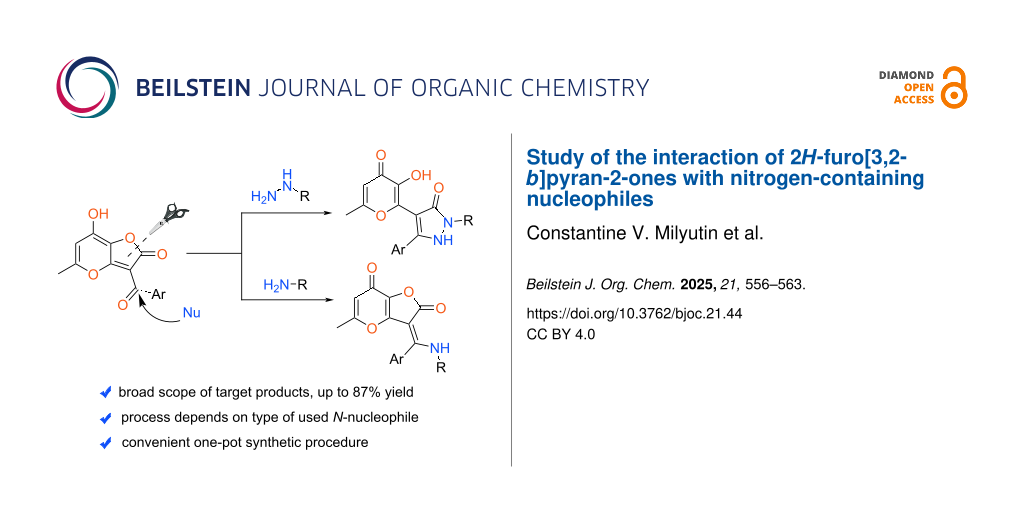
Abstract
For the first time, the reaction of substituted 2H-furo[3,2-b]pyran-2-ones with diverse N-nucleophiles was investigated. It was shown that the direction of the process depends on the type of employed nitrogen-containing reagent. For example, condensation with aliphatic amines leads to 2H-furo[3,2-b]pyran-2,7(3H)-diones bearing an exocyclic enamine moiety. At the same time, interaction with dinucleophiles results in recyclization accompanied by opening of the furan ring. Relied on the aforementioned process a general method for the synthesis of substituted pyrazol-3-ones with allomaltol fragment was designed. Structures of representatives of all obtained products were unambiguously confirmed by X-ray diffraction.
Graphical Abstract
Introduction
Substituted furan-2(5H)-ones (butenolides) are widely used as precursors for the preparation of diverse types of heterocyclic compounds possessing various biological activity [1-3]. Among the numerous approaches using considered furanones as starting compounds the recyclization processes are of significant interest [4,5]. The important subclass of such synthetic methods is the interaction with nitrogen-containing reagents. In this case depending on the structure of used N-nucleophile various types of a heterocyclic system can be obtained. A large number of examples of reactions with substituted amines have been reported in the literature. Generally, this process includes opening of the furanone ring followed by cyclization to the pyrrolone moiety [6-9]. Wherein, the wide variety of easily available starting butenolides and amines allows one to create a huge array of practically useful products. At the same time, the application of hydrazine derivatives expands the range of formed heterocycles. So, along with the aforementioned N-substituted pyrrolones such interaction can lead to pyridazinone systems [10,11]. Despite on the plenty of reactions with nitrogen-containing nucleophiles there is only one example of recyclization using furanone with a carbonyl group at position 3 (Scheme 1a, previous work) [6].
Scheme 1: Various examples of transformations of furanones.
Scheme 1: Various examples of transformations of furanones.
However, no work on this type of process with hydrazine derivatives is known in the literature. Therefore, the study of recyclizations of heterocyclic systems containing the 3-acylfuran-2(5H)-one core under action of various hydrazines is of a great interest.
Ongoing our research in the field of allomaltol chemistry in the present communication we investigated the interaction of substituted 2H-furo[3,2-b]pyran-2-ones 1 with nitrogen-containing nucleophiles (Scheme 1b, this work). As a result, the general approach to preparation of pyrazolones with a 3-hydroxy-4-pyranone unit was developed. It’s important to underline that both pyrazolone and allomaltol derivatives have the wide variety of biological activity [12-16]. This means that designing such hybrid products can significantly alter the pharmacological properties.
Results and Discussion
The starting compounds 1 were prepared from allomaltol employing a previously elaborated two-step method [17]. Relied on the obtained set of aroyl-substituted 2H-furo[3,2-b]pyran-2-ones 1 we investigated the interaction with diverse N-nucleophiles. Compound 1a was selected as a model object for realization of considered research. At first, reaction with benzylamine (2a) was tested under various conditions. Initially, we performed the studied process with equivalent amounts of starting materials in ethanol at reflux for 1 h. As a result, no products of condensation or recyclization have been obtained. At the same time, stable salt 3a was isolated in 95% yield (Scheme 2a). Besides that, the use of 3-fold excess of amine 2a led to the same results. Apparently, the stability of salt 3a is connected with high acidity of the starting furanone 1a and its recovery is only possible under the action of strong acids (HCl, H2SO4). Further, we investigated the chemical behavior of synthesized salt 3a at reflux in AcOH for 5 h. In this case the mixture of starting compound 3a and an unidentified product was obtained. Next, we increased the reaction time up to 24 h what allowed us to achieve the complete conversion of salt 3a. Wherein, the product 4a was isolated in pure form with 58% yield. Based on X-ray analysis data the synthesized compound 4a is 2H-furo[3,2-b]pyran-2,7(3H)-dione bearing an enamine fragment at the furanone ring (Scheme 2b). It’s interesting to note that prepared product 4a exists in form of the Z-isomer. This configuration is apparently stabilized by an intramolecular hydrogen bond between the NH-unit and the carbonyl group.
Scheme 2: Interaction of starting 2H-furo[3,2-b]pyran-2-ones with diverse amines.
Scheme 2: Interaction of starting 2H-furo[3,2-b]pyran-2-ones with diverse amines.
The presented results indicate that the reaction of the aliphatic amine doesn’t lead to recyclization of the studied heterocyclic system. Further, we hypothesized that synthesis of the enamine 4a can be realized in one-pot variant without isolation of intermediate salt 3a. Indeed, the reflux of starting compound 1a with amine 2a in AcOH for 24 h led to formation of product 4a with 62% yield.
Using various furanones 1 and amines we attempted to synthesize analogues of compound 4a based on the above procedure. It was found that the presence of an electron-donating group in the aroyl fragment deactivates the carbonyl moiety and as a consequence blocks the considered process. In this case only stable salt 3b was obtained at reflux in AcOH for 24 h with 90% yield (Scheme 2c). At the same time, various aliphatic amines can be applied in the considered transformation using 2H-furo[3,2-b]pyran-2-ones 1 without electron-donating substituents at the aromatic ring. Also, the considered protocol failed for interactions of furanone 1a with diverse anilines. In this case a complex mixture of products was obtained after 24 h reflux in AcOH (Scheme 2d). Apparently, for the realization of the presented condensation, the nucleophilicity of the aromatic amines is not sufficient. Wherein, the type of substituent in the aroyl fragment of furanone 1 doesn’t influence the result of this reaction. Thus, enamines 4 can be synthesized only using active aliphatic amines and 2H-furo[3,2-b]pyran-2-ones 1 without electron-donating units at the aryl fragment (Scheme 3).
Scheme 3: Synthesis of enamines 4. Reaction conditions: 1a (1 mmol, 0.38 g), amine 2 (1.2 mmol), AcOH (3 mL).
Scheme 3: Synthesis of enamines 4. Reaction conditions: 1a (1 mmol, 0.38 g), amine 2 (1.2 mmol), AcOH (3 mL).
Further, we investigated the interaction of furanones 1 with various hydrazine derivatives 6. For this purpose, we tested the model reaction of furanone 1a with phenylhydrazine 6a under various conditions and the obtained results are summarized in Table 1. It should be mentioned that all experiments were carried out at reflux due to low solubility of the starting compound 1a in all used solvents.
Table 1: Optimization of the reaction conditionsa.
|
|
||||
| Entry | Solvent | Reactant | Time, h | Yield, % |
| 1 | AcOH | 6a | 24 | 37 |
| 2 | AcOH | 6a | 48 | 36 |
| 3 | EtOH | 6a | 24 | – |
| 4 | MeCN | 6a | 24 | – |
| 5 | dioxane | 6a | 24 | – |
| 6 | AcOH | 7a | 24 | 55 |
| 7 | EtOH | 7a | 24 | 75 |
| 8 | MeCN | 7a | 24 | 51 |
| 9 | dioxane | 7a | 24 | 62 |
| 10 | EtOH | 7a | 8 | 76 |
| 11 | EtOH | 7a | 4 | 53 |
aReaction conditions: 1a (1 mmol, 0.38 g), reagent (1.1 mmol), solvent (5 mL).
At first, we carried out the process under conditions developed above for the synthesis of enamines 4. So, reflux of the mixture of starting materials in AcOH for 24 h led to pyrazolone 8a in 37% yield (Table 1, entry 1). Note that increasing the process time didn’t affect on the observed result (Table 1, entry 2). Next, we tested the studied reaction applying various solvents and in all cases the target product was not obtained (Table 1, entries 3–5). Apparently, the presence of acid reagent is necessary for implementation of considered recyclization. In this regard we tried to perform the process under study using phenylhydrazine in salt form. Indeed, reflux of furanone 1a with the corresponding hydrochloride 7a in AcOH for 24 h allows us to increase the yield of product 8a up to 55% (Table 1, entry 6). Further, we varied the solvents for reaction with phenylhydrazine hydrochloride (Table 1, entries 7–9). All used solvents are suitable for the considered transformation while among the tested conditions the best result was achieved in the case of EtOH (Table 1, entry 7). Then, we optimized the process time for the reaction with reagent 7a. It was shown that 8 h reflux is enough for the considered recyclization (Table 1, entry 10). Wherein, further shortening of the duration decreased the yield of product 8a (Table 1, entry 11). Thus, the optimal conditions for studied process are the application of phenylhydrazine hydrochloride at reflux in EtOH for 8 h.
Having in hands the protocol elaborated above we have synthesized the wide range of target pyrazolones 8 bearing the allomaltol fragment (Scheme 4). The suggested method allows one to utilize arylhydrazines both with donor and acceptor substituents in the aromatic ring. Besides that, heterocyclic hydrazines also can be used in the considered transformation.
Scheme 4: Synthesis of pyrazol-3-ones 8. Reaction conditions: 1 (1 mmol), hydrazine 7 (1.1 mmol), EtOH (5 mL).
Scheme 4: Synthesis of pyrazol-3-ones 8. Reaction conditions: 1 (1 mmol), hydrazine 7 (1.1 mmol), EtOH (5 mL)....
In addition, we have tried to carry out the process under investigation with unsubstituted hydrazine. So, reflux of furanone 1a with hydrazine monohydrochloride in EtOH for 8 h resulted in a complex mixture of products (Scheme 5a). Taking into account the fact that the nucleophilicity of hydrazine is higher than for arylhydrazines and similar to alkylamines we tested the conditions elaborated above for the preparation of enamines 4. Interaction of starting compound 1a with hydrazine was performed using acetic acid as a solvent at reflux for 8 h. As a result, the appropriate pyrazolone 10a, unsubstituted at both nitrogen atoms, was obtained with 73% yield (Scheme 5b).
Scheme 5: Synthesis of pyrazol-3-one 10a.
Scheme 5: Synthesis of pyrazol-3-one 10a.
Based on the elaborated protocol we synthesized the set of target products 10 (Scheme 6). It has been shown that in contrast to the reaction with amines described above, this process does not depend on the type of substituent in the aromatic fragment. It is interesting to note that the presented approach (AcOH, reflux 8 h) failed in the case of aliphatic hydrazines (methylhydrazine, tert-butylhydrazine) leading to a complex mixture of products. Besides that, the use of hydrochlorides of aforementioned alkylhydrazines and EtOH as a solvent gave analogous negative results. At the same time, the disclosed recyclization can be extended for the synthesis of relative isoxazolone 11. In this case the reaction of furanone 1c with hydroxylamine hydrochloride 12 also was carried out in refluxing EtOH for 8 h (Scheme 7).
Scheme 6: Synthesis of unsubstituted pyrazol-3-ones 10. Reaction conditions: 1 (1 mmol), hydrazine hydrate (2 mmol, 0.10 g), AcOH (5 mL).
Scheme 6: Synthesis of unsubstituted pyrazol-3-ones 10. Reaction conditions: 1 (1 mmol), hydrazine hydrate (2...
Scheme 7: Synthesis of isoxazolone 11. Reaction conditions: 1c (1 mmol, 0.30 g), hydroxylamine hydrochloride (1.2 mmol, 0.08 g), EtOH (5 mL).
Scheme 7: Synthesis of isoxazolone 11. Reaction conditions: 1c (1 mmol, 0.30 g), hydroxylamine hydrochloride ...
All prepared products 8, 10 and 11 are solid crystalline compounds whose structure was proved by 1H, 13C NMR spectroscopy and high-resolution MS. 1H NMR spectra of obtained products contain characteristic signals of the protons of the methyl group in the region δ 1.72–2.16 ppm and proton of pyranone fragment in the region δ 5.98–6.62 ppm. Besides that, the key structures of synthesized products were established by X-ray analysis.
The proposed mechanism of the considered processes is outlined at Scheme 8. Initially, the free nitrogen nucleophile is reversibly generated from the corresponding hydrochloride or acetate. Next, acid-catalyzed addition of the amine component to the carbon atom of the aroyl fragment leads to hemiaminal A. Then, enamine 4 is formed via dehydration of intermediate B. In the case of amines 2 the reaction stops at this stage while for other substrates the further recyclization proceeds. So, the additional NH or OH fragment attacks the lactone moiety leading to intermediate C. The subsequent opening of the furanone ring and proton transfer results in final compounds 8, 10 and 11.
The synthetic application of obtained pyrazolones 8 was demonstrated by its further derivatization. The interaction with electrophilic agents is determined by the presence of several nucleophilic centers in the molecule. In this regard we performed the acylation of starting compound 8o using pivaloyl chloride. The process was carried out with 3-fold excess of the aforementioned reagent at reflux in MeCN for 3 h. As a result, product 13 bearing two acyl fragments at oxygen atoms of pyranone and pyrazole units was isolated (Scheme 9). The structure of synthesized compound 13 was unambiguously confirmed by X-ray diffraction.
Scheme 9: Synthesis of product 13. Reaction conditions: 8o (1 mmol, 0.37 g), pivaloyl chloride (3 mmol, 0.36 g), MeCN (5 mL).
Scheme 9: Synthesis of product 13. Reaction conditions: 8o (1 mmol, 0.37 g), pivaloyl chloride (3 mmol, 0.36 ...
Conclusion
In summary, we studied the interaction of 2H-furo[3,2-b]pyran-2-one derivatives with various amines and hydrazines. It was demonstrated that, depending on the nature of the N-nucleophile used, two types of transformation are possible. So, 2H-furo[3,2-b]pyran-2,7(3H)-diones containing an exocyclic enamine unit are formed in the reaction with aliphatic amines. Wherein, condensation with hydrazines didn’t stop at the stage of enehydrazines and subsequent recyclization to pyrazolones occurred. The analogous process using hydroxylamine allowed us to prepare the similar isoxazolone with allomaltol fragment. Extensive studies have enabled the development of a straightforward approach to novel bifunctional products containing both allomaltol and pyrazolone cores. The structures of key synthesized products were unambiguously proved by X-ray diffraction.
Supporting Information
| Supporting Information File 1: General information, characterization data, NMR spectra and crystallographic data of synthesized compounds. | ||
| Format: PDF | Size: 5.0 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Hashem, A.; Kleinpeter, E. The Chemistry of 2(5H)-Furanones. In Advances in Heterocyclic Chemistry; Katrizky, A. R., Ed.; Academic Press: San Diego, CA, USA, 2001; Vol. 81, pp 107–165. doi:10.1016/s0065-2725(01)81011-4
Return to citation in text: [1] -
Rao, Y. S. Chem. Rev. 1964, 64, 353–388. doi:10.1021/cr60230a002
Return to citation in text: [1] -
De Souza, M. V. Mini-Rev. Org. Chem. 2005, 2, 139–145. doi:10.2174/1570193053544427
Return to citation in text: [1] -
Chen, Y.-S.; Yu, H.-M.; Shie, J.-J.; Cheng, T.-J. R.; Wu, C.-Y.; Fang, J.-M.; Wong, C.-H. Bioorg. Med. Chem. 2014, 22, 1766–1772. doi:10.1016/j.bmc.2014.01.009
Return to citation in text: [1] -
Liao, W.; Liao, Q.; Xu, C.; Wu, X.; Xiong, Y.; Li, Z.; Tang, H. ACS Appl. Polym. Mater. 2022, 4, 6466–6476. doi:10.1021/acsapm.2c00885
Return to citation in text: [1] -
Bekri, S.; Desriac, F.; Barreau, M.; Clamens, T.; Gallavardin, T.; Le Nahenec-Martel, P.; Vieillard, J.; Datoussaid, Y.; Choukchou-Braham, N.; Lesouhaitier, O.; Franck, X.; Leleu, S. Bioorg. Med. Chem. Lett. 2020, 30, 127580. doi:10.1016/j.bmcl.2020.127580
Return to citation in text: [1] [2] -
Liu, J.; Chen, Q.-X.; Wu, W.-F.; Wang, D.; Zhao, S.-Y.; Li, J.-H.; Chang, Y.-Q.; Zeng, S.-G.; Hu, J.-Y.; Li, Y.-J.; Du, J.-X.; Jiao, S.-M.; Xiao, H.-C.; Zhang, Q.; Xu, J.; Zhao, J.-F.; Zhou, H.-B.; Wang, Y.-H.; Zou, J.; Sun, P.-H. Eur. J. Med. Chem. 2024, 263, 115972. doi:10.1016/j.ejmech.2023.115972
Return to citation in text: [1] -
Choi, I.-S.; Kim, P.-S.; Ha, W.; Kim, Y. H.; Yoo, H. J.; Lee, J.; Youn, S. W. ACS Catal. 2023, 13, 15939–15947. doi:10.1021/acscatal.3c04631
Return to citation in text: [1] -
Lan, C. B.; Auclair, K. Eur. J. Org. Chem. 2024, 27, e202400071. doi:10.1002/ejoc.202400071
Return to citation in text: [1] -
Mykhaylychenko, S.; Harakat, D.; Dupas, G.; Shermolovich, Y. G.; Bouillon, J.-P. J. Fluorine Chem. 2009, 130, 418–427. doi:10.1016/j.jfluchem.2009.01.008
Return to citation in text: [1] -
Pathak, S.; Debnath, K.; Hossain, S. T.; Mukherjee, S. K.; Pramanik, A. Tetrahedron Lett. 2013, 54, 3137–3143. doi:10.1016/j.tetlet.2013.04.015
Return to citation in text: [1] -
Phasha, V.; Senabe, J.; Ndzotoyi, P.; Okole, B.; Fouche, G.; Chuturgoon, A. Cosmetics 2022, 9, 64. doi:10.3390/cosmetics9030064
Return to citation in text: [1] -
Brtko, J. Arch. Pharm. (Weinheim, Ger.) 2022, 355, 2200215. doi:10.1002/ardp.202200215
Return to citation in text: [1] -
Zilles, J. C.; dos Santos, F. L.; Kulkamp‐Guerreiro, I. C.; Contri, R. V. Exp. Dermatol. 2022, 31, 1500–1521. doi:10.1111/exd.14662
Return to citation in text: [1] -
Mustafa, G.; Zia-ur-Rehman, M.; Sumrra, S. H.; Ashfaq, M.; Zafar, W.; Ashfaq, M. J. Mol. Struct. 2022, 1262, 133044. doi:10.1016/j.molstruc.2022.133044
Return to citation in text: [1] -
Zhao, Z.; Dai, X.; Li, C.; Wang, X.; Tian, J.; Feng, Y.; Xie, J.; Ma, C.; Nie, Z.; Fan, P.; Qian, M.; He, X.; Wu, S.; Zhang, Y.; Zheng, X. Eur. J. Med. Chem. 2020, 186, 111893. doi:10.1016/j.ejmech.2019.111893
Return to citation in text: [1] -
Komogortsev, A. N.; Lichitsky, B. V.; Milyutin, C. V.; Melekhina, V. G. J. Heterocycl. Chem. 2024, 61, 86–92. doi:10.1002/jhet.4744
Return to citation in text: [1]
| 1. | Hashem, A.; Kleinpeter, E. The Chemistry of 2(5H)-Furanones. In Advances in Heterocyclic Chemistry; Katrizky, A. R., Ed.; Academic Press: San Diego, CA, USA, 2001; Vol. 81, pp 107–165. doi:10.1016/s0065-2725(01)81011-4 |
| 2. | Rao, Y. S. Chem. Rev. 1964, 64, 353–388. doi:10.1021/cr60230a002 |
| 3. | De Souza, M. V. Mini-Rev. Org. Chem. 2005, 2, 139–145. doi:10.2174/1570193053544427 |
| 6. | Bekri, S.; Desriac, F.; Barreau, M.; Clamens, T.; Gallavardin, T.; Le Nahenec-Martel, P.; Vieillard, J.; Datoussaid, Y.; Choukchou-Braham, N.; Lesouhaitier, O.; Franck, X.; Leleu, S. Bioorg. Med. Chem. Lett. 2020, 30, 127580. doi:10.1016/j.bmcl.2020.127580 |
| 10. | Mykhaylychenko, S.; Harakat, D.; Dupas, G.; Shermolovich, Y. G.; Bouillon, J.-P. J. Fluorine Chem. 2009, 130, 418–427. doi:10.1016/j.jfluchem.2009.01.008 |
| 11. | Pathak, S.; Debnath, K.; Hossain, S. T.; Mukherjee, S. K.; Pramanik, A. Tetrahedron Lett. 2013, 54, 3137–3143. doi:10.1016/j.tetlet.2013.04.015 |
| 6. | Bekri, S.; Desriac, F.; Barreau, M.; Clamens, T.; Gallavardin, T.; Le Nahenec-Martel, P.; Vieillard, J.; Datoussaid, Y.; Choukchou-Braham, N.; Lesouhaitier, O.; Franck, X.; Leleu, S. Bioorg. Med. Chem. Lett. 2020, 30, 127580. doi:10.1016/j.bmcl.2020.127580 |
| 7. | Liu, J.; Chen, Q.-X.; Wu, W.-F.; Wang, D.; Zhao, S.-Y.; Li, J.-H.; Chang, Y.-Q.; Zeng, S.-G.; Hu, J.-Y.; Li, Y.-J.; Du, J.-X.; Jiao, S.-M.; Xiao, H.-C.; Zhang, Q.; Xu, J.; Zhao, J.-F.; Zhou, H.-B.; Wang, Y.-H.; Zou, J.; Sun, P.-H. Eur. J. Med. Chem. 2024, 263, 115972. doi:10.1016/j.ejmech.2023.115972 |
| 8. | Choi, I.-S.; Kim, P.-S.; Ha, W.; Kim, Y. H.; Yoo, H. J.; Lee, J.; Youn, S. W. ACS Catal. 2023, 13, 15939–15947. doi:10.1021/acscatal.3c04631 |
| 9. | Lan, C. B.; Auclair, K. Eur. J. Org. Chem. 2024, 27, e202400071. doi:10.1002/ejoc.202400071 |
| 4. | Chen, Y.-S.; Yu, H.-M.; Shie, J.-J.; Cheng, T.-J. R.; Wu, C.-Y.; Fang, J.-M.; Wong, C.-H. Bioorg. Med. Chem. 2014, 22, 1766–1772. doi:10.1016/j.bmc.2014.01.009 |
| 5. | Liao, W.; Liao, Q.; Xu, C.; Wu, X.; Xiong, Y.; Li, Z.; Tang, H. ACS Appl. Polym. Mater. 2022, 4, 6466–6476. doi:10.1021/acsapm.2c00885 |
| 17. | Komogortsev, A. N.; Lichitsky, B. V.; Milyutin, C. V.; Melekhina, V. G. J. Heterocycl. Chem. 2024, 61, 86–92. doi:10.1002/jhet.4744 |
| 12. | Phasha, V.; Senabe, J.; Ndzotoyi, P.; Okole, B.; Fouche, G.; Chuturgoon, A. Cosmetics 2022, 9, 64. doi:10.3390/cosmetics9030064 |
| 13. | Brtko, J. Arch. Pharm. (Weinheim, Ger.) 2022, 355, 2200215. doi:10.1002/ardp.202200215 |
| 14. | Zilles, J. C.; dos Santos, F. L.; Kulkamp‐Guerreiro, I. C.; Contri, R. V. Exp. Dermatol. 2022, 31, 1500–1521. doi:10.1111/exd.14662 |
| 15. | Mustafa, G.; Zia-ur-Rehman, M.; Sumrra, S. H.; Ashfaq, M.; Zafar, W.; Ashfaq, M. J. Mol. Struct. 2022, 1262, 133044. doi:10.1016/j.molstruc.2022.133044 |
| 16. | Zhao, Z.; Dai, X.; Li, C.; Wang, X.; Tian, J.; Feng, Y.; Xie, J.; Ma, C.; Nie, Z.; Fan, P.; Qian, M.; He, X.; Wu, S.; Zhang, Y.; Zheng, X. Eur. J. Med. Chem. 2020, 186, 111893. doi:10.1016/j.ejmech.2019.111893 |
© 2025 Milyutin et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.