; Abdel-Hamid, H.; Ayoup, M. S. RSC Adv. 2020, 10, 42644–42681. doi:10.1039/d0ra07501a |
| 33. | Horak, Y. I.; Lytvyn, R. Z.; Homza, Y. V.; Zaytsev, V. P.; Mertsalov, D. F.; Babkina, M. N.; Nikitina, E. V.; Lis, T.; Kinzhybalo, V.; Matiychuk, V. S.; Zubkov, F. I.; Varlamov, A. V.; Obushak, M. D. Tetrahedron Lett. 2015, 56, 4499–4501. doi:10.1016/j.tetlet.2015.05.115 |
| 34. | Zubkov, F. I.; Zaytsev, V. P.; Mertsalov, D. F.; Nikitina, E. V.; Horak, Y. I.; Lytvyn, R. Z.; Homza, Y. V.; Obushak, M. D.; Dorovatovskii, P. V.; Khrustalev, V. N.; Varlamov, A. V. Tetrahedron 2016, 72, 2239–2253. doi:10.1016/j.tet.2016.03.023 |
| 8. | Ameta, K. L.; Dandia, A., Eds. Multicomponent Reactions: Synthesis of Bioactive Heterocycles; CRC Press: Boca Raton, FL, USA, 2017. doi:10.1201/9781315369754 |
| 35. | Voronov, A. A.; Alekseeva, K. A.; Ryzhkova, E. A.; Zarubaev, V. V.; Galochkina, A. V.; Zaytsev, V. P.; Majik, M. S.; Tilve, S. G.; Gurbanov, A. V.; Zubkov, F. I. Tetrahedron Lett. 2018, 59, 1108–1111. doi:10.1016/j.tetlet.2018.02.015 |
| 26. | Rifai, Y.; Tani, H. B.; Nur, M.; Aswad, M.; Lallo, S.; Wahyudin, E. Arch. Pharm. (Weinheim, Ger.) 2016, 349, 848–852. doi:10.1002/ardp.201600069 |
| 29. | Krishna, G.; Grudinin, D. G.; Nikitina, E. V.; Zubkov, F. I. Synthesis 2022, 54, 797–863. doi:10.1055/s-0040-1705983 |
| 30. | Yakovleva, E. D.; Shelukho, E. R.; Nadirova, M. A.; Erokhin, P. P.; Simakova, D. N.; Khrustalev, V. N.; Grigoriev, M. S.; Novikov, A. P.; Romanycheva, A. A.; Shetnev, A. A.; Bychkova, O. P.; Trenin, A. S.; Zubkov, F. I.; Zaytsev, V. P. Org. Biomol. Chem. 2024, 22, 2643–2653. doi:10.1039/d3ob01933k |
| 22. | Mishra, R.; Sachan, N.; Kumar, N.; Mishra, I.; Chand, P. J. Heterocycl. Chem. 2018, 55, 2019–2034. doi:10.1002/jhet.3249 |
| 23. | Tilve, S. G.; Torney, P. S.; Patre, R. E.; Kamat, D. P.; Srinivasan, B. R.; Zubkov, F. I. Tetrahedron Lett. 2016, 57, 2266–2268. doi:10.1016/j.tetlet.2016.04.038 |
| 24. | Huang, L.; Ye, L.; Li, X.-H.; Li, Z.-L.; Lin, J.-S.; Liu, X.-Y. Org. Lett. 2016, 18, 5284–5287. doi:10.1021/acs.orglett.6b02599 |
| 25. | Li, J.; Zhang, X.; Renata, H. Angew. Chem., Int. Ed. 2019, 58, 11657–11660. doi:10.1002/anie.201904102 |
| 31. | Nadirova, M. A.; Laba, Y.-O. V.; Zaytsev, V. P.; Sokolova, J. S.; Pokazeev, K. M.; Anokhina, V. A.; Khrustalev, V. N.; Horak, Y. I.; Lytvyn, R. Z.; Siczek, M.; Kinzhybalo, V.; Zubavichus, Y. V.; Kuznetsov, M. L.; Obushak, M. D.; Zubkov, F. I. Synthesis 2020, 52, 2196–2223. doi:10.1055/s-0039-1690833 |
| 32. | Horak, Y. I.; Lytvyn, R. Z.; Laba, Y.-O. V.; Homza, Y. V.; Zaytsev, V. P.; Nadirova, M. A.; Nikanorova, T. V.; Zubkov, F. I.; Varlamov, A. V.; Obushak, M. D. Tetrahedron Lett. 2017, 58, 4103–4106. doi:10.1016/j.tetlet.2017.09.038 |
| 20. | Pemberton, N.; Mogemark, M.; Arlbrandt, S.; Bold, P.; Cox, R. J.; Gardelli, C.; Holden, N. S.; Karabelas, K.; Karlsson, J.; Lever, S.; Li, X.; Lindmark, H.; Norberg, M.; Perry, M. W. D.; Petersen, J.; Blomqvist, S. R.; Thomas, M.; Tyrchan, C.; Eriksson, A. W.; Zlatoidsky, P.; Öster, L. J. Med. Chem. 2018, 61, 5435–5441. doi:10.1021/acs.jmedchem.8b00447 |
| 21. | Mariaule, G.; De Cesco, S.; Airaghi, F.; Kurian, J.; Schiavini, P.; Rocheleau, S.; Huskić, I.; Auclair, K.; Mittermaier, A.; Moitessier, N. J. Med. Chem. 2016, 59, 4221–4234. doi:10.1021/acs.jmedchem.5b01296 |
| 19. | Pokhodylo, N. T.; Тupychak, M. A.; Goreshnik, E. A.; Obushak, M. D. Synthesis 2023, 55, 977–988. doi:10.1055/s-0042-1751382 |
| 27. | Sarkar, T. K.; Panda, N.; Basak, S. J. Org. Chem. 2003, 68, 6919–6927. doi:10.1021/jo0344081 |
| 28. | Huang, J.; Du, X.; Van Hecke, K.; Van der Eycken, E. V.; Pereshivko, O. P.; Peshkov, V. A. Eur. J. Org. Chem. 2017, 4379–4388. doi:10.1002/ejoc.201700747 |
© 2025 Horak et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.