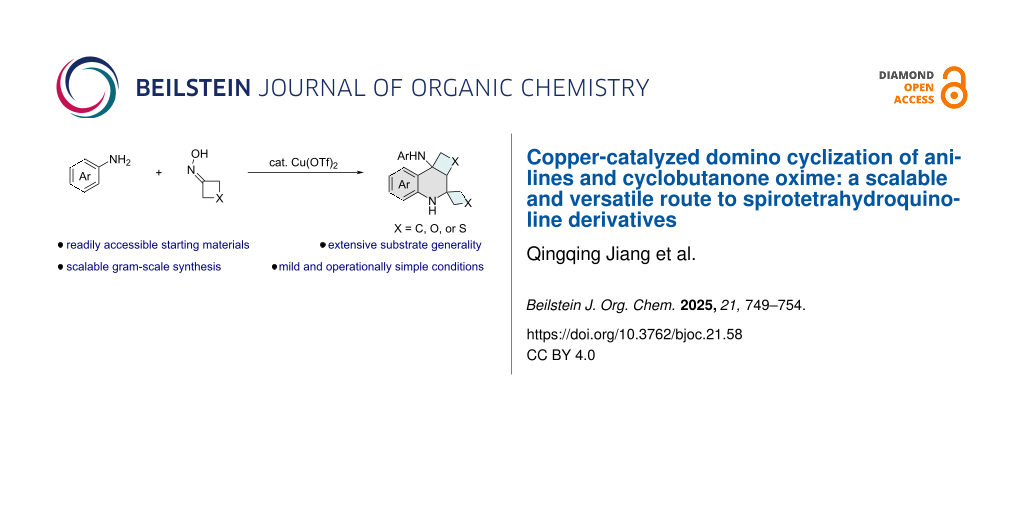
Abstract
In this study, we report the copper-catalyzed synthesis of tetrahydroquinoline derivatives via a domino reaction of aniline with cyclobutanone oxime. This method demonstrates a selective approach for generating bioactive tetrahydroquinoline scaffolds, which have broad applications in pharmaceutical chemistry. The reaction conditions were optimized for the effective formation of tetrahydroquinoline derivatives with varying substituents, showing high yields under mild conditions. Mechanistic studies suggest a catalytic cycle involving nucleophilic attack by the aniline on the cyclobutanone oxime, followed by cyclization to form the desired product.
Graphical Abstract
Introduction
Tetrahydroquinolines (THQs) represent a privileged scaffold in medicinal chemistry, exhibiting a broad spectrum of biological activities and serving as pivotal structural elements in drug discovery [1-4]. Notably, tetrahydroquinoline derivatives featuring a strain-inducing ring system are prevalent in numerous bioactive molecules, including those with promising therapeutic potential for neurological disorders, oncology, and various other medical conditions (Scheme 1a) [5-8]. Consequently, the development of efficient synthetic methodologies for constructing fused THQs is of paramount importance for advancing pharmaceutical research. Conventional synthetic strategies for THQs, which involve the formation of highly strained rings, typically employ catalytic cyclization [9-13], reductive aminations [14-17], and photochemical cyclization [18-20]. However, these approaches often necessitate multistep syntheses of starting materials and involve intricate experimental procedures, significantly impeding their practical utility and scalability [9-20].
Scheme 1: Synthetic strategies for the construction of spirotetrahydroquinoline (STHQ) scaffolds.
Scheme 1: Synthetic strategies for the construction of spirotetrahydroquinoline (STHQ) scaffolds.
Cyclobutane-fused tetrahydroquinolines (THQs) have garnered significant attention in drug discovery due to their inherent structural rigidity and enhanced pharmacological profiles [21], which render them highly desirable for therapeutic development. The strained cyclobutane ring, in particular, acts as a versatile and conformationally constrained building block, enabling the construction of complex molecular architectures with potent biological activities [22]. Despite their promise, the synthesis of cyclobutane-fused THQs remains a formidable challenge, primarily due to the inherent ring strain and the difficulties associated with achieving high diastereoselectivity during cyclization [4]. Recently, Chen and co-workers developed a chiral phosphoric acid (CPA)-catalyzed multicomponent reaction of anilines, aldehydes, and azetidinones, enabling the efficient and enantioselective synthesis of tetrahydroquinoline-fused azetidines with three contiguous stereocenters in a single step [23]. Later, Tanaka, Nagashima, and their co-workers established a chemo-, regio-, and diastereoselective dearomative transformation of quinolines into tetrahydroquinoline (THQ)-based 6-6-4-membered ring systems through a combination of nucleophilic addition and borate-mediated [2 + 2] photocycloaddition, offering a catalyst-free approach to construct conformationally constrained 2D/3D frameworks with high functional group compatibility and stereocontrol (Scheme 1b) [24]. In 2023, Zeng et al. reported the first example of a chromium-catalyzed spirocyclization between anilines and cyclobutanones, providing direct access to medicinally relevant cyclobutane-annulated and structurally constrained spirotetrahydroquinoline (STHQ) scaffolds [25]. Given the growing significance of cyclobutane-fused tetrahydroquinolines (THQs) in biochemistry and medicinal chemistry [26-28], we have developed an efficient and convenient method for synthesizing cyclobutane-fused and conformationally constrained spirotetrahydroquinolines (STHQs) from arylamines and cyclobutanone oxime using a copper-catalyzed reaction under ambient air conditions (Scheme 1c).
Results and Discussion
With these considerations in mind, we explored the feasibility of synthesizing cyclobutane-fused spirotetrahydroquinolines (STHQs) through the reaction of arylamines with cyclobutanone oxime under copper catalysis. After extensive optimization of the reaction parameters, the desired product 3aa was obtained in 92% yield under the following optimal conditions: the reaction between aniline (1a) and cyclobutanone oxime (2a) as the model system, hexane as the solvent, and copper(II) trifluoroacetate (Cu(TFA)2) as the catalyst (20 mol %) under ambient air at 80 °C for 12 hours; the product 3aa was isolated by chromatographic purification (Table 1, entry 1). The use of other solvents, including acetonitrile (MeCN), tetrahydrofuran (THF), toluene, acetone and methanol (MeOH), resulted in significantly lower yields of 3aa (Table 1, entry 2). Replacing the Cu(TFA)2 catalyst with other copper sources, such as cuprous chloride (CuCl), cuprous thiocyanate (CuSCN), copper bromide (CuBr2), copper trifluoromethanesulfonate (Cu(OTf)2), and copper powder resulted in diminished reaction efficiency (Table 1, entry 3). When iron(II) sulfate (FeSO4) and iron trifluoromethanesulfonate (Fe(OTf)2) were used as the catalyst instead of copper(II) trifluoroacetate (Cu(TFA)2), the yields of the product were decreased (Table 1, entries 4 and 5). Using palladium(II) acetate (Pd(OAc)2) as the catalyst provided a moderate yield (Table 1, entry 6). Conducting the reaction at room temperature (rt) instead of the optimal elevated temperature resulted in a lower yield (Table 1, entry 7). Increasing the reaction temperature to 100 °C did not improve the yield (Table 1, entry 8).
Table 1: Optimization of reaction conditions.a
|
|
||
| Entry | Deviation from "standard conditions" | Yield of 2a (%)b |
| 1 | none | 92 |
| 2 | MeCN, THF, toluene, acetone, or MeOH instead of hexane | 20–70 |
| 3 | CuCl, CuSCN, CuBr2, Cu(OTf)2 or Cu powder instead of Cu(TFA)2 | 31–60 |
| 4 | FeSO4 instead of Cu(TFA)2 | 76 |
| 5 | Fe(OTf)3 instead of Cu(TFA)2 | 74 |
| 6 | Pd(OAc)2 instead of Cu(TFA)2 | 67 |
| 7 | rt | 71 |
| 8 | 100 °C | 63 |
aReaction conditions: aniline (1a, 0.2 mmol), 2a (0.4 mmol), and Cu(TFA)2 (0.04 mmol) in hexane (2.0 mmol) under air atmosphere, 12 h, 80 °C. bIsolated yields after purification by column chromatography.
Having established the optimal reaction conditions, we proceeded to investigate the generality of this Cu-catalyzed system. Initially, a series of anilines bearing diverse substituents was examined, and the results are summarized in Scheme 2. When copper(II) trifluoroacetate was employed as the catalyst, para-halogen-substituted anilines 1b–e demonstrated excellent compatibility with the protocol, affording the desired products 3ba–ea in good yields. However, the introduction of strong electron-withdrawing groups, such as trifluoromethoxy, ester, and acetyl, at the para-position of the benzene ring (1f–h) led to a noticeable decrease in the yields of the corresponding STHQs 3fa–ha. In contrast, electron-donating groups, including 4-methylaniline (1i) and 4-methoxyaniline (1j), were well tolerated, delivering the expected products 3ia and 3ja in good yields. Additionally, a variety of meta-substituted anilines (3ka–ra) proved to be suitable substrates for this transformation. However, due to steric hindrance, ortho-substituted aniline 1s exhibited significantly lower reactivity, resulting in diminished yields of product 3sa. Notably, disubstituted anilines were also compatible with the protocol, furnishing the desired products 3ta–ya in moderate to good yields.
Scheme 2: Substrate scope. General reaction conditions: aniline 1 (0.2 mmol), 2 (0.4 mmol), and Cu(TFA)2 (0.04 mmol) in hexane (2.0 mmol) under air atmosphere, 12 h, 80 °C. Yields refer to isolated yields.
Scheme 2: Substrate scope. General reaction conditions: aniline 1 (0.2 mmol), 2 (0.4 mmol), and Cu(TFA)2 (0.0...
Subsequently, we investigated the scope of cyclobutanones and their analogues in the domino cyclization to access structurally diverse spirotetrahydroquinoline derivatives (Scheme 2). Heterocyclic analogues incorporating oxygen or sulfur atoms within the four-membered ring proved to be compatible substrates, affording cyclo-O/S-containing STHQ derivatives 3ab and 3ac in good yields. Additionally, ester-functionalized cyclobutanones exhibited smooth reactivity with aniline, enabling the synthesis of substituted STHQ motifs 3ad and 3ae in satisfactory yields. Notably, when cyclopentanone oxime (2f), cyclohexanone oxime (2g), or azetidinone oxime (2h) were employed as alternative substrates to cyclobutanone oxime, the corresponding spirotetrahydroquinoline products were not observed.
To showcase the practical utility of our Cu-catalyzed spirotetrahydroquinoline formation process, we conducted a 5.0 mmol scale reaction and obtained the target product 3aa in 82% yield (Scheme 3).
Based on previous reports, a plausible mechanism was proposed. In the presence of a copper catalyst, aniline reacts with cyclobutanone oxime to form an imine intermediate, which undergoes isomerization to generate an enamine intermediate. Subsequently, an intermolecular cyclization occurs between the enamine and imine intermediates, ultimately yielding the final target product through an aromatization process (Scheme 4).
Conclusion
In summary, we have developed an efficient and practical copper-catalyzed method for the synthesis of spirotetrahydroquinoline (STHQ) derivatives via the reaction of anilines with cyclobutanone oxime. This protocol offers a straightforward approach to constructing structurally diverse STHQ scaffolds under mild conditions, with broad substrate scope and high functional group tolerance. The optimized reaction conditions, utilizing copper(II) trifluoroacetate as the catalyst and hexane as the solvent, enabled the synthesis of the target products in good to excellent yields. Mechanistic studies suggest a catalytic cycle involving the formation of imine and enamine intermediates, followed by intermolecular cyclization and aromatization. The scalability of this method was demonstrated through a gram-scale reaction, highlighting its potential for practical applications in medicinal chemistry and drug discovery.
Supporting Information
| Supporting Information File 1: Experimental procedures, characterization data for all new compounds, and NMR spectra of products. | ||
| Format: PDF | Size: 6.7 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Katritzky, A. R.; Rachwal, S.; Rachwal, B. Tetrahedron 1996, 52, 15031–15070. doi:10.1016/s0040-4020(96)00911-8
Return to citation in text: [1] -
Nammalwar, B.; Bunce, R. A. Molecules 2014, 19, 204–232. doi:10.3390/molecules19010204
Return to citation in text: [1] -
Sridharan, V.; Suryavanshi, P. A.; Menéndez, J. C. Chem. Rev. 2011, 111, 7157–7259. doi:10.1021/cr100307m
Return to citation in text: [1] -
Muthukrishnan, I.; Sridharan, V.; Menéndez, J. C. Chem. Rev. 2019, 119, 5057–5191. doi:10.1021/acs.chemrev.8b00567
Return to citation in text: [1] [2] -
Goli, N.; Mainkar, P. S.; Kotapalli, S. S.; K, T.; Ummanni, R.; Chandrasekhar, S. Bioorg. Med. Chem. Lett. 2017, 27, 1714–1720. doi:10.1016/j.bmcl.2017.02.077
Return to citation in text: [1] -
Gore, V. K.; Ma, V. V.; Yin, R.; Ligutti, J.; Immke, D.; Doherty, E. M.; Norman, M. H. Bioorg. Med. Chem. Lett. 2010, 20, 3573–3578. doi:10.1016/j.bmcl.2010.04.125
Return to citation in text: [1] -
Chu, D. T. W.; Chen, J.; Zhang, W.; Li, X.; Song, J. G.; Wang, B.; Cong, Q.; James, D. R. Spiro Derivatives as Lipoxygenase Inhibitors. U.S. Pat. Appl. US20060128790A1, June 15, 2006.
Return to citation in text: [1] -
Zhong, W.; Hitchcock, S.; Albrecht, B. K.; Bartberger, M. D.; Brown, J.; Chaffee, S. C.; Cheng, Y.; Croghan, M.; Graceffa, R.; Harried, S.; Hickman, D.; Horne, D.; Hungate, R.; Judd, T.; Kaller, M.; Kreiman, C.; La, D.; Lopez, P.; Masse, C. E.; Nixey, T.; Patel, V. F.; Pennington, L.; Weiss, M.; Xue, Q.; Yang, B.; Monenschein, H.; Nguyen, T. Beta-Secretase Modulators and Methods of Use. WO Pat. Appl. WO2007061670A1, May 31, 2007.
Return to citation in text: [1] -
Zhao, Y.; Hu, Y.; Wang, H.; Li, X.; Wan, B. J. Org. Chem. 2016, 81, 4412–4420. doi:10.1021/acs.joc.6b00655
Return to citation in text: [1] [2] -
Yu, L.-Z.; Wei, Y.; Shi, M. Chem. Commun. 2017, 53, 8980–8983. doi:10.1039/c7cc04748g
Return to citation in text: [1] [2] -
Patil, D. V.; Cavitt, M. A.; Grzybowski, P.; France, S. Chem. Commun. 2012, 48, 10337–10339. doi:10.1039/c2cc34650h
Return to citation in text: [1] [2] -
Liu, L.; Wang, C.; Liu, Q.; Kong, Y.; Chang, W.; Li, J. Eur. J. Org. Chem. 2016, 3684–3690. doi:10.1002/ejoc.201600692
Return to citation in text: [1] [2] -
Kuppusamy, R.; Santhoshkumar, R.; Boobalan, R.; Wu, H.-R.; Cheng, C.-H. ACS Catal. 2018, 8, 1880–1883. doi:10.1021/acscatal.7b04087
Return to citation in text: [1] [2] -
Stepakov, A. V.; Boitsov, V. M.; Larina, A. G.; Molchanov, A. P. Russ. J. Org. Chem. 2014, 50, 389–393. doi:10.1134/s1070428014030154
Return to citation in text: [1] [2] -
Wang, H.; Wang, C.; Huang, K.; Liu, L.; Chang, W.; Li, J. Org. Lett. 2016, 18, 2367–2370. doi:10.1021/acs.orglett.6b00804
Return to citation in text: [1] [2] -
Ren, X.; Li, G.; Huang, J.; Wang, W.; Zhang, Y.; Xing, G.; Gao, C.; Zhao, G.; Zhao, J.; Tang, Z. Org. Lett. 2017, 19, 58–61. doi:10.1021/acs.orglett.6b03330
Return to citation in text: [1] [2] -
Li, L.-P.; Cai, X.; Xiang, Y.; Zhang, Y.; Song, J.; Yang, D.-C.; Guan, Z.; He, Y.-H. Green Chem. 2015, 17, 3148–3156. doi:10.1039/c4gc01123f
Return to citation in text: [1] [2] -
Umstead, W. J.; Mukhina, O. A.; Kutateladze, A. G. J. Photochem. Photobiol., A 2016, 329, 182–188. doi:10.1016/j.jphotochem.2016.07.004
Return to citation in text: [1] [2] -
Koolman, H. F.; Braje, W. M.; Haupt, A. Synlett 2016, 27, 2561–2566. doi:10.1055/s-0035-1562621
Return to citation in text: [1] [2] -
Xu, G.-Q.; Li, C.-G.; Liu, M.-Q.; Cao, J.; Luo, Y.-C.; Xu, P.-F. Chem. Commun. 2016, 52, 1190–1193. doi:10.1039/c5cc08833j
Return to citation in text: [1] [2] -
Zheng, Y.; Tice, C. M.; Singh, S. B. Bioorg. Med. Chem. Lett. 2014, 24, 3673–3682. doi:10.1016/j.bmcl.2014.06.081
Return to citation in text: [1] -
Bojack, G.; Baltz, R.; Dittgen, J.; Fischer, C.; Freigang, J.; Getachew, R.; Grill, E.; Helmke, H.; Hohmann, S.; Lange, G.; Lehr, S.; Porée, F.; Schmidt, J.; Schmutzler, D.; Yang, Z.; Frackenpohl, J. Eur. J. Org. Chem. 2021, 3442–3457. doi:10.1002/ejoc.202100415
Return to citation in text: [1] -
Qian, L.-L.; Hu, Y.-C.; Min, X.-T.; Yang, S.-N.; Shen, B.-X.; Wan, B.; Chen, Q.-A. Chem Catal. 2022, 2, 2024–2033. doi:10.1016/j.checat.2022.05.023
Return to citation in text: [1] -
Shimose, A.; Ishigaki, S.; Sato, Y.; Nogami, J.; Toriumi, N.; Uchiyama, M.; Tanaka, K.; Nagashima, Y. Angew. Chem., Int. Ed. 2024, 63, e202403461. doi:10.1002/anie.202403461
Return to citation in text: [1] -
Chen, C.; Wang, Z.; Wang, S.; Xu, L.; Zeng, X. Org. Lett. 2023, 25, 4241–4246. doi:10.1021/acs.orglett.3c01156
Return to citation in text: [1] -
Borthakur, I.; Nandi, S.; Pramanick, R.; Borpuzari, M. P.; Kundu, S. ACS Catal. 2025, 15, 3008–3022. doi:10.1021/acscatal.4c07103
Return to citation in text: [1] -
Devaraj, T.; Srinivasan, K. J. Org. Chem. 2024, 89, 13886–13893. doi:10.1021/acs.joc.4c00924
Return to citation in text: [1] -
Chen, D.-N.; Ye, D.-D.; Chen, L.-N.; Xia, P.-J. Org. Lett. 2024, 26, 9931–9936. doi:10.1021/acs.orglett.4c03761
Return to citation in text: [1]
| 1. | Katritzky, A. R.; Rachwal, S.; Rachwal, B. Tetrahedron 1996, 52, 15031–15070. doi:10.1016/s0040-4020(96)00911-8 |
| 2. | Nammalwar, B.; Bunce, R. A. Molecules 2014, 19, 204–232. doi:10.3390/molecules19010204 |
| 3. | Sridharan, V.; Suryavanshi, P. A.; Menéndez, J. C. Chem. Rev. 2011, 111, 7157–7259. doi:10.1021/cr100307m |
| 4. | Muthukrishnan, I.; Sridharan, V.; Menéndez, J. C. Chem. Rev. 2019, 119, 5057–5191. doi:10.1021/acs.chemrev.8b00567 |
| 18. | Umstead, W. J.; Mukhina, O. A.; Kutateladze, A. G. J. Photochem. Photobiol., A 2016, 329, 182–188. doi:10.1016/j.jphotochem.2016.07.004 |
| 19. | Koolman, H. F.; Braje, W. M.; Haupt, A. Synlett 2016, 27, 2561–2566. doi:10.1055/s-0035-1562621 |
| 20. | Xu, G.-Q.; Li, C.-G.; Liu, M.-Q.; Cao, J.; Luo, Y.-C.; Xu, P.-F. Chem. Commun. 2016, 52, 1190–1193. doi:10.1039/c5cc08833j |
| 14. | Stepakov, A. V.; Boitsov, V. M.; Larina, A. G.; Molchanov, A. P. Russ. J. Org. Chem. 2014, 50, 389–393. doi:10.1134/s1070428014030154 |
| 15. | Wang, H.; Wang, C.; Huang, K.; Liu, L.; Chang, W.; Li, J. Org. Lett. 2016, 18, 2367–2370. doi:10.1021/acs.orglett.6b00804 |
| 16. | Ren, X.; Li, G.; Huang, J.; Wang, W.; Zhang, Y.; Xing, G.; Gao, C.; Zhao, G.; Zhao, J.; Tang, Z. Org. Lett. 2017, 19, 58–61. doi:10.1021/acs.orglett.6b03330 |
| 17. | Li, L.-P.; Cai, X.; Xiang, Y.; Zhang, Y.; Song, J.; Yang, D.-C.; Guan, Z.; He, Y.-H. Green Chem. 2015, 17, 3148–3156. doi:10.1039/c4gc01123f |
| 9. | Zhao, Y.; Hu, Y.; Wang, H.; Li, X.; Wan, B. J. Org. Chem. 2016, 81, 4412–4420. doi:10.1021/acs.joc.6b00655 |
| 10. | Yu, L.-Z.; Wei, Y.; Shi, M. Chem. Commun. 2017, 53, 8980–8983. doi:10.1039/c7cc04748g |
| 11. | Patil, D. V.; Cavitt, M. A.; Grzybowski, P.; France, S. Chem. Commun. 2012, 48, 10337–10339. doi:10.1039/c2cc34650h |
| 12. | Liu, L.; Wang, C.; Liu, Q.; Kong, Y.; Chang, W.; Li, J. Eur. J. Org. Chem. 2016, 3684–3690. doi:10.1002/ejoc.201600692 |
| 13. | Kuppusamy, R.; Santhoshkumar, R.; Boobalan, R.; Wu, H.-R.; Cheng, C.-H. ACS Catal. 2018, 8, 1880–1883. doi:10.1021/acscatal.7b04087 |
| 26. | Borthakur, I.; Nandi, S.; Pramanick, R.; Borpuzari, M. P.; Kundu, S. ACS Catal. 2025, 15, 3008–3022. doi:10.1021/acscatal.4c07103 |
| 27. | Devaraj, T.; Srinivasan, K. J. Org. Chem. 2024, 89, 13886–13893. doi:10.1021/acs.joc.4c00924 |
| 28. | Chen, D.-N.; Ye, D.-D.; Chen, L.-N.; Xia, P.-J. Org. Lett. 2024, 26, 9931–9936. doi:10.1021/acs.orglett.4c03761 |
| 5. | Goli, N.; Mainkar, P. S.; Kotapalli, S. S.; K, T.; Ummanni, R.; Chandrasekhar, S. Bioorg. Med. Chem. Lett. 2017, 27, 1714–1720. doi:10.1016/j.bmcl.2017.02.077 |
| 6. | Gore, V. K.; Ma, V. V.; Yin, R.; Ligutti, J.; Immke, D.; Doherty, E. M.; Norman, M. H. Bioorg. Med. Chem. Lett. 2010, 20, 3573–3578. doi:10.1016/j.bmcl.2010.04.125 |
| 7. | Chu, D. T. W.; Chen, J.; Zhang, W.; Li, X.; Song, J. G.; Wang, B.; Cong, Q.; James, D. R. Spiro Derivatives as Lipoxygenase Inhibitors. U.S. Pat. Appl. US20060128790A1, June 15, 2006. |
| 8. | Zhong, W.; Hitchcock, S.; Albrecht, B. K.; Bartberger, M. D.; Brown, J.; Chaffee, S. C.; Cheng, Y.; Croghan, M.; Graceffa, R.; Harried, S.; Hickman, D.; Horne, D.; Hungate, R.; Judd, T.; Kaller, M.; Kreiman, C.; La, D.; Lopez, P.; Masse, C. E.; Nixey, T.; Patel, V. F.; Pennington, L.; Weiss, M.; Xue, Q.; Yang, B.; Monenschein, H.; Nguyen, T. Beta-Secretase Modulators and Methods of Use. WO Pat. Appl. WO2007061670A1, May 31, 2007. |
| 4. | Muthukrishnan, I.; Sridharan, V.; Menéndez, J. C. Chem. Rev. 2019, 119, 5057–5191. doi:10.1021/acs.chemrev.8b00567 |
| 24. | Shimose, A.; Ishigaki, S.; Sato, Y.; Nogami, J.; Toriumi, N.; Uchiyama, M.; Tanaka, K.; Nagashima, Y. Angew. Chem., Int. Ed. 2024, 63, e202403461. doi:10.1002/anie.202403461 |
| 22. | Bojack, G.; Baltz, R.; Dittgen, J.; Fischer, C.; Freigang, J.; Getachew, R.; Grill, E.; Helmke, H.; Hohmann, S.; Lange, G.; Lehr, S.; Porée, F.; Schmidt, J.; Schmutzler, D.; Yang, Z.; Frackenpohl, J. Eur. J. Org. Chem. 2021, 3442–3457. doi:10.1002/ejoc.202100415 |
| 25. | Chen, C.; Wang, Z.; Wang, S.; Xu, L.; Zeng, X. Org. Lett. 2023, 25, 4241–4246. doi:10.1021/acs.orglett.3c01156 |
| 21. | Zheng, Y.; Tice, C. M.; Singh, S. B. Bioorg. Med. Chem. Lett. 2014, 24, 3673–3682. doi:10.1016/j.bmcl.2014.06.081 |
| 9. | Zhao, Y.; Hu, Y.; Wang, H.; Li, X.; Wan, B. J. Org. Chem. 2016, 81, 4412–4420. doi:10.1021/acs.joc.6b00655 |
| 10. | Yu, L.-Z.; Wei, Y.; Shi, M. Chem. Commun. 2017, 53, 8980–8983. doi:10.1039/c7cc04748g |
| 11. | Patil, D. V.; Cavitt, M. A.; Grzybowski, P.; France, S. Chem. Commun. 2012, 48, 10337–10339. doi:10.1039/c2cc34650h |
| 12. | Liu, L.; Wang, C.; Liu, Q.; Kong, Y.; Chang, W.; Li, J. Eur. J. Org. Chem. 2016, 3684–3690. doi:10.1002/ejoc.201600692 |
| 13. | Kuppusamy, R.; Santhoshkumar, R.; Boobalan, R.; Wu, H.-R.; Cheng, C.-H. ACS Catal. 2018, 8, 1880–1883. doi:10.1021/acscatal.7b04087 |
| 14. | Stepakov, A. V.; Boitsov, V. M.; Larina, A. G.; Molchanov, A. P. Russ. J. Org. Chem. 2014, 50, 389–393. doi:10.1134/s1070428014030154 |
| 15. | Wang, H.; Wang, C.; Huang, K.; Liu, L.; Chang, W.; Li, J. Org. Lett. 2016, 18, 2367–2370. doi:10.1021/acs.orglett.6b00804 |
| 16. | Ren, X.; Li, G.; Huang, J.; Wang, W.; Zhang, Y.; Xing, G.; Gao, C.; Zhao, G.; Zhao, J.; Tang, Z. Org. Lett. 2017, 19, 58–61. doi:10.1021/acs.orglett.6b03330 |
| 17. | Li, L.-P.; Cai, X.; Xiang, Y.; Zhang, Y.; Song, J.; Yang, D.-C.; Guan, Z.; He, Y.-H. Green Chem. 2015, 17, 3148–3156. doi:10.1039/c4gc01123f |
| 18. | Umstead, W. J.; Mukhina, O. A.; Kutateladze, A. G. J. Photochem. Photobiol., A 2016, 329, 182–188. doi:10.1016/j.jphotochem.2016.07.004 |
| 19. | Koolman, H. F.; Braje, W. M.; Haupt, A. Synlett 2016, 27, 2561–2566. doi:10.1055/s-0035-1562621 |
| 20. | Xu, G.-Q.; Li, C.-G.; Liu, M.-Q.; Cao, J.; Luo, Y.-C.; Xu, P.-F. Chem. Commun. 2016, 52, 1190–1193. doi:10.1039/c5cc08833j |
| 23. | Qian, L.-L.; Hu, Y.-C.; Min, X.-T.; Yang, S.-N.; Shen, B.-X.; Wan, B.; Chen, Q.-A. Chem Catal. 2022, 2, 2024–2033. doi:10.1016/j.checat.2022.05.023 |
© 2025 Jiang et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.