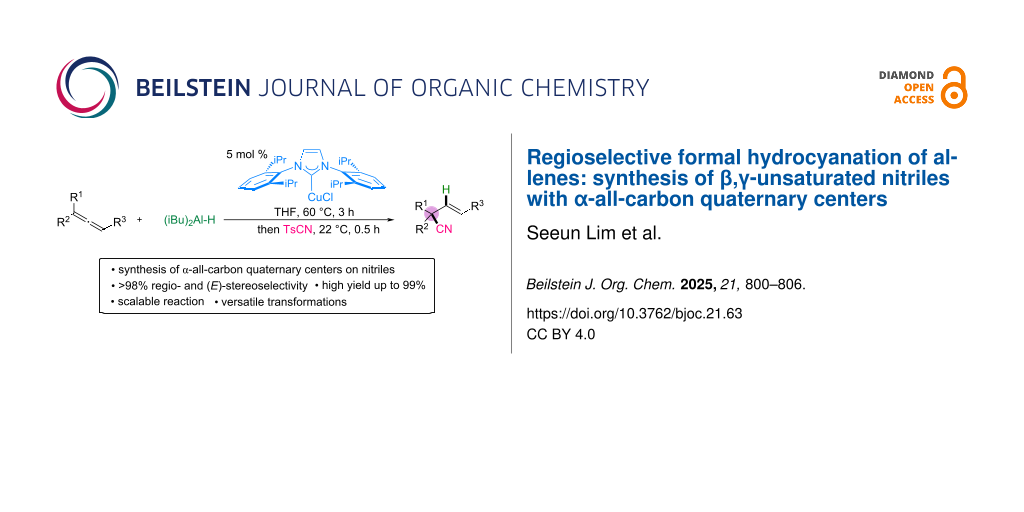
Abstract
This study introduces a highly selective hydrocyanation method based on copper-catalyzed hydroalumination of allenes with diisobutylaluminum hydride, followed by the regio- and stereoselective allylation with p-toluenesulfonyl cyanide. The proposed methodology is efficient for accessing acyclic β,γ-unsaturated nitriles with α-all-carbon quaternary centers and achieves yields up to 99% and excellent regio- and E-selectivity. The reaction proceeds under mild conditions and shows broad applicability to di- and trisubstituted allenes. Its practicality is demonstrated through the gram-scale synthesis and functional group transformations of amines, amides, and lactams, emphasizing its versatility and synthetic significance.
Graphical Abstract
Introduction
Acyclic nitriles that incorporate α-all-carbon quaternary centers are highly valuable structural motifs typically found in natural products, biologically active compounds, and synthetic pharmaceuticals [1-5]. These compounds are important intermediates in organic syntheses because of the versatility of the cyano group, which can be readily transformed into a wide range of functional groups, including amides, carboxylic acids, amines, aldehydes, ketones, and N-heterocycles [6-8]. However, the synthesis of all-carbon quaternary centers that contain functional groups is challenging mainly because of their sterically demanding property [9-11]. In this context, the incorporation of cyano groups at the quaternary carbon centers is promising for the development of versatile acyclic all-carbon quaternary stereocenters with diverse functional groups [12-14]. Consequently, the development of selective and predictable strategies for the introduction of cyano groups into quaternary carbon frameworks has become necessary in organic synthesis.
The transition-metal-catalyzed hydrocyanation of carbon–carbon double bonds is one of the most efficient and atom-economical approaches for synthesizing alkyl nitriles [15,16]. Among the potential substrates, allenes have attracted significant attention because of their unique structural features, which consist of two orthogonal and contiguous C=C bonds. This dual π-system configuration promotes selective functionalization, enabling the synthesis of various complex products through a single transformation [17-19]. Therefore, allenes have become versatile intermediates in numerous transition-metal-catalyzed reactions [20,21]. Despite extensive studies on the catalytic hydrocyanation of alkenes [22], including the industrially relevant DuPont adiponitrile process from 1,3-butadiene using nickel catalysts [23], the hydrocyanation of allenes to produce functionalized β,γ-unsaturated nitriles with quaternary carbon centers has not been investigated extensively [24]. The limited investigation of allene hydrocyanation can be attributed to the significant challenges posed by the two orthogonal π-systems in allenes. These challenges include achieving high regioselectivity and controlling (E)/(Z)-stereoselectivity, as 1,2-addition processes to allenes can generate up to four possible regioisomeric products.
Recent research has addressed some of these challenges. Arai [25,26], Fang [27] and Breit [28] investigated the nickel-catalyzed regio- and enantioselective hydrocyanation of 1,1-disubstituted allenes using acetone cyanohydrin or TMSCN/MeOH as the precursor for the in situ generation of hydrogen cyanide (Scheme 1a). This method achieved high regioselectivity and enantioselectivity, highlighting the potential of allene hydrocyanation for the synthesis of complex nitrile-containing products. In another approach, the Minakata group used electrophilic cyanating reagents, such as p-toluenesulfonyl cyanide (TsCN) and N-cyano-N-phenyl-p-toluenesulfonamide [29]. The hydroboration of allenes with 9-BBN (9-borabicyclo[3.3.1]nonane) as the hydride source, followed by regioselective cyanation with allylic boranes, provided nitrile-substituted quaternary carbon centers (Scheme 1b). Although both methodologies achieved high regioselectivity, their substrate scopes were limited, and most studies focused on the hydrocyanation of terminal allenes.
Scheme 1: Synthesis of acyclic nitrile-substituted quaternary carbon centers from allenes.
Scheme 1: Synthesis of acyclic nitrile-substituted quaternary carbon centers from allenes.
Given the synthetic importance of nitriles that bear all-carbon quaternary centers and the distinctive reactivity of allenes, the development of hydrocyanation methodologies with a broadened substrate scope and improved regio- and stereoselectivity is of significant interest. Inspired by our study on the construction of all-carbon quaternary centers via functionalized allylaluminum reagents obtained from the copper-catalyzed regioselective hydroalumination of allenes using diisobutylaluminum hydride (DIBAL-H), we envisioned that the nucleophilic attack of allylaluminum reagents on electrophilic cyanating reagents could provide a regioselective pathway for the synthesis of alkyl nitriles bearing quaternary carbon centers [30-33]. Herein, we report a mild and efficient method for the regio- and (E)-stereoselective formal hydrocyanation of di- and trisubstituted allenes. Using DIBAL-H as the hydride source and TsCN as a readily available and bench-stable cyanating agent in the presence of a copper catalyst, we synthesized new and versatile functionalized acyclic nitriles that include all-carbon quaternary centers with high selectivity (Scheme 1c). Compared to previous methodologies, our approach enables the efficient generation of tertiary nitrile products with a broader substrate scope, highlighting its synthetic utility and potential applicability in complex molecule synthesis.
Results and Discussion
We began by optimizing the hydrocyanation of allene 1a using DIBAL-H as the hydride source and p-toluenesulfonyl cyanide as the cyanating reagent (Scheme 2). Under previously established conditions, the hydride addition of DIBAL-H to allene 1a catalyzed by 5 mol % IPrCuCl as the optimal catalyst selectively generated the allylaluminum intermediate 2a with >98% conversion [30]. Subsequent addition of one equivalent of TsCN to 2a in a single vessel at room temperature proceeded regioselectively, achieving complete conversion within 30 min and yielding the desired α-quaternary nitrile 3a in 95% yield. Moreover, no byproducts, such as regioisomeric nitriles or derivatives from over-addition of allylaluminum, were observed.
Scheme 2: Hydrocyanation of allene 1a with tosyl cyanide.
Scheme 2: Hydrocyanation of allene 1a with tosyl cyanide.
After having established the optimized reaction conditions, the substrate scope for the formal hydrocyanation with 1,1-disubstituted and 1,1,3-trisubstituted allenes was examined (Scheme 3). All reactions were performed in the presence of 5 mol % IPrCuCl to generate the allylaluminum reagents in situ, followed by cyanation at room temperature for 30 min. This method efficiently constructed α-all-carbon quaternary centers on β,γ-unsaturated nitriles with excellent >98% regioselectivity and >98% (E)-selectivity. 1,1-Disubstituted allenes bearing silyl ether- and benzyl ether-tethered propyl groups were successfully converted into the desired nitriles 3a–c in yields ranging from 88% to 99%. Similarly, chloro-substituted allene 1d exhibited good tolerance under these conditions, affording the corresponding nitrile 3d in an 88% yield, whereas phenethyl-substituted allene 1e provided 3e in a 95% yield. Allenes 1f–i featuring phenyl and alkyl substituents, including methyl, ethyl, phenethyl, and allyl groups, also underwent smooth cyanation, resulting in α-quaternary nitriles 3f–i in yields of 85–94%. Furthermore, aryl-substituted allenes 1j–o, incorporating electron-donating or electron-withdrawing substituents such as methyl, fluoro, chloro, bromo, trifluoromethyl, or methoxy groups on the phenyl ring, were compatible with the reaction, producing nitriles 3j–o in 88–93% yields. In particular, thienyl-substituted allene 1p was efficiently transformed into the desired nitrile 3p.
Scheme 3: Hydrocyanation with various di- or trisubstituted allenes. Reaction conditions: allene 1 (0.3 mmol), (iBu)2Al-H (0.3 mmol), IPrCuCl (5 mol %), TsCN (0.25 mmol), THF (0.2 M), under N2. The yields of the isolated products are given.
Scheme 3: Hydrocyanation with various di- or trisubstituted allenes. Reaction conditions: allene 1 (0.3 mmol)...
We further demonstrated the versatility of this protocol using 1,1,3-trisubstituted allenes. Trisubstituted allenes 1q–s bearing phenyl and dialkyl groups, including a cyclohexyl moiety, underwent selective cyanation to deliver the (E)-isomers of the corresponding nitriles 3q–s in yields of 90–95%. In addition, aryl- and dialkyl-substituted allenes 1t–x containing substituents, such as fluoro, chloro, bromo, trifluoromethyl, or methoxy groups on the phenyl ring, were smoothly converted into β,γ-unsaturated nitriles 3t–x with high efficiency. Particularly, allenes 1y and 1z containing benzodioxane or naphthalene moieties were well-tolerated under these reaction conditions, affording nitriles 3y and 3z in 85% and 93% yield, respectively. Unfortunately, the 1,1,3-trialkyl-substituted allene 1aa was not suitable for Cu-catalyzed hydroalumination under the established conditions, resulting in less than 2% conversion to allylaluminum reagents.
In a previous study on the electrophilic cyanation of allylic boranes conducted by the Minakata group (Scheme 1b), only two examples of β,γ-unsaturated nitrile products bearing α-tertiary carbon centers were established, and the yields were moderate [29]. To broaden the applicability of the system, we extended it to the synthesis of nitriles containing both quaternary and tertiary carbon centers. The scope of monosubstituted allenes is illustrated in Scheme 4. Allenes 4a–c substituted with alkyl groups, including phenethyl, decyl, and cyclohexyl groups, smoothly underwent hydrocyanation, yielding the corresponding nitriles 5a–c in 79–90% yield with excellent regioselectivity (>98%). Functional groups such as silyl ether, benzyl ether, and chloro moieties on allenes 4d–f were well tolerated under the reaction conditions, producing nitrile-substituted tertiary carbon products 5d–f in yields ranging from 73% to 85%. Moreover, aryl-substituted allene 4g was efficiently converted to the desired nitrile 5g in high yield.
Scheme 4: Hydrocyanation with various monosubstituted allenes. Reaction conditions: allene 4 (0.3 mmol), (iBu)2Al-H (0.3 mmol), IPrCuCl (5 mol %), TsCN (0.25 mmol), THF (0.2 M), under N2. The yields of the isolated products are given.
Scheme 4: Hydrocyanation with various monosubstituted allenes. Reaction conditions: allene 4 (0.3 mmol), (iBu)...
Gram-scale reactions were conducted using allenes to demonstrate the practical applicability of this hydrocyanation method (Scheme 5). Allene 1q (1.04 g, 7.2 mmol) and allene 4b (1.08 g, 6.0 mmol) were effectively transformed into nitrile products 3q and 5b, achieving yields of 93% and 87%, respectively. When the catalyst loading was reduced to 3 mol % for the reaction of allene 4b, the hydroalumination did not reach full conversion even with an extended reaction time (6 h vs 3 h). As a result, the incomplete hydroalumination led to a side reaction between the remaining DIBAL-H and TsCN, ultimately yielding the cyanation product 5b in only 54%.
The synthetic potential of the obtained β,γ-unsaturated nitriles featuring α-quaternary carbon centers was further illustrated using a series of transformations (Scheme 6). Nitrile 3q was hydrolyzed to amide 6 in a 90% yield under basic conditions using sodium hydroxide and tert-butanol. The reduction of nitrile 3q with lithium aluminum hydride generated amine 7 in an 85% yield, whereas the selective hydrogenation of the alkene moiety of 3q using a Pd/C catalyst in a H2 gas environment smoothly produced product 8 in a 98% yield. Ortho-bromoaryl-substituted nitrile 3m also underwent tandem amidation and copper-catalyzed cyclization, efficiently producing lactam 9 in a 98% yield.
Scheme 7 illustrates a plausible reaction mechanism based on previous studies [34]. The process begins with the formation of NHC–copper hydride complex A through the reaction of IPrCuCl with DIBAL-H [35]. Copper hydride species A reacts regioselectively with allene 1 to form the allylcopper intermediate B. Subsequent transmetalation between allyl-Cu B and DIBAL-H generates allylaluminum species C and regenerates IPrCuH (A). The final step involves the regioselective nucleophilic attack of allylaluminum C on tosyl cyanide, which proceeds at the γ-position via six-membered ring transition state D, leading to the formation of the desired nitrile product. Transition state D is responsible for the E-selectivity observed in trisubstituted allenes, as it minimizes the allylic strain between the R and R'' groups.
Conclusion
In this study, we developed a highly regio- and (E)-selective formal hydrocyanation protocol for allenes using a copper-catalyzed hydroalumination/cyanation sequence with DIBAL-H and tosyl cyanide. This approach offers mild reaction conditions, broad functional group compatibility, and high efficiency, enabling the synthesis of new and versatile functionalized β,γ-unsaturated nitriles containing α-all-carbon quaternary centers with exceptional selectivity. The practicality of this approach was validated through gram-scale synthesis and the successful transformation of nitrile products into amines, amides, and lactams. Further studies are underway to broaden the scope and application of the proposed method.
Supporting Information
| Supporting Information File 1: General information, experimental procedures, characterization data and copies of spectra. | ||
| Format: PDF | Size: 2.2 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Zeng, X.-P.; Sun, J.-C.; Liu, C.; Ji, C.-B.; Peng, Y.-Y. Adv. Synth. Catal. 2019, 361, 3281–3305. doi:10.1002/adsc.201900015
Return to citation in text: [1] -
Fleming, F. F.; Yao, L.; Ravikumar, P. C.; Funk, L.; Shook, B. C. J. Med. Chem. 2010, 53, 7902–7917. doi:10.1021/jm100762r
Return to citation in text: [1] -
Fleming, F. F. Nat. Prod. Rep. 1999, 16, 597–606. doi:10.1039/a804370a
Return to citation in text: [1] -
Kern, C.; Wolf, C.; Bender, F.; Berger, M.; Noack, S.; Schmalz, S.; Ilg, T. Insect Mol. Biol. 2012, 21, 456–471. doi:10.1111/j.1365-2583.2012.01151.x
Return to citation in text: [1] -
Cheng, R. C. K.; Tikhonov, D. B.; Zhorov, B. S. J. Biol. Chem. 2009, 284, 28332–28342. doi:10.1074/jbc.m109.027326
Return to citation in text: [1] -
Nakao, Y. Chem. Rev. 2021, 121, 327–344. doi:10.1021/acs.chemrev.0c00301
Return to citation in text: [1] -
Kukushkin, V. Y.; Pombeiro, A. J. L. Chem. Rev. 2002, 102, 1771–1802. doi:10.1021/cr0103266
Return to citation in text: [1] -
Rappoport, Z., Ed. The Cyano Group; PATAI'S Chemistry of Functional Groups; John Wiley & Sons: London, UK, 1970. doi:10.1002/9780470771242
Return to citation in text: [1] -
Sun, X.; Li, B.-J. Synthesis 2022, 54, 2103–2118. doi:10.1055/s-0040-1719899
Return to citation in text: [1] -
Feng, J.; Holmes, M.; Krische, M. J. Chem. Rev. 2017, 117, 12564–12580. doi:10.1021/acs.chemrev.7b00385
Return to citation in text: [1] -
Das, J. P.; Marek, I. Chem. Commun. 2011, 47, 4593–4623. doi:10.1039/c0cc05222a
Return to citation in text: [1] -
Long, J.; Zhao, R.; Cheng, G.-J.; Fang, X. Angew. Chem., Int. Ed. 2023, 62, e202304543. doi:10.1002/anie.202304543
Return to citation in text: [1] -
Chen, L.; Pu, M.; Li, S.; Sang, X.; Liu, X.; Wu, Y.-D.; Feng, X. J. Am. Chem. Soc. 2021, 143, 19091–19098. doi:10.1021/jacs.1c08382
Return to citation in text: [1] -
Long, J.; Xia, S.; Wang, T.; Cheng, G.-J.; Fang, X. ACS Catal. 2021, 11, 13880–13890. doi:10.1021/acscatal.1c03729
Return to citation in text: [1] -
Zhang, H.; Su, X.; Dong, K. Org. Biomol. Chem. 2020, 18, 391–399. doi:10.1039/c9ob02374g
Return to citation in text: [1] -
Wu, W.-B.; Yu, J.-S.; Zhou, J. ACS Catal. 2020, 10, 7668–7690. doi:10.1021/acscatal.0c01918
Return to citation in text: [1] -
Li, G.; Huo, X.; Jiang, X.; Zhang, W. Chem. Soc. Rev. 2020, 49, 2060–2118. doi:10.1039/c9cs00400a
Return to citation in text: [1] -
Yu, S.; Ma, S. Angew. Chem., Int. Ed. 2012, 51, 3074–3112. doi:10.1002/anie.201101460
Return to citation in text: [1] -
Ma, S. Chem. Rev. 2005, 105, 2829–2872. doi:10.1021/cr020024j
Return to citation in text: [1] -
Blieck, R.; Taillefer, M.; Monnier, F. Chem. Rev. 2020, 120, 13545–13598. doi:10.1021/acs.chemrev.0c00803
Return to citation in text: [1] -
Liu, Y.; Bandini, M. Chin. J. Chem. 2019, 37, 431–441. doi:10.1002/cjoc.201800568
Return to citation in text: [1] -
Ahmad, M. S.; Meguellati, K.; Wang, Q. J. Saudi Chem. Soc. 2022, 26, 101483. doi:10.1016/j.jscs.2022.101483
Return to citation in text: [1] -
Tolman, C. A. J. Chem. Educ. 1986, 63, 199–201. doi:10.1021/ed063p199
Return to citation in text: [1] -
Arai, S. Chem. Pharm. Bull. 2019, 67, 397–403. doi:10.1248/cpb.c18-00953
Return to citation in text: [1] -
Arai, S.; Hori, H.; Amako, Y.; Nishida, A. Chem. Commun. 2015, 51, 7493–7496. doi:10.1039/c5cc01899d
Return to citation in text: [1] -
Hori, H.; Arai, S.; Nishida, A. Adv. Synth. Catal. 2017, 359, 1170–1176. doi:10.1002/adsc.201601400
Return to citation in text: [1] -
Ding, Y.; Long, J.; Fang, X. Org. Chem. Front. 2021, 8, 5852–5857. doi:10.1039/d1qo01099a
Return to citation in text: [1] -
Bury, T.; Kullmann, S.; Breit, B. Adv. Synth. Catal. 2023, 365, 335–341. doi:10.1002/adsc.202201189
Return to citation in text: [1] -
Kiyokawa, K.; Hata, S.; Kainuma, S.; Minakata, S. Chem. Commun. 2019, 55, 458–461. doi:10.1039/c8cc09229j
Return to citation in text: [1] [2] -
Lee, S.; Lee, S.; Lee, Y. Org. Lett. 2020, 22, 5806–5810. doi:10.1021/acs.orglett.0c01876
Return to citation in text: [1] [2] -
Yoon, S.; Lee, K.; Kamranifard, T.; Lee, Y. Bull. Korean Chem. Soc. 2022, 43, 1307–1311. doi:10.1002/bkcs.12629
Return to citation in text: [1] -
Lee, S.; Lee, Y. Adv. Synth. Catal. 2023, 365, 4641–4646. doi:10.1002/adsc.202300825
Return to citation in text: [1] -
Lee, K.; Cho, S.; Lim, S.; Lee, Y. Org. Chem. Front. 2024, 11, 1366–1371. doi:10.1039/d3qo01855e
Return to citation in text: [1] -
Nakamura, E.; Mori, S. Angew. Chem., Int. Ed. 2000, 39, 3750–3771. doi:10.1002/1521-3773(20001103)39:21<3750::aid-anie3750>3.0.co;2-l
Return to citation in text: [1] -
Jordan, A. J.; Lalic, G.; Sadighi, J. P. Chem. Rev. 2016, 116, 8318–8372. doi:10.1021/acs.chemrev.6b00366
Return to citation in text: [1]
| 35. | Jordan, A. J.; Lalic, G.; Sadighi, J. P. Chem. Rev. 2016, 116, 8318–8372. doi:10.1021/acs.chemrev.6b00366 |
| 29. | Kiyokawa, K.; Hata, S.; Kainuma, S.; Minakata, S. Chem. Commun. 2019, 55, 458–461. doi:10.1039/c8cc09229j |
| 34. | Nakamura, E.; Mori, S. Angew. Chem., Int. Ed. 2000, 39, 3750–3771. doi:10.1002/1521-3773(20001103)39:21<3750::aid-anie3750>3.0.co;2-l |
| 1. | Zeng, X.-P.; Sun, J.-C.; Liu, C.; Ji, C.-B.; Peng, Y.-Y. Adv. Synth. Catal. 2019, 361, 3281–3305. doi:10.1002/adsc.201900015 |
| 2. | Fleming, F. F.; Yao, L.; Ravikumar, P. C.; Funk, L.; Shook, B. C. J. Med. Chem. 2010, 53, 7902–7917. doi:10.1021/jm100762r |
| 3. | Fleming, F. F. Nat. Prod. Rep. 1999, 16, 597–606. doi:10.1039/a804370a |
| 4. | Kern, C.; Wolf, C.; Bender, F.; Berger, M.; Noack, S.; Schmalz, S.; Ilg, T. Insect Mol. Biol. 2012, 21, 456–471. doi:10.1111/j.1365-2583.2012.01151.x |
| 5. | Cheng, R. C. K.; Tikhonov, D. B.; Zhorov, B. S. J. Biol. Chem. 2009, 284, 28332–28342. doi:10.1074/jbc.m109.027326 |
| 15. | Zhang, H.; Su, X.; Dong, K. Org. Biomol. Chem. 2020, 18, 391–399. doi:10.1039/c9ob02374g |
| 16. | Wu, W.-B.; Yu, J.-S.; Zhou, J. ACS Catal. 2020, 10, 7668–7690. doi:10.1021/acscatal.0c01918 |
| 30. | Lee, S.; Lee, S.; Lee, Y. Org. Lett. 2020, 22, 5806–5810. doi:10.1021/acs.orglett.0c01876 |
| 31. | Yoon, S.; Lee, K.; Kamranifard, T.; Lee, Y. Bull. Korean Chem. Soc. 2022, 43, 1307–1311. doi:10.1002/bkcs.12629 |
| 32. | Lee, S.; Lee, Y. Adv. Synth. Catal. 2023, 365, 4641–4646. doi:10.1002/adsc.202300825 |
| 33. | Lee, K.; Cho, S.; Lim, S.; Lee, Y. Org. Chem. Front. 2024, 11, 1366–1371. doi:10.1039/d3qo01855e |
| 12. | Long, J.; Zhao, R.; Cheng, G.-J.; Fang, X. Angew. Chem., Int. Ed. 2023, 62, e202304543. doi:10.1002/anie.202304543 |
| 13. | Chen, L.; Pu, M.; Li, S.; Sang, X.; Liu, X.; Wu, Y.-D.; Feng, X. J. Am. Chem. Soc. 2021, 143, 19091–19098. doi:10.1021/jacs.1c08382 |
| 14. | Long, J.; Xia, S.; Wang, T.; Cheng, G.-J.; Fang, X. ACS Catal. 2021, 11, 13880–13890. doi:10.1021/acscatal.1c03729 |
| 30. | Lee, S.; Lee, S.; Lee, Y. Org. Lett. 2020, 22, 5806–5810. doi:10.1021/acs.orglett.0c01876 |
| 9. | Sun, X.; Li, B.-J. Synthesis 2022, 54, 2103–2118. doi:10.1055/s-0040-1719899 |
| 10. | Feng, J.; Holmes, M.; Krische, M. J. Chem. Rev. 2017, 117, 12564–12580. doi:10.1021/acs.chemrev.7b00385 |
| 11. | Das, J. P.; Marek, I. Chem. Commun. 2011, 47, 4593–4623. doi:10.1039/c0cc05222a |
| 28. | Bury, T.; Kullmann, S.; Breit, B. Adv. Synth. Catal. 2023, 365, 335–341. doi:10.1002/adsc.202201189 |
| 6. | Nakao, Y. Chem. Rev. 2021, 121, 327–344. doi:10.1021/acs.chemrev.0c00301 |
| 7. | Kukushkin, V. Y.; Pombeiro, A. J. L. Chem. Rev. 2002, 102, 1771–1802. doi:10.1021/cr0103266 |
| 8. | Rappoport, Z., Ed. The Cyano Group; PATAI'S Chemistry of Functional Groups; John Wiley & Sons: London, UK, 1970. doi:10.1002/9780470771242 |
| 29. | Kiyokawa, K.; Hata, S.; Kainuma, S.; Minakata, S. Chem. Commun. 2019, 55, 458–461. doi:10.1039/c8cc09229j |
| 25. | Arai, S.; Hori, H.; Amako, Y.; Nishida, A. Chem. Commun. 2015, 51, 7493–7496. doi:10.1039/c5cc01899d |
| 26. | Hori, H.; Arai, S.; Nishida, A. Adv. Synth. Catal. 2017, 359, 1170–1176. doi:10.1002/adsc.201601400 |
| 22. | Ahmad, M. S.; Meguellati, K.; Wang, Q. J. Saudi Chem. Soc. 2022, 26, 101483. doi:10.1016/j.jscs.2022.101483 |
| 27. | Ding, Y.; Long, J.; Fang, X. Org. Chem. Front. 2021, 8, 5852–5857. doi:10.1039/d1qo01099a |
| 20. | Blieck, R.; Taillefer, M.; Monnier, F. Chem. Rev. 2020, 120, 13545–13598. doi:10.1021/acs.chemrev.0c00803 |
| 21. | Liu, Y.; Bandini, M. Chin. J. Chem. 2019, 37, 431–441. doi:10.1002/cjoc.201800568 |
| 17. | Li, G.; Huo, X.; Jiang, X.; Zhang, W. Chem. Soc. Rev. 2020, 49, 2060–2118. doi:10.1039/c9cs00400a |
| 18. | Yu, S.; Ma, S. Angew. Chem., Int. Ed. 2012, 51, 3074–3112. doi:10.1002/anie.201101460 |
| 19. | Ma, S. Chem. Rev. 2005, 105, 2829–2872. doi:10.1021/cr020024j |
© 2025 Lim et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.