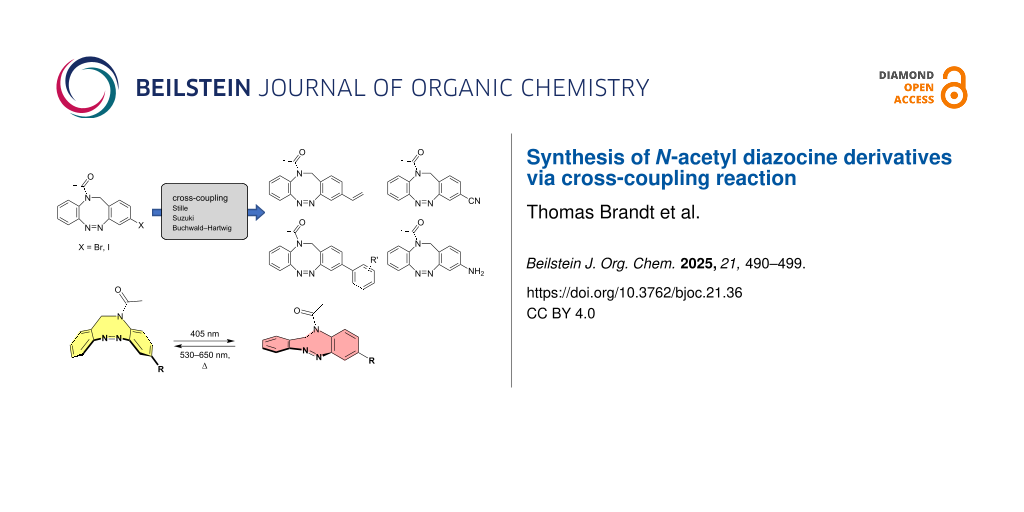
Abstract
Diazocines are photoswitches derived from azobenzenes by bridging the two phenyl rings in ortho position with a CH2CH2 group forming an eight membered (diazocine) ring. Diazocine is superior to most azobenzenes in almost all photophysical properties (switching efficiency, quantum yield, wavelengths etc.). The biggest advantage, especially in photopharmacology and when used in photoswitchable materials, is the inverted thermodynamic stability of the two switching states (isomers). The Z isomer is more stable than the E form. However, one disadvantage that it shares with the frequently used azobenzene is that the switching efficiency decreases sharply with increasing water content in the solvent. In a recently published paper, we reported that replacing one CH2 group in the bridge with NCOCH3 not only confers intrinsic water solubility, but also largely eliminates the problem of reduced switching efficiency in aqueous solutions. In order to investigate the chemistry of this promising photoswitch and to unlock further applications, we now investigate strategies for the synthesis of derivatives, which are based on cross-coupling reactions. Fourteen vinyl-, aryl-, cyano-, and amino-substituted diazocines were prepared via Stille, Suzuki, and Buchwald–Hartwig reactions. X-ray structures are presented for derivatives 1, 2 and 7.
Graphical Abstract
Introduction
Diazocines are frequently used photoswitches with superior photophysical properties. The parent ethylene-bridged diazocine shows excellent switching photoconversion between the Z and the E configurations (Γ(Z → E)385nm = 92% and Γ(E → Z)520nm > 99% in n-hexane) due to well-separated n–π*-transitions in the visible part of the electromagnetic spectra [1]. Moreover, the ethylene bridge creates a cyclic 8-membered core, inverting the thermodynamically stability in favor of the Z boat conformation compared to parent azobenzene, which has a stable E configuration [1-4]. Preceding studies including azobenzene-based photopharmacophores showed that, in most cases, the sterically demanding Z configuration is biologically inactive, while the stretched E configuration is biologically active [5-7]. Because of the inverted thermodynamic stability compared to azobenzene, the stable Z configuration of the diazocine can be administered and subsequently activated with light at the site of illness with high spatiotemporal resolution. Thus, collateral damage in the surrounding healthy tissue can be avoided. In addition, the quantitative thermal back-isomerization from the active E to the inactive Z configuration prevents contamination and accumulation in the environment after excretion [1,6,7]. These superior properties of diazocines have been exploited in several applications such as the control of protein folding by implementation as cross-linkers between protein side chains [8] or in peptide backbones [9], as photoswitchable neurotransmitters [10,11] or as switching units for potential dependent potassium channels [12]. Compared to the Z → E conversion rate of 92% (in n-hexane) of the parent diazocine the conversion in water/DMSO mixtures is decreasing with increasing water concentration (73% in water/DMSO 9:1) [8-12]. Moreover, the parent diazocine is insoluble in water (precipitation in water/DMSO > 9:1). Substitution with polar substituents such as CH2NH2 provides water solubility, however, it does not restore the high Z → E conversion rates of the parent system in organic solvents, which is a disadvantage, since biochemical reactions usually take place in aqueous environments [13]. The substitution of one CH2 group in the CH2CH2 bridge by N–C(=O)–CH3 leads to an intrinsic water solubility of the N-acetyl diazocine 1 (Figure 1) [3]. Furthermore the photoconversion of 1 shows no significant drop in pure water in contrast to the solubilized parent diazocine. These superior properties make N-acetyl diazocine 1 an ideal candidate for application in the field of photopharmacology especially in aqueous environments [13,14]. The same applies for the quantum yields. While the quantum yields ΦZ→E and ΦE→Z of the parent diazocine drop significantly in aqueous media, the corresponding quantum yields of the N-acetyl diazocine stay the same or even slightly improve (Table 1). In general, the quantum yields of parent diazocine and its’ nitrogen bridged derivative exceed the quantum yields of other molecular photoswitches like azobenzenes, spiropyranes and diarylethenes in organic solvents [3,13,15].
Table 1: Quantum yields of N-acetyl diazocine 1 in organic and aqueous media compared to parent diazocine [3,13].
There are two strategies of applying diazocines in photopharmacology. The first one exploits the structural similarity of the tricyclic diazocine framework to the tricyclic structure of, e.g., tetrahydrodibenzazocines [16,17] and tetracyclic steroid scaffolds such as 17β-estradiol [18], where the diazocine core mimics the framework of the bioactive compound (Figure 1a). The other option is to attach the diazocine photoswitch as a substituent (appendix) to the biologically active molecule (Figure 1b) [6,10,19-21]. The art of designing a photoswitchable drug is to place the switch at a position in the pharmacophore that allows switching of the biological effect by irradiation with light without greatly reducing the overall activity by unselective interference with the inhibitor–receptor interaction. This is a difficult task because the design of a photoswitchable agent usually starts with a known, non-switchable drug or a known biological molecule, which is already carefully "optimized" either by pharmaceutical industry or by nature. Hence, there is a high risk that any change in structure will also lead to a reduction in efficiency. In any case the light-induced geometry change via isomerization should selectively control the interaction between the inhibitor and the receptor [21].
Figure 1: a) Structural similarity of N-acetyl diazocine 1 with known 17βHSD3-inhibitor tetrahydrodibenzazocine (THB) [17] and parent diazocine with steroid scaffolds [18]. b) Parent diazocine attached to glutamate to form a photoswitchable neurotransmitter [10].
Figure 1: a) Structural similarity of N-acetyl diazocine 1 with known 17βHSD3-inhibitor tetrahydrodibenzazoci...
Currently there is only one example reported in the literature for the incorporation of N-acetyl diazocines into biologically active molecules [17]. As a starting point for further derivatization, the synthesis and characterization of monohalogenated N-acetyl diazocines 2 and 3 (Figure 1) have been performed [22]. Unfortunately, diazocines in general, and N-acetyl diazocines in particular cannot be derivatized by electrophilic aromatic substitution. Substituents such as halogen atoms must be introduced into the N-acetyl diazocine structure during the synthesis of the building blocks. In the present work we start from mono- and dihalogenated N-acetyl diazocine 2–4 (Figure 2) and focus on the further derivatization via cross-coupling reactions and the synthesis of a new dihalogenated N-acetyl diazocine 4 (Figure 2).
Figure 2: The halogen-substituted N-acetyl diazocines 2–4 were used as the starting compounds for further derivatization via Pd-catalyzed cross-coupling reactions. Solutions of the Z isomers are yellow. The E isomers are red.
Figure 2: The halogen-substituted N-acetyl diazocines 2–4 were used as the starting compounds for further der...
Results and Discussion
The monosubstituted N-acetyl diazocines 2 and 3 were synthesized according to the procedure published by our group recently [22]. The synthesis of disubstituted compound 4 followed the same procedure except the preparation of the aminoaniline building block tert-butyl (2-amino-5-bromophenyl)carbamate (5), which was prepared by Boc-protection of the 5-bromo-2-nitroaniline (6) and subsequent reduction of the nitro group (see Supporting Information File 1, section II.1).
Cross-coupling reactions
Stille cross-coupling reactions were performed by an organic halide reacting with an organotin compound. A great advantage of the used organostannanes is the easy accessibility, and their high air and moisture stability, so that usually a wide range of functional groups can be introduced under mild conditions [23]. Nevertheless, the arylation of monohalogenated N-acetyl diazocines via Stille coupling in our case gave unsatisfying results (Table 2). Reactions with tetrakis(triphenylphosphine)palladium(0) as catalyst resulted in no product 7 formation. Bis(tri-tert-butylphosphine)palladium(0) as catalyst gave rise to the product in very low yields independent from the used halogenated diazocine. In contrast to other cross coupling reactions described in this work, most of the starting material decomposed during the reaction and could not be re-isolated.
Table 2: Reaction conditions of the arylation of halogenated N-acetyl diazocines via Stille coupling reaction. Equivalents are normalized to the used amount of N-acetyl diazocine starting material.
|
|
|||
| cat. system | organotin compound | conditions | yield |
| Pd(OAc)2 (0.1 equiv) + PPh3 (0.8 equiv) | Ph3SnCl (1 equiv) | dry DMF, N2, 100 °C, 16 h | – |
| Pd(t-Bu3P)2 (0.1 equiv) | Ph3SnCl (1 equiv) | dry THF, N2, 65 °C, 16 h |
X = Br, 10%
X = I, 10% |
In contrast, the vinylation of diazocines 2 and 3 provides good yields of 65% and 71%, respectively, for the vinyl N-acetyl diazocine 8 (Table 3). An alternative way of vinylating N-acetyl diazocines is the Pd-catalyzed vinylation with polyvinylsiloxanes and TBAF as activating agent following the method by Denmark et al. giving rise to even higher yields of 74% for bromine 2 and 78% for iodo starting material 3 (Table 3) [24].
Table 3: Vinylation of halogenated N-acetyl diazocines via Pd-catalyzed coupling reactions. Equivalents are normalized to the used amount of N-acetyl diazocine starting material.
|
|
|||
| cat. system | reaction partner or additive | conditions | yield |
| Pd(OAc)2 (0.1 equiv) + PPh3 (0.8 equiv) | tributylvinyltin (1 equiv) | dry DMF, N2, 100 °C, 16 h |
X = Br, 65%
X = I, 71% |
| PdBr2 (0.1 equiv) + JohnPhos (0.1 equiv) |
D4Va (0.66 equiv)
TBAF (4 equiv) |
dry THF, N2, 50° C, 16 h |
X = Br, 74%
X = I, 78% |
aD4V: 1,3,5,7-tetramethyl-1,3,5,7-tetravinylcyclotetrasiloxane.
To overcome the problems of poor yields in the arylation of N-acetyl diazocines via Stille coupling we used Suzuki–Miyaura reactions of the diazocines 2 and 3 with different arylboronic acids [25,26]. There are several examples of last-step modifications of azobenzenes via Suzuki–Miyaura reactions in the current literature, which indicate that the reaction conditions are compatible with azo groups [27,28]. Suzuki–Miyaura reaction of 2 and 3 with different phenylboronic acids resulted in the formation of the corresponding arylated N-acetyl diazocines 7, and 9–13 in yields from 68 to 88% (Table 4). The yields increased slightly if boronic acids with electron-withdrawing groups were used. An influence of bulky substituents like carboxyl groups in ortho-position of the phenylboronic acids on the reaction was not observed. The synthesis of N-acetyl diazocines connected to heteroaromatic aromatic systems 14–16 was less successful. The pyridine-substituted N-acetyl diazocine 14 was formed in yields of 7% or 19% while furan- 15 and thiophene-substituted N-acetyl diazocine 16 could not be obtained. The reaction with benzylboronic acid gave the corresponding N-acetyl diazocine 17 (45%, Table 4). Interestingly, this reaction only took place if brominated N-acetyl diazocine 2 was used as starting material although iodoaryl compounds are in general more reactive [25]. The reaction of halogenated N-acetyl diazocines 2 and 3 with bis(pinacolato)diboron did not lead to the formation of the pinacolborane-substituted N-acetyl diazocine 18. Accordingly, the Suzuki–Miyaura reaction with inversed roles between N-acetyl diazocine boronic acid pinacol ester and aryl or alkyl halides could not be investigated.
Table 4: Derivatization of halogen-substituted N-acetyl diazocines via Suzuki–Miyaura reaction. Equivalents are normalized to the used amount of N-acetyl diazocine starting material.
|
|
||
| boronic acid | product | yield |
|
|
7 |
X = Br, 74%
X = I, 83% |
|
|
9 |
X = Br, 68%
X = I, 74% |
|
|
10 |
X = I, 82% |
|
|
11 |
X = Br, 81%
X = I, 88% |
|
|
12 |
X = Br, 70%
X = I, 77% |
|
|
13 |
X = Br, 76%a
X = I, 79%a |
|
|
14 |
X = Br, 7%
X = I, 19% |
|
|
15 |
X = Br, –
X = I, – |
|
|
16 |
X = Br, –
X = I, – |
|
|
17 |
X = Br, 45%
X = I, – |
|
|
18 |
X = Br, –
X = I, – |
aThe reaction was carried out in dry DMF at 100 °C because no reaction took place if the Suzuki–Miyaura standard procedure was applied.
The Buchwald–Hartwig amination is a versatile and powerful tool for C–N bond formation and widely applied in the synthesis of new pharmaceutical substances [29-31]. Furthermore, azobenzenes [32,33], as well as diazocines [34,35], have been derivatized via Buchwald–Hartwig amination. The Buchwald–Hartwig amination of halogenated N-acetyl diazocines according to the procedure of Maier et al. [34] with tert-butyl carbamate resulted in the formation of Boc-protected amino-substituted N-acetyl diazocine 19 in a yield of 72%. However, the reaction only took place if iodo N-acetyl diazocine 3 was used as starting material. Using diphenylamine as a more electron-rich amine resulted in the formation of diphenylamino-substituted N-acetyl diazocine 20 in a significantly lower yield of 25% starting from the bromide 2 and 47% starting from the iodo precursor 3 (Table 5).
Deprotection of carbamate 19 with trifluoroacetic acid provided the corresponding amino-substituted N-acetyl diazocine 21 (Scheme 1).
Scheme 1: Synthesis of amino-N-acetyl diazocine by deprotection of the carbamate.
Scheme 1: Synthesis of amino-N-acetyl diazocine by deprotection of the carbamate.
Another option for carbon–heteroatom bond formation reactions are copper-catalyzed Ullmann-type reactions, which have already been applied to the parent diazocine [36,37]. The attempted synthesis of azide-functionalized N-acetyl diazocine 22 under the conditions described by Hugenbusch et al. [37] showed no product formation and only starting material was isolated (Scheme 2).
Scheme 2: Reaction conditions for the attempted Ullmann-type reaction with sodium azide.
Scheme 2: Reaction conditions for the attempted Ullmann-type reaction with sodium azide.
The palladium-catalyzed introduction of cyano groups under mild conditions in analogy to Iqbal et al. [38] gave the cyano-substituted N-acetyl diazocine 23 in yields of 61% from bromide 2 and 81% from iodide 3 (Scheme 3). Nitriles are a good starting point for further functional group interconversions [39].
Scheme 3: Reaction conditions for the palladium-catalyzed introduction of a nitrile functionality.
Scheme 3: Reaction conditions for the palladium-catalyzed introduction of a nitrile functionality.
Photochemical characterization
With these new N-acetyl diazocine derivatives at hand we turned towards the photochemical characterization, in particular to gain insight into the effects of different substituents on UV spectra and switching behavior. For the determination of the n–π*-absorption maxima of the E and Z isomers 250 µM solutions of each compound in acetonitrile were prepared and measured at 25 °C. All compounds (4, 7–14, 17, 19–21, 23) exhibit an n–π*-transition at approximately 400 nm, matching the n–π*-transition of unsubstituted N-acetyl diazocine 1 (Table 6). Irradiation with light of 405 nm gives the metastable E isomers with photoconversion yields of 76–85% due to a very good separation of the n–π*-transitions. N-Acetyl diazocine derivatives containing electron-deficient groups (like 4, 10, 12, 14 and 23) show slightly but not significantly higher Z→E conversion rates than the other coupling products presented in this work. The nitrogen-substituted derivatives 19–21 show significantly lower conversion rates of 41–61%. This behavior has already been observed in other amino-substituted diazocines as well and is probably caused by the overlap of n–π*-transitions of the E and Z isomers and the electron-rich azo group [40]. An almost complete E→Z conversion (>99%) can be achieved by irradiation with light between 520 and 600 nm for all synthesized compounds.
Table 6: Photophysical properties of N-acetyl diazocines 1-4, 7–14, 17, 19–21, and 23 in acetonitrile.
| λmax (Z) | λmax (E) | t1/2 (25 °C) | k (25 °C) | ΓZ→E (405 nm)a | ΓE→Z (530 nm) | |
| nm | nm | min | 10−4 s−1 | |||
| 1 [20] | 397 | 513 | 29.5 | 3.916 | 88% | >99% |
| 2 [20] | 397 | 515 | 30.9 | 3.744 | 81% | >99% |
| 3 [20] | 397 | 517 | 28.6 | 4.036 | 82% | >99% |
| 4 | 396 | 519 | 16.9 ± 0.20 | 6.820 ± 0.096 | 85% | >99% |
| 7 | 399 | 516 | 29.3 ± 0.11 | 3.944 ± 0.017 | 79% | >99% |
| 8 | 398 | 515 | 29.6 ± 0.16 | 3.897 ± 0.025 | 76% | >99% |
| 9 | 394 | 515 | 34.2 ± 0.28 | 3.374 ± 0.032 | 77% | >99% |
| 10 | 396 | 516 | 34.2 ± 0.28 | 3.382 ± 0.033 | 82% | >99% |
| 11 | 397 | 515 | 33.8 ± 0.24 | 3.418 ± 0.029 | 78% | >99% |
| 12 | 396 | 517 | 29.8 ± 0.09 | 3.877 ± 0.014 | 80% | >99% |
| 13 | 399 | 517 | 31.2 ± 0.18 | 3.688 ± 0.024 | 77% | >99% |
| 14b | 396 | 515 | 39.9 ± 0.44 | 2.897 ± 0.038 | 79% | >99% |
| 17 | 398 | 515 | 29.3 ± 0.05 | 3.944 ± 0.008 | 82% | >99% |
| 19 | 397 | 514 | 30.6 ± 0.05 | 3.779 ± 0.008 | 61% | >99% |
| 20 | 389 | 510 | 31.5 ± 0.05 | 3.670 ± 0.008 | 49% | >99% |
| 21 | 396 | 511 | 38.9 ± 0.49 | 2.973 ± 0.043 | 41% | >99% |
| 23 | 396 | 518 | 39.8 ± 0.41 | 2.906 ± 0.035 | 83% | >99% |
aExtrapolated values (for details, see Supporting Information File 1, section IV) in deuterated acetonitrile 5 mM. bUV spectra measured with a concentration of 128 µM in acetonitrile and NMR spectra with a concentration of 2.55 mM.
Thermal half-lives (t1/2) were determined by monitoring the thermal relaxation of the synthesized diazocines at 25 °C in a UV spectrometer (see Supporting Information File 1, section III). The dihalogenated N-acetyl diazocine 4 shows a significantly reduced half-life compared to the mono-halogenated N-acetyl diazocines 2 and 3 and the unsubstituted N-acetyl diazocine 1. The substitution with a phenyl group does not exert a significant influence on the thermal half-life. The half-lives of compounds 7 and 17 are nearly identical compared to the parent N-acetyl diazocine 1. If electron-withdrawing substituents are added in ortho-position of the additional phenyl ring (12 and 13) the half-life is not affected significantly as well. An increase of about 10% of the half-lives has been observed for the weak +M substituents bromine (10) and fluorine (11) or methyl groups (9) in ortho- and para-positions. The increase is even stronger if electron-withdrawing pyridine- (14), cyano- (23) or the strong +M amino substituents (21) are attached to the N-acetyl diazocine in meta-position. In contrast to the extended half-life of the unsubstituted amine 21 the Boc-protected 19 and the diphenyl-substituted amine 20 show half-lives not significantly longer than the parent N-acetyl diazocine 1.
Given the water solubility and the excellent switching behavior of parent 1 in aqueous media [3,13,20], the photochemical properties of water-soluble substituted N-acetyl diazocines 13 and 21 were also investigated in aqueous solution (13 and 21 in aqueous PBS buffer solution at pH 7.4 250 µM, 21 at pH 3.5 250 µM, 13 at pH 9 250 µM). Benzoic acid derivative 13 was representatively chosen for polar aromatic substitution and the amino derivative 21 for non-aromatic substitution. Non- or less polar aromatic substituents were not characterized in detail since their solubility in pure water is decreased by the additional phenyl substituent. The pH values were chosen to make sure that the amine 21 is completely protonated and the carboxylic acid 13 is completely deprotonated. UV measurements revealed that the absorption maxima of the n–π*-transitions of the Z isomers of 13 and 21 (392–398 nm) are almost independent of solvent and pH, while the n–π*-transitions of the E isomers at ≈515 nm are significantly shifted to shorter wavelengths (Δλmax = 10–20 nm) in water (Table 7). At the same time the n–π*-transitions of the E isomers, which strongly overlap with the π–π*-transition in organic solvents are shifted to higher wavelengths (see Figures SIII.15–SIII.20 and SIII.29–SIII.36, Supporting Information File 1). For diazocine 13 this leads to a lower PSS of 53% E configuration in water at pH 7.4 and 48% at pH 9 while showing a PSS of 77% in acetonitrile for the Z to E photoisomerization. This is due to a higher overlap of the n–π*-transitions in both isomers in aqueous media. For the amino-substituted diazocine 21 the PSS (ΓZ→E) shows a slightly lower value of 37% E in water at pH 7.4 compared to 41% in acetonitrile, while in acidic aqueous media a PSS of 62% E for the Z→E photoisomerization was observed. This is due to the complete protonation of the amino group converting it to an electron-deficient substituent. The thermal half-lives of 13 and 21 increase by a factor of 2.5 and 4.2 when changing the solvent from acetonitrile to water at pH 7.4, which is consistent with the current literature for thermal half-lives of substituted parent diazocines in aqueous media [18,39].
Table 7: Photophysical properties of N-acetyl diazocines 1, 13, and 21 in water at various pH values.
| λmax (Z) | λmax (E) | t1/2 , 25 °C | k, 25 °C | ΓZ→E (405 nm)a | ΓE→Z (530 nm) | |
| nm | nm | min | 10−4 s−1 | |||
| 1 [20] | 393 | 502 | 72.8 | 1.587 | 72% | >99% |
| 13 at pH 9 | 392 | 502 | 106.4 ± 1.34 | 1.086 ± 0.016 | 48% | >99% |
| 13 at pH 7.4 | 392 | 505 | 78.2 ± 0.33 | 1.477 ± 0.007 | 53% | >99% |
| 21 at pH 3.5 | 392 | 495 | 118.0 ± 1.72 | 0.975 ± 0.017 | 62% | >99% |
| 21 at pH 7.4 | 392 | 489 | 162.5 ± 1.79 | 0.711 ± 0.009 | 37% | >99% |
aExtrapolated values (for details, see Supporting Information File 1, section IV) in deuterated water 5 mM.
Conclusion
Fourteen mono-meta-substituted (7–14, 17, 19–21, 23) and one di-meta-substituted (4) N-acetyl diazocines have been synthesized and characterized. The synthesis has been performed from halogenated precursors and cross-coupling reactions for further functionalization. The reaction conditions of various cross-coupling reactions have been correspondingly adjusted. The arylation of the N-acetyl diazocine system could be achieved via Suzuki coupling reactions in high yields (7, 9–14, 17) as well as the vinylation via Stille coupling (8). These compounds also exhibit excellent switching properties. Electron-withdrawing substituents at the aryl substituents have no significant influence on the switching behavior while weak +M substituents like bromine and fluorine as well as electron-poor heteroaromatic systems lead to increased thermal half-lives. An amino-substituted derivative 21 was obtained via Buchwald–Hartwig coupling with Boc-carbamate and subsequent deprotection. Amino-substituted N-acetyl diazocines 19–21 exhibit photostationary states (PSS) lower in their metastable configurations in analogy to amino-substituted azobenzenes and previously synthesized diazocines. We also investigated the switching properties of the water-soluble derivatives 13 and 21 in water at different pH values. The half-lives of the metastable E isomers are significantly longer in water than in less polar solvents like acetonitrile. For carboxylic acid-substituted 13, the Z→E conversion upon irradiation with 405 nm drops from 77% to 53% upon changing the solvent from acetonitrile to water (pH 7.4). The reverse effect was observed with amino-substituted 21.
The photophysical properties of photoswitchable drugs in photopharmacology are usually determined in organic solvents. Their natural environment, however, is the aqueous phase. There is a risk of overestimating the performance of photochromic drugs because photoconversion to the active state usually drops considerably in water, and also half-lives are different. Light-activatable drugs based on N-acetyl diazocines are more hydrophilic than those derived from the parent system diazocine and corresponding azobenzenes. They retain their switching properties even in an aqueous environment and are therefore promising switches in photopharmacological applications.
Supporting Information
CCDC-2329263 (1), CCDC-2329261 (2), and CCDC-2329262 (7) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
| Supporting Information File 1: Synthetic procedures, UV–vis and NMR switching experiments, copies of UV–vis and NMR spectra, and X-ray crystallographic data. | ||
| Format: PDF | Size: 10.7 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Siewertsen, R.; Neumann, H.; Buchheim-Stehn, B.; Herges, R.; Näther, C.; Renth, F.; Temps, F. J. Am. Chem. Soc. 2009, 131, 15594–15595. doi:10.1021/ja906547d
Return to citation in text: [1] [2] [3] -
Hammerich, M.; Schütt, C.; Stähler, C.; Lentes, P.; Röhricht, F.; Höppner, R.; Herges, R. J. Am. Chem. Soc. 2016, 138, 13111–13114. doi:10.1021/jacs.6b05846
Return to citation in text: [1] -
Lentes, P.; Stadler, E.; Röhricht, F.; Brahms, A.; Gröbner, J.; Sönnichsen, F. D.; Gescheidt, G.; Herges, R. J. Am. Chem. Soc. 2019, 141, 13592–13600. doi:10.1021/jacs.9b06104
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] -
Siewertsen, R.; Schönborn, J. B.; Hartke, B.; Renth, F.; Temps, F. Phys. Chem. Chem. Phys. 2011, 13, 1054–1063. doi:10.1039/c0cp01148g
Return to citation in text: [1] -
Beharry, A. A.; Woolley, G. A. Chem. Soc. Rev. 2011, 40, 4422–4437. doi:10.1039/c1cs15023e
Return to citation in text: [1] -
Hüll, K.; Morstein, J.; Trauner, D. Chem. Rev. 2018, 118, 10710–10747. doi:10.1021/acs.chemrev.8b00037
Return to citation in text: [1] [2] [3] -
Szymański, W.; Beierle, J. M.; Kistemaker, H. A. V.; Velema, W. A.; Feringa, B. L. Chem. Rev. 2013, 113, 6114–6178. doi:10.1021/cr300179f
Return to citation in text: [1] [2] -
Preußke, N.; Moormann, W.; Bamberg, K.; Lipfert, M.; Herges, R.; Sönnichsen, F. D. Org. Biomol. Chem. 2020, 18, 2650–2660. doi:10.1039/c9ob02442e
Return to citation in text: [1] [2] -
Albert, L.; Peñalver, A.; Djokovic, N.; Werel, L.; Hoffarth, M.; Ruzic, D.; Xu, J.; Essen, L.-O.; Nikolic, K.; Dou, Y.; Vázquez, O. ChemBioChem 2019, 20, 1417–1429. doi:10.1002/cbic.201800737
Return to citation in text: [1] [2] -
Cabré, G.; Garrido-Charles, A.; González-Lafont, À.; Moormann, W.; Langbehn, D.; Egea, D.; Lluch, J. M.; Herges, R.; Alibés, R.; Busqué, F.; Gorostiza, P.; Hernando, J. Org. Lett. 2019, 21, 3780–3784. doi:10.1021/acs.orglett.9b01222
Return to citation in text: [1] [2] [3] [4] -
Thapaliya, E. R.; Zhao, J.; Ellis-Davies, G. C. R. ACS Chem. Neurosci. 2019, 10, 2481–2488. doi:10.1021/acschemneuro.8b00734
Return to citation in text: [1] [2] -
Trads, J. B.; Hüll, K.; Matsuura, B. S.; Laprell, L.; Fehrentz, T.; Görldt, N.; Kozek, K. A.; Weaver, C. D.; Klöcker, N.; Barber, D. M.; Trauner, D. Angew. Chem., Int. Ed. 2019, 58, 15421–15428. doi:10.1002/anie.201905790
Return to citation in text: [1] [2] -
Lentes, P.; Frühwirt, P.; Freißmuth, H.; Moormann, W.; Kruse, F.; Gescheidt, G.; Herges, R. J. Org. Chem. 2021, 86, 4355–4360. doi:10.1021/acs.joc.1c00065
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] -
Volarić, J.; Szymanski, W.; Simeth, N. A.; Feringa, B. L. Chem. Soc. Rev. 2021, 50, 12377–12449. doi:10.1039/d0cs00547a
Return to citation in text: [1] -
Moormann, W.; Tellkamp, T.; Stadler, E.; Röhricht, F.; Näther, C.; Puttreddy, R.; Rissanen, K.; Gescheidt, G.; Herges, R. Angew. Chem., Int. Ed. 2020, 59, 15081–15086. doi:10.1002/anie.202005361
Return to citation in text: [1] -
Fink, B. E.; Gavai, A. V.; Tokarski, J. S.; Goyal, B.; Misra, R.; Xiao, H.-Y.; Kimball, S. D.; Han, W.-C.; Norris, D.; Spires, T. E.; You, D.; Gottardis, M. M.; Lorenzi, M. V.; Vite, G. D. Bioorg. Med. Chem. Lett. 2006, 16, 1532–1536. doi:10.1016/j.bmcl.2005.12.039
Return to citation in text: [1] -
Wages, F.; Lentes, P.; Griebenow, T.; Herges, R.; Peifer, C.; Maser, E. Chem.-Biol. Interact. 2022, 354, 109822. doi:10.1016/j.cbi.2022.109822
Return to citation in text: [1] [2] [3] -
Ewert, J.; Heintze, L.; Jordà-Redondo, M.; von Glasenapp, J.-S.; Nonell, S.; Bucher, G.; Peifer, C.; Herges, R. J. Am. Chem. Soc. 2022, 144, 15059–15071. doi:10.1021/jacs.2c03649
Return to citation in text: [1] [2] [3] -
Schoenberger, M.; Damijonaitis, A.; Zhang, Z.; Nagel, D.; Trauner, D. ACS Chem. Neurosci. 2014, 5, 514–518. doi:10.1021/cn500070w
Return to citation in text: [1] -
Gorostiza, P.; Isacoff, E. Y. Science 2008, 322, 395–399. doi:10.1126/science.1166022
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Velema, W. A.; Szymanski, W.; Feringa, B. L. J. Am. Chem. Soc. 2014, 136, 2178–2191. doi:10.1021/ja413063e
Return to citation in text: [1] [2] -
Lentes, P.; Rudtke, J.; Griebenow, T.; Herges, R. Beilstein J. Org. Chem. 2021, 17, 1503–1508. doi:10.3762/bjoc.17.107
Return to citation in text: [1] [2] -
Stille, J. K. Angew. Chem., Int. Ed. Engl. 1986, 25, 508–524. doi:10.1002/anie.198605081
Return to citation in text: [1] -
Denmark, S. E.; Butler, C. R. Org. Lett. 2006, 8, 63–66. doi:10.1021/ol052517r
Return to citation in text: [1] -
Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457–2483. doi:10.1021/cr00039a007
Return to citation in text: [1] [2] -
Suzuki, A. J. Organomet. Chem. 1999, 576, 147–168. doi:10.1016/s0022-328x(98)01055-9
Return to citation in text: [1] -
Walther, M.; Kipke, W.; Schultzke, S.; Ghosh, S.; Staubitz, A. Synthesis 2021, 53, 1213–1228. doi:10.1055/s-0040-1705999
Return to citation in text: [1] -
Walther, M.; Kipke, W.; Renken, R.; Staubitz, A. RSC Adv. 2023, 13, 15805–15809. doi:10.1039/d3ra02988c
Return to citation in text: [1] -
Guram, A. S.; Rennels, R. A.; Buchwald, S. L. Angew. Chem., Int. Ed. Engl. 1995, 34, 1348–1350. doi:10.1002/anie.199513481
Return to citation in text: [1] -
Louie, J.; Hartwig, J. F. Tetrahedron Lett. 1995, 36, 3609–3612. doi:10.1016/0040-4039(95)00605-c
Return to citation in text: [1] -
Brown, D. G.; Boström, J. J. Med. Chem. 2016, 59, 4443–4458. doi:10.1021/acs.jmedchem.5b01409
Return to citation in text: [1] -
Kanbara, T.; Oshima, M.; Imayasu, T.; Hasegawa, K. Macromolecules 1998, 31, 8725–8730. doi:10.1021/ma981085f
Return to citation in text: [1] -
Heindl, A. H.; Wegner, H. A. Beilstein J. Org. Chem. 2020, 16, 22–31. doi:10.3762/bjoc.16.4
Return to citation in text: [1] -
Maier, M. S.; Hüll, K.; Reynders, M.; Matsuura, B. S.; Leippe, P.; Ko, T.; Schäffer, L.; Trauner, D. J. Am. Chem. Soc. 2019, 141, 17295–17304. doi:10.1021/jacs.9b08794
Return to citation in text: [1] [2] -
Zhu, Q.; Wang, S.; Chen, P. Org. Lett. 2019, 21, 4025–4029. doi:10.1021/acs.orglett.9b01215
Return to citation in text: [1] -
Sambiagio, C.; Marsden, S. P.; Blacker, A. J.; McGowan, P. C. Chem. Soc. Rev. 2014, 43, 3525–3550. doi:10.1039/c3cs60289c
Return to citation in text: [1] -
Hugenbusch, D.; Lehr, M.; von Glasenapp, J.-S.; McConnell, A. J.; Herges, R. Angew. Chem., Int. Ed. 2023, 62, e202212571. doi:10.1002/anie.202212571
Return to citation in text: [1] [2] -
Iqbal, Z.; Lyubimtsev, A.; Hanack, M. Synlett 2008, 2287–2290. doi:10.1055/s-2008-1078269
Return to citation in text: [1] -
Xia, Y.; Jiang, H.; Wu, W. Eur. J. Org. Chem. 2021, 6658–6669. doi:10.1002/ejoc.202101196
Return to citation in text: [1] [2] -
Sell, H.; Näther, C.; Herges, R. Beilstein J. Org. Chem. 2013, 9, 1–7. doi:10.3762/bjoc.9.1
Return to citation in text: [1]
| 22. | Lentes, P.; Rudtke, J.; Griebenow, T.; Herges, R. Beilstein J. Org. Chem. 2021, 17, 1503–1508. doi:10.3762/bjoc.17.107 |
| 23. | Stille, J. K. Angew. Chem., Int. Ed. Engl. 1986, 25, 508–524. doi:10.1002/anie.198605081 |
| 24. | Denmark, S. E.; Butler, C. R. Org. Lett. 2006, 8, 63–66. doi:10.1021/ol052517r |
| 1. | Siewertsen, R.; Neumann, H.; Buchheim-Stehn, B.; Herges, R.; Näther, C.; Renth, F.; Temps, F. J. Am. Chem. Soc. 2009, 131, 15594–15595. doi:10.1021/ja906547d |
| 8. | Preußke, N.; Moormann, W.; Bamberg, K.; Lipfert, M.; Herges, R.; Sönnichsen, F. D. Org. Biomol. Chem. 2020, 18, 2650–2660. doi:10.1039/c9ob02442e |
| 3. | Lentes, P.; Stadler, E.; Röhricht, F.; Brahms, A.; Gröbner, J.; Sönnichsen, F. D.; Gescheidt, G.; Herges, R. J. Am. Chem. Soc. 2019, 141, 13592–13600. doi:10.1021/jacs.9b06104 |
| 34. | Maier, M. S.; Hüll, K.; Reynders, M.; Matsuura, B. S.; Leippe, P.; Ko, T.; Schäffer, L.; Trauner, D. J. Am. Chem. Soc. 2019, 141, 17295–17304. doi:10.1021/jacs.9b08794 |
| 1. | Siewertsen, R.; Neumann, H.; Buchheim-Stehn, B.; Herges, R.; Näther, C.; Renth, F.; Temps, F. J. Am. Chem. Soc. 2009, 131, 15594–15595. doi:10.1021/ja906547d |
| 6. | Hüll, K.; Morstein, J.; Trauner, D. Chem. Rev. 2018, 118, 10710–10747. doi:10.1021/acs.chemrev.8b00037 |
| 7. | Szymański, W.; Beierle, J. M.; Kistemaker, H. A. V.; Velema, W. A.; Feringa, B. L. Chem. Rev. 2013, 113, 6114–6178. doi:10.1021/cr300179f |
| 13. | Lentes, P.; Frühwirt, P.; Freißmuth, H.; Moormann, W.; Kruse, F.; Gescheidt, G.; Herges, R. J. Org. Chem. 2021, 86, 4355–4360. doi:10.1021/acs.joc.1c00065 |
| 36. | Sambiagio, C.; Marsden, S. P.; Blacker, A. J.; McGowan, P. C. Chem. Soc. Rev. 2014, 43, 3525–3550. doi:10.1039/c3cs60289c |
| 37. | Hugenbusch, D.; Lehr, M.; von Glasenapp, J.-S.; McConnell, A. J.; Herges, R. Angew. Chem., Int. Ed. 2023, 62, e202212571. doi:10.1002/anie.202212571 |
| 5. | Beharry, A. A.; Woolley, G. A. Chem. Soc. Rev. 2011, 40, 4422–4437. doi:10.1039/c1cs15023e |
| 6. | Hüll, K.; Morstein, J.; Trauner, D. Chem. Rev. 2018, 118, 10710–10747. doi:10.1021/acs.chemrev.8b00037 |
| 7. | Szymański, W.; Beierle, J. M.; Kistemaker, H. A. V.; Velema, W. A.; Feringa, B. L. Chem. Rev. 2013, 113, 6114–6178. doi:10.1021/cr300179f |
| 3. | Lentes, P.; Stadler, E.; Röhricht, F.; Brahms, A.; Gröbner, J.; Sönnichsen, F. D.; Gescheidt, G.; Herges, R. J. Am. Chem. Soc. 2019, 141, 13592–13600. doi:10.1021/jacs.9b06104 |
| 13. | Lentes, P.; Frühwirt, P.; Freißmuth, H.; Moormann, W.; Kruse, F.; Gescheidt, G.; Herges, R. J. Org. Chem. 2021, 86, 4355–4360. doi:10.1021/acs.joc.1c00065 |
| 15. | Moormann, W.; Tellkamp, T.; Stadler, E.; Röhricht, F.; Näther, C.; Puttreddy, R.; Rissanen, K.; Gescheidt, G.; Herges, R. Angew. Chem., Int. Ed. 2020, 59, 15081–15086. doi:10.1002/anie.202005361 |
| 32. | Kanbara, T.; Oshima, M.; Imayasu, T.; Hasegawa, K. Macromolecules 1998, 31, 8725–8730. doi:10.1021/ma981085f |
| 33. | Heindl, A. H.; Wegner, H. A. Beilstein J. Org. Chem. 2020, 16, 22–31. doi:10.3762/bjoc.16.4 |
| 1. | Siewertsen, R.; Neumann, H.; Buchheim-Stehn, B.; Herges, R.; Näther, C.; Renth, F.; Temps, F. J. Am. Chem. Soc. 2009, 131, 15594–15595. doi:10.1021/ja906547d |
| 2. | Hammerich, M.; Schütt, C.; Stähler, C.; Lentes, P.; Röhricht, F.; Höppner, R.; Herges, R. J. Am. Chem. Soc. 2016, 138, 13111–13114. doi:10.1021/jacs.6b05846 |
| 3. | Lentes, P.; Stadler, E.; Röhricht, F.; Brahms, A.; Gröbner, J.; Sönnichsen, F. D.; Gescheidt, G.; Herges, R. J. Am. Chem. Soc. 2019, 141, 13592–13600. doi:10.1021/jacs.9b06104 |
| 4. | Siewertsen, R.; Schönborn, J. B.; Hartke, B.; Renth, F.; Temps, F. Phys. Chem. Chem. Phys. 2011, 13, 1054–1063. doi:10.1039/c0cp01148g |
| 3. | Lentes, P.; Stadler, E.; Röhricht, F.; Brahms, A.; Gröbner, J.; Sönnichsen, F. D.; Gescheidt, G.; Herges, R. J. Am. Chem. Soc. 2019, 141, 13592–13600. doi:10.1021/jacs.9b06104 |
| 13. | Lentes, P.; Frühwirt, P.; Freißmuth, H.; Moormann, W.; Kruse, F.; Gescheidt, G.; Herges, R. J. Org. Chem. 2021, 86, 4355–4360. doi:10.1021/acs.joc.1c00065 |
| 34. | Maier, M. S.; Hüll, K.; Reynders, M.; Matsuura, B. S.; Leippe, P.; Ko, T.; Schäffer, L.; Trauner, D. J. Am. Chem. Soc. 2019, 141, 17295–17304. doi:10.1021/jacs.9b08794 |
| 35. | Zhu, Q.; Wang, S.; Chen, P. Org. Lett. 2019, 21, 4025–4029. doi:10.1021/acs.orglett.9b01215 |
| 8. | Preußke, N.; Moormann, W.; Bamberg, K.; Lipfert, M.; Herges, R.; Sönnichsen, F. D. Org. Biomol. Chem. 2020, 18, 2650–2660. doi:10.1039/c9ob02442e |
| 9. | Albert, L.; Peñalver, A.; Djokovic, N.; Werel, L.; Hoffarth, M.; Ruzic, D.; Xu, J.; Essen, L.-O.; Nikolic, K.; Dou, Y.; Vázquez, O. ChemBioChem 2019, 20, 1417–1429. doi:10.1002/cbic.201800737 |
| 10. | Cabré, G.; Garrido-Charles, A.; González-Lafont, À.; Moormann, W.; Langbehn, D.; Egea, D.; Lluch, J. M.; Herges, R.; Alibés, R.; Busqué, F.; Gorostiza, P.; Hernando, J. Org. Lett. 2019, 21, 3780–3784. doi:10.1021/acs.orglett.9b01222 |
| 11. | Thapaliya, E. R.; Zhao, J.; Ellis-Davies, G. C. R. ACS Chem. Neurosci. 2019, 10, 2481–2488. doi:10.1021/acschemneuro.8b00734 |
| 12. | Trads, J. B.; Hüll, K.; Matsuura, B. S.; Laprell, L.; Fehrentz, T.; Görldt, N.; Kozek, K. A.; Weaver, C. D.; Klöcker, N.; Barber, D. M.; Trauner, D. Angew. Chem., Int. Ed. 2019, 58, 15421–15428. doi:10.1002/anie.201905790 |
| 3. | Lentes, P.; Stadler, E.; Röhricht, F.; Brahms, A.; Gröbner, J.; Sönnichsen, F. D.; Gescheidt, G.; Herges, R. J. Am. Chem. Soc. 2019, 141, 13592–13600. doi:10.1021/jacs.9b06104 |
| 25. | Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457–2483. doi:10.1021/cr00039a007 |
| 12. | Trads, J. B.; Hüll, K.; Matsuura, B. S.; Laprell, L.; Fehrentz, T.; Görldt, N.; Kozek, K. A.; Weaver, C. D.; Klöcker, N.; Barber, D. M.; Trauner, D. Angew. Chem., Int. Ed. 2019, 58, 15421–15428. doi:10.1002/anie.201905790 |
| 13. | Lentes, P.; Frühwirt, P.; Freißmuth, H.; Moormann, W.; Kruse, F.; Gescheidt, G.; Herges, R. J. Org. Chem. 2021, 86, 4355–4360. doi:10.1021/acs.joc.1c00065 |
| 14. | Volarić, J.; Szymanski, W.; Simeth, N. A.; Feringa, B. L. Chem. Soc. Rev. 2021, 50, 12377–12449. doi:10.1039/d0cs00547a |
| 29. | Guram, A. S.; Rennels, R. A.; Buchwald, S. L. Angew. Chem., Int. Ed. Engl. 1995, 34, 1348–1350. doi:10.1002/anie.199513481 |
| 30. | Louie, J.; Hartwig, J. F. Tetrahedron Lett. 1995, 36, 3609–3612. doi:10.1016/0040-4039(95)00605-c |
| 31. | Brown, D. G.; Boström, J. J. Med. Chem. 2016, 59, 4443–4458. doi:10.1021/acs.jmedchem.5b01409 |
| 10. | Cabré, G.; Garrido-Charles, A.; González-Lafont, À.; Moormann, W.; Langbehn, D.; Egea, D.; Lluch, J. M.; Herges, R.; Alibés, R.; Busqué, F.; Gorostiza, P.; Hernando, J. Org. Lett. 2019, 21, 3780–3784. doi:10.1021/acs.orglett.9b01222 |
| 11. | Thapaliya, E. R.; Zhao, J.; Ellis-Davies, G. C. R. ACS Chem. Neurosci. 2019, 10, 2481–2488. doi:10.1021/acschemneuro.8b00734 |
| 25. | Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457–2483. doi:10.1021/cr00039a007 |
| 26. | Suzuki, A. J. Organomet. Chem. 1999, 576, 147–168. doi:10.1016/s0022-328x(98)01055-9 |
| 9. | Albert, L.; Peñalver, A.; Djokovic, N.; Werel, L.; Hoffarth, M.; Ruzic, D.; Xu, J.; Essen, L.-O.; Nikolic, K.; Dou, Y.; Vázquez, O. ChemBioChem 2019, 20, 1417–1429. doi:10.1002/cbic.201800737 |
| 13. | Lentes, P.; Frühwirt, P.; Freißmuth, H.; Moormann, W.; Kruse, F.; Gescheidt, G.; Herges, R. J. Org. Chem. 2021, 86, 4355–4360. doi:10.1021/acs.joc.1c00065 |
| 27. | Walther, M.; Kipke, W.; Schultzke, S.; Ghosh, S.; Staubitz, A. Synthesis 2021, 53, 1213–1228. doi:10.1055/s-0040-1705999 |
| 28. | Walther, M.; Kipke, W.; Renken, R.; Staubitz, A. RSC Adv. 2023, 13, 15805–15809. doi:10.1039/d3ra02988c |
| 16. | Fink, B. E.; Gavai, A. V.; Tokarski, J. S.; Goyal, B.; Misra, R.; Xiao, H.-Y.; Kimball, S. D.; Han, W.-C.; Norris, D.; Spires, T. E.; You, D.; Gottardis, M. M.; Lorenzi, M. V.; Vite, G. D. Bioorg. Med. Chem. Lett. 2006, 16, 1532–1536. doi:10.1016/j.bmcl.2005.12.039 |
| 17. | Wages, F.; Lentes, P.; Griebenow, T.; Herges, R.; Peifer, C.; Maser, E. Chem.-Biol. Interact. 2022, 354, 109822. doi:10.1016/j.cbi.2022.109822 |
| 13. | Lentes, P.; Frühwirt, P.; Freißmuth, H.; Moormann, W.; Kruse, F.; Gescheidt, G.; Herges, R. J. Org. Chem. 2021, 86, 4355–4360. doi:10.1021/acs.joc.1c00065 |
| 37. | Hugenbusch, D.; Lehr, M.; von Glasenapp, J.-S.; McConnell, A. J.; Herges, R. Angew. Chem., Int. Ed. 2023, 62, e202212571. doi:10.1002/anie.202212571 |
| 3. | Lentes, P.; Stadler, E.; Röhricht, F.; Brahms, A.; Gröbner, J.; Sönnichsen, F. D.; Gescheidt, G.; Herges, R. J. Am. Chem. Soc. 2019, 141, 13592–13600. doi:10.1021/jacs.9b06104 |
| 38. | Iqbal, Z.; Lyubimtsev, A.; Hanack, M. Synlett 2008, 2287–2290. doi:10.1055/s-2008-1078269 |
| 39. | Xia, Y.; Jiang, H.; Wu, W. Eur. J. Org. Chem. 2021, 6658–6669. doi:10.1002/ejoc.202101196 |
| 17. | Wages, F.; Lentes, P.; Griebenow, T.; Herges, R.; Peifer, C.; Maser, E. Chem.-Biol. Interact. 2022, 354, 109822. doi:10.1016/j.cbi.2022.109822 |
| 20. | Gorostiza, P.; Isacoff, E. Y. Science 2008, 322, 395–399. doi:10.1126/science.1166022 |
| 22. | Lentes, P.; Rudtke, J.; Griebenow, T.; Herges, R. Beilstein J. Org. Chem. 2021, 17, 1503–1508. doi:10.3762/bjoc.17.107 |
| 18. | Ewert, J.; Heintze, L.; Jordà-Redondo, M.; von Glasenapp, J.-S.; Nonell, S.; Bucher, G.; Peifer, C.; Herges, R. J. Am. Chem. Soc. 2022, 144, 15059–15071. doi:10.1021/jacs.2c03649 |
| 3. | Lentes, P.; Stadler, E.; Röhricht, F.; Brahms, A.; Gröbner, J.; Sönnichsen, F. D.; Gescheidt, G.; Herges, R. J. Am. Chem. Soc. 2019, 141, 13592–13600. doi:10.1021/jacs.9b06104 |
| 13. | Lentes, P.; Frühwirt, P.; Freißmuth, H.; Moormann, W.; Kruse, F.; Gescheidt, G.; Herges, R. J. Org. Chem. 2021, 86, 4355–4360. doi:10.1021/acs.joc.1c00065 |
| 20. | Gorostiza, P.; Isacoff, E. Y. Science 2008, 322, 395–399. doi:10.1126/science.1166022 |
| 10. | Cabré, G.; Garrido-Charles, A.; González-Lafont, À.; Moormann, W.; Langbehn, D.; Egea, D.; Lluch, J. M.; Herges, R.; Alibés, R.; Busqué, F.; Gorostiza, P.; Hernando, J. Org. Lett. 2019, 21, 3780–3784. doi:10.1021/acs.orglett.9b01222 |
| 18. | Ewert, J.; Heintze, L.; Jordà-Redondo, M.; von Glasenapp, J.-S.; Nonell, S.; Bucher, G.; Peifer, C.; Herges, R. J. Am. Chem. Soc. 2022, 144, 15059–15071. doi:10.1021/jacs.2c03649 |
| 39. | Xia, Y.; Jiang, H.; Wu, W. Eur. J. Org. Chem. 2021, 6658–6669. doi:10.1002/ejoc.202101196 |
| 21. | Velema, W. A.; Szymanski, W.; Feringa, B. L. J. Am. Chem. Soc. 2014, 136, 2178–2191. doi:10.1021/ja413063e |
| 20. | Gorostiza, P.; Isacoff, E. Y. Science 2008, 322, 395–399. doi:10.1126/science.1166022 |
| 17. | Wages, F.; Lentes, P.; Griebenow, T.; Herges, R.; Peifer, C.; Maser, E. Chem.-Biol. Interact. 2022, 354, 109822. doi:10.1016/j.cbi.2022.109822 |
| 20. | Gorostiza, P.; Isacoff, E. Y. Science 2008, 322, 395–399. doi:10.1126/science.1166022 |
| 18. | Ewert, J.; Heintze, L.; Jordà-Redondo, M.; von Glasenapp, J.-S.; Nonell, S.; Bucher, G.; Peifer, C.; Herges, R. J. Am. Chem. Soc. 2022, 144, 15059–15071. doi:10.1021/jacs.2c03649 |
| 40. | Sell, H.; Näther, C.; Herges, R. Beilstein J. Org. Chem. 2013, 9, 1–7. doi:10.3762/bjoc.9.1 |
| 6. | Hüll, K.; Morstein, J.; Trauner, D. Chem. Rev. 2018, 118, 10710–10747. doi:10.1021/acs.chemrev.8b00037 |
| 10. | Cabré, G.; Garrido-Charles, A.; González-Lafont, À.; Moormann, W.; Langbehn, D.; Egea, D.; Lluch, J. M.; Herges, R.; Alibés, R.; Busqué, F.; Gorostiza, P.; Hernando, J. Org. Lett. 2019, 21, 3780–3784. doi:10.1021/acs.orglett.9b01222 |
| 19. | Schoenberger, M.; Damijonaitis, A.; Zhang, Z.; Nagel, D.; Trauner, D. ACS Chem. Neurosci. 2014, 5, 514–518. doi:10.1021/cn500070w |
| 20. | Gorostiza, P.; Isacoff, E. Y. Science 2008, 322, 395–399. doi:10.1126/science.1166022 |
| 21. | Velema, W. A.; Szymanski, W.; Feringa, B. L. J. Am. Chem. Soc. 2014, 136, 2178–2191. doi:10.1021/ja413063e |
| 20. | Gorostiza, P.; Isacoff, E. Y. Science 2008, 322, 395–399. doi:10.1126/science.1166022 |
© 2025 Brandt et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.