Abstract
We report the synthesis and characterization of a new acyclic cucurbit[n]uril (CB[n]) host C1 that features four alkyl sulfate ionic groups. The X-ray crystal structure of the C1·Me6CHDA complex is reported. Host C1 is significantly less soluble in water (4 mM) compared to the analogous acyclic CB[n] host M1 which features sulfonate ionic groups (346 mM). Host C1 does not undergo significant self-association according to the results of 1H NMR dilution experiments. The molecular recognition behavior of the hosts C1 and M1 toward a panel of seven ammonium ions was explored by 1H NMR spectroscopy and isothermal titration calorimetry (ITC). We find that C1 generally binds slightly more tightly than M1 toward a specific guest. C1 binds more tightly to quaternary ammonium guests compared to the corresponding primary ammonium ions.
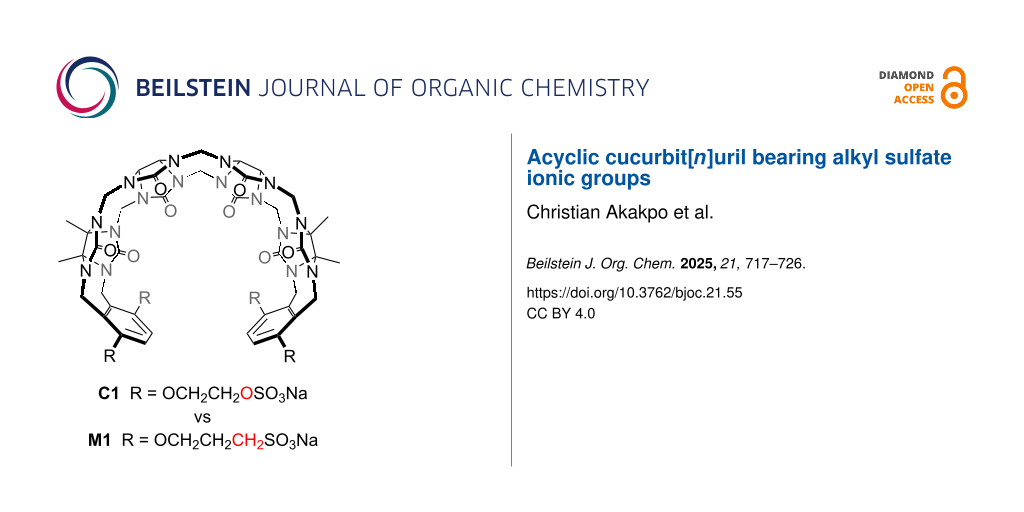
Graphical Abstract
Introduction
Molecular recognition interactions are key elements of life processes including self- versus non-self-recognition, biosynthesis, molecular and ion transport, and replication. Beginning with the pioneering works of Pedersen, Lehn, and Cram, supramolecular chemists have studied the fundamental aspects of non-covalent interactions in organic solvents and water [1-4]. Building on this fundamental knowledge, supramolecular chemists created a variety of functional systems including supramolecular polymers, sensing ensembles, molecular machines, supramolecular separation phases, and drug delivery systems [5-9]. A primary subfield of supramolecular chemistry involves the synthesis of macrocyclic hosts and studies of their molecular recognition properties. The most widely studied macrocyclic host systems include those created entirely by covalent bonds (crown ethers, cyclodextrins, calixarenes, cyclophanes, pillararenes, cucurbit[n]urils (CB[n])), and those prepared by metal ligands and H-bonding self-assembly processes [1,2,10-20]. Macrocycles have played key roles in important real-world products including the household deodorizer FebreezeTM, glucose monitors, and as solubilizing excipients [21-26]. Within these families of macrocyclic hosts, CB[n] molecular containers have proven particularly versatile because they form high affinity CB[n]–guest complexes in aqueous solution that are responsive to various stimuli (e.g., photochemical, electrochemical, chemical) [27-30]. For this reason, macrocyclic CB[n] have been used as key elements of separations processes [31,32], sensing systems [33,34], in pharmaceutical applications [35-38], in bioimaging systems [39,40], and even in household deodorizing products [41].
An important subclass of CB[n] hosts are acyclic CB[n]-type receptors which have been extensively studied by our lab and others over the past decade [42-52]. Figure 1 shows the chemical structure of the prototypical acyclic CB[n]-type known as M1 [53,54]. M1 features a central glycoluril tetramer, two aromatic o-xylylene walls, and four sulfonates as solubilizing ionic groups. In accord with these structural features, M1 binds a variety of hydrophobic and cationic guest molecules by the hydrophobic effect, π–π interactions, and electrostatic (ion–dipole and ion–ion) interactions. Although acyclic CB[n] are not macrocycles, they are preorganized into a C-shaped geometry by virtue of their polycyclic chemical structure and display binding affinities approaching those of macrocyclic CB[n]. M1 and analogues display outstanding biocompatibility and have been used for a number of in vivo biomedical applications including as a solubilizing excipient for anticancer agents and as an in vivo sequestrant to reverse the biological activity of neuromuscular blocking agents, anesthetics, and drugs of abuse (e.g., methamphetamine and fentanyl) [54-60].
Figure 1: Chemical structures of CB[n] and selected acyclic CB[n]-type molecular containers M1 and M0.
Figure 1: Chemical structures of CB[n] and selected acyclic CB[n]-type molecular containers M1 and M0.
As a result of their modular synthesis, acyclic CB[n] can be easily modified synthetically [42-47,61]. Acyclic CB[n]-type receptors featuring different length glycoluril oligomers (monomer–pentamer) and different aromatic walls (e.g., naphthalene, anthracene, triptycene) have been studied [42,62-67]. Previously, we have studied the influence of the length of the O(CH2)nSO3Na sidearm (n = 0, 2, 3, 4) and found that the M0 host – where the hydrophobic linker (CH2)n was completely removed – displayed higher binding affinity than M1 which we attributed to the location of the ionic group closer to the ureidyl C=O portals [68,69]. However, a close examination of the structures of M0 and M1 show that the ionic group for M1 is a sulfonate and for M0 is a sulfate. Accordingly, M1 and M0 differ in two ways: a) different (CH2)n linker length and b) different ionic group (sulfonate versus sulfate). In this paper, we present the synthesis and molecular recognition properties of a new acyclic CB[n]-type receptor C1 which allows us to disentangle these two effects.
Results and Discussion
This results and discussion section is organized as follows: First, we present the design, synthesis, and spectroscopic characterization of C1 along with determination of its inherent aqueous solubility and self-association properties. Next, we present the X-ray crystal structure of C1 as its C1·Me6CHDA complex. Subsequently, we describe a qualitative investigation of C1·guest and M1·guest complexation by 1H NMR spectroscopy and quantitative investigation by isothermal titration calorimetry (ITC). Finally, we discuss the trends in binding affinity observed for C1·guest and M1·guest complexation.
Design, synthesis and characterization of C1
In order to disentangle the effects of the ionic group (sulfonate versus sulfate) while maintaining the distance of the ionic group from the ureidyl C=O portal we designed acyclic CB[n]-type receptor C1 (Scheme 1). The only structural difference between M1 and C1 is the swapping of one CH2 group for one O atom in each alkyl chain which effectively changes the sulfonate group to a sulfate group. The synthetic route to C1 starts with the double electrophilic aromatic substitution reaction of methylene-bridged glycoluril tetramer (TetBCE) with W1 in TFA/Ac2O 1:1 which adds the sidewalls and transforms the OH groups into OAc groups to give TetW1OAc in 71% yield as described previously [70]. Saponification of TetW1OAc with LiOH at 50 °C followed by acidification with 0.1 M HCl gives TetW1 in 69% yield [70]. Finally, the sulfation of TetW1 occurs upon treatment with py·SO3 (20 equiv) in dry pyridine to yield C1 as a white solid in 68% yield. In accord with the depicted C2v-symmetric geometry (Scheme 1), the 1H NMR spectrum of C1 displays one aromatic resonance (Ha), two methyl resonances (CH3)j and (CH3)k, two equatorial methine doublets (Hl and Hm), along with three doublets for the diastereotopic methylene bridges around 5.5 ppm (Hd, Hf, Hh) in the expected 2:2:1 ratio (Figure 2a). The 4.0–4.5 ppm region is crowded which precludes precise assignments of the expected resonances for He, Hg, Hi, Hb, and Hc. Similarly, the 13C NMR spectrum recorded for C1 (Figure 2b) shows 15 of the 16 resonances expected based on time averaged C2v-symmetry in solution. For example, we observe two resonances for the C=O groups, three resonances for the aromatic C-atoms, two methyl resonances, three resonances for the bridging CH2 groups, and five of the six resonances for the sidearm (b and c) and equatorial glycoluril C-atoms. The negative-ion electrospray ionization mass spectrum shows an ion at m/z = 751.13 which corresponds to [C1 − 2Na]2−.
Scheme 1: Synthesis of C1. Conditions: a) TFA/Ac2O, 70 °C, 3.5 h, 71%; b) LiOH, 50 °C, 69%; c) dry pyridine, pyridine sulfur trioxide complex (20 equiv), 90 °C, 18 h, 68%.
Scheme 1: Synthesis of C1. Conditions: a) TFA/Ac2O, 70 °C, 3.5 h, 71%; b) LiOH, 50 °C, 69%; c) dry pyridine, ...
![[1860-5397-21-55-2]](/bjoc/content/figures/1860-5397-21-55-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: a) 1H NMR spectrum (600, D2O, rt) and b) 13C NMR spectrum recorded (150 MHz, D2O, rt) for C1.
Figure 2: a) 1H NMR spectrum (600, D2O, rt) and b) 13C NMR spectrum recorded (150 MHz, D2O, rt) for C1.
Inherent aqueous solubility of C1
After having firmly established the structure of C1 we decided to determine its inherent aqueous solubility. For this purpose, we added an excess of solid C1 to D2O and stirred the solution at room temperature overnight. Afterwards, the mixture was centrifuged (4400 rpm, 10 min) to pellet excess insoluble C1. An aliquot of the supernatant and a solution of dimethyl malonic acid as a non-binding internal standard of known concentration were transferred to an NMR tube followed by collection of a 1H NMR spectrum using a delay time between pulses of 20 seconds to ensure accurate integration. The inherent aqueous solubility of C1 was determined to be 3.97 mM by comparison of the integrals for Ha of C1 with that of the CH3-resonance for dimethyl malonic acid (Figure S5 in Supporting Information File 1).
Qualitative study of C1·guest recognition properties by 1H NMR spectroscopy
Next, we decided to perform a qualitative investigation of the host–guest properties of C1 by 1H NMR spectroscopy. Figure 3 shows the chemical structures of a panel of guests that were studied and the complexation-induced changes in chemical shift (Δδ) for C1·guest. As the central hydrophobic binding domain of the guests we selected alkylene, p-xylylene, cyclohexane, and adamantane moieties that are known to bind well to (acyclic) CB[n] receptors [71-73]. The cross-sectional area of this hydrophobic moiety increases as follows: PDA ≈ HDA < PXDA < CHDA < AdA. Given that (acyclic) CB[n] often bind to ammonium ion guests (e.g., NH3+ form) weaker than they do to the corresponding methonium ion guests (e.g., NMe3+ form) we elected to study both forms to elucidate related preferences for the sulfated C1 host relative to the sulfonated M1 host [69,71,74]. Figure 4 shows a 1H NMR stack plot created for uncomplexed C1 (Figure 4d), uncomplexed Me6PXDA (Figure 4d), and 1:1 and 1:2 mixtures of C1 and Me6PXDA. Several spectroscopic features are noteworthy. First, the Ar–H resonance for Me6PXDA undergoes a large upfield shift (Δδ = −1.33) upon formation of C1·Me6PXDA (Figure 4c) whereas the CH2 (Δδ = −0.68) and NMe3 (Δδ = −0.24) groups undergo smaller upfield shifts. This observation strongly suggests that the Ar–H protons are located nearer the center of the magnetically shielding cavity of C1 which is defined by the aromatic sidewalls and the ureidyl π-systems. The small changes in chemical shift for the methonium group suggests it is located near the ureidyl C=O portals and not inside the magnetically shielding cavity. Related complexation-induced changes in chemical shift are observed for the other C1·guest complexes (Figure 3 and Supporting Information File 1) which confirms that the hydrophobic central region of the guest binds inside the hydrophobic cavity of C1 whereas the hydrophilic ammonium and methonium groups reside at the electrostatically negative ureidyl C=O portals. Second, at a 1:2 C1/Me6PXDA ratio (Figure 4b), we observe separate resonances for free Me6PXDA and complexed C1·Me6PXDA which means that the rate of guest exchange is slow on the chemical shift timescale. Slow kinetics of guest exchange is commonly observed for tight host·guest complexes. In contrast, the kinetics of guest exchange are in the intermediate exchange regime on the chemical shift timescale for the complexes of C1 with CHDA, Me6CHDA, AdA, Me3AdA (Supporting Information File 1, Figures S10–S13) which is typical of weaker complexes. Third, we observe changes in the chemical shift for the Ha resonance of C1 upon formation of the C1·guest complexes. In uncomplexed C1 the tips of the aromatic rings are pointing toward each other which places Ha in the magnetically shielding region of the opposing sidewall. Upon formation of the C1·guest complexes, the tips of the aromatic sidewall change their orientation to accommodate the hydrophobic region of the guest which changes the orientation of Ha with respect to the magnetically shielding region [54,63,64].
Figure 3: Chemical structures of guests used in this study along with the complexation induced changes in chemical shift (Δδ) upon formation of the C1·guest complexes. Negative Δδ values represent upfield shifts upon complexation.
Figure 3: Chemical structures of guests used in this study along with the complexation induced changes in che...
![[1860-5397-21-55-4]](/bjoc/content/figures/1860-5397-21-55-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: 1H NMR spectra recorded (400 MHz, D2O, rt) for: a) Me6PXDA (0.5 mM), b) a mixture of C1 (0.5 mM) and Me6PXDA (1.0 mM), c) a mixture of C1 (0.5 mM) and Me6PXDA (0.5 mM), and d) C1 (0.5 mM).
Figure 4: 1H NMR spectra recorded (400 MHz, D2O, rt) for: a) Me6PXDA (0.5 mM), b) a mixture of C1 (0.5 mM) an...
X-ray crystal structure of C1
We were fortunate to obtain single crystals of the C1·Me6CHDA complex and solved the crystal structure by X-ray diffraction (CCDC 2411723). Figure 5 shows a cross-eyed stereoview of one C1·Me6CHDA complex in the crystal. Several features of this structure are noteworthy. First, the crystal structure confirms the molecular structure of C1 and its overall C-shaped geometry. Second, within the C1·Me6CHDA complex, the aromatic sidewalls are splayed away from the equator of C1 resulting in a helical geometry [63,65]. Both senses of helical chirality are present in the crystal; values in parenthesis given below refer to the complex with opposite helical chirality. The guest Me6CHDA possesses a mirror plane and is therefore achiral. In solution, host C1 is flexible and the two senses of helicity – and other conformations – undergo rapid equilibrium rendering the C1 and the C1·Me6CHDA complex achiral. The centroids of the aromatic sidewall are 0.9698 Å (1.1193 Å) above and 1.3090 Å (1.4832 Å) below the mean plane of the glycoluril methine and glycoluril quaternary C-atoms. Third, the Me6CHDA guest is not symmetrically oriented with respect to the ureidyl carbonyl portals of C1. Specifically, one of the methonium N-atoms is located inside the cavity of C1 at 1.4476 Å (0.6162 Å) below the mean plane of the ureidyl carbonyl O-atoms whereas the other methonium N-atom is located 1.7980 Å (0.9686 Å) outside the cavity.
![[1860-5397-21-55-5]](/bjoc/content/figures/1860-5397-21-55-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Cross-eyed stereoview of the C1·Me6CHDA complex in the crystal. Color code: C, gray; H, white; N, blue; O, red; S, yellow.
Figure 5: Cross-eyed stereoview of the C1·Me6CHDA complex in the crystal. Color code: C, gray; H, white; N, b...
Figure 6 shows the packing of four molecules of the C1·Me6CHDA complex in a single unit cell along with four molecules of Me6CHDA located outside the cavity of C1 to ensure overall charge neutrality. It is well known that CB[n]·guest complexation is driven by ion–dipole interactions at the ureidyl C=O portals [75]. Previously, we found that the Me3N+···O=C distances in the ultratight CB[7]·diamantane(NMe3)2 complex averaged 4.38(7) Å [74]. For comparison, a histogram of Me3N+···O=C distances drawn from 89 CCDC structures that contain an acetylcholine-type unit (Me3NCH2CH2O(C=O)R) range from 3.5 Å to 5 Å with a maximum probability of 4.4 Å [74]. Figure 6 shows Me3N+···O=C contacts that are less than 4.40 Å. The large number of contacts that are significantly shorter than 4.40 Å establishes that Me3N+···O=C cation–dipole interactions play an important role driving the inclusion of Me6CHDA inside of C1 to form the C1·Me6CHDA complex. Of course, the inclusion of the hydrophobic cyclohexyl moiety inside the cavity of C1 provides a hydrophobic driving force for complexation in water. Given that C1 is a tetraanion and that Me6CHDA is a dication, an additional molecule of Me6CHDA is present per molecule of C1 to ensure overall charge neutrality in the crystal. Among the four molecules of Me6CHDA outside the cavity of C1 in the molecular cell (Figure 6, only two external Me6CHDA are shown for clarity), only one Me3N+···O=C contact (4.548 Å) with a distance < 5.5 Å is observed. Given the anionic nature of the sulfate substituents, one might expect to observe Me3N+···−O3SO interactions in the crystal. Somewhat surprisingly, only a single short Me3N+···−O3SO contact (4.352 Å) is observed with distance < 4.4 Å. There are, however, numerous longer Me3N+···−O3SO contacts with distances in the 4.4–5.4 Å range which suggests they play a supporting role during crystallization.
![[1860-5397-21-55-6]](/bjoc/content/figures/1860-5397-21-55-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: Cross-eyed stereoview of the crystal packing observed in the molecular cell of C1·Me6CHDA. H-atoms are omitted for clarity. N···O distances less than 4.40 Å are indicated with dashed lines. Color code: C, gray; N, blue; O, red; S, yellow.
Figure 6: Cross-eyed stereoview of the crystal packing observed in the molecular cell of C1·Me6CHDA. H-atoms ...
Measurement of the self-association of C1
Before proceeding to investigate the molecular recognition properties of C1 by ITC, we wanted to determine whether C1 undergoes self-association in phosphate-buffered saline (PBS) which might impinge on guest binding and complicate the determination of C1·guest binding constants. For this purpose, we performed dilution experiments monitored by 1H NMR spectroscopy. We prepared a series of NMR samples of C1 in D2O (from 4 mM to 125 μM) and monitored the chemical shift of Ha (Supporting Information File 1, Figure S14). Over this dilution range, the resonance for Ha remains a sharp singlet at 6.94 ppm. Accordingly, we conclude that C1 remains monomeric at the low concentration (100 μM) typically employed for isothermal titration calorimetry measurements.
Use of isothermal titration calorimetry to measure the thermodynamic parameters of complexation
Acyclic CB[n]-type receptors are known to bind tightly (Ka > 106 M−1) to hydrophobic diammonium ions [42,65,71]. Accordingly, we elected to use isothermal titration calorimetry (ITC) to measure the binding between C1 or M1 with the panel of guests. A single ITC run is capable of delivering both the binding constant (Ka, M−1) and the enthalpy of complexation (ΔH, kcal mol−1). Direct ITC titrations are most appropriate for host·guest complexes with Ka ≤ 107 M−1 where Wiseman c-values from 5–500 can be achieved by changing the concentration of host in the ITC cell [76-78]. Figure 7a presents the thermogram recorded when a solution of C1 (100 μM) in phosphate-buffered saline (PBS) in the ITC cell was titrated with a solution of CHDA (1 mM) from the ITC injection syringe. The DP versus time data in Figure 6a was integrated and then plotted as ΔH versus molar ratio in Figure 7b. The ΔH versus molar ratio data was then fitted to the single-set-of-sites binding model in the PEAQ ITC data analysis software which delivered Ka = (6.49 ± 0.10) × 105 M−1 and ΔH = −7.82 ± 0.02 kcal mol−1 for the C1·CHDA complex (Table 1). All ITC experiments were performed in triplicate and the reported values represent the mean ± standard deviation. For stronger complexes, where the Wiseman c-value cannot be adjusted into the ideal range by reducing the host concentration in the ITC cell due to the insufficient heat evolved, competitive ITC titrations must be used. In competitive ITC titrations a solution of the host and an excess of a weak binding competitive guest in the ITC cell is titrated with a solution of the tighter binding guest from the ITC injection syringe [78]. The analysis of competitive titrations requires that the Ka and ΔH values for the host·competitor complexes have been previously determined and used as known inputs to the competitive binding model in the PEAQ data analysis software. To maximize the heat evolved in the competitive ITC titrations, the host·competitor and host·tight guest complexes should have very different ΔH values. Figure 7c shows the competitive ITC titration of a mixture of C1 (0.1 mM) and CHDA (0.8 mM) in the ITC cell with a solution of Me6PXDA (1.0 mM) from the syringe. The DP versus time plot was integrated and a plot of ΔH versus molar ratio was created (Figure 7d) and fitted to the competitive binding model in the PEAQ ITC data analysis software to determine Ka = (2.47 ± 0.06) × 108 M−1 and ΔH = −12.43 ± 0.02 kcal mol−1 for the C1·Me6PXDA complex. The Ka and ΔH values for the remaining C1·guest and M1·guest complexes were determined by analogous direct or competitive ITC titrations (Table 1 and Supporting Information File 1).
![[1860-5397-21-55-7]](/bjoc/content/figures/1860-5397-21-55-7.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 7: a) Representative plot of DP (μcal s−1) versus time from the titration of C1 (0.1 mM) in the ITC cell with a solution of CHDA (1.0 mM) from the ITC syringe. b) Plot of ΔH versus the C1:CHDA molar ratio. The solid line represents the best fit of the data to the single-set-of-sites binding model implemented in the PEAQ ITC data analysis software. The measurements were performed in triplicate and yielded Ka = (6.49 ± 0.10) × 105 M−1 and ΔH = −7.82 ± 0.02 kcal mol−1. c) Representative plot of DP versus time from the competitive titration of C1 (0.1 mM) and CHDA (0.8 mM) in the cell with a solution of Me6PXDA (1.0 mM) from the syringe. d) Plot of ΔH versus the C1:Me6PXDA molar ratio. The solid line represents the best fit of the data to the competitive binding model implemented in the PEAQ ITC data analysis software. The measurements were performed in triplicate and yielded Ka = (2.47 ± 0.06) × 108 M−1 and ΔH = −12.43 ± 0.02 kcal mol−1.
Figure 7: a) Representative plot of DP (μcal s−1) versus time from the titration of C1 (0.1 mM) in the ITC ce...
Table 1: Thermodynamic parameters (Ka (M−1), ∆H° (kcal/mol) determined for the C1·guest, M1·guest and M0·guest complexes by ITC. Conditions: 298.0 K, phosphate-buffered saline, pH 7.4.
| Guest | C1 | M1 | ||
| Ka | ∆H° | Ka (M−1) | ∆H° | |
| Me6PDA | (3.40 ± 0.09) × 107d | −13.47 ± 0.03 | (1.31 ± 0.05) × 106a | −5.98 ± 0.03 |
| Me6HDA | (6.54 ± 0.59) × 107b | −10.13 ± 0.02 | (2.95 ± 0.12) × 106a | −5.27 ± 0.02 |
| PXDA | (1.44 ± 0.03) × 108c | −10.07 ± 0.01 | (3.42 ± 0.05) × 107c | −5.67 ± 0.01 |
| Me6PXDA | (2.47 ± 0.06) × 108b | −12.43 ± 0.02 | (7.52 ± 0.18) × 107b | −8.64 ± 0.02 |
| CHDA | (6.49 ± 0.10) × 105a | −7.82 ± 0.02 | (2.79 ± 0.07) × 105a | −4.38 ± 0.02 |
| Me6CHDA | (1.75 ± 0.06) × 106a | −7.83 ± 0.03 | (1.20 ± 0.04) × 106a | −7.44 ± 0.03 |
| AdA | (2.41 ± 0.04) × 106a | −7.54 ± 0.03 | (1.99 ± 0.06) × 106a | −4.11 ± 0.03 |
| Me3AdA | (2.31 ± 0.07) × 106a | −11.00 ± 0.04 | (2.09 ± 0.07) × 106a | −7.42 ± 0.02 |
aMeasured by direct ITC titration of host (100 μM) in the cell with guest (1 mM) in the syringe. bMeasured by ITC competition assay using CHDA (0.8 mM) as competitor included in the cell. cMeasured by ITC competition assay using CHDA (0.5 mM) as competitor included in the cell. dMeasured by ITC competition assay using CHDA (0.1 mM) as competitor included in the cell.
Comparison of the thermodynamic parameters for C1·guest and M1·guest complexation
Overall, C1 is the more potent host with Ka values ranging from 2.41 × 105 (AdA) to 2.49 × 108 M−1 (Me6PXDA) relative to M1 whose Ka values range from 1.99 × 105 (AdA) to 7.52 × 107 M−1 (Me6PXDA). Similarly, the enthalpic contributions to binding are more favorable for C1 with ΔH values ranging from −7.54 (AdA) to −13.47 kcal mol−1 (Me6PDA) than for M1 with ΔH values ranging from −4.11 (AdA) to −8.64 kcal mol−1 (Me6PXDA). The more favorable enthalpic contributions to binding is likely due to stronger electrostatic interactions between the guest and the sulfate ionic groups. For both C1 and M1, the Me6HDA and Me6PXDA are the strongest binding guests whereas the cyclohexane and adamantane-based guests with a larger cross-sectional area bind 10–100-fold more weakly. The ratio of the binding constants of a common guest to C1 versus M1 is as follows: Me6PDA (26.0), Me6HDA (22.2), PXDA (4.2), Me6PXDA (3.3), CHDA (2.3), Me6CHDA (1.3), AdA (1.2), Me3AdA (1.1). The C1 host is both a tighter and more selective host for the narrower guests than M1. We can also tease out the effect of chain length by a comparison of Me6PDA with Me6HDA. We find that the longer and more hydrophobic Me6HDA guest binds 1.92-fold stronger to C1; similarly, Me6HDA binds 2.25-fold stronger to M1. These differences are likely due to the increased hydrophobicity of the additional CH2 group. Finally, we can compare the binding of the primary ammonium versus the corresponding quaternary ammonium ion guest toward C1 and separately M1. We find that C1 binds the quaternary ammoniums somewhat stronger: Me6PXDA vs PXDA (1.72-fold), Me6CHDA vs CHDA (2.42-fold), Me3AdA vs AdA (4.59-fold). A similar trend holds for M1: Me6PXDA vs PXDA (2.20-fold), Me6CHDA vs CHDA (4.30-fold), Me3AdA vs AdA (10.50-fold).
Conclusion
In summary, we have designed, synthesized, and characterized a new acyclic CB[n]-type receptor C1 that bears sulfate ionic groups and compared its properties with M1 which features sulfonate ionic groups. We find that C1 is much less soluble (4 mM) than M1 (346 mM) in water. Host C1 does not undergo self-association in PBS buffer according to 1H NMR dilution experiments. Analysis of complexation-induced changes in chemical shifts establish that the hydrophobic regions of the guests bind within the anisotropic shielding cavity of C1 whereas the ionic groups reside closer to the ureidyl carbonyl portals of C1. Direct and competitive ITC titrations were used to measure the thermodynamic parameters of binding for C1·guest and M1·guest complexes in PBS solution. Overall, we find that C1 – with its sulfate ionic groups – binds tighter than M1 toward each member of the guest panel with largest differences observed for the narrowest Me6PDA (26-fold) and Me6HDA (22.2-fold) guests. Similarly, we find that C1 binds the quaternary ammonium stronger than the corresponding primary ammonium ion guest by 1.72 to 9.59-fold. In conclusion, we find that C1 displays somewhat enhanced molecular recognition properties than M1 but possesses less desirable aqueous solubility properties.
Experimental
General experimental details
All chemicals were purchased from commercial suppliers and were used without further purification. Guest molecules were available from previous studies [65,71]. Compounds TetW1OAc and TetW1 were prepared according to the literature procedures with slight modifications [70]. NMR spectra were recorded using commercial spectrometers operating at 600 or 400 MHz for 1H and 150 or 100 MHz for 13C. Melting points were measured on a Meltemp apparatus in open capillary tubes and are uncorrected. IR spectra were measured on a Thermo Nicolet NEXUS 670 FT/IR spectrometer by attenuated total reflectance (ATR) and are reported in cm−1. Mass spectrometry was performed using a JEOL AccuTOF electrospray instrument. ITC data was collected on a Malvern Microcal PEAQ-ITC instrument with a cell volume of 200 µL and an injection syringe with a capacity of 40 μL. For ITC experiments, the host and guest solutions were prepared in a 20 mM phosphate-buffered water (pH 7.4). The sample cell was filled (200 μL) with the host solution and the guest solution was titrated (first injection = 0.4 μL, subsequent 18 injections = 2 μL) into the cell. All ITC experiments were analyzed using the MicroCal PEAQ-ITC data analysis software.
Compound C1
A mixture of TetW1 (0.430 g, 0.376 mmol) and pyridine sulfur trioxide (1.1838 g, 7.437 mmol) was dissolved in dry pyridine (57 mL). The resulting mixture was heated at 90 °C under N2 for 18 h and then cooled to rt. The precipitate was collected by first decanting some of the solvent and then the remaining mixture was transferred to a 50 mL centrifuge tube and centrifuged (7200 rpm, 5 min). The supernatant was carefully poured off. Next, the crude solid was dissolved in 1 M NaOH (25 mL) which results in a yellow and then red solution. Afterwards, EtOH (144 mL) was added which gave a white precipitate that was collected by centrifugation (7200 rpm, 10 min). The crude solid was analyzed by 1H NMR which showed residual pyridine. The crude solid was subsequently dissolved in water (150 mL) and re-precipitated by the addition of EtOH (144 mL) followed by centrifugation (7200 rpm, 5 min) to obtain a white solid. The solid was dried overnight under high vacuum to yield C1 as a white solid (0.3444 g, 68% yield). Mp > 300 °C; IR (ATR, cm−1): 3456 (w), 1720 (m), 1472 (m), 1378 (w), 1226 (m), 1101 (s), 1023 (m), 972 (w), 790 (w); 1H NMR (400 MHz, D2O) 6.71 (s, 4H), 5.67 (d, J = 15.4 Hz, 2H), 5.57 (d, J = 15.8 Hz, 4H), 5.46 (d, J = 8.9 Hz, 2H), 5.42 (d, J = 8.9 Hz, 2H), 5.41 (d, J = 16.3 Hz, 4H), 4.45–4.40 (m, 4H), 4.35–4.30 (m, 4H), 4.27 (d, J = 15.8 Hz, 2 × 4H), 4.25–4.18 (m, 4H), 4.18–4.13 (m, 4H), 4.12 (d, J = 15.4 Hz, 2H), 1.78 (s, 6H), 1.75 (s, 6H); 13C NMR (100 MHz, D2O/acetone-d6 6:1 (v:v)) 156.7, 155.9, 150.5, 129.3, 116.1, 78.6, 77.4, 71.2, 69.5, 67.4, 52.5, 48.4, 35.0, 16.3, 15.4 ppm; ESIMS (m/z): 751.13 ([M − 2Na]2−), calcd for [C50H56N16Na2S4O28]2−, 751.1064.
Supporting Information
The X-ray crystal structure of C1 is deposited with the Cambridge Crystallographic Data Centre (CCDC 2411723).
| Supporting Information File 1: Synthesis and characterization of compounds, solubility determination, 1H NMR dilution experiments, 1H NMR and ITC binding studies. | ||
| Format: PDF | Size: 4.7 MB | Download |
Data Availability Statement
Data generated and analyzed during this study is openly available in Digital Repository at the University of Maryland (UMD DRUM) at https://doi.org/10.13016/4gal-sini.
References
-
Pedersen, C. J. Angew. Chem., Int. Ed. Engl. 1988, 27, 1021–1027. doi:10.1002/anie.198810211
Return to citation in text: [1] [2] -
Cram, D. J. Angew. Chem., Int. Ed. Engl. 1988, 27, 1009–1020. doi:10.1002/anie.198810093
Return to citation in text: [1] [2] -
Lehn, J.-M. Angew. Chem., Int. Ed. Engl. 1988, 27, 89–112. doi:10.1002/anie.198800891
Return to citation in text: [1] -
Anslyn, E. V. J. Org. Chem. 2007, 72, 687–699. doi:10.1021/jo0617971
Return to citation in text: [1] -
Kolesnichenko, I. V.; Anslyn, E. V. Chem. Soc. Rev. 2017, 46, 2385–2390. doi:10.1039/c7cs00078b
Return to citation in text: [1] -
Aida, T.; Meijer, E. W.; Stupp, S. I. Science 2012, 335, 813–817. doi:10.1126/science.1205962
Return to citation in text: [1] -
Stoddart, J. F. Angew. Chem., Int. Ed. 2017, 56, 11094–11125. doi:10.1002/anie.201703216
Return to citation in text: [1] [2] -
Borsley, S.; Leigh, D. A.; Roberts, B. M. W. Angew. Chem., Int. Ed. 2024, 63, e202400495. doi:10.1002/anie.202400495
Return to citation in text: [1] -
Feringa, B. L. Angew. Chem., Int. Ed. 2017, 56, 11060–11078. doi:10.1002/anie.201702979
Return to citation in text: [1] -
Rekharsky, M. V.; Inoue, Y. Chem. Rev. 1998, 98, 1875–1918. doi:10.1021/cr970015o
Return to citation in text: [1] -
Gutsche, C. D. Acc. Chem. Res. 1983, 16, 161–170. doi:10.1021/ar00089a003
Return to citation in text: [1] -
Dale, E. J.; Vermeulen, N. A.; Juríček, M.; Barnes, J. C.; Young, R. M.; Wasielewski, M. R.; Stoddart, J. F. Acc. Chem. Res. 2016, 49, 262–273. doi:10.1021/acs.accounts.5b00495
Return to citation in text: [1] -
Jordan, J. H.; Gibb, B. C. Chem. Soc. Rev. 2015, 44, 547–585. doi:10.1039/c4cs00191e
Return to citation in text: [1] -
Diederich, F. Angew. Chem., Int. Ed. Engl. 1988, 27, 362–386. doi:10.1002/anie.198803621
Return to citation in text: [1] -
Rebek, J., Jr. Acc. Chem. Res. 2009, 42, 1660–1668. doi:10.1021/ar9001203
Return to citation in text: [1] -
Ogoshi, T.; Yamagishi, T.-a.; Nakamoto, Y. Chem. Rev. 2016, 116, 7937–8002. doi:10.1021/acs.chemrev.5b00765
Return to citation in text: [1] -
Xue, M.; Yang, Y.; Chi, X.; Zhang, Z.; Huang, F. Acc. Chem. Res. 2012, 45, 1294–1308. doi:10.1021/ar2003418
Return to citation in text: [1] -
Wu, J.-R.; Yang, Y.-W. Chem. Commun. 2019, 55, 1533–1543. doi:10.1039/c8cc09374a
Return to citation in text: [1] -
Harris, K.; Fujita, D.; Fujita, M. Chem. Commun. 2013, 49, 6703–6712. doi:10.1039/c3cc43191f
Return to citation in text: [1] -
Zarra, S.; Wood, D. M.; Roberts, D. A.; Nitschke, J. R. Chem. Soc. Rev. 2015, 44, 419–432. doi:10.1039/c4cs00165f
Return to citation in text: [1] -
Febreze.com “Ingredients”. https://www.febreze.com/en-us/ingredients-safety/our-ingredients (accessed March 27, 2025).
Return to citation in text: [1] -
Meadows, M. K.; Anslyn, E. V. Three Tales of Supramolecular Analytical Chemistry. In Macrocyclic and Supramolecular Chemistry: How Izatt–Christensen Award Winners Shaped the Field; Izatt, R. M., Ed.; John Wiley & Sons: Chichester, UK, 2016; pp 92–126. doi:10.1002/9781119053859.ch5
Return to citation in text: [1] -
Rajewski, R. A.; Stella, V. J. J. Pharm. Sci. 1996, 85, 1142–1169. doi:10.1021/js960075u
Return to citation in text: [1] -
Stella, V. J.; Rajewski, R. A. Pharm. Res. 1997, 14, 556–567. doi:10.1023/a:1012136608249
Return to citation in text: [1] -
Davis, A. P. Chem. Soc. Rev. 2020, 49, 2531–2545. doi:10.1039/c9cs00391f
Return to citation in text: [1] -
Tromans, R. A.; Samanta, S. K.; Chapman, A. M.; Davis, A. P. Chem. Sci. 2020, 11, 3223–3227. doi:10.1039/c9sc05406e
Return to citation in text: [1] -
Isaacs, L. Acc. Chem. Res. 2014, 47, 2052–2062. doi:10.1021/ar500075g
Return to citation in text: [1] -
Sindelar, V.; Silvi, S.; Kaifer, A. E. Chem. Commun. 2006, 2185–2187. doi:10.1039/b601959e
Return to citation in text: [1] -
Ko, Y. H.; Kim, E.; Hwang, I.; Kim, K. Chem. Commun. 2007, 1305–1315. doi:10.1039/b615103e
Return to citation in text: [1] -
del Barrio, J.; Ryan, S. T. J.; Jambrina, P. G.; Rosta, E.; Scherman, O. A. J. Am. Chem. Soc. 2016, 138, 5745–5748. doi:10.1021/jacs.5b11642
Return to citation in text: [1] -
Zhang, G.; Emwas, A.-H.; Shahul Hameed, U. F.; Arold, S. T.; Yang, P.; Chen, A.; Xiang, J.-F.; Khashab, N. M. Chem 2020, 6, 1082–1096. doi:10.1016/j.chempr.2020.03.003
Return to citation in text: [1] -
Zhang, G.; Lin, W.; Huang, F.; Sessler, J.; Khashab, N. M. J. Am. Chem. Soc. 2023, 145, 19143–19163. doi:10.1021/jacs.3c06175
Return to citation in text: [1] -
Ghale, G.; Nau, W. M. Acc. Chem. Res. 2014, 47, 2150–2159. doi:10.1021/ar500116d
Return to citation in text: [1] -
Sinn, S.; Biedermann, F. Isr. J. Chem. 2018, 58, 357–412. doi:10.1002/ijch.201700118
Return to citation in text: [1] -
Sun, C.; Zhang, H.; Li, S.; Zhang, X.; Cheng, Q.; Ding, Y.; Wang, L.-H.; Wang, R. ACS Appl. Mater. Interfaces 2018, 10, 25090–25098. doi:10.1021/acsami.8b06598
Return to citation in text: [1] -
Zou, L.; Braegelman, A. S.; Webber, M. J. ACS Cent. Sci. 2019, 5, 1035–1043. doi:10.1021/acscentsci.9b00195
Return to citation in text: [1] -
Walker, S.; Oun, R.; McInnes, F. J.; Wheate, N. J. Isr. J. Chem. 2011, 51, 616–624. doi:10.1002/ijch.201100033
Return to citation in text: [1] -
Gu, A.; Wheate, N. J. J. Inclusion Phenom. Macrocyclic Chem. 2021, 100, 55–69. doi:10.1007/s10847-021-01055-9
Return to citation in text: [1] -
Sasmal, R.; Das Saha, N.; Pahwa, M.; Rao, S.; Joshi, D.; Inamdar, M. S.; Sheeba, V.; Agasti, S. S. Anal. Chem. (Washington, DC, U. S.) 2018, 90, 11305–11314. doi:10.1021/acs.analchem.8b01851
Return to citation in text: [1] -
Sasmal, R.; Som, A.; Kumari, P.; Nair, R. V.; Show, S.; Barge, N. S.; Pahwa, M.; Das Saha, N.; Rao, S.; Vasu, S.; Agarwal, R.; Agasti, S. S. ACS Cent. Sci. 2024, 10, 1945–1959. doi:10.1021/acscentsci.4c01080
Return to citation in text: [1] -
Aqdot Limited Home Page, “Odour & VOC Elimination Technology”. https://aqdot.com/our-technology/ (accessed March 27, 2025).
Return to citation in text: [1] -
Ganapati, S.; Isaacs, L. Isr. J. Chem. 2018, 58, 250–263. doi:10.1002/ijch.201700098
Return to citation in text: [1] [2] [3] [4] -
Bauer, D.; Andrae, B.; Gaß, P.; Trenz, D.; Becker, S.; Kubik, S. Org. Chem. Front. 2019, 6, 1555–1560. doi:10.1039/c9qo00156e
Return to citation in text: [1] [2] -
Jiang, S.; Lan, S.; Mao, D.; Yang, X.; Shi, K.; Ma, D. Chem. Commun. 2018, 54, 9486–9489. doi:10.1039/c8cc05552a
Return to citation in text: [1] [2] -
Wu, Y.; Yang, J.; Zhuang, S.-Y.; Yu, S.-B.; Zong, Y.; Liu, Y.-Y.; Wu, G.; Qi, Q.-Y.; Wang, H.; Tian, J.; Zhou, W.; Ma, D.; Zhang, D.-W.; Li, Z.-T. J. Med. Chem. 2024, 67, 2176–2187. doi:10.1021/acs.jmedchem.3c02110
Return to citation in text: [1] [2] -
Zhang, S.; Zhou, C.; Gao, C.; Yang, J.; Liao, X.; Yang, B. J. Mol. Liq. 2023, 390, 122942. doi:10.1016/j.molliq.2023.122942
Return to citation in text: [1] [2] -
Zhu, P.; Kong, L.; Zhang, Y.; Liu, Q.; Liao, X.; Song, Y.; Yang, B. J. Mol. Liq. 2023, 372, 121198. doi:10.1016/j.molliq.2023.121198
Return to citation in text: [1] [2] -
Peng, W.-C.; Lei, Z.; Lin, Q.-H.; Wu, Y.; Yang, J.-Y.; Wang, H.; Zhou, W.; Zhang, D.-W.; Li, Z.-T.; Ma, D. ChemPlusChem 2023, 88, e202300465. doi:10.1002/cplu.202300465
Return to citation in text: [1] -
Feng, K.; Liu, Y.-Y.; Zong, Y.; Lei, Z.; Wu, Y.; Yang, J.; Lin, F.; Qi, Q.-Y.; Li, Q.; Zhuang, S.-Y.; Zhang, J.; Tian, J.; Zhou, W.; Ma, D.; Zhang, D.-W.; Li, Z.-T.; Yu, S.-B. J. Med. Chem. 2024, 67, 17905–17918. doi:10.1021/acs.jmedchem.4c01960
Return to citation in text: [1] -
Huo, M.; Song, S.-Q.; Dai, X.-Y.; Li, F.-F.; Hu, Y.-Y.; Liu, Y. Chem. Sci. 2024, 15, 5163–5173. doi:10.1039/d4sc00160e
Return to citation in text: [1] -
Stancl, M.; Hodan, M.; Sindelar, V. Org. Lett. 2009, 11, 4184–4187. doi:10.1021/ol9017886
Return to citation in text: [1] -
Gilberg, L.; Zhang, B.; Zavalij, P. Y.; Sindelar, V.; Isaacs, L. Org. Biomol. Chem. 2015, 13, 4041–4050. doi:10.1039/c5ob00184f
Return to citation in text: [1] -
Ma, D.; Hettiarachchi, G.; Nguyen, D.; Zhang, B.; Wittenberg, J. B.; Zavalij, P. Y.; Briken, V.; Isaacs, L. Nat. Chem. 2012, 4, 503–510. doi:10.1038/nchem.1326
Return to citation in text: [1] -
Ma, D.; Zhang, B.; Hoffmann, U.; Sundrup, M. G.; Eikermann, M.; Isaacs, L. Angew. Chem., Int. Ed. 2012, 51, 11358–11362. doi:10.1002/anie.201206031
Return to citation in text: [1] [2] [3] -
Hoffmann, U.; Grosse-Sundrup, M.; Eikermann-Haerter, K.; Zaremba, S.; Ayata, C.; Zhang, B.; Ma, D.; Isaacs, L.; Eikermann, M. Anesthesiology 2013, 119, 317–325. doi:10.1097/aln.0b013e3182910213
Return to citation in text: [1] -
Haerter, F.; Simons, J. C. P.; Foerster, U.; Moreno Duarte, I.; Diaz-Gil, D.; Ganapati, S.; Eikermann-Haerter, K.; Ayata, C.; Zhang, B.; Blobner, M.; Isaacs, L.; Eikermann, M. Anesthesiology 2015, 123, 1337–1349. doi:10.1097/aln.0000000000000868
Return to citation in text: [1] -
Diaz-Gil, D.; Haerter, F.; Falcinelli, S.; Ganapati, S.; Hettiarachchi, G. K.; Simons, J. C. P.; Zhang, B.; Grabitz, S. D.; Moreno Duarte, I.; Cotten, J. F.; Eikermann-Haerter, K.; Deng, H.; Chamberlin, N. L.; Isaacs, L.; Briken, V.; Eikermann, M. Anesthesiology 2016, 125, 333–345. doi:10.1097/aln.0000000000001199
Return to citation in text: [1] -
Ganapati, S.; Grabitz, S. D.; Murkli, S.; Scheffenbichler, F.; Rudolph, M. I.; Zavalij, P. Y.; Eikermann, M.; Isaacs, L. ChemBioChem 2017, 18, 1583–1588. doi:10.1002/cbic.201700289
Return to citation in text: [1] -
Thevathasan, T.; Grabitz, S. D.; Santer, P.; Rostin, P.; Akeju, O.; Boghosian, J. D.; Gill, M.; Isaacs, L.; Cotten, J. F.; Eikermann, M. Br. J. Anaesth. 2020, 125, e140–e147. doi:10.1016/j.bja.2020.02.019
Return to citation in text: [1] -
Brockett, A. T.; Deng, C.; Shuster, M.; Perera, S.; DiMaggio, D.; Cheng, M.; Murkli, S.; Briken, V.; Roesch, M. R.; Isaacs, L. Chem. – Eur. J. 2021, 27, 17476–17486. doi:10.1002/chem.202102919
Return to citation in text: [1] -
Mao, D.; Liang, Y.; Liu, Y.; Zhou, X.; Ma, J.; Jiang, B.; Liu, J.; Ma, D. Angew. Chem., Int. Ed. 2017, 56, 12614–12618. doi:10.1002/anie.201707164
Return to citation in text: [1] -
Liu, W.; Lu, X.; Meng, Z.; Isaacs, L. Org. Biomol. Chem. 2018, 16, 6499–6506. doi:10.1039/c8ob01575a
Return to citation in text: [1] -
Lu, X.; Samanta, S. K.; Zavalij, P. Y.; Isaacs, L. Angew. Chem., Int. Ed. 2018, 57, 8073–8078. doi:10.1002/anie.201803132
Return to citation in text: [1] [2] [3] -
Zhang, B.; Isaacs, L. J. Med. Chem. 2014, 57, 9554–9563. doi:10.1021/jm501276u
Return to citation in text: [1] [2] -
Murkli, S.; Klemm, J.; King, D.; Zavalij, P. Y.; Isaacs, L. Chem. – Eur. J. 2020, 26, 15249–15258. doi:10.1002/chem.202002874
Return to citation in text: [1] [2] [3] [4] -
DiMaggio, D.; Brockett, A. T.; Shuster, M.; Murkli, S.; Zhai, C.; King, D.; O'Dowd, B.; Cheng, M.; Brady, K.; Briken, V.; Roesch, M. R.; Isaacs, L. ChemMedChem 2022, 17, e202200046. doi:10.1002/cmdc.202200046
Return to citation in text: [1] -
Brady, K. G.; Gilberg, L.; Sigwalt, D.; Bistany-Riebman, J.; Murkli, S.; Klemm, J.; Kulhánek, P.; Šindelář, V.; Isaacs, L. Supramol. Chem. 2020, 32, 479–494. doi:10.1080/10610278.2020.1795173
Return to citation in text: [1] -
Sigwalt, D.; Moncelet, D.; Falcinelli, S.; Mandadapu, V.; Zavalij, P. Y.; Day, A.; Briken, V.; Isaacs, L. ChemMedChem 2016, 11, 980–989. doi:10.1002/cmdc.201600090
Return to citation in text: [1] -
Lu, X.; Zebaze Ndendjio, S. A.; Zavalij, P. Y.; Isaacs, L. Org. Lett. 2020, 22, 4833–4837. doi:10.1021/acs.orglett.0c01637
Return to citation in text: [1] [2] -
Zhang, B.; Zavalij, P. Y.; Isaacs, L. Org. Biomol. Chem. 2014, 12, 2413–2422. doi:10.1039/c3ob42603c
Return to citation in text: [1] [2] [3] -
Xue, W.; Zavalij, P. Y.; Isaacs, L. Org. Biomol. Chem. 2019, 17, 5561–5569. doi:10.1039/c9ob00906j
Return to citation in text: [1] [2] [3] [4] -
Barrow, S. J.; Kasera, S.; Rowland, M. J.; del Barrio, J.; Scherman, O. A. Chem. Rev. 2015, 115, 12320–12406. doi:10.1021/acs.chemrev.5b00341
Return to citation in text: [1] -
Masson, E.; Ling, X.; Joseph, R.; Kyeremeh-Mensah, L.; Lu, X. RSC Adv. 2012, 2, 1213–1247. doi:10.1039/c1ra00768h
Return to citation in text: [1] -
Cao, L.; Šekutor, M.; Zavalij, P. Y.; Mlinarić‐Majerski, K.; Glaser, R.; Isaacs, L. Angew. Chem., Int. Ed. 2014, 53, 988–993. doi:10.1002/anie.201309635
Return to citation in text: [1] [2] [3] -
Mock, W. L.; Shih, N.-Y. J. Org. Chem. 1986, 51, 4440–4446. doi:10.1021/jo00373a018
Return to citation in text: [1] -
Wiseman, T.; Williston, S.; Brandts, J. F.; Lin, L.-N. Anal. Biochem. 1989, 179, 131–137. doi:10.1016/0003-2697(89)90213-3
Return to citation in text: [1] -
Broecker, J.; Vargas, C.; Keller, S. Anal. Biochem. 2011, 418, 307–309. doi:10.1016/j.ab.2011.07.027
Return to citation in text: [1] -
Velazquez-Campoy, A.; Freire, E. Nat. Protoc. 2006, 1, 186–191. doi:10.1038/nprot.2006.28
Return to citation in text: [1] [2]
| 76. | Wiseman, T.; Williston, S.; Brandts, J. F.; Lin, L.-N. Anal. Biochem. 1989, 179, 131–137. doi:10.1016/0003-2697(89)90213-3 |
| 77. | Broecker, J.; Vargas, C.; Keller, S. Anal. Biochem. 2011, 418, 307–309. doi:10.1016/j.ab.2011.07.027 |
| 78. | Velazquez-Campoy, A.; Freire, E. Nat. Protoc. 2006, 1, 186–191. doi:10.1038/nprot.2006.28 |
| 78. | Velazquez-Campoy, A.; Freire, E. Nat. Protoc. 2006, 1, 186–191. doi:10.1038/nprot.2006.28 |
| 65. | Murkli, S.; Klemm, J.; King, D.; Zavalij, P. Y.; Isaacs, L. Chem. – Eur. J. 2020, 26, 15249–15258. doi:10.1002/chem.202002874 |
| 71. | Xue, W.; Zavalij, P. Y.; Isaacs, L. Org. Biomol. Chem. 2019, 17, 5561–5569. doi:10.1039/c9ob00906j |
| 1. | Pedersen, C. J. Angew. Chem., Int. Ed. Engl. 1988, 27, 1021–1027. doi:10.1002/anie.198810211 |
| 2. | Cram, D. J. Angew. Chem., Int. Ed. Engl. 1988, 27, 1009–1020. doi:10.1002/anie.198810093 |
| 3. | Lehn, J.-M. Angew. Chem., Int. Ed. Engl. 1988, 27, 89–112. doi:10.1002/anie.198800891 |
| 4. | Anslyn, E. V. J. Org. Chem. 2007, 72, 687–699. doi:10.1021/jo0617971 |
| 27. | Isaacs, L. Acc. Chem. Res. 2014, 47, 2052–2062. doi:10.1021/ar500075g |
| 28. | Sindelar, V.; Silvi, S.; Kaifer, A. E. Chem. Commun. 2006, 2185–2187. doi:10.1039/b601959e |
| 29. | Ko, Y. H.; Kim, E.; Hwang, I.; Kim, K. Chem. Commun. 2007, 1305–1315. doi:10.1039/b615103e |
| 30. | del Barrio, J.; Ryan, S. T. J.; Jambrina, P. G.; Rosta, E.; Scherman, O. A. J. Am. Chem. Soc. 2016, 138, 5745–5748. doi:10.1021/jacs.5b11642 |
| 42. | Ganapati, S.; Isaacs, L. Isr. J. Chem. 2018, 58, 250–263. doi:10.1002/ijch.201700098 |
| 62. | Liu, W.; Lu, X.; Meng, Z.; Isaacs, L. Org. Biomol. Chem. 2018, 16, 6499–6506. doi:10.1039/c8ob01575a |
| 63. | Lu, X.; Samanta, S. K.; Zavalij, P. Y.; Isaacs, L. Angew. Chem., Int. Ed. 2018, 57, 8073–8078. doi:10.1002/anie.201803132 |
| 64. | Zhang, B.; Isaacs, L. J. Med. Chem. 2014, 57, 9554–9563. doi:10.1021/jm501276u |
| 65. | Murkli, S.; Klemm, J.; King, D.; Zavalij, P. Y.; Isaacs, L. Chem. – Eur. J. 2020, 26, 15249–15258. doi:10.1002/chem.202002874 |
| 66. | DiMaggio, D.; Brockett, A. T.; Shuster, M.; Murkli, S.; Zhai, C.; King, D.; O'Dowd, B.; Cheng, M.; Brady, K.; Briken, V.; Roesch, M. R.; Isaacs, L. ChemMedChem 2022, 17, e202200046. doi:10.1002/cmdc.202200046 |
| 67. | Brady, K. G.; Gilberg, L.; Sigwalt, D.; Bistany-Riebman, J.; Murkli, S.; Klemm, J.; Kulhánek, P.; Šindelář, V.; Isaacs, L. Supramol. Chem. 2020, 32, 479–494. doi:10.1080/10610278.2020.1795173 |
| 21. | Febreze.com “Ingredients”. https://www.febreze.com/en-us/ingredients-safety/our-ingredients (accessed March 27, 2025). |
| 22. | Meadows, M. K.; Anslyn, E. V. Three Tales of Supramolecular Analytical Chemistry. In Macrocyclic and Supramolecular Chemistry: How Izatt–Christensen Award Winners Shaped the Field; Izatt, R. M., Ed.; John Wiley & Sons: Chichester, UK, 2016; pp 92–126. doi:10.1002/9781119053859.ch5 |
| 23. | Rajewski, R. A.; Stella, V. J. J. Pharm. Sci. 1996, 85, 1142–1169. doi:10.1021/js960075u |
| 24. | Stella, V. J.; Rajewski, R. A. Pharm. Res. 1997, 14, 556–567. doi:10.1023/a:1012136608249 |
| 25. | Davis, A. P. Chem. Soc. Rev. 2020, 49, 2531–2545. doi:10.1039/c9cs00391f |
| 26. | Tromans, R. A.; Samanta, S. K.; Chapman, A. M.; Davis, A. P. Chem. Sci. 2020, 11, 3223–3227. doi:10.1039/c9sc05406e |
| 68. | Sigwalt, D.; Moncelet, D.; Falcinelli, S.; Mandadapu, V.; Zavalij, P. Y.; Day, A.; Briken, V.; Isaacs, L. ChemMedChem 2016, 11, 980–989. doi:10.1002/cmdc.201600090 |
| 69. | Lu, X.; Zebaze Ndendjio, S. A.; Zavalij, P. Y.; Isaacs, L. Org. Lett. 2020, 22, 4833–4837. doi:10.1021/acs.orglett.0c01637 |
| 1. | Pedersen, C. J. Angew. Chem., Int. Ed. Engl. 1988, 27, 1021–1027. doi:10.1002/anie.198810211 |
| 2. | Cram, D. J. Angew. Chem., Int. Ed. Engl. 1988, 27, 1009–1020. doi:10.1002/anie.198810093 |
| 10. | Rekharsky, M. V.; Inoue, Y. Chem. Rev. 1998, 98, 1875–1918. doi:10.1021/cr970015o |
| 11. | Gutsche, C. D. Acc. Chem. Res. 1983, 16, 161–170. doi:10.1021/ar00089a003 |
| 12. | Dale, E. J.; Vermeulen, N. A.; Juríček, M.; Barnes, J. C.; Young, R. M.; Wasielewski, M. R.; Stoddart, J. F. Acc. Chem. Res. 2016, 49, 262–273. doi:10.1021/acs.accounts.5b00495 |
| 13. | Jordan, J. H.; Gibb, B. C. Chem. Soc. Rev. 2015, 44, 547–585. doi:10.1039/c4cs00191e |
| 14. | Diederich, F. Angew. Chem., Int. Ed. Engl. 1988, 27, 362–386. doi:10.1002/anie.198803621 |
| 15. | Rebek, J., Jr. Acc. Chem. Res. 2009, 42, 1660–1668. doi:10.1021/ar9001203 |
| 16. | Ogoshi, T.; Yamagishi, T.-a.; Nakamoto, Y. Chem. Rev. 2016, 116, 7937–8002. doi:10.1021/acs.chemrev.5b00765 |
| 17. | Xue, M.; Yang, Y.; Chi, X.; Zhang, Z.; Huang, F. Acc. Chem. Res. 2012, 45, 1294–1308. doi:10.1021/ar2003418 |
| 18. | Wu, J.-R.; Yang, Y.-W. Chem. Commun. 2019, 55, 1533–1543. doi:10.1039/c8cc09374a |
| 19. | Harris, K.; Fujita, D.; Fujita, M. Chem. Commun. 2013, 49, 6703–6712. doi:10.1039/c3cc43191f |
| 20. | Zarra, S.; Wood, D. M.; Roberts, D. A.; Nitschke, J. R. Chem. Soc. Rev. 2015, 44, 419–432. doi:10.1039/c4cs00165f |
| 54. | Ma, D.; Zhang, B.; Hoffmann, U.; Sundrup, M. G.; Eikermann, M.; Isaacs, L. Angew. Chem., Int. Ed. 2012, 51, 11358–11362. doi:10.1002/anie.201206031 |
| 55. | Hoffmann, U.; Grosse-Sundrup, M.; Eikermann-Haerter, K.; Zaremba, S.; Ayata, C.; Zhang, B.; Ma, D.; Isaacs, L.; Eikermann, M. Anesthesiology 2013, 119, 317–325. doi:10.1097/aln.0b013e3182910213 |
| 56. | Haerter, F.; Simons, J. C. P.; Foerster, U.; Moreno Duarte, I.; Diaz-Gil, D.; Ganapati, S.; Eikermann-Haerter, K.; Ayata, C.; Zhang, B.; Blobner, M.; Isaacs, L.; Eikermann, M. Anesthesiology 2015, 123, 1337–1349. doi:10.1097/aln.0000000000000868 |
| 57. | Diaz-Gil, D.; Haerter, F.; Falcinelli, S.; Ganapati, S.; Hettiarachchi, G. K.; Simons, J. C. P.; Zhang, B.; Grabitz, S. D.; Moreno Duarte, I.; Cotten, J. F.; Eikermann-Haerter, K.; Deng, H.; Chamberlin, N. L.; Isaacs, L.; Briken, V.; Eikermann, M. Anesthesiology 2016, 125, 333–345. doi:10.1097/aln.0000000000001199 |
| 58. | Ganapati, S.; Grabitz, S. D.; Murkli, S.; Scheffenbichler, F.; Rudolph, M. I.; Zavalij, P. Y.; Eikermann, M.; Isaacs, L. ChemBioChem 2017, 18, 1583–1588. doi:10.1002/cbic.201700289 |
| 59. | Thevathasan, T.; Grabitz, S. D.; Santer, P.; Rostin, P.; Akeju, O.; Boghosian, J. D.; Gill, M.; Isaacs, L.; Cotten, J. F.; Eikermann, M. Br. J. Anaesth. 2020, 125, e140–e147. doi:10.1016/j.bja.2020.02.019 |
| 60. | Brockett, A. T.; Deng, C.; Shuster, M.; Perera, S.; DiMaggio, D.; Cheng, M.; Murkli, S.; Briken, V.; Roesch, M. R.; Isaacs, L. Chem. – Eur. J. 2021, 27, 17476–17486. doi:10.1002/chem.202102919 |
| 5. | Kolesnichenko, I. V.; Anslyn, E. V. Chem. Soc. Rev. 2017, 46, 2385–2390. doi:10.1039/c7cs00078b |
| 6. | Aida, T.; Meijer, E. W.; Stupp, S. I. Science 2012, 335, 813–817. doi:10.1126/science.1205962 |
| 7. | Stoddart, J. F. Angew. Chem., Int. Ed. 2017, 56, 11094–11125. doi:10.1002/anie.201703216 |
| 8. | Borsley, S.; Leigh, D. A.; Roberts, B. M. W. Angew. Chem., Int. Ed. 2024, 63, e202400495. doi:10.1002/anie.202400495 |
| 9. | Feringa, B. L. Angew. Chem., Int. Ed. 2017, 56, 11060–11078. doi:10.1002/anie.201702979 |
| 42. | Ganapati, S.; Isaacs, L. Isr. J. Chem. 2018, 58, 250–263. doi:10.1002/ijch.201700098 |
| 43. | Bauer, D.; Andrae, B.; Gaß, P.; Trenz, D.; Becker, S.; Kubik, S. Org. Chem. Front. 2019, 6, 1555–1560. doi:10.1039/c9qo00156e |
| 44. | Jiang, S.; Lan, S.; Mao, D.; Yang, X.; Shi, K.; Ma, D. Chem. Commun. 2018, 54, 9486–9489. doi:10.1039/c8cc05552a |
| 45. | Wu, Y.; Yang, J.; Zhuang, S.-Y.; Yu, S.-B.; Zong, Y.; Liu, Y.-Y.; Wu, G.; Qi, Q.-Y.; Wang, H.; Tian, J.; Zhou, W.; Ma, D.; Zhang, D.-W.; Li, Z.-T. J. Med. Chem. 2024, 67, 2176–2187. doi:10.1021/acs.jmedchem.3c02110 |
| 46. | Zhang, S.; Zhou, C.; Gao, C.; Yang, J.; Liao, X.; Yang, B. J. Mol. Liq. 2023, 390, 122942. doi:10.1016/j.molliq.2023.122942 |
| 47. | Zhu, P.; Kong, L.; Zhang, Y.; Liu, Q.; Liao, X.; Song, Y.; Yang, B. J. Mol. Liq. 2023, 372, 121198. doi:10.1016/j.molliq.2023.121198 |
| 61. | Mao, D.; Liang, Y.; Liu, Y.; Zhou, X.; Ma, J.; Jiang, B.; Liu, J.; Ma, D. Angew. Chem., Int. Ed. 2017, 56, 12614–12618. doi:10.1002/anie.201707164 |
| 39. | Sasmal, R.; Das Saha, N.; Pahwa, M.; Rao, S.; Joshi, D.; Inamdar, M. S.; Sheeba, V.; Agasti, S. S. Anal. Chem. (Washington, DC, U. S.) 2018, 90, 11305–11314. doi:10.1021/acs.analchem.8b01851 |
| 40. | Sasmal, R.; Som, A.; Kumari, P.; Nair, R. V.; Show, S.; Barge, N. S.; Pahwa, M.; Das Saha, N.; Rao, S.; Vasu, S.; Agarwal, R.; Agasti, S. S. ACS Cent. Sci. 2024, 10, 1945–1959. doi:10.1021/acscentsci.4c01080 |
| 42. | Ganapati, S.; Isaacs, L. Isr. J. Chem. 2018, 58, 250–263. doi:10.1002/ijch.201700098 |
| 43. | Bauer, D.; Andrae, B.; Gaß, P.; Trenz, D.; Becker, S.; Kubik, S. Org. Chem. Front. 2019, 6, 1555–1560. doi:10.1039/c9qo00156e |
| 44. | Jiang, S.; Lan, S.; Mao, D.; Yang, X.; Shi, K.; Ma, D. Chem. Commun. 2018, 54, 9486–9489. doi:10.1039/c8cc05552a |
| 45. | Wu, Y.; Yang, J.; Zhuang, S.-Y.; Yu, S.-B.; Zong, Y.; Liu, Y.-Y.; Wu, G.; Qi, Q.-Y.; Wang, H.; Tian, J.; Zhou, W.; Ma, D.; Zhang, D.-W.; Li, Z.-T. J. Med. Chem. 2024, 67, 2176–2187. doi:10.1021/acs.jmedchem.3c02110 |
| 46. | Zhang, S.; Zhou, C.; Gao, C.; Yang, J.; Liao, X.; Yang, B. J. Mol. Liq. 2023, 390, 122942. doi:10.1016/j.molliq.2023.122942 |
| 47. | Zhu, P.; Kong, L.; Zhang, Y.; Liu, Q.; Liao, X.; Song, Y.; Yang, B. J. Mol. Liq. 2023, 372, 121198. doi:10.1016/j.molliq.2023.121198 |
| 48. | Peng, W.-C.; Lei, Z.; Lin, Q.-H.; Wu, Y.; Yang, J.-Y.; Wang, H.; Zhou, W.; Zhang, D.-W.; Li, Z.-T.; Ma, D. ChemPlusChem 2023, 88, e202300465. doi:10.1002/cplu.202300465 |
| 49. | Feng, K.; Liu, Y.-Y.; Zong, Y.; Lei, Z.; Wu, Y.; Yang, J.; Lin, F.; Qi, Q.-Y.; Li, Q.; Zhuang, S.-Y.; Zhang, J.; Tian, J.; Zhou, W.; Ma, D.; Zhang, D.-W.; Li, Z.-T.; Yu, S.-B. J. Med. Chem. 2024, 67, 17905–17918. doi:10.1021/acs.jmedchem.4c01960 |
| 50. | Huo, M.; Song, S.-Q.; Dai, X.-Y.; Li, F.-F.; Hu, Y.-Y.; Liu, Y. Chem. Sci. 2024, 15, 5163–5173. doi:10.1039/d4sc00160e |
| 51. | Stancl, M.; Hodan, M.; Sindelar, V. Org. Lett. 2009, 11, 4184–4187. doi:10.1021/ol9017886 |
| 52. | Gilberg, L.; Zhang, B.; Zavalij, P. Y.; Sindelar, V.; Isaacs, L. Org. Biomol. Chem. 2015, 13, 4041–4050. doi:10.1039/c5ob00184f |
| 35. | Sun, C.; Zhang, H.; Li, S.; Zhang, X.; Cheng, Q.; Ding, Y.; Wang, L.-H.; Wang, R. ACS Appl. Mater. Interfaces 2018, 10, 25090–25098. doi:10.1021/acsami.8b06598 |
| 36. | Zou, L.; Braegelman, A. S.; Webber, M. J. ACS Cent. Sci. 2019, 5, 1035–1043. doi:10.1021/acscentsci.9b00195 |
| 37. | Walker, S.; Oun, R.; McInnes, F. J.; Wheate, N. J. Isr. J. Chem. 2011, 51, 616–624. doi:10.1002/ijch.201100033 |
| 38. | Gu, A.; Wheate, N. J. J. Inclusion Phenom. Macrocyclic Chem. 2021, 100, 55–69. doi:10.1007/s10847-021-01055-9 |
| 53. | Ma, D.; Hettiarachchi, G.; Nguyen, D.; Zhang, B.; Wittenberg, J. B.; Zavalij, P. Y.; Briken, V.; Isaacs, L. Nat. Chem. 2012, 4, 503–510. doi:10.1038/nchem.1326 |
| 54. | Ma, D.; Zhang, B.; Hoffmann, U.; Sundrup, M. G.; Eikermann, M.; Isaacs, L. Angew. Chem., Int. Ed. 2012, 51, 11358–11362. doi:10.1002/anie.201206031 |
| 33. | Ghale, G.; Nau, W. M. Acc. Chem. Res. 2014, 47, 2150–2159. doi:10.1021/ar500116d |
| 34. | Sinn, S.; Biedermann, F. Isr. J. Chem. 2018, 58, 357–412. doi:10.1002/ijch.201700118 |
| 70. | Zhang, B.; Zavalij, P. Y.; Isaacs, L. Org. Biomol. Chem. 2014, 12, 2413–2422. doi:10.1039/c3ob42603c |
| 31. | Zhang, G.; Emwas, A.-H.; Shahul Hameed, U. F.; Arold, S. T.; Yang, P.; Chen, A.; Xiang, J.-F.; Khashab, N. M. Chem 2020, 6, 1082–1096. doi:10.1016/j.chempr.2020.03.003 |
| 32. | Zhang, G.; Lin, W.; Huang, F.; Sessler, J.; Khashab, N. M. J. Am. Chem. Soc. 2023, 145, 19143–19163. doi:10.1021/jacs.3c06175 |
| 41. | Aqdot Limited Home Page, “Odour & VOC Elimination Technology”. https://aqdot.com/our-technology/ (accessed March 27, 2025). |
| 71. | Xue, W.; Zavalij, P. Y.; Isaacs, L. Org. Biomol. Chem. 2019, 17, 5561–5569. doi:10.1039/c9ob00906j |
| 72. | Barrow, S. J.; Kasera, S.; Rowland, M. J.; del Barrio, J.; Scherman, O. A. Chem. Rev. 2015, 115, 12320–12406. doi:10.1021/acs.chemrev.5b00341 |
| 73. | Masson, E.; Ling, X.; Joseph, R.; Kyeremeh-Mensah, L.; Lu, X. RSC Adv. 2012, 2, 1213–1247. doi:10.1039/c1ra00768h |
| 70. | Zhang, B.; Zavalij, P. Y.; Isaacs, L. Org. Biomol. Chem. 2014, 12, 2413–2422. doi:10.1039/c3ob42603c |
| 70. | Zhang, B.; Zavalij, P. Y.; Isaacs, L. Org. Biomol. Chem. 2014, 12, 2413–2422. doi:10.1039/c3ob42603c |
| 74. | Cao, L.; Šekutor, M.; Zavalij, P. Y.; Mlinarić‐Majerski, K.; Glaser, R.; Isaacs, L. Angew. Chem., Int. Ed. 2014, 53, 988–993. doi:10.1002/anie.201309635 |
| 42. | Ganapati, S.; Isaacs, L. Isr. J. Chem. 2018, 58, 250–263. doi:10.1002/ijch.201700098 |
| 65. | Murkli, S.; Klemm, J.; King, D.; Zavalij, P. Y.; Isaacs, L. Chem. – Eur. J. 2020, 26, 15249–15258. doi:10.1002/chem.202002874 |
| 71. | Xue, W.; Zavalij, P. Y.; Isaacs, L. Org. Biomol. Chem. 2019, 17, 5561–5569. doi:10.1039/c9ob00906j |
| 7. | Stoddart, J. F. Angew. Chem., Int. Ed. 2017, 56, 11094–11125. doi:10.1002/anie.201703216 |
| 74. | Cao, L.; Šekutor, M.; Zavalij, P. Y.; Mlinarić‐Majerski, K.; Glaser, R.; Isaacs, L. Angew. Chem., Int. Ed. 2014, 53, 988–993. doi:10.1002/anie.201309635 |
| 63. | Lu, X.; Samanta, S. K.; Zavalij, P. Y.; Isaacs, L. Angew. Chem., Int. Ed. 2018, 57, 8073–8078. doi:10.1002/anie.201803132 |
| 65. | Murkli, S.; Klemm, J.; King, D.; Zavalij, P. Y.; Isaacs, L. Chem. – Eur. J. 2020, 26, 15249–15258. doi:10.1002/chem.202002874 |
| 75. | Mock, W. L.; Shih, N.-Y. J. Org. Chem. 1986, 51, 4440–4446. doi:10.1021/jo00373a018 |
| 69. | Lu, X.; Zebaze Ndendjio, S. A.; Zavalij, P. Y.; Isaacs, L. Org. Lett. 2020, 22, 4833–4837. doi:10.1021/acs.orglett.0c01637 |
| 71. | Xue, W.; Zavalij, P. Y.; Isaacs, L. Org. Biomol. Chem. 2019, 17, 5561–5569. doi:10.1039/c9ob00906j |
| 74. | Cao, L.; Šekutor, M.; Zavalij, P. Y.; Mlinarić‐Majerski, K.; Glaser, R.; Isaacs, L. Angew. Chem., Int. Ed. 2014, 53, 988–993. doi:10.1002/anie.201309635 |
| 54. | Ma, D.; Zhang, B.; Hoffmann, U.; Sundrup, M. G.; Eikermann, M.; Isaacs, L. Angew. Chem., Int. Ed. 2012, 51, 11358–11362. doi:10.1002/anie.201206031 |
| 63. | Lu, X.; Samanta, S. K.; Zavalij, P. Y.; Isaacs, L. Angew. Chem., Int. Ed. 2018, 57, 8073–8078. doi:10.1002/anie.201803132 |
| 64. | Zhang, B.; Isaacs, L. J. Med. Chem. 2014, 57, 9554–9563. doi:10.1021/jm501276u |
© 2025 Akakpo et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.