Abstract
Cinnamic acid derivatives represent a significant class of biologically active compounds exhibiting a broad spectrum of activities, such as antifungal, antidengue, antimetastatic, antimicrobial, antibacterial, and anticancer properties. Their preparation has attracted considerable attention due to their versatile applications across the pharmaceutical, food, and chemical sectors. This review elucidates the functional groups of cinnamic acid that are instrumental in the rational design of biologically active derivatives. A comprehensive representative of recent advancements in synthetic methodologies over the past five years is presented, particularly emphasizing the active scaffolds of bioactive cinnamic acid derivatives. The review provides a strategic overview of alternative synthetic routes and highlights the latest innovations, including more efficient, highly selective, and environmentally sustainable approaches. Given the widespread incorporation of the cinnamic acid framework in various therapeutic agents, this review delivers critical insights into a molecular design for hit-to-lead optimization, offering detailed synthetic strategies for diverse functional modifications. By critically examining these methodologies, the paper underscores their role in expanding the utility of cinnamic acid derivatives and addressing prevailing challenges.

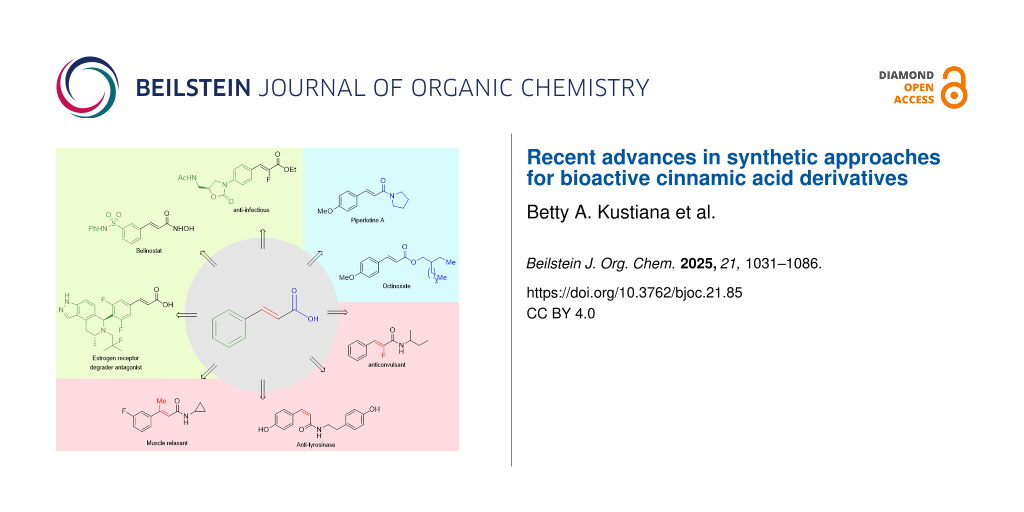
Graphical Abstract
Review
1 Introduction
Cinnamic acid is a naturally occurring plant metabolite frequently found in honey, fruits, and vegetables [1]. Cinnamic acid is biosynthesized through a shikimate pathway, catalyzed by the phenylalanine ammonia-lyase (PAL) enzyme. Generally, cinnamic acid derivatives possess a wide range of bioactivities, such as flavor, fragrance, and therapeutic activities (Figure 1). Several reported activities of cinnamic acid include antibacterial and antifungal properties [1-4], antidengue [5], antimetastatic [6], neuroprotective synergy and angiogenesis effects [7], antileishmaniasis [8], anticancer [9], thromboxane (TXA2) synthetase inhibition [10], antinociceptive [11], histone deacetylase inhibition [12], α-glucosidase inhibition [13] , tyrosinase inhibition [14], allelochemical [15], anticonvulsant [16], antioxidant [17], antiplatelet aggregation [18], anti-inflammatory [19], and UV absorption [20].
Figure 1: Biologically active cinnamic acid derivatives.
Figure 1: Biologically active cinnamic acid derivatives.
Cinnamic acid possesses three distinct functional groups: carboxyl, alkenyl, and aromatic (Scheme 1). Therefore, highly selective reaction strategies are immensely desired for cinnamic acid derivatizations, preventing other functional groups from interfering. This review is partitioned into specific methodologies based on the functional groups of cinnamic acid reported in the last five years: modifying the carboxyl group can involve several pathways, such as O/N-acylation, oxidative acylation, alkenyl/alkynyl carboxylation, and other reactions. Altering the double bond can be approached through double-bond construction, alkyne hydrogenation, ylide and carbene reaction, metathesis, E/Z isomerization, and other methods, including Cα and Cβ functionalizations. Preparing various functional group-tethered aromatic groups can be achieved by directly installing an aromatic group via cross-coupling reactions and other reaction types.
Scheme 1: General synthetic strategies for cinnamic acid derivatizations.
Scheme 1: General synthetic strategies for cinnamic acid derivatizations.
Recent advancements in synthetic chemistry and molecular engineering have created significant opportunities to modify the structural framework of cinnamic acid, resulting in novel derivatives with improved therapeutic potential and enhanced biological efficacy, thus triggering their broader applications in the pharmaceutical industry. In addition, innovative synthetic methodologies, including environmentally sustainable approaches [21-23], advanced catalytic systems [24-26], photocatalysis [27-29], and cutting-edge technologies, such as flow chemistry [30,31], have contributed significantly to the efficient and cost-effective production of cinnamic acid derivatives.
This review focuses on diverse, one-step strategies to access cinnamic acid derivatives with more efficient, highly selective, and sustainable approaches. By examining recent advancements in the design and synthesis of these derivatives, this study aims to offer direct synthetic guidance and important insights into the rational design of novel cinnamate molecules with promising potential as future drug candidates. The reaction mechanisms will be discussed briefly.
2 Carboxyl group functionalization
2.1 O/N-acylations
2.1.1 Stoichiometric reagents: Anhydride formation is one reliable method to activate the carboxylic group of cinnamic acid. For instance, in 2020, Longobardo and DellaGreca utilized isobutyl chloroformate in water to construct an O-protected amide derivative 2 of hydroxycinnamic acid 1 with an excellent yield (Scheme 2) [32]. The formed anhydride 3 was smoothly converted to the amide at room temperature. This method provides a green approach by allowing cinnamic acid derivatization in water as a benign solvent.
Scheme 2: Cinnamic acid coupling via isobutyl anhydride formation.
Scheme 2: Cinnamic acid coupling via isobutyl anhydride formation.
Similarly, Rajendran and Rajan (2023) reported a one-pot transamidation of cinnamamide 4 by utilizing pivaloyl chloride via the N-pivaloyl-activated amide 6 to give piperlotine A (5), the secondary metabolite of black pepper (Piper nigrum) reported to show antibacterial and bioinsecticidal activities, in good yield (Scheme 3A) [33,34]. In the akin process, Xu and co-workers (2023) reported a carboxyl group activation of cinnamic acid (7) by applying pivalic anhydride in a single step to afford the corresponding amide 8 in excellent yield (Scheme 3B) [35]. Moreover, pivalic anhydride is easier to handle than its chloride counterpart.
Scheme 3: Amidation reaction via O/N-pivaloyl activation.
Scheme 3: Amidation reaction via O/N-pivaloyl activation.
Wang and co-workers (2022) utilized 5-nitro-4,6-dithiocyanatopyrimidine (NDTP) as the coupling reagent for cinnamic acid amidation with swift reaction time (Scheme 4) [22]. The reaction of cinnamic acid (7) with NDTP resulted in the active acyl thioester 11 followed by a reaction with an amine to give the corresponding amide 10. The byproduct of the coupling reagent could be recycled by adding POCl3 and KSCN, thus promoting the reagent’s sustainability by reducing waste.
Scheme 4: Cinnamic acid amidation using TCCA/PPh3 reagent.
Scheme 4: Cinnamic acid amidation using TCCA/PPh3 reagent.
Carboxyl group activation can also be achieved by using triazine-based reagents. For example, Saberi and Salimiyan (2019) applied 2,4,6-trichloro-1,3,5-triazine (TCT) to perform amidation of cinnamic acid (7) in a deep eutectic solvent of choline chloride/urea (ChCl/urea) to give amides 12 and 13 in moderate yields via triacylated triazine 14 as the active ester (Scheme 5A) [36]. The TCT reagent and ChCl/urea solvent are known for their non-toxicity and low cost, promoting their wide applications in organic reactions. Similarly, Kunishima and co-workers (2021) utilized (N,N’-dialkyl)triazinedione-4-(dimethylamino)pyridine (ATD-DMAP) for the amidation of cinnamic acid (7) to generate the corresponding amide 10 in excellent yield (Scheme 5B) [37]. Mechanistically, the carboxyl group attacks the electrophilic triazinedione, releasing DMAP to give ester 15 which reacts with DMAP to afford the active N-acylpyridinium species 16.
Scheme 5: Cinnamic acid amidation using triazine-based reagents.
Scheme 5: Cinnamic acid amidation using triazine-based reagents.
In addition, N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDC·HCl), a common coupling reagent, has been applied for a continuous flow mechanochemistry synthesis of cinnamic acid derivatives. Herein, Kulkarni and Atapalkar (2023) converted cinnamic acids into the corresponding amides 17 and 18 and hydrazide 19 in moderate yields (Scheme 6) [30]. Impressively, the reaction capacity could be increased to produce 100 g of the amide products with 90% yields.
Scheme 6: Cinnamic acid amidation using continuous flow mechanochemistry.
Scheme 6: Cinnamic acid amidation using continuous flow mechanochemistry.
Müller and co-workers (2019) reported the synthesis of 6-amino-5-carboxamidouracil derivatives 20 and 21, precursors for A2A antagonists, in good yields by using non-hazardous (1-cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino(morpholino)carbenium hexafluorophosphate (COMU) as the coupling reagent (Scheme 7) [38].
Scheme 7: Cinnamic acid amidation using COMU as coupling reagent.
Scheme 7: Cinnamic acid amidation using COMU as coupling reagent.
By mimicking the electrophilic sp-carbon center of common coupling reagents (e.g., DCC, EDC), Zhao and co-workers (2021) employed allenone 22 as a coupling reagent for amidation [39]. Herein, cinnamic acid was smoothly converted to its corresponding amide 10 in a one-pot two-step amidation in 5 min reaction time via the formation of the isolable enol ester intermediate 23 (Scheme 8A). Similarly, Feng and co-workers (2019) studied one-pot two-step esterification of cinnamic acid (7) by applying electrophilic sp carbon center of methyl propiolate (25) as the coupling reagent via in situ formation of an active enol ester 26. The phenol was formed in situ during the second step from phenylboronic acid oxidation utilizing H2O2 (30%) as green oxidant (Scheme 8B) [40].
Scheme 8: Cinnamic acid amidation using allenone coupling reagent.
Scheme 8: Cinnamic acid amidation using allenone coupling reagent.
Sureshbabu and co-workers (2023) activated the carboxyl group of 4-hydroxycinnamic acid (1) by selectively reacting it with a triflate surrogate, 4-acetamidophenyl triflimide (AITF), to generate the intermediate reactive acyl triflic anhydride 28 which afforded the corresponding amide 27 in good yield (Scheme 9) [41].
Scheme 9: Cinnamic acid amidation using 4-acetamidophenyl triflimide as reagent.
Scheme 9: Cinnamic acid amidation using 4-acetamidophenyl triflimide as reagent.
On the other hand, Braddock and co-workers (2022) employed methyltrimethoxysilane (MTM) to activate the carboxyl group of cinnamic acid (7) to generate the reactive silyl ester 30, which converted to the corresponding amide 29 upon reaction with an amine on a gram-scale operation (Scheme 10) [42].
Scheme 10: Cinnamic acid amidation using methyltrimethoxysilane (MTM).
Scheme 10: Cinnamic acid amidation using methyltrimethoxysilane (MTM).
Ramachandran and co-workers (2020) performed the amidation of cinnamic acid (7) by utilizing stoichiometric amine–BH3 reagent via triacyloxyborane–amine complex 33 to obtain the corresponding amides 31 and 32 in good yields (Scheme 11) [43].
Scheme 11: Cinnamic acid amidation utilizing amine–borane reagent.
Scheme 11: Cinnamic acid amidation utilizing amine–borane reagent.
Moreover, acid halides are widely applied for esterification and amidation. For example, Pattarawarapan and co-workers (2020) reported the amidation of cinnamic acid (7) by using trichloroisocyanuric acid/triphenylphosphine (TCCA/PPh3) assisted by ultrasound to give the corresponding amide 34 in good yield (Scheme 12) [44]. Herein, PPh3 attacks chloride atoms in TCCA to subsequently generate phosphonium intermediate 35, followed by the formation of reactive acid chloride 36.
Scheme 12: Cinnamic acid amidation using TCCA/PPh3 reagent.
Scheme 12: Cinnamic acid amidation using TCCA/PPh3 reagent.
On the other hand, Ma and co-workers (2019) converted cinnamic acids to the corresponding amides 37 and 38 in good yields via the active acyloxyphosphonium iodide species 39 in equilibrium with the acid iodide 40 (Scheme 13) [45]. Subsequently, 39/40 was coupled with 41 prepared via in situ nitro reduction using Mn powder to afford the amide intermediate 42.
Scheme 13: Cinnamic acid amidation using PPh3/I2 reagent.
Scheme 13: Cinnamic acid amidation using PPh3/I2 reagent.
The acid halide once again was applied for cinnamic acid amidation. Xiao and co-workers (2021) performed a transesterification and aminolysis of the tert-butyl ester 43 simply by using PCl3 through in situ generation of the active acid chloride 46 to obtain the corresponding ester 44 and amide products 13 and 45 in good yields (Scheme 14) [46]. In addition, this method has successfully been conducted in gram-scale reactions.
Scheme 14: Cinnamic acid amidation using PCl3 reagent.
Scheme 14: Cinnamic acid amidation using PCl3 reagent.
Cobb and Brittain (2021) reported the amidation of cinnamic acid (7) by applying electrophilic pentafluoropyridine (PFP) via in situ formation of an active acid fluoride 48 to afford the corresponding amides 13 and 47 in moderate yields (Scheme 15) [47]. In addition, the amidation process could be scaled up to a gram scale to give 47 in 90% yield.
Scheme 15: Cinnamic acid amidation utilizing pentafluoropyridine (PFP) as reagent.
Scheme 15: Cinnamic acid amidation utilizing pentafluoropyridine (PFP) as reagent.
Maruoka and co-workers (2021) converted cinnamic esters into active acid fluorides 48 by utilizing hypervalent iodine(III) of PhI(OPiv)2 and py·HF as the fluoride source to afford the corresponding amides 49 and 50 in excellent yields (Scheme 16) [48]. Herein, the hypervalent iodine(III) reagent reacted with the phenol group to give intermediates 51 and 52 followed by the fluoride attack.
Scheme 16: Cinnamic acid amidation using hypervalent iodine(III).
Scheme 16: Cinnamic acid amidation using hypervalent iodine(III).
Shibata and co-workers (2024) developed an amidation process by utilizing 1,1,2,2-tetrafluoroethyl-N,N-dimethylamine (TFEDMA) proceeding via an active acid fluoride in a mechanochemical fashion [49]. In this method, cinnamic acid was reacted with TFEDMA under solvent-free conditions to afford the corresponding acid fluoride 53 in good yield. This was followed by the amidation step to give the corresponding amide 54 in good yield (Scheme 17). The method was also conducted on a gram-scale operation.
Scheme 17: Mechanochemical amidation using 1,1,2,2-tetrafluoroethyl-N,N-dimethylamine (TFEDMA) reagent.
Scheme 17: Mechanochemical amidation using 1,1,2,2-tetrafluoroethyl-N,N-dimethylamine (TFEDMA) reagent.
Nishihara and co-workers (2020) performed methoxylation of acid fluoride 53 by using tris(2,4,6-trimethoxyphenyl)phosphine (TMPP) to obtain methyl cinnamate (44) in good yield (Scheme 18) [50]. Herein, the phosphine group attacks the acid fluoride to give intermediate 55, fluoride attack then triggers methoxy group release.
Scheme 18: Methyl ester preparation using tris(2,4,6-trimethoxyphenyl)phosphine (TMPP).
Scheme 18: Methyl ester preparation using tris(2,4,6-trimethoxyphenyl)phosphine (TMPP).
Toste and co-workers (2021) synthesized N-trifluoromethyl amide 57 from the corresponding acid chloride 46 by employing isothiocyanate in the presence of AgF (Scheme 19) [51]. Herein, fluoride ions attack the isothiocyanate to afford the reactive N-trifluoromethylated secondary amine intermediates 58.
Scheme 19: N-Trifluoromethyl amide preparation using isothiocyanate and AgF.
Scheme 19: N-Trifluoromethyl amide preparation using isothiocyanate and AgF.
Xiao and co-workers (2019) prepared cinnamamide 13 from cinnamic acid (7) and N,N’-dimethylformamide (DMF)-mediated by POCl3 via acid chloride 36 formation (Scheme 20) [52].
Scheme 20: POCl3-mediated amide coupling of carboxylic acid and DMF.
Scheme 20: POCl3-mediated amide coupling of carboxylic acid and DMF.
On top of the carboxyl activation approaches demonstrated above, O/N-acylation could also be achieved by employing an electrophilic alkylating agent by exploiting the nucleophilicity of the O atom of the carboxylate group. For instance, Coote and co-workers (2019) reported electrochemical methylation of cinnamic acid 7 using the TEMPO-Me reagent via reactive radical cation 59 to give the corresponding methyl ester 44 in moderate yield (Scheme 21A) [53]. Wang and co-workers (2019) studied a novel trideuteromethylation reagent, trideuteromethylsulfoxonium iodide (TDMSOI), to convert cinnamic acid (7) into its corresponding trideuteromethyl ester 60 in a one-pot two-step setup with excellent yield and D incorporation (Scheme 21B) [54]. The reagent was prepared by reacting trimethylsulfoxonium iodide (TMSOI) with DMSO-d6, resulting in CH3/CD3 exchange. Furthermore, Chisholm and co-workers (2019) synthesized bulky cinnamate esters 61–64 utilizing a trichloroacetimidate-based alkylating agent in moderate to excellent yields via carbocation 65 formation upon trichloroacetamide release (Scheme 21C) [55].
Scheme 21: O-Alkylation of cinnamic acid using alkylating agents.
Scheme 21: O-Alkylation of cinnamic acid using alkylating agents.
Kawabata and co-workers (2020) prepared the β-glycoside 66 from α-ᴅ-glucose and cinnamic acid (7) in good yield through the Mitsunobu reaction (Scheme 22) [56]. The 13C kinetic isotope effect experiment (KIE = 1.028) showed that the glycosylation proceeded via SN2 substitution (67).
Scheme 22: Glycoside preparation via Mitsunobu reaction.
Scheme 22: Glycoside preparation via Mitsunobu reaction.
Sun and co-workers (2021) utilized oxime chloride and cinnamic acid to synthesize O-acylhydroxamate 68 in good yield (Scheme 23A) [57]. In the presence of a base, oxime chloride was converted to electrophilic nitrile oxide 69 and reacted with carboxylic acid to form the cyclic intermediate 70. On the other hand, Fan and co-workers (2020) prepared α-amidoketone 71 by employing vinyl azide and cinnamic acid (7) in good yield via cascade reaction (Scheme 23B) [58]. The thermal decomposition of the azide led to the generation of the reactive azirine intermediate 72.
Scheme 23: O/N-Acylation via rearrangement reactions.
Scheme 23: O/N-Acylation via rearrangement reactions.
Moreover, Li and co-workers (2020) utilized isothiocyanate and cinnamic acid (7) to prepare the corresponding amide 47 in good yield via a carbamothioic anhydride 74 formation followed by carbonyl sulfide (COS) release (Scheme 24A) [59]. Similarly, Zhao and co-workers (2020) employed tetraalkylthiuram disulfides to synthesize amides via COS release (82). Numerous cinnamic acid derivatives with electron-withdrawing and -donating groups were converted to the corresponding amides 76–81 in moderate to excellent yields (Scheme 24B) [60].
Scheme 24: Amidation reactions using sulfur-based alkylating agents.
Scheme 24: Amidation reactions using sulfur-based alkylating agents.
2.1.2 Transition-metal catalysis: Several transition metals have been exploited to catalyze O/N-acylations of cinnamic acid. For example, Chen and co-workers (2020) reported the Pd-catalyzed N-acylation of cinnamic acids using tertiary amines to generate the corresponding amides 83 and 84 in good yields via C–N cleavage (Scheme 25) [61]. The active Pd0 species was inserted into the carboxylate group to afford the intermediate 85 followed by reductive elimination with tertiary amine to give intermediate 86.
Scheme 25: Amidation reaction catalyzed by Pd0 via C–N cleavage.
Scheme 25: Amidation reaction catalyzed by Pd0 via C–N cleavage.
The utilization of earth-abundant transition metals for O/N-acylation has emerged due to their low cost. For instance, Son and co-workers (2023) utilized a more cost-efficient Cu salt to access N-acyliminophosphorane 89 from the corresponding dioxazolone 88 in excellent yields via reductive elimination from intermediate 90 (Scheme 26) [62].
Scheme 26: Amidation reaction catalyzed by CuCl/PPh3.
Scheme 26: Amidation reaction catalyzed by CuCl/PPh3.
Hu and co-workers (2019) also employed a Cu salt (Cu(OTf)2) to synthesize N-difluoroethylimide 91 from cinnamic acid (7) and tert-butyl nitrite (TBN) in good yield via nitrilium salt 92 followed by carboxylate attack (93) and Mumm rearrangement (Scheme 27) [63].
Scheme 27: Cu(II) triflate-catalyzed N-difluoroethylimide synthesis.
Scheme 27: Cu(II) triflate-catalyzed N-difluoroethylimide synthesis.
Furthermore, Maruoka and co-workers (2020) developed a one-pot transamidation reaction catalyzed by Cu via acid fluoride 48 (Scheme 28) [64]. In this work, single-electron transfer (SET) between Selectfluor and CuBr promoted hydrogen atom abstraction from the amide 94 resulting in the benzylic radical species 95, followed by oxidation to give acyliminium species 96.
Scheme 28: Cu/Selectfluor-catalyzed transamidation reaction.
Scheme 28: Cu/Selectfluor-catalyzed transamidation reaction.
Luque and co-workers (2020) developed a biogenic carbonate of CuO–CaCO3 to catalyze solvent- and additive-free amidation reactions in air, promoting ecocompatibility by minimizing waste. In this study, Cu-incorporated CaCO3 catalyzed the amidation of cinnamic acid (7) to give the corresponding amide 12 in good yield (Scheme 29) [65].
Scheme 29: CuO–CaCO3-catalyzed amidation reaction.
Scheme 29: CuO–CaCO3-catalyzed amidation reaction.
Moreover, Li and co-workers (2022) employed a Ni salt to catalyze the reductive amidation of nitrobenzene and N-acylbenzotriazole 97 via the Ni(II)-nitrene species 98 to afford its corresponding amide 47 in moderate yield (Scheme 30) [66].
Scheme 30: Ni-catalyzed reductive amidation.
Scheme 30: Ni-catalyzed reductive amidation.
Shimizu and co-workers (2019) reported a CeO2-catalyzed esterification of unactivated cinnamamide (99) and phenol in solvent-free conditions to afford the corresponding amide 24 in good yield. The reaction proceeds via a CeO2-coordinated carboxylate mode (Scheme 31A) [67]. In addition, the catalyst also offered high reusability for up to 4 runs thus further promoting the eco-friendliness.
Scheme 31: Lewis acidic transition-metal-catalyzed O/N-acylations.
Scheme 31: Lewis acidic transition-metal-catalyzed O/N-acylations.
Carbonyl activation via Lewis acid–O=C interaction has also been achieved using other transition metals, such as Yb and Ti. Salunke and co-workers (2020) utilized Yb(OTf)3 to catalyze the Boc-protection of cinnamic acid (7) via the formation of the chelate complex 101 between Boc2O and Yb(OTf)3 (Scheme 31B) [68]. Recently, Ramachandran and Alawaed (2024) reported a Ti-catalyzed direct amidation of cinnamic acid (7) to give amide 12 in excellent yield via Ti(IV)–O=C complex 102 (Scheme 31C) [69].
2.1.3 Photocatalysis: Photoredox catalysis has gained much attention as a sustainable alternative approach to performing O/N-acylation by utilizing light as a renewable source. For example, Li and co-workers (2022) investigated the visible-light-mediated amidation of cinnamic acid (7) by using ethyl 2-diazoacetate and acetonitrile to give its corresponding amide 103 in excellent yield (Scheme 32) [29]. The reactive free carbene 104 was released upon light exposure of the diazo ester leading to the nitrilium ion 105 formation via its reaction with acetonitrile.
Scheme 32: Visible-light-promoted amidation of cinnamic acid.
Scheme 32: Visible-light-promoted amidation of cinnamic acid.
Kokotos and co-workers (2023) prepared Weinreb amide 107 mediated by sunlight or LED 370 nm from cinnamic acid (7) via light-activated DMAP 108, leading to electrophilic iminium 109 formation (Scheme 33) [28].
Scheme 33: Sunlight/LED-promoted amidation of cinnamic acid.
Scheme 33: Sunlight/LED-promoted amidation of cinnamic acid.
On the other hand, Gilmour and co-workers (2022) functionalized Weinreb amides through organophotocatalytic N–O cleavage via 114 and 115 to give the corresponding primary amides 111–113 in good yields (Scheme 34) [27].
Scheme 34: Organophotocatalyst-promoted N–O cleavage of Weinreb amides to synthesize primary amides.
Scheme 34: Organophotocatalyst-promoted N–O cleavage of Weinreb amides to synthesize primary amides.
Xie and co-workers (2022) synthesized cinnamamide 83 mediated by [Ir(dF(CF3)ppy)2(dtbbpy)]PF6 (PC-1) as photocatalyst proceeding via C–N-bond cleavage of the oxidized tertiary amine 116 (Scheme 35) [70]. Cinnamic acid (7) was activated by forming the acyl radical 118 after −OPyf group cleavage from 117.
Scheme 35: Cinnamamide synthesis through [Ir] photocatalyst-promoted C–N-bond cleavage of tertiary amines.
Scheme 35: Cinnamamide synthesis through [Ir] photocatalyst-promoted C–N-bond cleavage of tertiary amines.
Recently, Li and co-workers (2024) studied visible-light-mediated FeCl3-catalyzed reductive transamidation of nitro compounds and N-acylbenzotriazole 97 (Scheme 36) [71]. In this work, the photoactive [FeCl4]− formed in situ triggered silyl radical 119 generation, leading to N-silylamine 120 as the active amine nucleophile.
Scheme 36: Blue LED-promoted FeCl3-catalyzed reductive transamidation.
Scheme 36: Blue LED-promoted FeCl3-catalyzed reductive transamidation.
2.1.4 Metal-free catalysis: Despite the wide applications of metal-based catalysts in developing O/N-acylation reactions, metal catalysts, particularly precious transition metals, are considered less sustainable due to their limited availability. Therefore, metal-free catalysis methods have emerged as an alternative to respond to the green chemistry agenda. For instance, Huy and Mbouhom (2019) employed N-formylpyrrolidine (FPyr) and trichlorotriazine (TCT) to catalyze the amidation of cinnamic acid derivative 121. The reaction proceeds via formation of the reactive acid chloride 36 through cascade reaction involving reactive intermediates 123 and 124 (Scheme 37) [72].
Scheme 37: FPyr/TCT-catalyzed amidation of cinnamic acid derivative 121.
Scheme 37: FPyr/TCT-catalyzed amidation of cinnamic acid derivative 121.
Zeng and co-workers (2019) used activated amide 125 to access ester 126 via formation of the reactive carboxyl radical 128 mediated by DMAP and Cs2CO3 (Scheme 38) [73].
Scheme 38: Cs2CO3/DMAP-mediated esterification.
Scheme 38: Cs2CO3/DMAP-mediated esterification.
Dong and co-workers (2020) reported an atroposelective N-acylation of mixed anhydride 129 catalyzed by the isothiourea organocatalyst homobenzotetramisole (HBTM) (Scheme 39) [74]. In this work, HBTM reacted with the anhydride 129 to give the acylisothiouronium intermediate followed by amine attack (131). The reaction capacity has been successfully increased to a gram scale. In the same year, a similar work was reported by Zhao and co-workers [75].
Scheme 39: HBTM organocatalyzed atroposelective N-acylation.
Scheme 39: HBTM organocatalyzed atroposelective N-acylation.
On the other hand, carboxyl activation via Lewis acid–O=C interaction has also been developed. Ramachandran and Hamann (2021) directly prepared the amide 12 from cinnamic acid (7) catalyzed by BH3. The reaction proceeds via formation of the triacyloxyborane–amine complex intermediate 132 (Scheme 40A) [76]. The same group (2024) also investigated BH3·pyridine to catalyze amidation reactions with lower catalyst loading (Scheme 40B) [77].
Scheme 40: BH3-catalyzed N-acylation reactions.
Scheme 40: BH3-catalyzed N-acylation reactions.
Whiting and co-workers (2019) also used boranes to catalyze the direct amidation of carboxylic acids. In this work, they co-polymerized styrene, divinylbenzene and vinylphenylboronic acid to synthesize the solid-supported phenylboronic acid catalyst (cat 1) which was used to convert cinnamic acid (7) to its corresponding amide 12 in moderate yield. The reaction involves dicarboxylate complex 135 formed through Lewis acid B–O=C interaction (Scheme 41A) [23]. The catalyst could be reused multiple times without significant loss of activity.
Scheme 41: Borane-catalyzed N-acylation reactions.
Scheme 41: Borane-catalyzed N-acylation reactions.
Using B(C6F5)3 as catalyst, Wu and co-workers (2021) developed a borane-catalyzed Fischer esterification of cinnamic acids with methanol to afford the corresponding methyl cinnamates 136 and 137 via (C6F5)3B–O=C interaction (138) (Scheme 41B) [78].
Shankarling and co-workers (2020) directly prepared amide 12 from cinnamic acid (7) under solvent-free conditions catalyzed by graphene oxide via hydrogen-bonding activation (139) (Scheme 42A) [79]. The catalyst could be recycled multiple times without significant activity loss. Similarly, Božić and co-workers (2022) reported the microwave-assisted direct amidation reaction of cinnamic acid (7) catalyzed by N-fluorobenzenesulfonimide (NFSi) via halogen bonding activation (Scheme 42B) [80]. In addition, the method has been successfully scaled up to a gram scale.
Scheme 42: Catalytic N-acylation reactions via H/F bonding activation.
Scheme 42: Catalytic N-acylation reactions via H/F bonding activation.
Activating a nucleophile with a catalytic base has also been applied to prepare cinnamate esters. For example, Heller and co-workers (2021) synthesized the ester 141 catalyzed by DBU which functioned as a Brønsted base for the alcohol (Scheme 43A) [81]. Impressively, the method could be scaled up to a multigram scale. Similarly, Rajendran and Rajan (2024) utilized catalytic DABCO to perform the esterification of cinnamamide 99, where DABCO acted as a hydrogen-bond acceptor for phenol (Scheme 43B) [82].
Scheme 43: Brønsted base-catalyzed synthesis of cinnamic acid esters.
Scheme 43: Brønsted base-catalyzed synthesis of cinnamic acid esters.
Yuan-Yong and co-workers (2022) reported a DABCO/Fe3O4-catalyzed N-methyl amidation of cinnamic acid 122 via cooperation reaction (143) to activate the isothiocyanate as the coupling partner (Scheme 44) [83].
Scheme 44: DABCO/Fe3O4-catalyzed N-methyl amidation of cinnamic acid 122.
Scheme 44: DABCO/Fe3O4-catalyzed N-methyl amidation of cinnamic acid 122.
On the other hand, nucleophile activation could also be achieved via a catalytic oxidation reaction. For instance, Ablajan and co-workers (2024) reported the preparation of phenyl cinnamate (24) starting from the acid chloride 46 and phenylboronic acid in the presence of TBHP and catalytic amounts of K2S2O8. Under these conditions, phenol is formed through oxidative hydroxylation which reacts with 46 to give product 24 in good yield (Scheme 45A) [84]. The method has been scaled up to a gram scale. Similarly, Chi and co-workers (2019) reported an enantioselective esterification via oxidative N-heterocyclic carbene (NHC)-catalyzed phthalaldehyde activation to form azolium ester intermediate 147 to give the chiral phthalidyl ester 146 with excellent enantiomeric ratio (Scheme 45B) [85]. Additionally, the reaction capacity could be increased to a gram scale.
Scheme 45: Catalytic oxidation reactions of acylating agents.
Scheme 45: Catalytic oxidation reactions of acylating agents.
Moreover, catalytic oxidation was also applied to prepare an electrophilic coupling partner. Gilmour and co-workers (2023) demonstrated the I(I)–I(III) catalytic cycle by using p-iodotoluene (cat 2) as the catalyst to activate 2-phenethyl-substituted 1,3-diene 148 with nucleophilic cinnamonitrile 149 to give benzocyclooctene 150 via iodonium intermediate 151 (Scheme 46) [86].
Scheme 46: Preparation of cinnamamide-substituted benzocyclooctene using I(I)/I(III) catalysis.
Scheme 46: Preparation of cinnamamide-substituted benzocyclooctene using I(I)/I(III) catalysis.
2.2 Oxidative acylations
Cinnamic ester or amide preparation could also be achieved by oxidizing cinnamyl alcohol, aldehyde, imine, and ketone as an alternative to the traditional O/N-acylation of cinnamic acid above.
2.2.1 Alcohol oxidation: Kapdi and co-workers (2019) reported Pd-colloids-catalyzed esterification via Ag2O-catalyzed alcohol oxidation. Herein, cinnamyl alcohols were oxidized to the corresponding cinnamaldehydes catalyzed by Ag2O, followed by oxidative addition to Pd via 153 and 154 to give the corresponding esters 44 and 152 driven by MeOH attack (Scheme 47) [87].
Scheme 47: Pd-colloids-catalyzed oxidative esterification of cinnamyl alcohol.
Scheme 47: Pd-colloids-catalyzed oxidative esterification of cinnamyl alcohol.
Hu and co-workers (2021) developed an N-doped carbon black-supported PdBi bimetallic catalyst (Pd5Bi5/NCB) for the oxidative esterification of cinnamyl alcohols via hemiacetal 156 oxidation (Scheme 48A) [88]. A similar reaction, reported by Zheng and co-workers (2020) utilized a graphene-supported Au/Pd catalyst to achieve the aerobic oxidative esterification of cinnamyl alcohol 157 via oxidation of the hemiacetal embedded in the catalyst surface 158 to obtain the corresponding ester 44 in quantitative yield (Scheme 48B) [89].
Scheme 48: Graphene-supported Pd/Au alloy-catalyzed oxidative esterification via hemiacetal intermediate.
Scheme 48: Graphene-supported Pd/Au alloy-catalyzed oxidative esterification via hemiacetal intermediate.
Furthermore, Doris and co-workers (2019) also utilized a Au catalyst for oxidative esterification reactions. Herein, Au nanoparticles supported on carbon nanotubes (AuCNT) catalyzed the conversion of cinnamyl alcohol 157 to the corresponding ester 44 via hemiacetal-anchored on the catalyst surface 159 using air as O2 source (Scheme 49A) [24]. On the other hand, Wang and co-workers (2019) employed a porous boron nitride (pBN)-supported Au catalyst (Au/pBN) to prepare the ester 44, also via the formation of hemiacetal under O2 atmosphere (Scheme 49B) [25].
Scheme 49: Au-supported on A) carbon nanotubes (CNT) and B) on porous boron nitride (pBN) as catalyst for the oxidative esterification of cinnamyl alcohol.
Scheme 49: Au-supported on A) carbon nanotubes (CNT) and B) on porous boron nitride (pBN) as catalyst for the ...
Wei and co-workers (2021) developed a Cr-based catalyst stabilized by a pentaerythritol-decorated Anderson-type polyoxometalate, [N(C4H9)4]3[CrMo6O18(OH)3C{(OCH2)3CH2-OH}] (cat 3), to catalyze the oxidative esterification of cinnamyl alcohols using H2O2. The reaction proceeds also via a hemiacetal intermediate 155 (Scheme 50) [90].
Scheme 50: Cr-based catalyzed oxidative esterification of cinnamyl alcohols with H2O2 as the oxidant.
Scheme 50: Cr-based catalyzed oxidative esterification of cinnamyl alcohols with H2O2 as the oxidant.
Utilizing earth-abundant transition metals, such as Fe, Cu, Ni, and Co, has been considered more sustainable due to their abundance. Several studies employing earth-abundant transition metals for oxidative esterifications of cinnamyl alcohols have flourished in recent years. For instance, Zheng and co-workers (2022) prepared the ester 44 via oxidative esterification of cinnamyl alcohol 157 catalyzed by an N-doped porous carbon-encapsulated Au-doped Co catalyst (AuxCo@NC) via the formation of hemiacetal intermediate 156 (Scheme 51A) [91]. Similarly, Leng and co-workers (2020) designed a Co/Cu nanoparticle-co-decorated nitrogen-doped carbon catalyst (CoCu@NCn) which was used to catalyze the oxidative esterification of cinnamyl alcohol (157) without the need of a base additive (Scheme 51B) [26].
Scheme 51: Co-based catalysts used for oxidative esterification of cinnamyl alcohol.
Scheme 51: Co-based catalysts used for oxidative esterification of cinnamyl alcohol.
2.2.2 Aldehyde/ketone oxidation: By employing non-precious transition metals, Wei and co-workers (2019) prepared a ring-like polyoxometalate (POM) inorganic ligand-supported Fe(III) catalyst (FePOM) to convert cinnamaldehyde (162) into methyl cinnamate (44) in good yield. The reaction proceeds through hemiacetal-attached on the catalyst surface 163 (Scheme 52A) [92]. Similarly, Erande and co-workers (2023) employed a Cu(II) square-planar complex {[CuIIL] LH2, 9,9’-(ethane-1,2-diylbis(azanediyl))bis(1H-phenalen-1-one} to convert cinnamaldehyde (162) into methyl cinnamate (44) in the presence of H2O2 as green oxidant via intermediate 164 formation (Scheme 52B) [93].
Scheme 52: Iron (A) and copper (B)-catalyzed oxidative esterification of cinnamaldehyde.
Scheme 52: Iron (A) and copper (B)-catalyzed oxidative esterification of cinnamaldehyde.
Using a different earth-abundant transition metal, Patel and Patel (2020) utilized a Ni salt of phosphomolybdic acid (NiHPMA) to synthesize methyl cinnamate (44) from cinnamaldehyde (162) via a reactive peroxo species 165 (Scheme 53) [94].
Scheme 53: NiHPMA-catalyzed oxidative esterification of cinnamaldehyde.
Scheme 53: NiHPMA-catalyzed oxidative esterification of cinnamaldehyde.
Beyond transition metals, metal-free oxidative esterifications, such as carbene-based reactions, have also been explored, thus boosting sustainability value. For instance, Wang and co-workers (2019) synthesized benzyl cinnamate (166) from cinnamaldehyde (162) catalyzed by an NHC catalyst (cat 4) in the presence of the low-cost oxidant CCl3CN. The reaction involves formation of acyl azolium intermediate 168 formed through hydride transfer from 167 (Scheme 54A) [95]. Similarly, Huang and co-workers (2020) employed the same NHC catalyst but used ambient air as the external oxidant for acyl azolium intermediate formation (Scheme 54B) [96]. On the other hand, Ohshima and co-workers (2020) directly used acylimidazole 170 to prepare methyl cinnamate (44) without preactivation (Scheme 54C) [97].
Scheme 54: Synthesis of cinammic acid esters through NHC-catalyzed oxidative esterification via intermolecular oxidation.
Scheme 54: Synthesis of cinammic acid esters through NHC-catalyzed oxidative esterification via intermolecular...
Recently, Sundén and co-workers (2024) applied a hybrid NHC-based catalyst (cat 5) which, in its active form ox-cat 5, converts cinnamaldehyde (162) to the corresponding esters 24, 171–173 via internal oxidation of azolium intermediate 174 under ambient air conditions (Scheme 55). The reduced catalyst red-cat 5 is reoxidized by the co-catalyst Fe(III) phthalocyanine (FePc), closing the catalytic cycle [98].
Scheme 55: Redox-active NHC-catalyzed esterification via intramolecular oxidation.
Scheme 55: Redox-active NHC-catalyzed esterification via intramolecular oxidation.
On the other hand, Syaikh and co-workers (2021) converted cinnamaldehyde (162) into methyl cinnamate (44) via an electrochemical method using TBAF as the supporting electrolyte (Scheme 56) [99]. Under these conditions, the aldehyde was oxidized to give an oxonium cation intermediate 176.
Scheme 56: Electrochemical conversion of cinnamaldehyde to methyl cinnamate.
Scheme 56: Electrochemical conversion of cinnamaldehyde to methyl cinnamate.
Moreover, Babu and co-workers (2024) oxidized an imine, cinnamalaldehyde N-tosylhydrazone (177), by using TBHP and a catalytic amount of Bu4NI to synthesize bisamide 178. The reaction proceeds through intermediates 179–181 (Scheme 57) [100]. In addition, the method has been successfully scaled up to a gram scale.
Scheme 57: Bu4NI/TBHP-catalyzed synthesis of bisamides from cinnamalaldehyde N-tosylhydrazone.
Scheme 57: Bu4NI/TBHP-catalyzed synthesis of bisamides from cinnamalaldehyde N-tosylhydrazone.
Han and co-workers (2021) converted ketone 182 into methyl cinnamate (44) catalyzed by Zn(II)-coordinated to microporous N-doped carbon (Zn/NC-950). the reaction proceeds through homolytic β-scission involving intermediates 183 and 184 (Scheme 58) [101].
Scheme 58: Zn/NC-950-catalyzed oxidative esterification of ketone 182.
Scheme 58: Zn/NC-950-catalyzed oxidative esterification of ketone 182.
2.3 Alkenyl/alkynyl carboxylation
2.3.1 Alkenyl carboxylation: Li and co-workers (2021) investigated a photoinduced oxidative alkoxycarbonylation of styrenes with alkyl formates and the oxidant 4-cyano-1-(1-methylethoxy)pyridinium trifluoromethanesulfonate to give the corresponding cinnamate esters 185–192 via intermolecular addition of styrene to an alkoxyradical forming radical adduct 193 (Scheme 59) [102].
Scheme 59: Ru-catalyzed oxidative carboxylation of terminal alkenes.
Scheme 59: Ru-catalyzed oxidative carboxylation of terminal alkenes.
Iwasawa and co-workers (2020) employed CO2 to perform the carboxylation of alkenylpyrazole 194 to the corresponding cinnamic ester 195 catalyzed by Rh(III) via a pyrazole-directed oxidative addition of the alkenyl C–H (196) accompanied by CO2 insertion to give Rh(I) carboxylate intermediate 197 (Scheme 60A) [103]. A gram scale operation has been successfully done for this method. Similarly, Hou and co-workers (2022) also used CO2 to carry out an auto-tandem Cu-catalyzed carboxylation of styrenes via β-hydride elimination (208) (Scheme 60B) [104]. Impressively, several natural product-like compounds (e.g., 207) were successfully prepared using this method.
Scheme 60: Direct carboxylation of alkenes using CO2.
Scheme 60: Direct carboxylation of alkenes using CO2.
Furthermore, Dai and co-workers (2020) employed alkenylboronic acid 209 and O-methyl S-p-tolyl thiocarbonate to prepare methyl cinnamate (44) catalyzed by Pd2dba3 in the presence of Cu(I) thiophene-2-carboxylate (CuTC) and the ligand tri(2-furyl)phosphine (TFP). The reaction proceeds via oxidative Pd insertion into the Cu-activated thioester followed by transmetalation with alkenylboronic acid to give complex 210 (Scheme 61A) [105]. The method has been scaled up to a gram scale. Similarly, Hu and co-workers (2021) utilized alkenylboronic ester 211 and Boc2O to synthesize methyl cinnamate (44). The reaction is catalyzed by Cu(I) and 4,4’-dimethyl-2,2’-bipyridine as the ligand and proceeds via organocopper intermediate 212 followed by carbonate 213 formation (Scheme 61B) [106]. In addition, a gram scale reaction has been smoothly conducted.
Scheme 61: Carboxylation of alkenylboronic acid/ester.
Scheme 61: Carboxylation of alkenylboronic acid/ester.
Yu and co-workers (2019) reported a Cu-catalyzed carboxylation of gem-difluoroalkenes with CO2 to give the corresponding α-fluoro methyl cinnamates 214–217 via transmetallation and carboxylation (219 and 218) (Scheme 62A) [107]. The thus-obtained α-fluorocinnamic acid was successfully converted into the bioactive compounds 220 and 221. In addition, the reaction capacity could be increased to a gram-scale operation. Also utilizing gem-difluoroalkenes, Zhou and co-workers (2020) performed a direct electrochemical carboxylation with CO2 to the corresponding α-fluoro methyl cinnamates 222–225 via formation of radical anion 226 followed by carboxylation (227) (Scheme 62B) [108].
Scheme 62: Carboxylation of gem-difluoroalkenes with CO2.
Scheme 62: Carboxylation of gem-difluoroalkenes with CO2.
Moreover, Feng and co-workers (2019) employed gem-difluoroalkenes and CO2 to perform a photoredox/Pd dual-catalyzed carboxylation reaction affording the corresponding methyl cinnamates 223, 228–232. The reaction proceeds via photochemical-induced formation of fluoroalkenyl radical 233, followed by Pd insertion (234) and carboxylation (235) (Scheme 63A) [109]. Recently, Wang and co-workers (2024) reported a photoredox-promoted carboxylation of gem-difluoroalkene 236 by using formate salts, which also involves formation of a fluoroalkenyl radical intermediate 238 (Scheme 63B) [110].
Scheme 63: Photoredox-catalyzed carboxylation of difluoroalkenes.
Scheme 63: Photoredox-catalyzed carboxylation of difluoroalkenes.
On the other hand, Yao and co-workers (2023) used alkenyl halides 240 and formate salts to prepare cinnamic ester 24 catalyzed by a Ru complex via an oxidative addition/reductive elimination cycle involving intermediates 241–243 (Scheme 64) [111]. The method has been successfully scaled up to a gram scale.
Scheme 64: Ru-catalyzed carboxylation of alkenyl halide.
Scheme 64: Ru-catalyzed carboxylation of alkenyl halide.
Uozumi and co-workers (2019) reported a carbonylation under aqueous flow conditions using alkenyl halide 240 to prepare cinnamic acid (7) catalyzed by an amphiphilic polystyrene-poly(ethylene glycol) resin-supported Pd-diphenylphosphine catalyst (cat 6) (Scheme 65A) [31]. Also using flow conditions, Kim and co-workers (2021) reported the cis–trans isomerization of α-functionalized stilbenes in a flow microreactor (Scheme 65B) [112]. The isomerization could be regioselectively controlled in an incredibly short time within milliseconds (247, 248) to give either the E-isomeric product 245 or the corresponding Z-isomeric product 246, both in good yields.
Scheme 65: Carboxylation of alkenyl halides under flow conditions.
Scheme 65: Carboxylation of alkenyl halides under flow conditions.
Alkenyl sulfides have also been used for the preparation of cinnamic acid esters. For example, Wu and co-workers (2020) studied the Pd-catalyzed carbonylation of alkenyl sulfides in the presence of NHC ligands via C–S cleavage (252) to afford the corresponding cinnamic esters 249–251 (Scheme 66A) [113]. Similarly, Chen and co-workers (2023) utilized alkenyl sulfones and CO2 to synthesize cinnamic acid ester 253–255 via an electrochemical set-up by generating the reactive intermediate 256 or 257 (Scheme 66B) [114].
Scheme 66: Cinnamic acid ester syntheses through carboxylation of alkenyl sulfides/sulfones.
Scheme 66: Cinnamic acid ester syntheses through carboxylation of alkenyl sulfides/sulfones.
An interesting transformation of cinnamic acid to its derivatives can be achieved through decarboxylative cross-coupling. Recently, Wang and co-workers (2024) reported the Ag-catalyzed decarboxylative cross-coupling of cinnamic acids with isocyanide to give the corresponding amides 258–260. The reaction involves a vinylic radical intermediate 261 (Scheme 67) [115].
Scheme 67: Cinnamic acid derivatives synthesis through a Ag-catalyzed decarboxylative cross-coupling proceeding via a radical mechanism.
Scheme 67: Cinnamic acid derivatives synthesis through a Ag-catalyzed decarboxylative cross-coupling proceedin...
2.3.2 Alkynyl carboxylation: Zhu and co-workers (2023) reported a Pd-catalyzed hydrocarbonylation of phenylacetylene (264) using CO and hydrosilane as the hydrogen source to prepare cinnamamides 8, 265–269 in good yields with high linear-to-branched (L/B) selectivity of >20:1. The reaction involves the formation of palladium hydride (Pd–H) 270 as the key species followed by alkyne and CO insertion reactions via 271 and 272 (Scheme 68A) [116]. On the one hand, Jia and co-workers (2021) utilized environmentally benign water as the hydrogen source to perform the Pd-catalyzed alkyne hydrocarbonylation with CO via 276 to obtain the corresponding cinnamic acids 273–275 in good yields (Scheme 68B) [117]. In addition, a gram scale operation has been carried out.
Scheme 68: Pd-catalyzed alkyne hydrocarbonylation.
Scheme 68: Pd-catalyzed alkyne hydrocarbonylation.
Li and co-workers (2019) employed a non-precious transition metal, ligand-free Fe3(CO)12, to catalyze the alkyne hydrocarbonylation using CO and ZrF4 as the co-catalyst. The reaction afforded the corresponding amides 133, 277, and 278 in good yields via acyl carbonyl iron intermediate 280, which was observed by NMR (Scheme 69) [118].
Scheme 69: Fe-catalyzed alkyne hydrocarbonylation.
Scheme 69: Fe-catalyzed alkyne hydrocarbonylation.
By applying less toxic and safer CO2 instead of CO, Jiang and co-workers (2020) reported a Pd-catalyzed hydrocarboxylation of alkynes with high regioselectivity to obtain the corresponding cinnamic acids 281 and 282 in good yields via cyclopalladation intermediate 283 (Scheme 70A) [119]. The method has been scaled up to a gram scale operation. Furthermore, Sato and co-workers (2019) employed an air-stable Ni nanoparticle supported on sulfur-modified gold (SANi) to convert alkynes to the corresponding cinnamic acids 285–287 (Scheme 70B) [120]. In addition, the SANi catalyst could be recycled without significant loss of activity.
Scheme 70: Alkyne hydrocarboxylation using CO2.
Scheme 70: Alkyne hydrocarboxylation using CO2.
By generating CO in situ from HCO2H, He and co-workers (2022) developed a Pd-catalyzed hydrocarboxylation of alkynes to the corresponding cinnamic acids 288 and 289 in good yields (Scheme 71A) [121]. Mechanistically, HCO2H protonates the Pd-coordinated alkyne to give the alkenyl–Pd intermediate 290, accompanied by CO insertion to afford the acylpalladium intermediate 291. The same group (2021) also developed a Cu/Pd dual catalysis method to carry out the alkyne hydrocarboxylation using HCO2H as the CO surrogate to give the corresponding cinnamic acids 292 and 293 via the generation of vinylcopper species 294 followed by CO insertion (295) and transmetalation with palladium hydride to afford the acylpalladium species 296 (Scheme 71B) [122].
Scheme 71: Alkyne hydrocarboxylation using HCO2H as CO surrogate.
Scheme 71: Alkyne hydrocarboxylation using HCO2H as CO surrogate.
Utilizing a non-precious transition metal as an alternative for Pd, Yoshikai and co-workers (2020) reported the cooperative cobalt/Lewis acidic AlMe3-catalyzed hydrocarboxylation of alkynes 297 with N,N’-dimethylformamide (DMF) to afford the corresponding cinnamamide 298 via ligand-to-ligand hydrogen transfer 299 (Scheme 72) [123].
Scheme 72: Co/AlMe3-catalyzed alkyne hydrocarboxylation using DMF.
Scheme 72: Co/AlMe3-catalyzed alkyne hydrocarboxylation using DMF.
Furthermore, Song and co-workers (2023) employed propargylic ester 300 to prepare the corresponding cinnamic esters/amides with excellent anti-Markovnikov selectivity catalyzed by Au(III) in the presence of dimethylaminopyridine N-oxide (DMAPO) as oxidant. The reaction proceeds via Au–allenylidene species 307 (Scheme 73) [124]. Various cinnamic esters and amides with natural product-based alkoxy groups 301–304 and sulfoximines 305 and 306, a relatively new prima donna of functional groups explored in drug design, were smoothly prepared.
Scheme 73: Au-catalyzed oxidation of Au–allenylidenes.
Scheme 73: Au-catalyzed oxidation of Au–allenylidenes.
2.4 Miscellaneous reactions
Cyclopropenone, the smallest Hückel aromatic system, has been subjected to the ring opening reactions, driven by the release of ring strain. Numerous transformations of cyclopropenone based on C–C bond activations have been explored. For instance, Ravikumar and co-workers (2021) employed diphenylcyclopropenone (309) to synthesize the corresponding esters 310–313 and amides 314 and 315. The reaction was catalyzed by Pd/N-heterocyclic carbene via oxidative Pd insertion into the C–C bond of cyclopropenone to give a cyclic intermediate 316 (Scheme 74A) [125]. On the other hand, Wu and co-workers (2022) developed a Pd-catalyzed selective ring-opening of cyclopropenones and vinyl epoxide 318 to give the corresponding esters 319–321 in good yields via a π–allyl palladium intermediate 323 (Scheme 74B) [126].
Scheme 74: Pd-catalyzed C–C-bond activation of cyclopropenones to synthesize unsaturated esters and amides.
Scheme 74: Pd-catalyzed C–C-bond activation of cyclopropenones to synthesize unsaturated esters and amides.
In addition, Sun and co-workers (2020) utilized diphenylcyclopropenone (309) and nitrones to access the corresponding imides 324–327 via a Ag-mediated β-carbon elimination promoted ring-opening reaction involving species 328 and 329 (Scheme 75A) [127]. The method could be scaled up to a gram scale operation. Similarly, Liu and co-workers (2020) reported a Ag2O-catalyzed ring-opening of cylopropenone 309 with oximes to give the corresponding series of novel 1,3-oxazinones 330–332 in good yields. The reaction involves a [4 + 2] cycloaddition (334) of the fragmented cyclopropenone 333 followed by the second cyclopropenone ring-opening (335) (Scheme 75B) [128].
Scheme 75: Ag-catalyzed C–C-bond activation of diphenylcyclopropenone.
Scheme 75: Ag-catalyzed C–C-bond activation of diphenylcyclopropenone.
Leyva-Pérez and co-workers (2022) reported the esterification and amidation of diphenylcyclopropenone (309) with alcohols and amines, respectively, catalyzed by Cu(II) (Scheme 76A) [129]. In addition, they prepared a multimetal-organic framework (M-MOF, M= Cu, Ni) which successfully catalyzed the one-pot cyclopropenone hydration/Chan–Lam coupling reaction of 309 and boronic acid 341 to give the corresponding ester 310 in good yield via the formation of acid 342 (Scheme 76B).
Scheme 76: Cu-catalyzed C–C bond activation of diphenylcyclopropenone.
Scheme 76: Cu-catalyzed C–C bond activation of diphenylcyclopropenone.
Cyclopropenone was also subjected to non-metal-catalyzed ring-opening reactions, e.g., by using PPh3. For instance, Reddy and co-workers (2024) utilized PPh3 to catalyze the esterification of diphenylcyclopropenone (309) with a series of natural products of the coumarin family to give the corresponding esters 311, 343, and 344 in good yields via the formation of an active α-ketenyl phosphorus ylide 345 (Scheme 77A) [130]. Similarly, Lin and co-workers (2024) employed PPh3 to selectively catalyze the esterification reaction of cyclopropenone 309 and amides to give the corresponding esters 346–348 containing an oxime ether in good yields (Scheme 77B) [131]. In addition, a gram scale operation has been successfully demonstrated.
Scheme 77: PPh3-catalyzed C–C-bond activation of diphenylcyclopropenone.
Scheme 77: PPh3-catalyzed C–C-bond activation of diphenylcyclopropenone.
In addition, Hu and co-workers (2020) studied a catalyst-free multicomponent coupling reaction of benzynes derived from alkyne 349, cyclopropenone 309, and sulfoxides to give the corresponding o-(methylthio)phenyl acrylates 350–352 in good yields via benzyne intermediate 353 followed by a [2 + 2] cycloaddition with the sulfoxide affording an o-quinone intermediate 354 (Scheme 78) [132].
Scheme 78: Catalyst-free C–C-bond activation of diphenylcyclopropenone.
Scheme 78: Catalyst-free C–C-bond activation of diphenylcyclopropenone.
Furthermore, dioxolane compounds have also been subjected to synthesize cinnamic acid derivatives through CO2 release. For example, Chen and co-workers (2022) prepared N-acyl sulfenamide 355 in good yield from dioxolane 88 and thiols through Cu catalysis via Cu–acyl-nitrenoid 357 (Scheme 79A) [133]. Recently, Son and co-workers (2024) also employed a Cu catalyst to cleave dioxolane 88 in the presence of silanes as the reductant affording cinnamamide (99) in excellent yield. The reaction proceeds via the decarboxylated amide–copper complex 359 (Scheme 79B) [134] and could be scaled-up to a gram scale reaction.
Scheme 79: Cu-catalyzed dioxolane cleavage.
Scheme 79: Cu-catalyzed dioxolane cleavage.
Cinnamic acid derivatives could also be accessed via multicomponent reactions. For example, Jana and Ghosh (2019) utilized Meldrum’s acid (361), aldehyde 360, and an amine to prepare the corresponding cinnamamides 362–364, natural products of the piperamide family. The multicomponent reaction involves consecutive acetone and CO2 release via 365 and 366 (Scheme 80A) [135]. A multigram scale operation has been achieved smoothly. Similarly, Silvani and co-workers (2022) also reported a multicomponent Ugi-type reaction of cinnamic acid (7), aldehyde/ketone, (S)-β-phenyl β-aminoboronate 367, and tert-butyl isocyanide to give the corresponding pharmacophoric β-substituted β-amido boronates 368–370 in moderate yields (Scheme 80B) [136].
Scheme 80: Multicomponent coupling reactions.
Scheme 80: Multicomponent coupling reactions.
3 Double-bond functionalization
3.1 Double-bond constructions
3.1.1 Conjugated alkyne hydrogenation: Mei and co-workers (2019) developed a Pd-catalyzed partial hydrogenation of conjugated alkynes in the presence of water as the hydrogen source and Mn as the reductant to give the corresponding cinnamamides 13, 77, 99, and 371 in good yields via the active palladium–hydride species 372 (Scheme 81A) [137]. The Z-to-E-selectivity could be effectively tuned by changing the solvent and temperature from CH3CN to DMF and rt to 80 °C, respectively. Similarly, Wang and co-workers (2023) employed Pd to catalyze the partial hydrogenation of the conjugated alkyne 374, also using water as the hydrogen source to give trans-methyl cinnamate (44). The reaction also proceeds via a palladium–hydride species (Scheme 81B) [138]. The method has been smoothly conducted in a gram scale operation.
Scheme 81: Pd-catalyzed partial hydrogenation of electrophilic alkynes.
Scheme 81: Pd-catalyzed partial hydrogenation of electrophilic alkynes.
More sustainable metal catalysts, such as earth-abundant transition metals (Ni and Co), have also been used to catalyze partial hydrogenation reactions of conjugated alkynes using environmentally benign water as the hydrogen source. For instance, Fan and co-workers (2019) reported the Co-catalyzed partial hydrogenation of the conjugated alkyne 374 to give cis-methyl cinnamate (375) in moderate yield via cobalt–hydride species (Scheme 82A) [139]. Similarly, Liu and co-workers (2023) utilized Ni to catalyze partial hydrogenation of conjugated alkynes to generate the corresponding trans-cinnamate esters 44 and 376 via nickel–hydride species (Scheme 82B) [140].
Scheme 82: Nickel and cobalt as earth-abundant transition metals used as catalysts for the partial hydrogenation of conjugated alkynes.
Scheme 82: Nickel and cobalt as earth-abundant transition metals used as catalysts for the partial hydrogenati...
Furthermore, metal-free catalyzed partial hydrogenations of alkynes have also been explored. For instance, Santos and co-workers (2019) employed B2Pin2 to mediate the partial hydrogenation of alkynoic acids to generate the corresponding trans-cinnamic acids 377–380 via an α-borylation–protodeborylaton mechanism involving intermediates 381 and 382 (Scheme 83A) [141]. On the one hand, Vilotijevic and co-workers (2021) reported a phosphine-mediated partial hydrogenation of conjugated alkynes using water as the hydrogen source to obtain the corresponding cis-cinnamic acid esters 383–385 and amides 386 and 387 via zwitterionic allene species 389 (Scheme 83B) [142].
Scheme 83: Metal-free-catalyzed partial hydrogenation of conjugated alkynes.
Scheme 83: Metal-free-catalyzed partial hydrogenation of conjugated alkynes.
3.1.2 Ylide reactions: The construction of double bond ylide reactions (e.g. Wittig and Horner–Wadsworth–Emmons reactions) is one of the most reliable and stereoselective methods. Several advancements in this area have recently been demonstrated to access conjugated alkenes, particularly cinnamic acid derivatives, with high stereoselectivity. For instance, Reeves and co-workers (2023) performed a Horner–Wadsworth–Emmons reaction of triethyl 2-fluoro-2-phosphonoacetate (390) and aldehydes to give the corresponding ethyl α-fluorocinnamates 391–394. When using MeMgBr the products 391, 392 were obtained with high (Z)-selectivity (Scheme 84). However, when applying n-BuLi as the base instead of MeMgBr the reaction afforded the corresponding (E)-configured products 393, 394 [143].
Scheme 84: Horner–Wadsworth–Emmons reaction between triethyl 2-fluoro-2-phosphonoacetate and aldehydes with either MeMgBr or n-BuLi as the base.
Scheme 84: Horner–Wadsworth–Emmons reaction between triethyl 2-fluoro-2-phosphonoacetate and aldehydes with ei...
Moreover, Maulide and co-workers (2022) utilized a novel thiouronium ylide 396 and 2-(tert-butyl)-1,1,3,3-tetramethylguanidine (395) for the olefination of aldehydes to generate the corresponding cinnamates 398 and 399 with excellent (Z)-selectivity (Scheme 85). Interestingly, exchanging aldehydes for tosylimines and using ylide 397 altered the stereoselectivity to E-isomeric products 400 and 401 [144].
Scheme 85: Preparation of E/Z-cinnamates using thiouronium ylides.
Scheme 85: Preparation of E/Z-cinnamates using thiouronium ylides.
Wu and co-workers (2020) employed Pd to mediate the cross-coupling reaction of sulfoxonium ylide 402 and benzyl bromides to give the corresponding (Z)-ethyl cinnamates 403–406 in good yields via carbene migratory insertion (407) (Scheme 86A) [145]. On the other hand, Werner and Liu (2020) reported a Mn-catalyzed coupling of alcohols and phosphorus ylide 408 to afford the corresponding E-isomers of cinnamate esters 409–411 via an acceptorless dehydrogenative coupling mechanism (Scheme 86B) [146]. In addition, the method could be scaled up to a gram scale.
Scheme 86: Transition-metal-catalyzed ylide reactions.
Scheme 86: Transition-metal-catalyzed ylide reactions.
Redox-driven ylide reactions have gained increasing attention to tackle several disadvantages of traditional ylide-type reactions, such as use of reagents in excess and harsh reaction conditions. For example, Werner and co-workers (2019) reported a catalytic Wittig reaction using phosphetane oxide 412 as redox cycling catalyst using silanes as the reductant to convert aldehydes and α-bromoesters into the corresponding cinnamate esters 44, 154, and 413. The reaction proceeds via formation of nucleophilic phosphane 414 (Scheme 87A) [147]. Moreover, Suryavanshi and co-workers (2020) utilized PhI(OAc)2 to mediate the oxidative olefination of amines and Wittig reagents 415 to give the corresponding cinnamate esters 416–419 via formation of imines 421 (Scheme 87B) [148]. In addition, a gram scale operation has been conducted smoothly.
3.1.3 Carbene/carbenoid reactions: α-Diazocarbonyl compounds are useful reagents especially to synthesize aziridine compounds through reactive carbene species. A few examples of utilizing α-diazocarbonyl compounds to prepare olefins have also been reported, either mediated by metal catalysts or through a more sustainable way via a non-metal strategy. For instance, Liu and Kardile (2019) reported Ag(I)-catalyzed olefinations of α-diazoester 422 and N-Boc-protected imines to afford the corresponding β-aryl-β-aminoacrylates 423–426 via formation of a Mannich–addition intermediate 428 which undergoes 1,2-hydride migration to 429 (Scheme 88A) [149]. On the other hand, Novikov and co-workers (2019) utilized Rh(II) to catalyze the [2 + 1 + 1] assembly of spiro β-lactams 431–434 from diazocarbonyl compound 430 and azirines via Rh carbenoid 435 followed by aziridine ring-opening (436 and 437) (Scheme 88B) [150].
Scheme 88: Noble transition-metal-catalyzed olefination via carbenoid species.
Scheme 88: Noble transition-metal-catalyzed olefination via carbenoid species.
Lv and co-workers (2019) employed a non-metal Lewis acid tritylium salt (TrBF4) to catalyze the stereoselective olefination of α-diazocarbonyl compounds 438 to access Z-cinnamate esters 439–442 via 1,2-hydride migration (443) (Scheme 89) [151]. The ion pair of carbocation, BF4− anions, and the trityldiazene group are cis-coplanar resulting in the Z-isomer product.
Scheme 89: TrBF4-catalyzed olefination via carbene species.
Scheme 89: TrBF4-catalyzed olefination via carbene species.
3.1.4 Metathesis reactions: Metathesis reactions are one of the most crucial approaches to preparing olefins. Grubbs and Grubbs–Hoveyda catalysts are among the most frequently used catalysts for the stereoselective construction of C=C bonds. Moreover, metathesis reactions have also been applied to stereoselectively synthesize cinnamic acid derivatives. For example, Lakhdar and co-workers (2022) combined E-selective Grubbs second-generation catalysts (cat 7) with photocatalyst (PC-4) to convert styrenes and methyl acrylate (444) into the corresponding (Z)-cinnamic acid esters 445–448 in excellent yields via E-to-Z photoisomerization mediated by the photocatalyst (Scheme 90) [152].
Scheme 90: Grubbs catalyst (cat 7)/photocatalyst-mediated metathesis reactions.
Scheme 90: Grubbs catalyst (cat 7)/photocatalyst-mediated metathesis reactions.
Nguyen and co-workers (2019) employed iodine to catalyze the intermolecular olefin-carbonyl metathesis reaction of benzaldehyde (449) and acrylate 450 to give the corresponding methyl cinnamate (44) via Lewis acidic I+-activated carbonyl intermediate 451 followed by formation of oxetane intermediate 452 (Scheme 91) [153]. Despite the low yield, this approach offers an alternative for catalyst simplicity, potentially leading to more advancements in applications using iodine as catalyst.
Scheme 91: Elemental I2-catalyzed carbonyl-olefin metathesis.
Scheme 91: Elemental I2-catalyzed carbonyl-olefin metathesis.
3.1.5 E/Z isomerization: Numerous bioactive cinnamic acid derivatives are not limited to E-isomers. Instead, multiple studies have reported high activities of (Z)-cinnamic acid derivatives, such as anti-tyrosinase activity and plant growth stimulation [14,154]. Several methods to access (Z)-cinnamic acid derivatives have been occasionally demonstrated in the previous chapters, however, direct methods to convert E- to Z-isomers have gained more interest due to their sustainable values, such as catalytic transformation and atom economy. Although the E-to-Z conversion is thermodynamically counterproductive, some strategies have been reported to achieve the desired (Z)-cinnamic acid derivatives. For instance, Poisson and co-workers (2020) reported a Cu-catalyzed E-to-Z isomerization of α/β-substituted cinnamamides under blue LED irradiation via a singlet-state mechanism (Scheme 92A) [155]. On the other hand, Collins and co-workers (2021) reported a heteroleptic Cu-based photosensitizer for the E-to-Z isomerization of cinnamate esters 458–461 with excellent yields via energy transfer (Scheme 92B) [156].
Scheme 92: Cu-photocatalyzed E-to-Z isomerization of cinnamic acid derivatives.
Scheme 92: Cu-photocatalyzed E-to-Z isomerization of cinnamic acid derivatives.
Recently, Akkarasamiyo and co-workers (2024) reported a Ni-catalyzed E-to-Z isomerization of (E)-epoxycinnamamides to (Z)-cinnamamides 462–465. The reaction proceeds via a Ni-induced epoxide-ring opening (466, 467) following phosphine attack (Scheme 93) [157]. In addition, a gram-scale reaction has been successfully demonstrated.
Scheme 93: Ni-catalyzed E-to-Z isomerization.
Scheme 93: Ni-catalyzed E-to-Z isomerization.
3.1.6 Elimination reactions: Elimination reactions are usually straightforward due to the considerably low substrate or reagent pretreatment and frequently, this simple procedure leads to highly stereoselective transformations. For example, Kawasaki and co-workers (2019) reported the highly E-selective dehydration of α-substituted-β-hydroxyesters in the presence of a base to give the corresponding (E)-cinnamate esters 468–471 in excellent yields. The reaction follows an E1cB mechanism (Scheme 94) [158].
Scheme 94: Dehydration of β-hydroxy esters via an E1cB mechanism to access (E)-cinnamic acid esters.
Scheme 94: Dehydration of β-hydroxy esters via an E1cB mechanism to access (E)-cinnamic acid esters.
Bakthadoss and co-workers (2021) employed Baylis–Hillman acetates 472 and 2-arylchromanones 473 to afford the corresponding β-substituted cinnamate esters 474–476 in the presence of a base via base-induced C–O bond cleavage (477, 478) followed by addition reaction towards the acetate (Scheme 95) [159].
Scheme 95: Domino ring-opening reaction induced by a base.
Scheme 95: Domino ring-opening reaction induced by a base.
Furthermore, elimination reactions could also be achieved through photocatalysis. For example, El-Sepelgy and co-workers (2023) reported a Co-catalyzed dehydroamination of α-aminoester derivatives 479 to give the corresponding cinnamate esters 44, 480, and 481 with excellent E-selectivity (>20:1) under blue LED irradiation via SET (482 and 483) followed by Co(II) insertion (484) (Scheme 96) [160].
Scheme 96: Dehydroamination of α-aminoester derivatives.
Scheme 96: Dehydroamination of α-aminoester derivatives.
Similarly, Chen and co-workers (2021) reported a transition-metal-free deamination of α-aminoester 485 mediated by NaI in the presence of a pyrylium salt under blue LED irradiation to give methyl cinnamate (44). The reaction demonstrates excellent E-selectivity and proceeds via Katritzky salt intermediate 487, followed by radical cleavage (Scheme 97). The same method could also be applied to the decarboxylation of carboxylic acid 486 by using N-hydroxyphthalimide (NHPI) via NHPI ester intermediate 488 [161].
Scheme 97: Accessing methyl cinnamate (44) via metal-free deamination or decarboxylation.
Scheme 97: Accessing methyl cinnamate (44) via metal-free deamination or decarboxylation.
3.1.7 Miscellaneous reactions: Direct condensation of amines with 1,3-dicarbonyl compounds has been widely implemented to access β-enaminones, particularly β-amino-cinnamic acid derivatives. Some developments have been reported in this well-established area. For example, Sharma and co-workers (2023) developed an in situ-generated naphthoquinone–Co complex covalently immobilized on a silica-coated magnetite nanosupport to catalyze condensation reactions of amines and β-carbonylester 489 to give the corresponding β-enamino esters 490 and 491 in solvent-free conditions (Scheme 98A) [162]. The core-shell magnetic silica catalyst worked as a nanoreactor with high recyclability (TON up to 357) thus promoting sustainability goals. On the other hand, using a metal-free approach, Li and co-workers (2019) reported a diphenylammonium triflate (DPAT)-catalyzed condensation of β-carbonylesters with amines or 4-methoxybenzenesulfonamide to give the corresponding β-enaminones 492 and 493, respectively, via a Brønsted acid–O=C activation mode (Scheme 98B) [163].
Scheme 98: The core–shell magnetic nanosupport-catalyzed condensation reaction.
Scheme 98: The core–shell magnetic nanosupport-catalyzed condensation reaction.
Moreover, the α-olefination of acetate esters or amides to synthesize the corresponding α,β-unsaturated esters and amides has been well documented. For instance, Gunanathan and Pandia (2021) reported a Mn-catalyzed α-olefination of acetamide 494 and alcohols to give the corresponding cinnamamides 495–497 through alcohol oxidation promoted by Mn resulting in the electrophilic aldehyde 498 and H2 (Scheme 99A) [164]. On the other hand, Rao and Padder (2020) performed a Blaise reaction of dinitrile ester 499 and ethyl α-bromoacetate (500) to afford the corresponding cinnamate ester 501 (Scheme 99B) [165].
Scheme 99: Accessing cinnamic acid derivatives from acetic acid esters/amides through α-olefination.
Scheme 99: Accessing cinnamic acid derivatives from acetic acid esters/amides through α-olefination.
Cinnamic acid derivatives could also be obtained through a catalytic α,β-dehydrogenation strategy catalyzed predominantly by noble metals [166,167]. Recent developments in this area have utilized more sustainable catalyst sources, such as earth-abundant transition metals. For instance, Newhouse and co-workers (2019) reported a Ni-catalyzed acceptorless α,β-dehydrogenation of amides to give the corresponding cinnamamides 502 and 503 via a β-hydride elimination mechanism (Scheme 100A) [168]. Similarly, Huang and co-workers (2021) employed an organophotoredox/Co dual catalyst to mediate the acceptorless α,β-dehydrogenation of esters or amides to give the corresponding cinnamate ester 24 or cinnamamide 504, respectively, under visible light irradiation via a β-hydride elimination process (Scheme 100B) [169].
Scheme 100: Accessing cinnamic acid derivatives via acceptorless α,β-dehydrogenation.
Scheme 100: Accessing cinnamic acid derivatives via acceptorless α,β-dehydrogenation.
Cycloaddition reactions have also been applied to prepare cinnamic acid derivatives. For instance, Hu and co-workers (2020) reported a Cu-catalyzed formal propargylic [3 + 2] cycloaddition of 3-(2-tosylhydrazono)propanoate 505 with propargylic acetates 506 to afford the corresponding β-pyrazolyl acrylates 507–509 with excellent E-selectivity (Scheme 101) [170].
Scheme 101: Cu-catalyzed formal [3 + 2] cycloaddition.
Scheme 101: Cu-catalyzed formal [3 + 2] cycloaddition.
3.2 Cα functionalization
The direct Cα functionalization of cinnamic acid derivatives may offer a convenient strategy for preparing various α-substituted cinnamic acid derivatives, particularly during the hit-to-lead stage of drug discovery. Several methods employing metal and metal-free catalysts have been developed to fulfill this purpose. For example, Baudoin and Rocaboy (2019) reported the conversion of cinnamamides 510 to the corresponding α-arylidene γ-lactams 511–514 via a 1,4-Pd shift (515, 516) enabling C(sp3)–H activation (517) (Scheme 102A) [171]. Similarly, Zhang and co-workers (2021) conducted a Pd-catalyzed synthesis of 6-membered lactams 519–521 from alkyne-tethered aryl iodides 518 through 1,4-Pd shift (522, 523) followed by C(sp2)–H silylation (524) (Scheme 102B) [172].
Scheme 102: Pd-catalyzed C–C bond formation via 1,4-Pd-shift.
Scheme 102: Pd-catalyzed C–C bond formation via 1,4-Pd-shift.
In addition, the metal-free catalyzed synthesis of 6-membered lactam formation has been explored through Rauhut–Currier reactions. For instance, Lupton and co-workers (2019) utilized N-heterocyclic carbene (NHC-1) to catalyze the Rauhut–Currier reaction of bis(enoate) 525 to afford the corresponding hydrocoumarins 526–528 with excellent enantioselectivity (>20:1 dr) via intermediate 529 (Scheme 103A) [173]. The same group (2021) also reported an NHC (NHC-2)-catalyzed Rauhut–Currier reaction towards β-substituted acrylamides 530 to give the corresponding 2-quinolones 531–533 with good diastereoselectivity (Scheme 103B) [174].
Scheme 103: NHC-catalyzed Rauhut–Currier reactions.
Scheme 103: NHC-catalyzed Rauhut–Currier reactions.
The intermolecular Cα functionalization of cinnamic acid derivatives has been accomplished through several strategies, including Mizoroki–Heck coupling reactions and photocatalysis. For example, Chou and co-workers (2021) performed a regioselective Mizoroki–Heck coupling of β-cyclohexadienyl acrylate 534 with aryl iodides followed by decarboxylative aromatization to give the corresponding α-arylated cinnamate esters 535–538 (Scheme 104A) [175]. In this work, the carboxylate group directed the Pd catalyst insertion into the conjugated double bond via coordination 539. Furthermore, Xia and co-workers (2020) performed a metal-free-photochemical Heck-type coupling of hydroxylated cinnamate esters and 4’-bromoacetophenone under blue LED irradiation to obtain the corresponding α-arylated cinnamate esters 540–542 in good yields. The reaction proceeds through visible light excitation followed by a single-electron transfer (SET) process (543 and 544) (Scheme 104B) [176].
Scheme 104: Heck-type reaction for Cα arylation.
Scheme 104: Heck-type reaction for Cα arylation.
Dai and co-workers (2019) reported a Cu-catalyzed α-selective trifluoromethylation of cinnamamides using TMSCF3 (Scheme 105) [177]. In the presence of Ag(I), TMSCF3 was converted into CF3 radical species leading to the Cα attacked via single electron transfer (SET) (548) followed by benzylic carbocation 549 formation promoted by Cu(II).
Scheme 105: Cu-catalyzed trifluoromethylation of cinnamamide.
Scheme 105: Cu-catalyzed trifluoromethylation of cinnamamide.
In addition, alkynes have been subjected to hydroarylation reactions to access α-substituted cinnamic acid derivatives. For example, Larrosa and co-workers (2020) utilized CO2 as a traceless carboxylate directing group for meta-selective Ru-catalyzed olefination of fluoroarene 550 with α,β-alkynyl ester 551 to generate the corresponding (Z)-α-arylated cinnamate ester 552 (Scheme 106A) [178]. Herein, the Ru complex with the carboxylated fluoroarene led to alkyne insertion via 553 followed by decarboxylation to form metallacycle intermediate 554. Similarly, Koley and co-workers (2021) reported a Ru-catalyzed alkenylation of indoline 555 with α,β-alkynyl ester 551 to give the corresponding (Z)-α-arylated cinnamate ester 556 using N-protected pivalic group as the internal directing group (557) (Scheme 106B) [179]. Later, the same group (2022) also reported the Ru-catalyzed alkenylation of pirfenidone (558) with α,β-alkynyl ester 374 to give the corresponding (Z)-α-arylated cinnamate ester 559 with pyridone acting as the directing group (Scheme 106C) [180].
Scheme 106: Ru-catalyzed alkenylation of arenes using directing groups.
Scheme 106: Ru-catalyzed alkenylation of arenes using directing groups.
On the other hand, earth-abundant transition metals, such as Mn and Ni, have also been applied to catalyze the hydroarylation of alkynes. In this context, Larrosa and co-workers (2022) utilized a Mn catalyst to achieve the hydroarylation of α,β-alkynyl ester 374 with 2-phenylpyridine (560) to afford the corresponding α-arylated cinnamate ester 561. The reaction proceeds via pyridine–Mn coordination complex 562 as an off-cycle catalyst, subsequently leading to the active catalyst species 563 upon alkyne ligation (Scheme 107A) [181]. Recently, Wen and co-workers (2024) reported a Ni-catalyzed hydroarylation of α,β-alkynyl ester 374 with arylboronic acid to generate the corresponding (E)-α-arylated cinnamate esters 564–566 via formation of the active ArNi(I) species 567, 568 after insertion into the alkynyl group (Scheme 107B) [182].
Scheme 107: Earth-abundant transition-metal-catalyzed hydroarylation of α,β-alkynyl ester 374.
Scheme 107: Earth-abundant transition-metal-catalyzed hydroarylation of α,β-alkynyl ester 374.
3.3 Cβ functionalization
The direct Cβ-functionalization of cinnamic acid derivatives has also been studied in recent years, dominated by C–C/C–N cross-coupling methods. For instance, Lu and co-workers (2021) reported a Rh-catalyzed β-arylation of cinnamamide 569 with aryl pinacol boronates proceeding via an initial Rh–amidyl coordination triggering Cβ-activation to generate the five-membered rhodacycle species 571, accompanied by transmetalation with the boronate to form 572 (Scheme 108A) [183]. In 2023, Bakthadoss and Reddy reported a Pd-catalyzed β-arylation of cinnamate ester 44 with an α-substituted cinnamate ester 573 as the coupling partner to give product 574 (Scheme 108B). In this study, the coupling reaction took place selectively at the meta position of 573 directed by the cyanide group, thus leading to the generation of a 12-membered palladacycle species 575 (Scheme 108B) [184].
Scheme 108: Precious transition-metal-catalyzed β-arylation of cinnamic acid amide/ester.
Scheme 108: Precious transition-metal-catalyzed β-arylation of cinnamic acid amide/ester.
On the other hand, Shi and co-workers (2023) performed Cβ–N bond coupling of cinnamamide 577 with di-tert-butyldiaziridinone (578) catalyzed by Pd to give the corresponding β-amination products 579–581 (Scheme 109) [185]. The Cβ–H activation was driven by a ligand exchange with the cinnamamide 577 to form the palladacycle species 582, which undergoes oxidative addition with di-tert-butyldiaziridinone (583).
Scheme 109: Pd-catalyzed β-amination of cinnamamide.
Scheme 109: Pd-catalyzed β-amination of cinnamamide.
In the absence of a metal catalyst, Phan and co-workers (2020) utilized S8 to mediate the β-amination of methyl cinnamate (44) via radical sulfuration of the cinnamate at the Cα-position (Scheme 110). Herein, a trisulfur radical anion reacted with the Cα/β of cinnamate to give a five-membered cyclic transition state followed by formation of thiirane 585 and subsequent NH3 attack at the Cβ position to give 586 [186].
Scheme 110: S8-mediated β-amination of methyl cinnamate (44).
Scheme 110: S8-mediated β-amination of methyl cinnamate (44).
In addition to the electron-deficient alkenes discussed above, electron-deficient alkynes have also been used to prepare β-substituted cinnamic acid derivatives. Recently, Huang and co-workers (2024) investigated a Pd-catalyzed β-arylation of alkynyl esters with phenylsilanes as the coupling partner under microwave conditions to give the corresponding β-arylated cinnamate esters 587–589 (Scheme 111) [187]. Herein, the phenylsilane was activated by fluoride ions (590) and subsequently entered the cross-coupling reaction cycle.
Scheme 111: Pd-catalyzed cross-coupling reaction of alkynyl esters with phenylsilanes.
Scheme 111: Pd-catalyzed cross-coupling reaction of alkynyl esters with phenylsilanes.
Fang and co-workers (2023) studied the Pd-catalyzed hydrocyanation of alkynyl amides using acetone cyanohydrin (591) as the cyanide source to give the corresponding β-cyanocinnamamides 592–595 with E/Z-selectivity depending on the ligand employed. The reaction proceeds via (E)-β-cyanocinnamamide as the intermediate for Z-isomer products (Scheme 112A) [188]. The E-to-Z isomerization occurred when Cα and Cβ rotated due to the steric effect promoted by the five-membered cyclopalladium ring in 596. Recently, the same group also investigated the Pd-catalyzed hydrocyanation of alkynyl ester 597 using butanone cyanohydrin (598) as the cyanide reagent to generate the corresponding β-cyanocinnamate esters 599 and 600 with E/Z-selectivity depending on the ligand used (Scheme 112B) [189].
Scheme 112: Pd-catalyzed β-cyanation of alkynyl amide/ester.
Scheme 112: Pd-catalyzed β-cyanation of alkynyl amide/ester.
Furthermore, Nolan and co-workers (2019) reported a Au-catalyzed regioselective hydroamination of alkynyl ester 374 and azole 601 to give the corresponding (Z)-β-substituted cinnamate esters 602, 603 via the formation of a gem-diaurated species 604 equilibrated with s-monoaurated species 605 (Scheme 113A) [190]. Similarly, Matsuya and co-workers (2021) utilized Au(I) to catalyze the preparation of substituted pyrazolines 607 and 608 from alkynyl ester 374 and 606 via aza-enyne metathesis intermediate 609 followed by addition–6π-electrocyclization to afford the final product (Scheme 113B) [191].
Scheme 113: Au-catalyzed β-amination of alkynyl ester 374.
Scheme 113: Au-catalyzed β-amination of alkynyl ester 374.
In addition, a metal-free catalysis method was used to functionalize the Cβ position of electron-deficient alkynes, particularly through Michael addition. For instance, Kore and co-workers (2019) performed a Michael addition of alkynyl ester 551 with pseudouridine 610 using DBU as the base to furnish selective N1-acrylated product 611, a useful building block for in vitro enzymatic introduction (Scheme 114A) [192]. The authors assumed that the bulkiness and non-nucleophilic nature of DBU played a major role in the observe N1- over N3-anion regioselectivity. On the other hand, Paradies and co-workers (2022) reported a frustrated Lewis pair-catalyzed hydroboration of nitriles to generate the nucleophilic diborylated amine 617, followed by Cβ-addition of alkynyl ester 551 to give the final 3-amino acrylate products 612–614 in good yields (Scheme 114B) [193].
Scheme 114: Metal-free-catalyzed Cβ-functionalizations of alkynyl esters.
Scheme 114: Metal-free-catalyzed Cβ-functionalizations of alkynyl esters.
4 Aromatic group functionalization
4.1 Cross-coupling reactions
The cross-coupling reaction is a versatile method for constructing various building blocks through C–C-bond formation, particularly aromatic group attachment, as demonstrated by its vast utilization in industrial setups and growing studies recorded over the years. Among other things, numerous improvements in the sustainability of the coupling methods, such as catalyst recyclability and waste reduction, have been the main focus of the development in this area. In addition, direct coupling reactions via C(sp2)–H activation using arenes instead of the common haloarenes have been developed with highly regioselective manners. Despite heavy dependency on rare transition metals utilization for the catalysts, many recent studies have demonstrated the application of more abundant metals, such as Ni, Co, and Cu. Herein, we selectively compile recent developments of cross-coupling reactions to access cinnamic acid derivatives in highly selective manners and diversely functional group-substituted aromatics with sustainability approaches.
4.1.1 C(sp2)–X alkenylation (X = halo, N2BF4, SO2NR2, CNR): Khan and Parveen (2021) reported the palladium-catalyzed Mizoroki–Heck reaction of aryl halides/tosylates and methyl acrylate (618) in combination with the chroman-4-one-based ligand L1 in water to give the corresponding methyl cinnamic acid derivatives 159, 168, and 619 (Scheme 115A) [194]. On the other hand, Brunner and Vedder (2019) utilized calcium-phyllosilicates (circosil/circolit) as the base for the Matsuda–Heck reaction of 4-methoxybenzenediazonium tetrafluoroborate (620) and the acrylate 621 to afford the commercial sunscreen incredient 622 in good yield (Scheme 115B) [195].
Several studies have explored non-conventional functionalized arenes as the aryl donor for Mizoroki–Heck coupling reactions. For instance, Panda and Ojha (2022) employed N-methoxysulfonamides 623 as aryl donor and acrylamide 624 to perform a palladium-catalyzed Mizoroki–Heck desulfitative arylation to give the corresponding cinnamamide 625. The reaction was performed in the presence of CuCl2 as additive which is involved in a Cu-mediated SET mechanism (626–628) (Scheme 116A) [196]. Moreover, Dai and co-workers (2021) developed the Mizoroki–Heck reaction of aryl ketone 629 through C–C-bond cleavage (Scheme 116B) [197]. In this work, the aryl ketone 629 was converted into oxime ester 630 followed by Pd-catalyzed olefination to afford the corresponding cinnamate esters 631 and 632. This method has been successfully scaled up to a gram-scale operation.
Scheme 116: Mizoroki–Heck coupling reactions using unconventional functionalized arenes.
Scheme 116: Mizoroki–Heck coupling reactions using unconventional functionalized arenes.
Furthermore, (Z)-cinnamic acid derivatives could also be accessed through functional group-directed olefination of aryl halides. For example, Das and co-workers (2021) studied the Pd-catalyzed Z-selective arylation of acrylamide 633 using aminoimidazole[1,2-a]pyridine as the directing group (638) to give the corresponding (Z)-cinnamamides 634–637 (Scheme 117A) [198]. In addition, a gram-scale operation has been successfully conducted. Similarly, Gooßen and co-workers (2022) reported a Ru-catalyzed Z-selective arylation of acrylic acid 639 to obtain the corresponding methyl (Z)-cinnamates 640–642 after methylation (Scheme 117B) [199]. Herein, the stereoselectivity was directed by the -CO2H group to form metallacycle intermediate 643. Wang and co-workers (2023) reported an Ir-catalyzed Z-selective arylation of acrylic acid 644 with arylboronate 645 to give the corresponding (Z)-cinnamate ester 646 following alkylation (Scheme 117C) [200].
Scheme 117: Functional group-directed Mizoroki–Heck coupling reactions.
Scheme 117: Functional group-directed Mizoroki–Heck coupling reactions.
Another attractive strategy employed material-supported Pd nanoparticles to catalyze the Mizoroki–Heck coupling reaction. This approach provided thermal stability, easy separation, and milder reaction conditions. For instance, Bagherzadeh and co-workers (2019) developed magnetic Pd-supported graphene oxide, carbon nanotube and ferrite (Pd-rGO/CNT/CaFe2O4) nanocomposites which were used as photocatalyst for the Mizoroki–Heck coupling reaction of aryl halides and methyl acrylate (618) to give the corresponding methyl cinnamates 160 and 648 (Scheme 118A) [201]. In addition, the catalyst could be recycled for up to six consecutive operations without significant activity loss. Similarly, Hasan (2020) prepared methyl salicylate-embedded magnetic chitosan-immobilized Pd nanoparticles (Fe3O4@CS@MS@Pd) to catalyze the Mizoroki–Heck coupling reaction of aryl halides and acrylate ester 649 in water to give the corresponding cinnamate esters 251 and 650 (Scheme 118B) [202]. Moreover, Sobhani and co-workers (2021) developed a Pd–Co bimetallic alloy immobilized on a melamine-based dendrimer on magnetic nanoparticles g-Fe2O3@MBD/Pd-Co and used it to catalyze the Mizoroki–Heck coupling reaction of aryl halides and acrylate ester 651 to obtain the corresponding cinnamate esters 652 and 653 (Scheme 118C) [203]. Similar studies utilizing Pd nanoparticles for Heck-type reactions have flourished in recent years [204-206].
Scheme 118: Pd nanoparticles-catalyzed Mizoroki–Heck coupling reactions.
Scheme 118: Pd nanoparticles-catalyzed Mizoroki–Heck coupling reactions.
Furthermore, multicomponent Heck-type reactions have been investigated to synthesize cinnamic acid derivatives with multifunctionalized arene core, particularly Catellani-type reactions. Catellani reactions allow multiple functional groups to be installed in the aryl ring in one pot. For instance, Cheng and co-workers (2020) reported the stereoselective synthesis of C-aryl glycosides using aryl iodides, tetrabenzyl-protected α-mannosyl chloride 654, and methyl acrylate (618) through a Catellani mechanism (Scheme 119A) [207]. Herein, norbornenes N1 are crucial to facilitate the multi-substitution of the arene ring through arylnorbornylpalladacycle 657 as the key species. In addition, a gram-scale operation has been successfully carried out. Similarly, Luan and co-workers (2020) conducted a four-component Catellani reaction using aryl iodide 658, anhydrides 659, acrylate ester 618, and benzyl bromide to give the corresponding methyl cinnamates with multifunctionalized arenes 660 and 661 (Scheme 119B) [208].
Scheme 119: Catellani-type reactions to access methyl cinnamate with multifunctionalized arene.
Scheme 119: Catellani-type reactions to access methyl cinnamate with multifunctionalized arene.
In addition, Müller and co-workers (2020) studied a three-component Sonogashira coupling reaction of aryl iodides, ethyl propiolate (662), followed by Michael addition of pyrrolidine to obtain β-amino enoates 663–665 (Scheme 120A) [209]. On the other hand, Yao and co-workers (2021) investigated a tandem intramolecular Heck coupling reaction/aryne dicarbofunctionalization (via 670 and 671) of olefin-tethered aryl iodide 666, aryne precursor 667, and n-butyl acrylate (651) to give the corresponding o-alkylated arylacrylates 668 and 669 in excellent yields (Scheme 120B) [210]. As shown here, these multicomponent methods enabled the preparation of intricate and complex cinnamic acid derivatives more efficiently.
Scheme 120: Multicomponent coupling reactions.
Scheme 120: Multicomponent coupling reactions.
Müllen and co-workers (2023) reported a single atom Pt-catalyzed Heck coupling reaction of aryl iodides and ethyl acrylate (672) to give the corresponding cinnamate esters 673, 674 (Scheme 121) [211]. The computational study revealed that a migratory insertion mechanism assisted by a base was operative.
Scheme 121: Single atom Pt-catalyzed Heck coupling reaction.
Scheme 121: Single atom Pt-catalyzed Heck coupling reaction.
Despite massive dependency on rare transition metal catalysts applied for cross-coupling reactions, significant developments using earth-abundant transition metals have materialized in recent years to fulfill the sustainability agenda. For instance, Patel and co-workers (2019) utilized NiBr2(PPh3)2 to catalyze the Heck coupling reaction of aryl iodides and methyl acrylate (618) in the presence of Zn powder to obtain the corresponding methyl cinnamates 648 and 675 (Scheme 122A) [212]. The reducing agent (Zn) is necessary to recycle the Ni catalyst effectively. On the other hand, Chu and co-workers (2019) studied Mizoroki–Heck coupling reactions of arylboronic acids 676 and methyl acrylate (618) catalyzed by bis-quinoline-2-carboxylic acid Cu salt (cat 9) to give the corresponding methyl cinnamates 619, 677 in excellent yields via phenyl radical 678 (Scheme 122B) [21]. In this study, air was used as the oxidant to recycle the catalyst.
Scheme 122: Earth-abundant transition metal-catalyzed Heck coupling reactions.
Scheme 122: Earth-abundant transition metal-catalyzed Heck coupling reactions.
Furthermore, Sobhani and co-workers (2020) developed a hydrophilic heterogenous Co catalyst on chitosan (mTEG-CS-Co-Schiff-base) to catalyze Heck coupling reactions of aryl iodides and n-butyl acrylate (651) in water as environmentally benign solvent. The corresponding n-butyl cinnamates 652 and 653 were obtained in excellent yields via formation of the organocobalt species 679 (Scheme 123A) [213]. In addition, the catalyst could be successfully reused for up to 6 runs. On the other hand, Anas and co-workers (2020) reported Heck coupling reactions of aryl iodides and methyl acrylate 618 catalyzed by an amidoxime-modified PAN-immobilized Cu salt to give the corresponding methyl cinnamates 160 and 675 via the migratory insertion of Cu, generating organocopper species 680 (Scheme 123B) [214]. The catalyst was smoothly recycled for up to 5 runs without significant loss of its activity.
Scheme 123: Polymer-coated earth-abundant transition metals-catalyzed Heck coupling reactions.
Scheme 123: Polymer-coated earth-abundant transition metals-catalyzed Heck coupling reactions.
Moreover, Alavi and co-workers (2021) prepared bimetallic nanoparticles supported on magnetic NiFe2O4 nanoparticles (NiFe2O4@SiO2@ZrO2/SO42–/Cu/Co) to catalyze the Heck coupling reaction of aryl iodides and n-butyl acrylate (651) in water to afford the corresponding n-butyl cinnamates 681 and 682. The catalyst could be smoothly recycled for up to 11 runs (Scheme 124A) [215]. The coupling mechanism was thought to proceed via migratory insertion of the Cu site to give an organocopper species 683. Similarly, Koukabi and co-workers (2019) developed a polyamidoamine-Co complex immobilized on magnetic nanoparticles (MNP@PAMAM-Co) to catalyze Mizoroki–Heck coupling reactions of aryl iodides and methyl acrylate (618) giving rise to the corresponding methyl cinnamates 675, 684 (Scheme 124B) [216]. The catalyst could be recycled for up to 5 operations without significant activity diminution.
Scheme 124: Earth-abundant transition-metal-based nanoparticles as catalysts for Heck coupling reactions.
Scheme 124: Earth-abundant transition-metal-based nanoparticles as catalysts for Heck coupling reactions.
4.1.2 C(sp2)–H alkenylation: The direct C(sp2)–H alkenylation of arenes has significantly flourished as the need to use or prepare haloarenes or heteroatom-substituted arenes involved in the coupling process diminishes, thus promoting reaction efficiency and atom economy. However, due to the weaker activity of the C(sp2)–H bond than its heteroatom-functionalized counterparts, its application was hampered by its low reactivity and poor regioselectivity. In this case, utilizing directing group-tethered arenes could overcome this issue elegantly. For instance, Jin and co-workers (2019) developed consecutive activation of ortho-C(sp2)–H bond and ipso-C(sp3)–O bond utilizing a nitrile-based directing group via CN–[Pd] coordination (688). In this study, a directing group-functionalized arene 685 was reacted with acrylamide 624 to give the corresponding ortho-selective substituted cinnamamide 686 followed by an ipso-C(sp3)–O arylation to obtain the final cinnamamide product 687 in good yield (Scheme 125A) [217]. Similarly, Xu and co-workers (2019) utilized a silane-functionalized arene 689 and reacted it with acrylate 651 to conduct an ortho-selective C(sp2)–H alkenylation to obtain the corresponding cinnamate esters 690 and 691 with good enantiomeric ratio (Scheme 125B) [218]. In addition, the reaction scale could be raised to a gram-scale operation.
Scheme 125: CN- and Si-based directing groups to access o-selective cinnamic acid derivatives.
Scheme 125: CN- and Si-based directing groups to access o-selective cinnamic acid derivatives.
Fu and co-workers (2019) employed Ir to catalyze the o-selective C(sp2)–H alkenylation of 2,3,4,5,6-pentafluorobenzoyl (PFB)-anchored benzylamine 692 using silver acetate as oxidant to obtain the corresponding o-substituted ethyl cinnamates 693 and 694 via a five-membered iridacycle 695 (Scheme 126A) [219]. On the other hand, Kumar and co-workers (2021) investigated the Ru-catalyzed o-selective C(sp2)–H alkenylation of benzamide 696 in the presence of copper acetate as oxidant to give the corresponding o-substituted ethyl cinnamates 697, 698 (Scheme 126B) [220]. The amide group directed the Ru catalyst to activate o-C(sp2)–H by forming a five-membered ruthenacycle 699. In addition, a gram-scale operation has been successfully achieved. Similarly, Yang and co-workers (2023) reported a Rh-catalyzed multicomponent coupling reaction using natural products-based fragments via dual C–H activation to generate the corresponding ketoxime 702 in good yield (Scheme 126C) [221]. Initially, the Rh catalyst activates the C(sp3)–H bond in oxime 701 enabling C–C bond formation with dioxazolone 700 (703 and 704). The newly installed amide group in intermediate 704, then acts as directing group for the Rh catalyst to activate the o-C(sp2)–H bond of the arene by forming metalacycle species 705.
Scheme 126: Amide-based directing group to access o-selective cinnamic acid derivatives.
Scheme 126: Amide-based directing group to access o-selective cinnamic acid derivatives.
Beyond N-based directing groups, C=O groups could also direct C–H functionalization. For example, Bakthadoss and co-workers (2020) employed Ru to catalyze the o-C(sp2)–H alkenylation of azaflavanone 706 to give the corresponding methyl cinnamates 707 and 708 via C=O–[Ru] directing mode 709 (Scheme 127A) [222]. Similarly, Kumar and co-workers (2021) reported weakly chelating lactone-directed o-C(sp2)–H alkenylation (via 713) of 3-arylcoumarins 710 and acrylate 672 to afford the corresponding methyl cinnamates 711 and 712 (Scheme 127B) [223]. In addition, a gram-scale operation has been successfully conducted.
Scheme 127: Carbonyl-based directing group to access o-selective cinnamic acid derivatives.
Scheme 127: Carbonyl-based directing group to access o-selective cinnamic acid derivatives.
However, the direct o-selective C(sp2)–H alkenylation catalyzed by earth-abundant transition metals is still extremely underdeveloped. One rare example was reported by Maji and co-workers in 2019. In this work, aromatic ketones 714 were selectively o-alkenylated catalyzed by Co(III) to give the corresponding o-ketocinnamate esters 715 and 716 in good yields via metallacycle species (Scheme 127C) [224].
Numerous atropisomeric compounds are known for their bioactivities. Significant efforts have been dedicated to the stereoselective synthesis of atropisomers, by, for example, selective C–H functionalization and the ortho-selective C(sp2)–H functionalization is a powerful tool for achieving this goal. Shi and co-workers (2019) developed a Pd-catalyzed C(sp2)–H alkenylation of rac-quinoline-derived biaryl 717 with acrylate 651 to give the corresponding axially chiral cinnamate ester-anchored biaryls 718 and 719 with excellent enantiomeric excess utilizing chiral spiro phosphoric acids ((R)-STRIP) as ligands (Scheme 128A) [225]. DFT calculations revealed a concerted metalation–deprotonation (CMD)-type transition state of Spinol-ts-S 720 as the main pathway due to the less steric repulsion between quinolone and isopropyl groups. The same group (2019) also reported the preparation of axially chiral styrenes 722, 723 from arenes 721 and a chiral auxiliary tert-leucine-derived amino amide via a palladacycle species 724 (Scheme 128B) [226]. In addition, the method was used for a gram-scale operation.
Scheme 128: Stereoselective preparation of atropisomers via o-selective C(sp2)–H functionalization.
Scheme 128: Stereoselective preparation of atropisomers via o-selective C(sp2)–H functionalization.
On the other hand, meta-selective C(sp2)–H alkenylation reactions to access m-substituted cinnamic acid derivatives using directing groups are considerably less explored than their ortho-counterparts due to more remote activations. In this context, the formation of a thermodynamically stable, larger metallacycle during C–H activation was the key to achieving m-selectivity. Despite the challenges, several studies have been published in recent years to access meta-position functionalizations. Yu and co-workers (2019) investigated a one-pot Pd-catalyzed m-selective alkenylation/ipso-selective C(sp2)–O functionalization of directing group-tethered phenols 725 to give the corresponding m-functionalized cinnamate esters 726, 727 in moderate yields (Scheme 129A) [227]. The nitrile-tethered template facilitated remote C(sp2)–H activation via a macrocyclic palladacycle species. Similarly, Maiti and co-workers (2021) successfully achieved m-selective C(sp2)–H functionalization of biphenyl aldehydes 728 to afford the corresponding m-arylated cinnamate esters 729 and 730 using a pyrimidine-based directing group (TDG1) via a macrocyclic C=N–[Pd] species (Scheme 129B) [228]. Moreover, Li and co-workers (2020) utilized carboxyl group-tethered benzylsulfonamides 731 to access m-sulfamoylmethyl cinnamate esters 732 and 733 via κ2 coordination (734) of the pendant carboxyl group with the Pd catalyst (Scheme 129C) [229].
Scheme 129: meta-Selective C(sp2)–H functionalization using directing group-tethered arenes.
Scheme 129: meta-Selective C(sp2)–H functionalization using directing group-tethered arenes.
Furthermore, Maiti and co-workers (2019) studied the first distal para-selective C(sp2)–H alkenylation of silyl-tethered arenes 735 to afford the corresponding p-silylmethyl cinnamate esters 736 and 737 via remote CN–[Rh] coordination (738) (Scheme 130) [230].
Scheme 130: para-Selective C(sp2)–H functionalization using directing group-tethered arenes.
Scheme 130: para-Selective C(sp2)–H functionalization using directing group-tethered arenes.
The same group (2022) also reported a non-directed C(sp2)–H functionalization of arenes through an electrooxidative Fujiwara–Moritani reaction to obtain the corresponding cinnamate esters 739 and 740 with high regioselectivity via a PdII/PdIV cycle (Scheme 131) [231]. In addition, a gram-scale operation has been successfully achieved.
Scheme 131: Non-directed C(sp2)–H functionalization via electrooxidative Fujiwara–Moritani reaction.
Scheme 131: Non-directed C(sp2)–H functionalization via electrooxidative Fujiwara–Moritani reaction.
4.2 Miscellaneous reactions
Li and co-workers (2023) developed a photocatalyzed cyanation of p-methoxycinnamate salt 741 utilizing TMSCN as the cyanide source and photocatalyst PC7 to give the corresponding p-cyano product 742 in moderate yield via an arene cation radical species (Scheme 132A) [232]. The product 742 was then converted into a nitrile analog of cinepazide 743, a drug used for treating cardio- and cerebrovascular diseases, in ≈37% isolated yield over two steps. On the other hand, Topf and co-workers (2021) utilized Co corrole (cat 10) to catalyze the hydrogenation of m-nitrocinnamic acid (744) to afford the HCl salt of m-aminocinnamic acid 745 upon acidification in excellent yield (Scheme 132B) [233].
Scheme 132: Interconversion of functional groups attached to cinnamic acid.
Scheme 132: Interconversion of functional groups attached to cinnamic acid.
Moreover, a Pd-catalyzed direct C(sp2)–H functionalization of cinnamic acid derivatives has been recently reported. Bakthadoss and Reddy (2023) developed a protocol to functionalize the meta-position of a nitrile-based directing group-anchored cinnamate ester 746 with various functional groups to the corresponding m-substituted cinnamate esters 747–752 in moderate to good yields (Scheme 133) [184].
Scheme 133: meta-Selective C(sp2)–H functionalization of cinnamate ester.
Scheme 133: meta-Selective C(sp2)–H functionalization of cinnamate ester.
Tsui and co-workers (2022) reacted Grignard reagents with monofluoroalkene 753 or gem-difluoroalkene 755 to give the corresponding β-arylated cinnamate esters 754 or 756, respectively, via a concerted mechanism (757) (Scheme 134) [234].
Scheme 134: C(sp2)–F arylation using Grignard reagents.
Scheme 134: C(sp2)–F arylation using Grignard reagents.
Furthermore, Zhou and co-workers (2021) reported an NHC-mediated Truce–Smiles rearrangement of N-aryl metacrylamide 758 to prepare the corresponding (E)-cinnamamides 759 and 760 via lactam intermediate 761 (Scheme 135) [235].
Scheme 135: Truce–Smiles rearrangement of N-aryl metacrylamides.
Scheme 135: Truce–Smiles rearrangement of N-aryl metacrylamides.
In addition, Huang and co-workers (2022) investigated a one-pot two-step phosphine-catalyzed cyclization of γ-vinyl allenoate 763 with enamino ester 762 to give the corresponding 2,3,6-trisubstituted pyridines 764 and 765 after oxidative aromatization of intermediate 766 by DDQ (Scheme 136) [236]. The method has been successfully scaled up to a gram scale.
Scheme 136: Phosphine-catalyzed cyclization of γ-vinyl allenoate with enamino esters.
Scheme 136: Phosphine-catalyzed cyclization of γ-vinyl allenoate with enamino esters.
Conclusion
Exploiting cinnamic acid building blocks for various purposes, especially for drug design, has led to more efficient, selective, and greener approaches in recent years. As shown in this review, plenty elegant one-step strategies have been developed to access not only common functional groups-anchored cinnamic acid derivatives, but also more exotic functional groups, thus potentially boosting hit-to-lead stages of drug discovery studies by providing new strategies with versatile functional groups. In addition, many methods shown here have been successfully carried out on a gram scale, potentially promoting scalable productions of bioactive cinnamic acid derivatives using these approaches.
However, the issue of strong dependency on rare transition metals in many areas should be addressed by increasing the exploration of novel strategies utilizing more sustainable sources, such as earth-abundant transition metals, and even metal-free transformations. In addition, designing recyclable catalysts (e.g., in cross-coupling reactions) is gaining considerable attention in the synthetic chemistry community. Similarly, tackling excessive reaction waste has been executed by designing recyclable coupling reagents. As cinnamic acid scaffolds are among prima donnas in drug design, there is no doubt that more advances will continue to emerge.
Funding
The authors express their gratitude to the Research Center for Pharmaceutical Ingredients and Traditional Medicine – Research Organization for Health at the National Research and Innovation Agency of the Republic of Indonesia (BRIN) for funding this research through the Research and Innovation for Indonesia Advanced to Wave 2 (RIIM Stage 2) for the 2024 fiscal year. This work was supported by Research and Innovation for Indonesia Advanced to Wave 2 (RIIM Stage 2) through the National Research and Innovation Agency (BRIN/LPDP) under No. 82/II.7/HK/2022 (T.E) and No. B-960/II.7.5/FR.06.00/2/2024, dated February 2, 2024 concerning Notification of Implementation of RIIM Wave 2 Continuation Year.
Data Availability Statement
Data sharing is not applicable as no new data was generated or analyzed in this study.
References
-
Wang, S.; Chen, J.; Shi, J.; Wang, Z.; Hu, D.; Song, B. J. Agric. Food Chem. 2021, 69, 11804–11815. doi:10.1021/acs.jafc.1c03087
Return to citation in text: [1] [2] -
Wiedeman, P. E.; Djuric, S. W.; Pilushchev, M.; Sciotti, R. J.; Madar, D. J.; Kopecka, H. Oxazolidinone Chemotherapeutic Agents. U.S. Pat. Appl. US2002/0115669A1, Aug 22, 2002.
Return to citation in text: [1] -
Zhou, Y.; Zhou, X. E.; Gong, Y.; Zhu, Y.; Cao, X.; Brunzelle, J. S.; Xu, H. E.; Zhou, M.; Melcher, K.; Zhang, F. PLoS Pathog. 2020, 16, e1008323. doi:10.1371/journal.ppat.1008323
Return to citation in text: [1] -
Yap, P. S. X.; Krishnan, T.; Chan, K.-G.; Lim, S. H. E. J. Microbiol. Biotechnol. 2015, 25, 1299–1306. doi:10.4014/jmb.1407.07054
Return to citation in text: [1] -
Bezerra França, S.; Carine Barros de Lima, L.; Rychard da Silva Cunha, C.; Santos Anunciação, D.; Ferreira da Silva-Júnior, E.; Ester de Sá Barreto Barros, M.; José da Paz Lima, D. Bioorg. Med. Chem. 2021, 44, 116299. doi:10.1016/j.bmc.2021.116299
Return to citation in text: [1] -
Lima, G. D. d. A.; Rodrigues, M. P.; Mendes, T. A. d. O.; Moreira, G. A.; Siqueira, R. P.; da Silva, A. M.; Vaz, B. G.; Fietto, J. L. R.; Bressan, G. C.; Machado-Neves, M.; Teixeira, R. R. Toxicol. In Vitro 2018, 53, 1–9. doi:10.1016/j.tiv.2018.07.015
Return to citation in text: [1] -
Zhang, W.-X.; Wang, H.; Cui, H.-R.; Guo, W.-B.; Zhou, F.; Cai, D.-S.; Xu, B.; Jia, X.-H.; Huang, X.-M.; Yang, Y.-Q.; Chen, H.-S.; Qi, J.-C.; Wang, P.-L.; Lei, H.-M. Eur. J. Med. Chem. 2019, 183, 111695. doi:10.1016/j.ejmech.2019.111695
Return to citation in text: [1] -
Rodrigues, M. P.; Tomaz, D. C.; Ângelo de Souza, L.; Onofre, T. S.; Aquiles de Menezes, W.; Almeida-Silva, J.; Suarez-Fontes, A. M.; Rogéria de Almeida, M.; Manoel da Silva, A.; Bressan, G. C.; Vannier-Santos, M. A.; Rangel Fietto, J. L.; Teixeira, R. R. Eur. J. Med. Chem. 2019, 183, 111688. doi:10.1016/j.ejmech.2019.111688
Return to citation in text: [1] -
Scott, J. S.; Bailey, A.; Buttar, D.; Carbajo, R. J.; Curwen, J.; Davey, P. R. J.; Davies, R. D. M.; Degorce, S. L.; Donald, C.; Gangl, E.; Greenwood, R.; Groombridge, S. D.; Johnson, T.; Lamont, S.; Lawson, M.; Lister, A.; Morrow, C. J.; Moss, T. A.; Pink, J. H.; Polanski, R. J. Med. Chem. 2019, 62, 1593–1608. doi:10.1021/acs.jmedchem.8b01837
Return to citation in text: [1] -
Uchida, Y.; Murao, S. Jpn. Heart J. 1981, 22, 971–975. doi:10.1536/ihj.22.971
Return to citation in text: [1] -
Vivier, D.; Soussia, I. B.; Rodrigues, N.; Lolignier, S.; Devilliers, M.; Chatelain, F. C.; Prival, L.; Chapuy, E.; Bourdier, G.; Bennis, K.; Lesage, F.; Eschalier, A.; Busserolles, J.; Ducki, S. J. Med. Chem. 2017, 60, 1076–1088. doi:10.1021/acs.jmedchem.6b01285
Return to citation in text: [1] -
Marquard, L.; Petersen, K. D.; Persson, M.; Hoff, K. D.; Jensen, P. B.; Sehested, M. APMIS 2008, 116, 382–392. doi:10.1111/j.1600-0463.2008.00957.x
Return to citation in text: [1] -
Xu, X.-T.; Deng, X.-Y.; Chen, J.; Liang, Q.-M.; Zhang, K.; Li, D.-L.; Wu, P.-P.; Zheng, X.; Zhou, R.-P.; Jiang, Z.-Y.; Ma, A.-J.; Chen, W.-H.; Wang, S.-H. Eur. J. Med. Chem. 2020, 189, 112013. doi:10.1016/j.ejmech.2019.112013
Return to citation in text: [1] -
Yang, H. H.; Oh, K.-E.; Jo, Y. H.; Ahn, J. H.; Liu, Q.; Turk, A.; Jang, J. Y.; Hwang, B. Y.; Lee, K. Y.; Lee, M. K. Bioorg. Med. Chem. 2018, 26, 509–515. doi:10.1016/j.bmc.2017.12.011
Return to citation in text: [1] [2] -
Abe, M.; Nishikawa, K.; Fukuda, H.; Nakanishi, K.; Tazawa, Y.; Taniguchi, T.; Park, S.-y.; Hiradate, S.; Fujii, Y.; Okuda, K.; Shindo, M. Phytochemistry 2012, 84, 56–67. doi:10.1016/j.phytochem.2012.08.001
Return to citation in text: [1] -
Dagang, Y.; Jing, L.; Sishun, Y.; Dongshan, W.; Jianheng, Y.; Li, G.; Xin, Z.; Chuankun, R.; Yongyuan, G. Fluorine-Containing Carboxylic Acid Compound and Preparation Method Thereof. Chin. Pat. Appl. CN111592519A, Aug 28, 2020.
Return to citation in text: [1] -
Thiyam, U.; Stöckmann, H.; Zum Felde, T.; Schwarz, K. Eur. J. Lipid Sci. Technol. 2006, 108, 239–248. doi:10.1002/ejlt.200500292
Return to citation in text: [1] -
Li, C.-Y.; Tsai, W.-J.; Damu, A. G.; Lee, E.-J.; Wu, T.-S.; Dung, N. X.; Thang, T. D.; Thanh, L. J. Agric. Food Chem. 2007, 55, 9436–9442. doi:10.1021/jf071963l
Return to citation in text: [1] -
Henrique, T.; Zanon, C. d. F.; Girol, A. P.; Stefanini, A. C. B.; Contessoto, N. S. d. A.; da Silveira, N. J. F.; Bezerra, D. P.; Silveira, E. R.; Barbosa-Filho, J. M.; Cornélio, M. L.; Oliani, S. M.; Tajara, E. H. Sci. Rep. 2020, 10, 22283. doi:10.1038/s41598-020-78220-6
Return to citation in text: [1] -
Bonin, A. M.; Arlauskas, A. P.; Angus, D. S..; Baker, R. S. U.; Gallagher, C. H.; Greenoak, G.; Brown, M. M. L.; Meher-Homji, K. M.; Reeve, V. Mutat. Res. Lett. 1982, 105, 303–308. doi:10.1016/0165-7992(82)90097-5
Return to citation in text: [1] -
Zuo, M.; Li, Z.; Fu, W.; Guo, R.; Hou, C.; Guo, W.; Sun, Z.; Chu, W. Catal. Lett. 2019, 149, 3217–3223. doi:10.1007/s10562-019-02885-6
Return to citation in text: [1] [2] -
Li, Y.; Li, J.; Bao, G.; Yu, C.; Liu, Y.; He, Z.; Wang, P.; Ma, W.; Xie, J.; Sun, W.; Wang, R. Org. Lett. 2022, 24, 1169–1174. doi:10.1021/acs.orglett.1c04258
Return to citation in text: [1] [2] -
Du, Y.; Barber, T.; Lim, S. E.; Rzepa, H. S.; Baxendale, I. R.; Whiting, A. Chem. Commun. 2019, 55, 2916–2919. doi:10.1039/c8cc09913h
Return to citation in text: [1] [2] -
Gopi, E.; Gravel, E.; Doris, E. Nanoscale Adv. 2019, 1, 1181–1185. doi:10.1039/c8na00192h
Return to citation in text: [1] [2] -
Zhang, R.; Yang, X.; Tao, Z.; Wang, X.; Wang, H.; Wang, L.; Lv, B. ACS Appl. Mater. Interfaces 2019, 11, 46678–46687. doi:10.1021/acsami.9b14460
Return to citation in text: [1] [2] -
Jiang, J.; Li, X.; Du, S.; Shi, L.; Jiang, P.; Zhang, P.; Dong, Y.; Leng, Y. New J. Chem. 2020, 44, 7780–7785. doi:10.1039/d0nj00172d
Return to citation in text: [1] [2] -
Soika, J.; McLaughlin, C.; Neveselý, T.; Daniliuc, C. G.; Molloy, J. J.; Gilmour, R. ACS Catal. 2022, 12, 10047–10056. doi:10.1021/acscatal.2c02991
Return to citation in text: [1] [2] -
Bourboula, A.; Mountanea, O. G.; Krasakis, G.; Mantzourani, C.; Kokotou, M. G.; Kokotos, C. G.; Kokotos, G. Eur. J. Org. Chem. 2023, 26, e202300008. doi:10.1002/ejoc.202300008
Return to citation in text: [1] [2] -
Zhu, K.; Cao, M.; Zhao, G.; Zhao, J.; Li, P. Org. Lett. 2022, 24, 5855–5859. doi:10.1021/acs.orglett.2c02426
Return to citation in text: [1] [2] -
Atapalkar, R. S.; Kulkarni, A. A. Chem. Commun. 2023, 59, 9231–9234. doi:10.1039/d3cc02402d
Return to citation in text: [1] [2] -
Osako, T.; Kaiser, R.; Torii, K.; Uozumi, Y. Synlett 2019, 30, 961–966. doi:10.1055/s-0037-1611769
Return to citation in text: [1] [2] -
DellaGreca, M.; Longobardo, L. ChemistrySelect 2020, 5, 4588–4591. doi:10.1002/slct.202000176
Return to citation in text: [1] -
Samuel Rajan, I. A. P.; Rajendran, S. Org. Biomol. Chem. 2023, 21, 4760–4765. doi:10.1039/d3ob00418j
Return to citation in text: [1] -
Samuel Rajan, I. A. P.; Rajendran, S. New J. Chem. 2023, 47, 10480–10483. doi:10.1039/d3nj01490h
Return to citation in text: [1] -
Mao, F.; Jin, C.; Wang, J.; Yang, H.; Yan, X.; Li, X.; Xu, X. Org. Biomol. Chem. 2023, 21, 3825–3828. doi:10.1039/d3ob00452j
Return to citation in text: [1] -
Salimiyan, K.; Saberi, D. ChemistrySelect 2019, 4, 3985–3989. doi:10.1002/slct.201804066
Return to citation in text: [1] -
Liu, J.; Fujita, H.; Kitamura, M.; Shimada, D.; Kunishima, M. Org. Biomol. Chem. 2021, 19, 4712–4719. doi:10.1039/d1ob00450f
Return to citation in text: [1] -
Marx, D.; Wingen, L. M.; Schnakenburg, G.; Müller, C. E.; Scholz, M. S. Front. Chem. (Lausanne, Switz.) 2019, 7, 56. doi:10.3389/fchem.2019.00056
Return to citation in text: [1] -
Wang, Z.; Wang, X.; Wang, P.; Zhao, J. J. Am. Chem. Soc. 2021, 143, 10374–10381. doi:10.1021/jacs.1c04614
Return to citation in text: [1] -
Xu, X.; Feng, H.; Li, H.; Huang, L. Eur. J. Org. Chem. 2019, 3921–3928. doi:10.1002/ejoc.201900137
Return to citation in text: [1] -
Chetankumar, E.; Bharamawadeyar, S.; Srinivasulu, C.; Sureshbabu, V. V. Org. Biomol. Chem. 2023, 21, 8875–8882. doi:10.1039/d3ob01351k
Return to citation in text: [1] -
Braddock, D. C.; Davies, J. J.; Lickiss, P. D. Org. Lett. 2022, 24, 1175–1179. doi:10.1021/acs.orglett.1c04265
Return to citation in text: [1] -
Ramachandran, P. V.; Hamann, H. J.; Choudhary, S. Org. Lett. 2020, 22, 8593–8597. doi:10.1021/acs.orglett.0c03184
Return to citation in text: [1] -
Phakhodee, W.; Yamano, D.; Pattarawarapan, M. Synlett 2020, 31, 703–707. doi:10.1055/s-0039-1691583
Return to citation in text: [1] -
Wang, S.-P.; Cheung, C. W.; Ma, J.-A. J. Org. Chem. 2019, 84, 13922–13934. doi:10.1021/acs.joc.9b02068
Return to citation in text: [1] -
Wu, X.; Zhou, L.; Li, F.; Xiao, J. J. Chem. Res. 2021, 45, 491–497. doi:10.1177/1747519820987530
Return to citation in text: [1] -
Brittain, W. D. G.; Cobb, S. L. Org. Lett. 2021, 23, 5793–5798. doi:10.1021/acs.orglett.1c01953
Return to citation in text: [1] -
Lee, H.-J.; Huang, X.; Sakaki, S.; Maruoka, K. Green Chem. 2021, 23, 848–855. doi:10.1039/d0gc03912h
Return to citation in text: [1] -
Zhao, Z.; Ikawa, S.; Mori, S.; Sumii, Y.; Adachi, H.; Kagawa, T.; Shibata, N. ACS Sustainable Chem. Eng. 2024, 12, 3565–3574. doi:10.1021/acssuschemeng.3c06417
Return to citation in text: [1] -
Wang, X.; Wang, Z.; Ishida, T.; Nishihara, Y. J. Org. Chem. 2020, 85, 7526–7533. doi:10.1021/acs.joc.0c00640
Return to citation in text: [1] -
Liu, J.; Parker, M. F. L.; Wang, S.; Flavell, R. R.; Toste, F. D.; Wilson, D. M. Chem 2021, 7, 2245–2255. doi:10.1016/j.chempr.2021.07.005
Return to citation in text: [1] -
Bi, X.; Li, J.; Shi, E.; Li, Y.; Liu, Y.; Wang, H.; Xiao, J. Phosphorus, Sulfur Silicon Relat. Elem. 2019, 194, 236–240. doi:10.1080/10426507.2018.1539991
Return to citation in text: [1] -
Norcott, P. L.; Hammill, C. L.; Noble, B. B.; Robertson, J. C.; Olding, A.; Bissember, A. C.; Coote, M. L. J. Am. Chem. Soc. 2019, 141, 15450–15455. doi:10.1021/jacs.9b08634
Return to citation in text: [1] -
Shen, Z.; Zhang, S.; Geng, H.; Wang, J.; Zhang, X.; Zhou, A.; Yao, C.; Chen, X.; Wang, W. Org. Lett. 2019, 21, 448–452. doi:10.1021/acs.orglett.8b03641
Return to citation in text: [1] -
Mahajani, N. S.; Meador, R. I. L.; Smith, T. J.; Canarelli, S. E.; Adhikari, A. A.; Shah, J. P.; Russo, C. M.; Wallach, D. R.; Howard, K. T.; Millimaci, A. M.; Chisholm, J. D. J. Org. Chem. 2019, 84, 7871–7882. doi:10.1021/acs.joc.9b00745
Return to citation in text: [1] -
Takeuchi, H.; Fujimori, Y.; Ueda, Y.; Shibayama, H.; Nagaishi, M.; Yoshimura, T.; Sasamori, T.; Tokitoh, N.; Furuta, T.; Kawabata, T. Org. Lett. 2020, 22, 4754–4759. doi:10.1021/acs.orglett.0c01549
Return to citation in text: [1] -
Wang, K.-K.; Li, Y.-L.; Zhao, Y.-C.; Zhang, S.-S.; Chen, R.; Sun, A. RSC Adv. 2021, 11, 40193–40196. doi:10.1039/d1ra06860a
Return to citation in text: [1] -
Gao, C.; Zhou, Q.; Yang, L.; Zhang, X.; Fan, X. J. Org. Chem. 2020, 85, 13710–13720. doi:10.1021/acs.joc.0c01871
Return to citation in text: [1] -
Li, J.-S.; Xie, X.-Y.; Jiang, S.; Yang, P.-P.; Li, Z.-W.; Lu, C.-H.; Liu, W.-D. Org. Chem. Front. 2021, 8, 697–701. doi:10.1039/d0qo01264e
Return to citation in text: [1] -
Lai, M.; Wu, Z.; Su, F.; Yu, Y.; Jing, Y.; Kong, J.; Wang, Z.; Wang, S.; Zhao, M. Eur. J. Org. Chem. 2020, 198–208. doi:10.1002/ejoc.201901630
Return to citation in text: [1] -
Li, Z.; Liu, L.; Xu, K.; Huang, T.; Li, X.; Song, B.; Chen, T. Org. Lett. 2020, 22, 5517–5521. doi:10.1021/acs.orglett.0c01869
Return to citation in text: [1] -
Park, J.; Krishnapriya, A. U.; Park, Y.; Kim, M.; Reidl, T. W.; Kuniyil, R.; Son, J. Adv. Synth. Catal. 2023, 365, 4495–4501. doi:10.1002/adsc.202300693
Return to citation in text: [1] -
Gao, Y.; Peng, S.-Q.; Liu, D.-Y.; Rui, P.-X.; Hu, X.-G. Eur. J. Org. Chem. 2019, 1715–1721. doi:10.1002/ejoc.201801334
Return to citation in text: [1] -
Wang, Z.; Matsumoto, A.; Maruoka, K. Chem. Sci. 2020, 11, 12323–12328. doi:10.1039/d0sc05137c
Return to citation in text: [1] -
Chaparro, S.; Rojas, H. A.; Castillo, J. C.; Portilla, J.; Romanelli, G. P.; Pineda, A.; Elsharif, A. M.; Martinez, J. J.; Luque, R. ACS Sustainable Chem. Eng. 2020, 8, 13139–13146. doi:10.1021/acssuschemeng.0c04429
Return to citation in text: [1] -
Qu, E.; Li, S.; Bai, J.; Zheng, Y.; Li, W. Org. Lett. 2022, 24, 58–63. doi:10.1021/acs.orglett.1c03535
Return to citation in text: [1] -
Rashed, M. N.; Siddiki, S. M. A. H.; Touchy, A. S.; Jamil, M. A. R.; Poly, S. S.; Toyao, T.; Maeno, Z.; Shimizu, K.-i. Chem. – Eur. J. 2019, 25, 10594–10605. doi:10.1002/chem.201901446
Return to citation in text: [1] -
Kaur, A.; Pannu, A.; Brar, D. S.; Mehta, S. K.; Salunke, D. B. ACS Omega 2020, 5, 21007–21014. doi:10.1021/acsomega.0c02516
Return to citation in text: [1] -
Alawaed, A. A.; Ramachandran, P. V. Org. Biomol. Chem. 2024, 22, 1915–1919. doi:10.1039/d3ob01943h
Return to citation in text: [1] -
Gu, C.; Wang, S.; Zhang, Q.; Xie, J. Chem. Commun. 2022, 58, 5873–5876. doi:10.1039/d2cc01655a
Return to citation in text: [1] -
Zhu, R.; Li, Y.; Shen, Y.; Pan, M.; Dong, W.; Li, W. Org. Chem. Front. 2024, 11, 2095–2101. doi:10.1039/d3qo02111d
Return to citation in text: [1] -
Huy, P. H.; Mbouhom, C. Chem. Sci. 2019, 10, 7399–7406. doi:10.1039/c9sc02126d
Return to citation in text: [1] -
Jian, J.; Wang, Z.; Chen, L.; Gu, Y.; Miao, L.; Liu, Y.; Zeng, Z. Synthesis 2019, 51, 4078–4084. doi:10.1055/s-0039-1690178
Return to citation in text: [1] -
Li, D.; Wang, S.; Ge, S.; Dong, S.; Feng, X. Org. Lett. 2020, 22, 5331–5336. doi:10.1021/acs.orglett.0c01581
Return to citation in text: [1] -
Ong, J.-Y.; Ng, X. Q.; Lu, S.; Zhao, Y. Org. Lett. 2020, 22, 6447–6451. doi:10.1021/acs.orglett.0c02266
Return to citation in text: [1] -
Ramachandran, P. V.; Hamann, H. J. Org. Lett. 2021, 23, 2938–2942. doi:10.1021/acs.orglett.1c00591
Return to citation in text: [1] -
Ramachandran, P. V.; Singh, A.; Walker, H.; Hamann, H. J. Molecules 2024, 29, 268. doi:10.3390/molecules29010268
Return to citation in text: [1] -
Li, Y.-L.; Pang, J.-Y.; Lou, J.-C.; Sun, W.-W.; Liu, J.-K.; Wu, B. Asian J. Org. Chem. 2021, 10, 1424–1427. doi:10.1002/ajoc.202100188
Return to citation in text: [1] -
Patel, K. P.; Gayakwad, E. M.; Shankarling, G. S. ChemistrySelect 2020, 5, 8295–8300. doi:10.1002/slct.202000870
Return to citation in text: [1] -
Božić, B.; Lađarević, J.; Petković, M.; Mijin, D.; Stavber, S. Catalysts 2022, 12, 1413. doi:10.3390/catal12111413
Return to citation in text: [1] -
Nelson, H.; Richard, W.; Brown, H.; Medlin, A.; Light, C.; Heller, S. T. Angew. Chem., Int. Ed. 2021, 60, 22818–22825. doi:10.1002/anie.202107438
Return to citation in text: [1] -
Samuel Rajan, I. A. P.; Rajendran, S. Org. Biomol. Chem. 2024, 22, 5170–5180. doi:10.1039/d4ob00752b
Return to citation in text: [1] -
Yingxian, L.; Wei, C.; Linchun, Z.; Ji-Quan, Z.; Yonglong, Z.; Chun, L.; Bing, G.; Lei, T.; Yuan-Yong, Y. RSC Adv. 2022, 12, 20550–20554. doi:10.1039/d2ra03255d
Return to citation in text: [1] -
Liu, F.; Sohail, A.; Ablajan, K. J. Org. Chem. 2024, 89, 27–33. doi:10.1021/acs.joc.3c01151
Return to citation in text: [1] -
Liu, Y.; Chen, Q.; Mou, C.; Pan, L.; Duan, X.; Chen, X.; Chen, H.; Zhao, Y.; Lu, Y.; Jin, Z.; Chi, Y. R. Nat. Commun. 2019, 10, 1675. doi:10.1038/s41467-019-09445-x
Return to citation in text: [1] -
Yu, Y. J.; Häfliger, J.; Wang, Z. X.; Daniliuc, C. G.; Gilmour, R. Angew. Chem., Int. Ed. 2023, 62, e202309789. doi:10.1002/anie.202309789
Return to citation in text: [1] -
Sable, V.; Shah, J.; Sharma, A.; Kapdi, A. R. Chem. – Asian J. 2019, 14, 2639–2647. doi:10.1002/asia.201900566
Return to citation in text: [1] -
Hu, Y.; Xia, J.; Li, J.; Li, H.; Li, Y.; Li, S.; Duanmu, C.; Li, B.; Wang, X. J. Mater. Sci. 2021, 56, 7308–7320. doi:10.1007/s10853-020-05726-9
Return to citation in text: [1] -
Wang, R.; Liu, H.; Fan, C.; Gao, J.; Chen, C.; Zheng, Z. Mol. Catal. 2020, 484, 110687. doi:10.1016/j.mcat.2019.110687
Return to citation in text: [1] -
Wang, J.; Jiang, F.; Tao, C.; Yu, H.; Ruhlmann, L.; Wei, Y. Green Chem. 2021, 23, 2652–2657. doi:10.1039/d1gc00161b
Return to citation in text: [1] -
Wang, R.; Lu, K.; Zhang, J.; Li, X.; Zheng, Z. ACS Catal. 2022, 12, 14290–14303. doi:10.1021/acscatal.2c04837
Return to citation in text: [1] -
Yu, H.; Wang, J.; Wu, Z.; Zhao, Q.; Dan, D.; Han, S.; Tang, J.; Wei, Y. Green Chem. 2019, 21, 4550–4554. doi:10.1039/c9gc02053e
Return to citation in text: [1] -
Mali, G.; Tiwari, G.; Kamboj, N.; Metre, R. K.; Erande, R. D. Tetrahedron Lett. 2023, 124, 154608. doi:10.1016/j.tetlet.2023.154608
Return to citation in text: [1] -
Patel, A.; Patel, J. RSC Adv. 2020, 10, 22146–22155. doi:10.1039/d0ra04119j
Return to citation in text: [1] -
Wu, Z.; Jiang, D.; Wang, J. Org. Chem. Front. 2019, 6, 688–693. doi:10.1039/c8qo01420e
Return to citation in text: [1] -
Wang, G.; Wei, C.; Hong, X.; Fu, Z.; Huang, W. Green Chem. 2020, 22, 6819–6826. doi:10.1039/d0gc02555k
Return to citation in text: [1] -
Xin, H.-L.; Pang, B.; Choi, J.; Akkad, W.; Morimoto, H.; Ohshima, T. J. Org. Chem. 2020, 85, 11592–11606. doi:10.1021/acs.joc.0c01458
Return to citation in text: [1] -
Bacaicoa, S.; Stenkvist, S.; Sundén, H. Org. Lett. 2024, 26, 3114–3118. doi:10.1021/acs.orglett.4c00731
Return to citation in text: [1] -
Smeyne, D.; Verboom, K.; Bryan, M.; LoBue, J.; Shaikh, A. Tetrahedron Lett. 2021, 68, 152898. doi:10.1016/j.tetlet.2021.152898
Return to citation in text: [1] -
Shamnad, A.; Nayak, K. H.; Babu, B. P. J. Org. Chem. 2024, 89, 6545–6554. doi:10.1021/acs.joc.4c00156
Return to citation in text: [1] -
Xie, C.; Lin, L.; Huang, L.; Wang, Z.; Jiang, Z.; Zhang, Z.; Han, B. Nat. Commun. 2021, 12, 4823. doi:10.1038/s41467-021-25118-0
Return to citation in text: [1] -
Tang, W.-Y.; Chen, L.; Zheng, M.; Zhan, L.-W.; Hou, J.; Li, B.-D. Org. Lett. 2021, 23, 3939–3943. doi:10.1021/acs.orglett.1c01096
Return to citation in text: [1] -
Saitou, T.; Jin, Y.; Isobe, K.; Suga, T.; Takaya, J.; Iwasawa, N. Chem. – Asian J. 2020, 15, 1941–1944. doi:10.1002/asia.202000476
Return to citation in text: [1] -
Sahoo, H.; Zhang, L.; Cheng, J.; Nishiura, M.; Hou, Z. J. Am. Chem. Soc. 2022, 144, 23585–23594. doi:10.1021/jacs.2c10754
Return to citation in text: [1] -
Cao, Y.-F.; Li, L.-J.; Liu, M.; Xu, H.; Dai, H.-X. J. Org. Chem. 2020, 85, 4475–4481. doi:10.1021/acs.joc.0c00198
Return to citation in text: [1] -
Xu, J.-D.; Su, X.-B.; Wang, C.; Yao, L.-W.; Liu, J.-H.; Hu, G.-Q. Synlett 2021, 32, 833–837. doi:10.1055/a-1377-7369
Return to citation in text: [1] -
Yan, S.-S.; Wu, D.-S.; Ye, J.-H.; Gong, L.; Zeng, X.; Ran, C.-K.; Gui, Y.-Y.; Li, J.; Yu, D.-G. ACS Catal. 2019, 9, 6987–6992. doi:10.1021/acscatal.9b02351
Return to citation in text: [1] -
Xie, S.-L.; Gao, X.-T.; Wu, H.-H.; Zhou, F.; Zhou, J. Org. Lett. 2020, 22, 8424–8429. doi:10.1021/acs.orglett.0c03051
Return to citation in text: [1] -
Zhu, C.; Zhang, Y.-F.; Liu, Z.-Y.; Zhou, L.; Liu, H.; Feng, C. Chem. Sci. 2019, 10, 6721–6726. doi:10.1039/c9sc01336a
Return to citation in text: [1] -
Sun, C.; Zhou, Q.; Li, C.-Y.; Hou, Z.-W.; Wang, L. Org. Lett. 2024, 26, 883–888. doi:10.1021/acs.orglett.3c04071
Return to citation in text: [1] -
Yang, L.; Guan, A.-L.; Xu, Z.-Y.; Yao, Z.-J. Inorg. Chem. 2023, 62, 18198–18208. doi:10.1021/acs.inorgchem.3c02663
Return to citation in text: [1] -
Lee, H.-J.; Yonekura, Y.; Kim, N.; Yoshida, J.-i.; Kim, H. Org. Lett. 2021, 23, 2904–2910. doi:10.1021/acs.orglett.1c00538
Return to citation in text: [1] -
Xu, J.-X.; Zhao, F.; Wu, X.-F. Org. Biomol. Chem. 2020, 18, 9796–9799. doi:10.1039/d0ob02043e
Return to citation in text: [1] -
Yang, Z.-X.; Lai, L.; Chen, J.; Yan, H.; Ye, K.-Y.; Chen, F.-E. Chin. Chem. Lett. 2023, 34, 107956. doi:10.1016/j.cclet.2022.107956
Return to citation in text: [1] -
Yan, Q.; Yuan, Q.-J.; Shatskiy, A.; Alvey, G. R.; Stepanova, E. V.; Liu, J.-Q.; Kärkäs, M. D.; Wang, X.-S. Org. Lett. 2024, 26, 3380–3385. doi:10.1021/acs.orglett.4c00872
Return to citation in text: [1] -
Luo, Y.; Wang, X.; Liu, Q.; He, Y.; Li, J.; Luo, S.; Zhu, Q. Green Chem. 2023, 25, 1120–1127. doi:10.1039/d2gc04689j
Return to citation in text: [1] -
Lv, J.; Zong, L.; Zhang, J.; Song, J.; Zhao, J.; Zhang, K.; Zhou, Z.; Gao, M.; Xie, C.; Jia, X.; Ren, X. Catal. Sci. Technol. 2021, 11, 4708–4713. doi:10.1039/d1cy00699a
Return to citation in text: [1] -
Huang, Z.; Dong, Y.; Li, Y.; Makha, M.; Li, Y. ChemCatChem 2019, 11, 5236–5240. doi:10.1002/cctc.201900995
Return to citation in text: [1] -
Xiong, W.; Shi, F.; Cheng, R.; Zhu, B.; Wang, L.; Chen, P.; Lou, H.; Wu, W.; Qi, C.; Lei, M.; Jiang, H. ACS Catal. 2020, 10, 7968–7978. doi:10.1021/acscatal.0c01687
Return to citation in text: [1] -
Taniguchi, T.; Saito, N.; Doi, R.; Kimoto, A.; Hoshiya, N.; Fujiki, K.; Shuto, S.; Fujioka, H.; Arisawa, M.; Sato, Y. Chem. Lett. 2019, 48, 1406–1409. doi:10.1246/cl.190650
Return to citation in text: [1] -
Xia, S.-M.; Yang, Z.-W.; Chen, K.-H.; Wang, N.; He, L.-N. Chin. J. Catal. 2022, 43, 1642–1651. doi:10.1016/s1872-2067(21)63848-2
Return to citation in text: [1] -
Xia, S.-M.; Yang, Z.-W.; Yao, X.-Y.; Chen, K.-H.; Qiu, L.-Q.; He, L.-N. Green Chem. 2021, 23, 8089–8095. doi:10.1039/d1gc02735b
Return to citation in text: [1] -
Wang, C.-S.; Di Monaco, S.; Thai, A. N.; Rahman, M. S.; Pang, B. P.; Wang, C.; Yoshikai, N. J. Am. Chem. Soc. 2020, 142, 12878–12889. doi:10.1021/jacs.0c06412
Return to citation in text: [1] -
Sun, X.; Duan, X.; Zheng, N.; Song, W. Org. Lett. 2023, 25, 2798–2805. doi:10.1021/acs.orglett.3c00682
Return to citation in text: [1] -
Nanda, T.; Biswal, P.; Pati, B. V.; Banjare, S. K.; Ravikumar, P. C. J. Org. Chem. 2021, 86, 2682–2695. doi:10.1021/acs.joc.0c02700
Return to citation in text: [1] -
Lu, L.-G.; Chen, J.-H.; Huang, X.-B.; Liu, M.-C.; Zhou, Y.-B.; Wu, H.-Y. J. Org. Chem. 2022, 87, 16851–16859. doi:10.1021/acs.joc.2c01976
Return to citation in text: [1] -
Xu, J.-L.; Tian, H.; Kang, J.-H.; Kang, W.-X.; Sun, W.; Sun, R.; Li, Y.-M.; Sun, M. Org. Lett. 2020, 22, 6739–6743. doi:10.1021/acs.orglett.0c02099
Return to citation in text: [1] -
Yang, Y.-F.; Huang, X.-B.; Gao, W.-X.; Zhou, Y.-B.; Liu, M.-C.; Wu, H.-Y. Org. Biomol. Chem. 2020, 18, 5822–5825. doi:10.1039/d0ob00601g
Return to citation in text: [1] -
Greco, R.; Tiburcio, E.; Palomar-De Lucas, B.; Ferrando-Soria, J.; Armentano, D.; Pardo, E.; Leyva-Pérez, A. Mol. Catal. 2022, 522, 112228. doi:10.1016/j.mcat.2022.112228
Return to citation in text: [1] -
Sahoo, T.; Sankaram, G. S.; Madhu, M.; Sridhar, B.; Reddy, B. V. S. Eur. J. Org. Chem. 2024, e202400036. doi:10.1002/ejoc.202400036
Return to citation in text: [1] -
Wang, H.; Wei, Y.; He, Y.; He, T.-J.; Lin, Y.-W. J. Org. Chem. 2024, 89, 10093–10098. doi:10.1021/acs.joc.4c00941
Return to citation in text: [1] -
Yao, L.; Hu, Q.; Lei, Y.; Bao, L.; Hu, Y. Org. Chem. Front. 2020, 7, 3633–3637. doi:10.1039/d0qo00967a
Return to citation in text: [1] -
Bai, Z.; Zhu, S.; Hu, Y.; Yang, P.; Chu, X.; He, G.; Wang, H.; Chen, G. Nat. Commun. 2022, 13, 6445. doi:10.1038/s41467-022-34223-7
Return to citation in text: [1] -
Bae, H.; Park, J.; Yoon, R.; Lee, S.; Son, J. RSC Adv. 2024, 14, 9440–9444. doi:10.1039/d4ra00320a
Return to citation in text: [1] -
Ghosh, S.; Jana, C. K. Green Chem. 2019, 21, 5803–5807. doi:10.1039/c9gc02937k
Return to citation in text: [1] -
Manenti, M.; Gusmini, S.; Lo Presti, L.; Silvani, A. Eur. J. Org. Chem. 2022, e202200435. doi:10.1002/ejoc.202200435
Return to citation in text: [1] -
Zhao, C.-Q.; Chen, Y.-G.; Qiu, H.; Wei, L.; Fang, P.; Mei, T.-S. Org. Lett. 2019, 21, 1412–1416. doi:10.1021/acs.orglett.9b00148
Return to citation in text: [1] -
Shi, J.; Ye, T.; Dong, J.; Liu, A.; Xu, T.; Tai, M.; Zhang, L.; Wang, C. ACS Omega 2023, 8, 11492–11502. doi:10.1021/acsomega.3c00287
Return to citation in text: [1] -
Li, K.; Khan, R.; Zhang, X.; Gao, Y.; Zhou, Y.; Tan, H.; Chen, J.; Fan, B. Chem. Commun. 2019, 55, 5663–5666. doi:10.1039/c9cc01970g
Return to citation in text: [1] -
Wu, Y.; Ao, Y.; Li, Z.; Liu, C.; Zhao, J.; Gao, W.; Li, X.; Wang, H.; Liu, Y.; Liu, Y. Nat. Commun. 2023, 14, 1655. doi:10.1038/s41467-023-37022-w
Return to citation in text: [1] -
Verma, A.; Grams, R. J.; Rastatter, B. P.; Santos, W. L. Tetrahedron 2019, 75, 2113–2117. doi:10.1016/j.tet.2019.02.030
Return to citation in text: [1] -
Seifert, F.; Drikermann, D.; Steinmetzer, J.; Zi, Y.; Kupfer, S.; Vilotijevic, I. Org. Biomol. Chem. 2021, 19, 6092–6097. doi:10.1039/d1ob00909e
Return to citation in text: [1] -
Volchkov, I.; Powell, B. V.; Zatolochnaya, O. V.; Leung, J. C.; Pennino, S.; Wu, L.; Gonnella, N. C.; Bhaskararao, B.; Kozlowski, M. C.; Reeves, J. T. J. Org. Chem. 2023, 88, 10881–10904. doi:10.1021/acs.joc.3c00917
Return to citation in text: [1] -
Merad, J.; Grant, P. S.; Stopka, T.; Sabbatani, J.; Meyrelles, R.; Preinfalk, A.; Matyasovsky, J.; Maryasin, B.; González, L.; Maulide, N. J. Am. Chem. Soc. 2022, 144, 12536–12543. doi:10.1021/jacs.2c05637
Return to citation in text: [1] -
Yan, K.; He, M.; Li, J.; He, H.; Lai, R.; Luo, Y.; Guo, L.; Wu, Y. Chem. Commun. 2020, 56, 14287–14290. doi:10.1039/d0cc06236g
Return to citation in text: [1] -
Liu, X.; Werner, T. Adv. Synth. Catal. 2021, 363, 1096–1104. doi:10.1002/adsc.202001209
Return to citation in text: [1] -
Longwitz, L.; Spannenberg, A.; Werner, T. ACS Catal. 2019, 9, 9237–9244. doi:10.1021/acscatal.9b02456
Return to citation in text: [1] -
Ramavath, V.; Rupanawar, B. D.; More, S. G.; Bansode, A. H.; Suryavanshi, G. New J. Chem. 2021, 45, 8806–8813. doi:10.1039/d1nj01170g
Return to citation in text: [1] -
Kardile, R. D.; Liu, R.-S. Org. Lett. 2019, 21, 6452–6456. doi:10.1021/acs.orglett.9b02343
Return to citation in text: [1] -
Golubev, A. A.; Smetanin, I. A.; Agafonova, A. V.; Rostovskii, N. V.; Khlebnikov, A. F.; Starova, G. L.; Novikov, M. S. Org. Biomol. Chem. 2019, 17, 6821–6830. doi:10.1039/c9ob01301f
Return to citation in text: [1] -
Shang, W.; Duan, D.; Liu, Y.; Lv, J. Org. Lett. 2019, 21, 8013–8017. doi:10.1021/acs.orglett.9b03005
Return to citation in text: [1] -
Chérif, S. E.; Ghosh, A.; Chelli, S.; Dixon, I. M.; Kraiem, J.; Lakhdar, S. Chem. Sci. 2022, 13, 12065–12070. doi:10.1039/d2sc03961c
Return to citation in text: [1] -
Tran, U. P. N.; Oss, G.; Breugst, M.; Detmar, E.; Pace, D. P.; Liyanto, K.; Nguyen, T. V. ACS Catal. 2019, 9, 912–919. doi:10.1021/acscatal.8b03769
Return to citation in text: [1] -
Steenackers, W.; El Houari, I.; Baekelandt, A.; Witvrouw, K.; Dhondt, S.; Leroux, O.; Gonzalez, N.; Corneillie, S.; Cesarino, I.; Inzé, D.; Boerjan, W.; Vanholme, B. J. Exp. Bot. 2019, 70, 6293–6304. doi:10.1093/jxb/erz392
Return to citation in text: [1] -
Brégent, T.; Bouillon, J.-P.; Poisson, T. Org. Lett. 2020, 22, 7688–7693. doi:10.1021/acs.orglett.0c02894
Return to citation in text: [1] -
Cruché, C.; Neiderer, W.; Collins, S. K. ACS Catal. 2021, 11, 8829–8836. doi:10.1021/acscatal.1c01983
Return to citation in text: [1] -
Akkarasamiyo, S.; Chitsomkhuan, S.; Buakaew, S.; Samec, J. S. M.; Songsri, C.; Kuntiyong, P. Asian J. Org. Chem. 2024, 13, e202400009. doi:10.1002/ajoc.202400009
Return to citation in text: [1] -
Ozeki, M.; Hachino, A.; Shigeta, T.; Niki, A.; Kobayashi, N.; Mizutani, H.; Nakamura, A.; Horie, A.; Arimitsu, K.; Kajimoto, T.; Hosoi, S.; Iwasaki, H.; Kojima, N.; Yamashita, M.; Kawasaki, I. Chem. Pharm. Bull. 2019, 67, 71–74. doi:10.1248/cpb.c18-00666
Return to citation in text: [1] -
Bakthadoss, M.; Agarwal, V.; Tadiparthi, T. R.; Mohammad, M. Mol. Diversity 2021, 25, 2467–2478. doi:10.1007/s11030-020-10105-2
Return to citation in text: [1] -
Wang, C.; Dam, P.; Elghobashy, M.; Brückner, A.; Rabeah, J.; Azofra, L. M.; El-Sepelgy, O. ACS Catal. 2023, 13, 14205–14212. doi:10.1021/acscatal.3c04305
Return to citation in text: [1] -
Chen, K.-Q.; Shen, J.; Wang, Z.-X.; Chen, X.-Y. Chem. Sci. 2021, 12, 6684–6690. doi:10.1039/d1sc01024g
Return to citation in text: [1] -
Dutta, S.; Kumar, P.; Sharma, S.; Yadav, S.; Priyanka; Dixit, R.; Srivastava, A.; Sharma, R. K. RSC Sustainability 2023, 1, 975–986. doi:10.1039/d3su00073g
Return to citation in text: [1] -
Zhao, T.-T.; Song, J.-L.; Hong, F.-Q.; Xia, J.-S.; Li, J.-J. Chem. Pap. 2019, 73, 2857–2868. doi:10.1007/s11696-019-00838-2
Return to citation in text: [1] -
Pandia, B. K.; Gunanathan, C. J. Org. Chem. 2021, 86, 9994–10005. doi:10.1021/acs.joc.1c00685
Return to citation in text: [1] -
Surya Prakash Rao, H.; Padder, A. H. New J. Chem. 2020, 44, 9010–9017. doi:10.1039/d0nj00231c
Return to citation in text: [1] -
Chen, Y.; Turlik, A.; Newhouse, T. R. J. Am. Chem. Soc. 2016, 138, 1166–1169. doi:10.1021/jacs.5b12924
Return to citation in text: [1] -
Chen, Y.; Romaire, J. P.; Newhouse, T. R. J. Am. Chem. Soc. 2015, 137, 5875–5878. doi:10.1021/jacs.5b02243
Return to citation in text: [1] -
Huang, D.; Szewczyk, S. M.; Zhang, P.; Newhouse, T. R. J. Am. Chem. Soc. 2019, 141, 5669–5674. doi:10.1021/jacs.9b02552
Return to citation in text: [1] -
Zhou, M.-J.; Zhang, L.; Liu, G.; Xu, C.; Huang, Z. J. Am. Chem. Soc. 2021, 143, 16470–16485. doi:10.1021/jacs.1c05479
Return to citation in text: [1] -
Han, J.; Xu, Y.-W.; Wang, X.-M.; Zhang, L.; Hu, X.-P. Tetrahedron Lett. 2020, 61, 151409. doi:10.1016/j.tetlet.2019.151409
Return to citation in text: [1] -
Rocaboy, R.; Baudoin, O. Org. Lett. 2019, 21, 1434–1437. doi:10.1021/acs.orglett.9b00187
Return to citation in text: [1] -
Cheng, C.; Zhu, Q.; Zhang, Y. Chem. Commun. 2021, 57, 9700–9703. doi:10.1039/d1cc03677g
Return to citation in text: [1] -
Bae, S.; Zhang, C.; Gillard, R. M.; Lupton, D. W. Angew. Chem., Int. Ed. 2019, 58, 13370–13374. doi:10.1002/anie.201907111
Return to citation in text: [1] -
Pitchumani, V.; Breugst, M.; Lupton, D. W. Org. Lett. 2021, 23, 9413–9418. doi:10.1021/acs.orglett.1c03554
Return to citation in text: [1] -
Tsai, J.-J.; Huang, Y.-H.; Chou, C.-M. Org. Lett. 2021, 23, 9468–9473. doi:10.1021/acs.orglett.1c03650
Return to citation in text: [1] -
Liang, K.; Li, T.; Li, N.; Zhang, Y.; Shen, L.; Ma, Z.; Xia, C. Chem. Sci. 2020, 11, 2130–2135. doi:10.1039/c9sc06184c
Return to citation in text: [1] -
Sun, S.-Z.; Xu, H.; Dai, H.-X. Chin. Chem. Lett. 2019, 30, 969–972. doi:10.1016/j.cclet.2019.02.011
Return to citation in text: [1] -
Spencer, A. R. A.; Korde, R.; Font, M.; Larrosa, I. Chem. Sci. 2020, 11, 4204–4208. doi:10.1039/d0sc01138j
Return to citation in text: [1] -
Raziullah; Kumar, M.; Khan, A. A.; Dutta, H. S.; Ahmad, A.; Vaishnav, J.; Kant, R.; Ampapathi, R. S.; Koley, D. Eur. J. Org. Chem. 2021, 2107–2113. doi:10.1002/ejoc.202100085
Return to citation in text: [1] -
Raziullah; Kumar, M.; Ahmad, A.; Dutta, H. S.; Rastogi, A.; Gangwar, M. K.; Koley, D. Chem. Commun. 2022, 58, 3481–3484. doi:10.1039/d2cc00257d
Return to citation in text: [1] -
Choudhary, S.; Cannas, D. M.; Wheatley, M.; Larrosa, I. Chem. Sci. 2022, 13, 13225–13230. doi:10.1039/d2sc04295a
Return to citation in text: [1] -
Li, H.; Tian, X.; Dang, Q.-Q.; Zhang, J.; Wen, Z.-K. ACS Catal. 2024, 14, 9985–9992. doi:10.1021/acscatal.4c01944
Return to citation in text: [1] -
Wang, H.-W.; Qiao, Y.-H.; Wu, J.-X.; Wang, Q.-P.; Tian, M.-X.; Li, Y.-F.; Yao, Q.-X.; Li, D.-C.; Dou, J.-M.; Lu, Y. Org. Lett. 2021, 23, 656–662. doi:10.1021/acs.orglett.0c03688
Return to citation in text: [1] -
Bakthadoss, M.; Reddy, T. T. Chem. Sci. 2023, 14, 5880–5886. doi:10.1039/d2sc06206b
Return to citation in text: [1] [2] -
Wang, J.; Hong, H.; Hu, D.; Liu, J.; Liu, W.; Shi, Y. Org. Chem. Front. 2024, 11, 484–488. doi:10.1039/d3qo01472j
Return to citation in text: [1] -
Nguyen, K. X.; Pham, P. H.; Nguyen, T. T.; Yang, C.-H.; Pham, H. T. B.; Nguyen, T. T.; Wang, H.; Phan, N. T. S. Org. Lett. 2020, 22, 9751–9756. doi:10.1021/acs.orglett.0c03846
Return to citation in text: [1] -
Liao, M.; Liu, C.; Feng, H.; You, Q.; Huang, J. Mol. Catal. 2024, 558, 114031. doi:10.1016/j.mcat.2024.114031
Return to citation in text: [1] -
Long, J.; Zhao, R.; Cheng, G.-J.; Fang, X. Angew. Chem., Int. Ed. 2023, 62, e202304543. doi:10.1002/anie.202304543
Return to citation in text: [1] -
Long, J.; Wang, L.; Liu, X.; Liu, J.; Luo, S.-P.; Fang, X. Org. Lett. 2024, 26, 3945–3950. doi:10.1021/acs.orglett.4c01192
Return to citation in text: [1] -
Michon, C.; Gilbert, J.; Trivelli, X.; Nahra, F.; Cazin, C. S. J.; Agbossou-Niedercorn, F.; Nolan, S. P. Org. Biomol. Chem. 2019, 17, 3805–3811. doi:10.1039/c9ob00587k
Return to citation in text: [1] -
Sugimoto, K.; Kosuge, S.; Sugita, T.; Miura, Y.; Tsuge, K.; Matsuya, Y. Org. Lett. 2021, 23, 3981–3985. doi:10.1021/acs.orglett.1c01171
Return to citation in text: [1] -
Shanmugasundaram, M.; Senthilvelan, A.; Kore, A. R. Tetrahedron Lett. 2019, 60, 157–160. doi:10.1016/j.tetlet.2018.12.001
Return to citation in text: [1] -
Sieland, B.; Hoppe, A.; Stepen, A. J.; Paradies, J. Adv. Synth. Catal. 2022, 364, 3143–3148. doi:10.1002/adsc.202200525
Return to citation in text: [1] -
Khan, D.; Parveen, I. Eur. J. Org. Chem. 2021, 4946–4957. doi:10.1002/ejoc.202100866
Return to citation in text: [1] -
Brunner, H.; Vedder, L. ChemCatChem 2019, 11, 698–702. doi:10.1002/cctc.201801519
Return to citation in text: [1] -
Ojha, S.; Panda, N. Org. Biomol. Chem. 2022, 20, 1292–1298. doi:10.1039/d1ob02360h
Return to citation in text: [1] -
Wang, M.-L.; Xu, H.; Li, H.-Y.; Ma, B.; Wang, Z.-Y.; Wang, X.; Dai, H.-X. Org. Lett. 2021, 23, 2147–2152. doi:10.1021/acs.orglett.1c00296
Return to citation in text: [1] -
Mondal, B.; Ghosh, P.; Kundu, M.; Das, T. K.; Das, S. Org. Biomol. Chem. 2021, 19, 360–364. doi:10.1039/d0ob02134b
Return to citation in text: [1] -
Belitz, F.; Seitz, A.-K.; Goebel, J. F.; Hu, Z.; Gooßen, L. J. Org. Lett. 2022, 24, 3466–3470. doi:10.1021/acs.orglett.2c01043
Return to citation in text: [1] -
Wu, J.-X.; Chi, R.; Li, D.-C.; Cui, J.-C.; Dou, J.-M.; Wang, H.-W. Tetrahedron Lett. 2023, 125, 154619. doi:10.1016/j.tetlet.2023.154619
Return to citation in text: [1] -
Bagherzadeh, M.; Kaveh, R.; Mahmoudi, H. J. Mater. Chem. A 2019, 7, 16257–16266. doi:10.1039/c9ta02882j
Return to citation in text: [1] -
Hasan, K. ChemistrySelect 2020, 5, 7129–7140. doi:10.1002/slct.202001933
Return to citation in text: [1] -
Sobhani, S.; Zarei, H.; Sansano, J. M. Sci. Rep. 2021, 11, 17025. doi:10.1038/s41598-021-95931-6
Return to citation in text: [1] -
Lei, L. Appl. Organomet. Chem. 2019, 33, e5158. doi:10.1002/aoc.5158
Return to citation in text: [1] -
Dohendou, M.; Dekamin, M. G.; Namaki, D. Nanoscale Adv. 2023, 5, 3463–3484. doi:10.1039/d3na00157a
Return to citation in text: [1] -
Eslahi, H.; Sardarian, A. R.; Esmaeilpour, M. Appl. Organomet. Chem. 2021, 35, e6260. doi:10.1002/aoc.6260
Return to citation in text: [1] -
Lv, W.; Chen, Y.; Wen, S.; Ba, D.; Cheng, G. J. Am. Chem. Soc. 2020, 142, 14864–14870. doi:10.1021/jacs.0c07634
Return to citation in text: [1] -
Wang, J.; Qin, C.; Lumb, J.-P.; Luan, X. Chem 2020, 6, 2097–2109. doi:10.1016/j.chempr.2020.06.021
Return to citation in text: [1] -
Niedballa, J.; Reiss, G. J.; Müller, T. J. J. Eur. J. Org. Chem. 2020, 5019–5024. doi:10.1002/ejoc.202000823
Return to citation in text: [1] -
Yao, T.; Hui, J.; Liu, T.; Li, T. J. Org. Chem. 2021, 86, 5477–5488. doi:10.1021/acs.joc.0c02994
Return to citation in text: [1] -
Li, B.; Ju, C.-W.; Wang, W.; Gu, Y.; Chen, S.; Luo, Y.; Zhang, H.; Yang, J.; Liang, H.-w.; Bonn, M.; Müllen, K.; Goddard, W. A., III; Zhou, Y. J. Am. Chem. Soc. 2023, 145, 24126–24135. doi:10.1021/jacs.3c07851
Return to citation in text: [1] -
Mishra, S.; Halpani, C. G.; Patel, S. ChemistrySelect 2019, 4, 6913–6916. doi:10.1002/slct.201901131
Return to citation in text: [1] -
Sobhani, S.; Hosseini Moghadam, H.; Skibsted, J.; Sansano, J. M. Green Chem. 2020, 22, 1353–1365. doi:10.1039/c9gc03455b
Return to citation in text: [1] -
Sruthi, P. R.; Anjali, S.; Varghese, N.; Anas, S. J. Organomet. Chem. 2020, 921, 121354. doi:10.1016/j.jorganchem.2020.121354
Return to citation in text: [1] -
Alavi G., S. A.; Nasseri, M. A.; Kazemnejadi, M.; Allahresani, A.; HussainZadeh, M. New J. Chem. 2021, 45, 7741–7757. doi:10.1039/d0nj06208a
Return to citation in text: [1] -
Arghan, M.; Koukabi, N.; Kolvari, E. Appl. Organomet. Chem. 2019, 33, e4823. doi:10.1002/aoc.4823
Return to citation in text: [1] -
Chen, J.; Xu, J.; Zhou, Y.; Xie, S.; Gao, F.; Xu, X.; Xu, X.; Jin, Z. Org. Lett. 2019, 21, 7928–7932. doi:10.1021/acs.orglett.9b02910
Return to citation in text: [1] -
Lin, Y.; Ma, W.-Y.; Xu, Z.; Zheng, Z.-J.; Cao, J.; Yang, K.-F.; Cui, Y.-M.; Xu, L.-W. Chem. – Asian J. 2019, 14, 2082–2085. doi:10.1002/asia.201900408
Return to citation in text: [1] -
Yang, X.; Sun, R.; Zhang, C.; Zheng, X.; Yuan, M.; Fu, H.; Li, R.; Chen, H. Org. Lett. 2019, 21, 1002–1006. doi:10.1021/acs.orglett.8b04005
Return to citation in text: [1] -
Baghel, A. S.; Aghi, A.; Kumar, A. J. Org. Chem. 2021, 86, 9744–9754. doi:10.1021/acs.joc.1c01090
Return to citation in text: [1] -
Wang, H.; Li, Z.; Chen, X.; Wong, J. J.; Bi, T.; Tong, X.; Xu, Z.; Zhen, M.; Wan, Y.; Tang, L.; Liu, B.; Zong, X.; Xu, D.; Zuo, J.; Yang, L.; Huang, W.; Houk, K. N.; Yang, W. Chem 2023, 9, 607–623. doi:10.1016/j.chempr.2022.10.019
Return to citation in text: [1] -
Bakthadoss, M.; Reddy, T. T.; Sharada, D. S. RSC Adv. 2020, 10, 31570–31574. doi:10.1039/d0ra06580c
Return to citation in text: [1] -
Shinde, V. N.; Rangan, K.; Kumar, D.; Kumar, A. J. Org. Chem. 2021, 86, 9755–9770. doi:10.1021/acs.joc.1c01097
Return to citation in text: [1] -
Sk, M. R.; Bera, S. S.; Maji, M. S. Adv. Synth. Catal. 2019, 361, 585–590. doi:10.1002/adsc.201801385
Return to citation in text: [1] -
Luo, J.; Zhang, T.; Wang, L.; Liao, G.; Yao, Q.-J.; Wu, Y.-J.; Zhan, B.-B.; Lan, Y.; Lin, X.-F.; Shi, B.-F. Angew. Chem., Int. Ed. 2019, 58, 6708–6712. doi:10.1002/anie.201902126
Return to citation in text: [1] -
Song, H.; Li, Y.; Yao, Q.-J.; Jin, L.; Liu, L.; Liu, Y.-H.; Shi, B.-F. Angew. Chem., Int. Ed. 2020, 59, 6576–6580. doi:10.1002/anie.201915949
Return to citation in text: [1] -
Xu, J.; Chen, J.; Gao, F.; Xie, S.; Xu, X.; Jin, Z.; Yu, J.-Q. J. Am. Chem. Soc. 2019, 141, 1903–1907. doi:10.1021/jacs.8b13403
Return to citation in text: [1] -
Bag, S.; Jana, S.; Pradhan, S.; Bhowmick, S.; Goswami, N.; Sinha, S. K.; Maiti, D. Nat. Commun. 2021, 12, 1393. doi:10.1038/s41467-021-21633-2
Return to citation in text: [1] -
Cai, L.; Li, S.; Zhou, C.; Li, G. Org. Lett. 2020, 22, 7791–7796. doi:10.1021/acs.orglett.0c02528
Return to citation in text: [1] -
Dutta, U.; Maiti, S.; Pimparkar, S.; Maiti, S.; Gahan, L. R.; Krenske, E. H.; Lupton, D. W.; Maiti, D. Chem. Sci. 2019, 10, 7426–7432. doi:10.1039/c9sc01824g
Return to citation in text: [1] -
Panja, S.; Ahsan, S.; Pal, T.; Kolb, S.; Ali, W.; Sharma, S.; Das, C.; Grover, J.; Dutta, A.; Werz, D. B.; Paul, A.; Maiti, D. Chem. Sci. 2022, 13, 9432–9439. doi:10.1039/d2sc03288k
Return to citation in text: [1] -
Wu, X.; Chen, W.; Holmberg-Douglas, N.; Bida, G. T.; Tu, X.; Ma, X.; Wu, Z.; Nicewicz, D. A.; Li, Z. Chem 2023, 9, 343–362. doi:10.1016/j.chempr.2022.12.007
Return to citation in text: [1] -
Timelthaler, D.; Schöfberger, W.; Topf, C. Eur. J. Org. Chem. 2021, 2114–2120. doi:10.1002/ejoc.202100073
Return to citation in text: [1] -
Wang, Y.; Tang, Y.; Zong, Y.; Tsui, G. C. Org. Lett. 2022, 24, 4087–4092. doi:10.1021/acs.orglett.2c01639
Return to citation in text: [1] -
Hu, Y.; Wang, Z.; Luo, H.; Jin, H.; Liu, Y.; Zhou, B. Org. Biomol. Chem. 2021, 19, 3834–3837. doi:10.1039/d1ob00443c
Return to citation in text: [1] -
Li, X.; Cai, W.; Huang, Y. Adv. Synth. Catal. 2022, 364, 1879–1883. doi:10.1002/adsc.202200071
Return to citation in text: [1]
| 23. | Du, Y.; Barber, T.; Lim, S. E.; Rzepa, H. S.; Baxendale, I. R.; Whiting, A. Chem. Commun. 2019, 55, 2916–2919. doi:10.1039/c8cc09913h |
| 78. | Li, Y.-L.; Pang, J.-Y.; Lou, J.-C.; Sun, W.-W.; Liu, J.-K.; Wu, B. Asian J. Org. Chem. 2021, 10, 1424–1427. doi:10.1002/ajoc.202100188 |
| 76. | Ramachandran, P. V.; Hamann, H. J. Org. Lett. 2021, 23, 2938–2942. doi:10.1021/acs.orglett.1c00591 |
| 77. | Ramachandran, P. V.; Singh, A.; Walker, H.; Hamann, H. J. Molecules 2024, 29, 268. doi:10.3390/molecules29010268 |
| 180. | Raziullah; Kumar, M.; Ahmad, A.; Dutta, H. S.; Rastogi, A.; Gangwar, M. K.; Koley, D. Chem. Commun. 2022, 58, 3481–3484. doi:10.1039/d2cc00257d |
| 75. | Ong, J.-Y.; Ng, X. Q.; Lu, S.; Zhao, Y. Org. Lett. 2020, 22, 6447–6451. doi:10.1021/acs.orglett.0c02266 |
| 179. | Raziullah; Kumar, M.; Khan, A. A.; Dutta, H. S.; Ahmad, A.; Vaishnav, J.; Kant, R.; Ampapathi, R. S.; Koley, D. Eur. J. Org. Chem. 2021, 2107–2113. doi:10.1002/ejoc.202100085 |
| 176. | Liang, K.; Li, T.; Li, N.; Zhang, Y.; Shen, L.; Ma, Z.; Xia, C. Chem. Sci. 2020, 11, 2130–2135. doi:10.1039/c9sc06184c |
| 175. | Tsai, J.-J.; Huang, Y.-H.; Chou, C.-M. Org. Lett. 2021, 23, 9468–9473. doi:10.1021/acs.orglett.1c03650 |
| 83. | Yingxian, L.; Wei, C.; Linchun, Z.; Ji-Quan, Z.; Yonglong, Z.; Chun, L.; Bing, G.; Lei, T.; Yuan-Yong, Y. RSC Adv. 2022, 12, 20550–20554. doi:10.1039/d2ra03255d |
| 178. | Spencer, A. R. A.; Korde, R.; Font, M.; Larrosa, I. Chem. Sci. 2020, 11, 4204–4208. doi:10.1039/d0sc01138j |
| 177. | Sun, S.-Z.; Xu, H.; Dai, H.-X. Chin. Chem. Lett. 2019, 30, 969–972. doi:10.1016/j.cclet.2019.02.011 |
| 81. | Nelson, H.; Richard, W.; Brown, H.; Medlin, A.; Light, C.; Heller, S. T. Angew. Chem., Int. Ed. 2021, 60, 22818–22825. doi:10.1002/anie.202107438 |
| 172. | Cheng, C.; Zhu, Q.; Zhang, Y. Chem. Commun. 2021, 57, 9700–9703. doi:10.1039/d1cc03677g |
| 82. | Samuel Rajan, I. A. P.; Rajendran, S. Org. Biomol. Chem. 2024, 22, 5170–5180. doi:10.1039/d4ob00752b |
| 171. | Rocaboy, R.; Baudoin, O. Org. Lett. 2019, 21, 1434–1437. doi:10.1021/acs.orglett.9b00187 |
| 79. | Patel, K. P.; Gayakwad, E. M.; Shankarling, G. S. ChemistrySelect 2020, 5, 8295–8300. doi:10.1002/slct.202000870 |
| 174. | Pitchumani, V.; Breugst, M.; Lupton, D. W. Org. Lett. 2021, 23, 9413–9418. doi:10.1021/acs.orglett.1c03554 |
| 80. | Božić, B.; Lađarević, J.; Petković, M.; Mijin, D.; Stavber, S. Catalysts 2022, 12, 1413. doi:10.3390/catal12111413 |
| 173. | Bae, S.; Zhang, C.; Gillard, R. M.; Lupton, D. W. Angew. Chem., Int. Ed. 2019, 58, 13370–13374. doi:10.1002/anie.201907111 |
| 181. | Choudhary, S.; Cannas, D. M.; Wheatley, M.; Larrosa, I. Chem. Sci. 2022, 13, 13225–13230. doi:10.1039/d2sc04295a |
| 88. | Hu, Y.; Xia, J.; Li, J.; Li, H.; Li, Y.; Li, S.; Duanmu, C.; Li, B.; Wang, X. J. Mater. Sci. 2021, 56, 7308–7320. doi:10.1007/s10853-020-05726-9 |
| 89. | Wang, R.; Liu, H.; Fan, C.; Gao, J.; Chen, C.; Zheng, Z. Mol. Catal. 2020, 484, 110687. doi:10.1016/j.mcat.2019.110687 |
| 86. | Yu, Y. J.; Häfliger, J.; Wang, Z. X.; Daniliuc, C. G.; Gilmour, R. Angew. Chem., Int. Ed. 2023, 62, e202309789. doi:10.1002/anie.202309789 |
| 87. | Sable, V.; Shah, J.; Sharma, A.; Kapdi, A. R. Chem. – Asian J. 2019, 14, 2639–2647. doi:10.1002/asia.201900566 |
| 84. | Liu, F.; Sohail, A.; Ablajan, K. J. Org. Chem. 2024, 89, 27–33. doi:10.1021/acs.joc.3c01151 |
| 85. | Liu, Y.; Chen, Q.; Mou, C.; Pan, L.; Duan, X.; Chen, X.; Chen, H.; Zhao, Y.; Lu, Y.; Jin, Z.; Chi, Y. R. Nat. Commun. 2019, 10, 1675. doi:10.1038/s41467-019-09445-x |
| 190. | Michon, C.; Gilbert, J.; Trivelli, X.; Nahra, F.; Cazin, C. S. J.; Agbossou-Niedercorn, F.; Nolan, S. P. Org. Biomol. Chem. 2019, 17, 3805–3811. doi:10.1039/c9ob00587k |
| 187. | Liao, M.; Liu, C.; Feng, H.; You, Q.; Huang, J. Mol. Catal. 2024, 558, 114031. doi:10.1016/j.mcat.2024.114031 |
| 186. | Nguyen, K. X.; Pham, P. H.; Nguyen, T. T.; Yang, C.-H.; Pham, H. T. B.; Nguyen, T. T.; Wang, H.; Phan, N. T. S. Org. Lett. 2020, 22, 9751–9756. doi:10.1021/acs.orglett.0c03846 |
| 189. | Long, J.; Wang, L.; Liu, X.; Liu, J.; Luo, S.-P.; Fang, X. Org. Lett. 2024, 26, 3945–3950. doi:10.1021/acs.orglett.4c01192 |
| 188. | Long, J.; Zhao, R.; Cheng, G.-J.; Fang, X. Angew. Chem., Int. Ed. 2023, 62, e202304543. doi:10.1002/anie.202304543 |
| 90. | Wang, J.; Jiang, F.; Tao, C.; Yu, H.; Ruhlmann, L.; Wei, Y. Green Chem. 2021, 23, 2652–2657. doi:10.1039/d1gc00161b |
| 183. | Wang, H.-W.; Qiao, Y.-H.; Wu, J.-X.; Wang, Q.-P.; Tian, M.-X.; Li, Y.-F.; Yao, Q.-X.; Li, D.-C.; Dou, J.-M.; Lu, Y. Org. Lett. 2021, 23, 656–662. doi:10.1021/acs.orglett.0c03688 |
| 91. | Wang, R.; Lu, K.; Zhang, J.; Li, X.; Zheng, Z. ACS Catal. 2022, 12, 14290–14303. doi:10.1021/acscatal.2c04837 |
| 182. | Li, H.; Tian, X.; Dang, Q.-Q.; Zhang, J.; Wen, Z.-K. ACS Catal. 2024, 14, 9985–9992. doi:10.1021/acscatal.4c01944 |
| 24. | Gopi, E.; Gravel, E.; Doris, E. Nanoscale Adv. 2019, 1, 1181–1185. doi:10.1039/c8na00192h |
| 185. | Wang, J.; Hong, H.; Hu, D.; Liu, J.; Liu, W.; Shi, Y. Org. Chem. Front. 2024, 11, 484–488. doi:10.1039/d3qo01472j |
| 25. | Zhang, R.; Yang, X.; Tao, Z.; Wang, X.; Wang, H.; Wang, L.; Lv, B. ACS Appl. Mater. Interfaces 2019, 11, 46678–46687. doi:10.1021/acsami.9b14460 |
| 184. | Bakthadoss, M.; Reddy, T. T. Chem. Sci. 2023, 14, 5880–5886. doi:10.1039/d2sc06206b |
| 192. | Shanmugasundaram, M.; Senthilvelan, A.; Kore, A. R. Tetrahedron Lett. 2019, 60, 157–160. doi:10.1016/j.tetlet.2018.12.001 |
| 191. | Sugimoto, K.; Kosuge, S.; Sugita, T.; Miura, Y.; Tsuge, K.; Matsuya, Y. Org. Lett. 2021, 23, 3981–3985. doi:10.1021/acs.orglett.1c01171 |
| 59. | Li, J.-S.; Xie, X.-Y.; Jiang, S.; Yang, P.-P.; Li, Z.-W.; Lu, C.-H.; Liu, W.-D. Org. Chem. Front. 2021, 8, 697–701. doi:10.1039/d0qo01264e |
| 60. | Lai, M.; Wu, Z.; Su, F.; Yu, Y.; Jing, Y.; Kong, J.; Wang, Z.; Wang, S.; Zhao, M. Eur. J. Org. Chem. 2020, 198–208. doi:10.1002/ejoc.201901630 |
| 58. | Gao, C.; Zhou, Q.; Yang, L.; Zhang, X.; Fan, X. J. Org. Chem. 2020, 85, 13710–13720. doi:10.1021/acs.joc.0c01871 |
| 67. | Rashed, M. N.; Siddiki, S. M. A. H.; Touchy, A. S.; Jamil, M. A. R.; Poly, S. S.; Toyao, T.; Maeno, Z.; Shimizu, K.-i. Chem. – Eur. J. 2019, 25, 10594–10605. doi:10.1002/chem.201901446 |
| 198. | Mondal, B.; Ghosh, P.; Kundu, M.; Das, T. K.; Das, S. Org. Biomol. Chem. 2021, 19, 360–364. doi:10.1039/d0ob02134b |
| 197. | Wang, M.-L.; Xu, H.; Li, H.-Y.; Ma, B.; Wang, Z.-Y.; Wang, X.; Dai, H.-X. Org. Lett. 2021, 23, 2147–2152. doi:10.1021/acs.orglett.1c00296 |
| 65. | Chaparro, S.; Rojas, H. A.; Castillo, J. C.; Portilla, J.; Romanelli, G. P.; Pineda, A.; Elsharif, A. M.; Martinez, J. J.; Luque, R. ACS Sustainable Chem. Eng. 2020, 8, 13139–13146. doi:10.1021/acssuschemeng.0c04429 |
| 200. | Wu, J.-X.; Chi, R.; Li, D.-C.; Cui, J.-C.; Dou, J.-M.; Wang, H.-W. Tetrahedron Lett. 2023, 125, 154619. doi:10.1016/j.tetlet.2023.154619 |
| 66. | Qu, E.; Li, S.; Bai, J.; Zheng, Y.; Li, W. Org. Lett. 2022, 24, 58–63. doi:10.1021/acs.orglett.1c03535 |
| 199. | Belitz, F.; Seitz, A.-K.; Goebel, J. F.; Hu, Z.; Gooßen, L. J. Org. Lett. 2022, 24, 3466–3470. doi:10.1021/acs.orglett.2c01043 |
| 63. | Gao, Y.; Peng, S.-Q.; Liu, D.-Y.; Rui, P.-X.; Hu, X.-G. Eur. J. Org. Chem. 2019, 1715–1721. doi:10.1002/ejoc.201801334 |
| 194. | Khan, D.; Parveen, I. Eur. J. Org. Chem. 2021, 4946–4957. doi:10.1002/ejoc.202100866 |
| 64. | Wang, Z.; Matsumoto, A.; Maruoka, K. Chem. Sci. 2020, 11, 12323–12328. doi:10.1039/d0sc05137c |
| 193. | Sieland, B.; Hoppe, A.; Stepen, A. J.; Paradies, J. Adv. Synth. Catal. 2022, 364, 3143–3148. doi:10.1002/adsc.202200525 |
| 61. | Li, Z.; Liu, L.; Xu, K.; Huang, T.; Li, X.; Song, B.; Chen, T. Org. Lett. 2020, 22, 5517–5521. doi:10.1021/acs.orglett.0c01869 |
| 196. | Ojha, S.; Panda, N. Org. Biomol. Chem. 2022, 20, 1292–1298. doi:10.1039/d1ob02360h |
| 62. | Park, J.; Krishnapriya, A. U.; Park, Y.; Kim, M.; Reidl, T. W.; Kuniyil, R.; Son, J. Adv. Synth. Catal. 2023, 365, 4495–4501. doi:10.1002/adsc.202300693 |
| 195. | Brunner, H.; Vedder, L. ChemCatChem 2019, 11, 698–702. doi:10.1002/cctc.201801519 |
| 29. | Zhu, K.; Cao, M.; Zhao, G.; Zhao, J.; Li, P. Org. Lett. 2022, 24, 5855–5859. doi:10.1021/acs.orglett.2c02426 |
| 28. | Bourboula, A.; Mountanea, O. G.; Krasakis, G.; Mantzourani, C.; Kokotou, M. G.; Kokotos, C. G.; Kokotos, G. Eur. J. Org. Chem. 2023, 26, e202300008. doi:10.1002/ejoc.202300008 |
| 68. | Kaur, A.; Pannu, A.; Brar, D. S.; Mehta, S. K.; Salunke, D. B. ACS Omega 2020, 5, 21007–21014. doi:10.1021/acsomega.0c02516 |
| 69. | Alawaed, A. A.; Ramachandran, P. V. Org. Biomol. Chem. 2024, 22, 1915–1919. doi:10.1039/d3ob01943h |
| 73. | Jian, J.; Wang, Z.; Chen, L.; Gu, Y.; Miao, L.; Liu, Y.; Zeng, Z. Synthesis 2019, 51, 4078–4084. doi:10.1055/s-0039-1690178 |
| 74. | Li, D.; Wang, S.; Ge, S.; Dong, S.; Feng, X. Org. Lett. 2020, 22, 5331–5336. doi:10.1021/acs.orglett.0c01581 |
| 71. | Zhu, R.; Li, Y.; Shen, Y.; Pan, M.; Dong, W.; Li, W. Org. Chem. Front. 2024, 11, 2095–2101. doi:10.1039/d3qo02111d |
| 72. | Huy, P. H.; Mbouhom, C. Chem. Sci. 2019, 10, 7399–7406. doi:10.1039/c9sc02126d |
| 27. | Soika, J.; McLaughlin, C.; Neveselý, T.; Daniliuc, C. G.; Molloy, J. J.; Gilmour, R. ACS Catal. 2022, 12, 10047–10056. doi:10.1021/acscatal.2c02991 |
| 70. | Gu, C.; Wang, S.; Zhang, Q.; Xie, J. Chem. Commun. 2022, 58, 5873–5876. doi:10.1039/d2cc01655a |
| 139. | Li, K.; Khan, R.; Zhang, X.; Gao, Y.; Zhou, Y.; Tan, H.; Chen, J.; Fan, B. Chem. Commun. 2019, 55, 5663–5666. doi:10.1039/c9cc01970g |
| 138. | Shi, J.; Ye, T.; Dong, J.; Liu, A.; Xu, T.; Tai, M.; Zhang, L.; Wang, C. ACS Omega 2023, 8, 11492–11502. doi:10.1021/acsomega.3c00287 |
| 135. | Ghosh, S.; Jana, C. K. Green Chem. 2019, 21, 5803–5807. doi:10.1039/c9gc02937k |
| 134. | Bae, H.; Park, J.; Yoon, R.; Lee, S.; Son, J. RSC Adv. 2024, 14, 9440–9444. doi:10.1039/d4ra00320a |
| 137. | Zhao, C.-Q.; Chen, Y.-G.; Qiu, H.; Wei, L.; Fang, P.; Mei, T.-S. Org. Lett. 2019, 21, 1412–1416. doi:10.1021/acs.orglett.9b00148 |
| 136. | Manenti, M.; Gusmini, S.; Lo Presti, L.; Silvani, A. Eur. J. Org. Chem. 2022, e202200435. doi:10.1002/ejoc.202200435 |
| 131. | Wang, H.; Wei, Y.; He, Y.; He, T.-J.; Lin, Y.-W. J. Org. Chem. 2024, 89, 10093–10098. doi:10.1021/acs.joc.4c00941 |
| 130. | Sahoo, T.; Sankaram, G. S.; Madhu, M.; Sridhar, B.; Reddy, B. V. S. Eur. J. Org. Chem. 2024, e202400036. doi:10.1002/ejoc.202400036 |
| 133. | Bai, Z.; Zhu, S.; Hu, Y.; Yang, P.; Chu, X.; He, G.; Wang, H.; Chen, G. Nat. Commun. 2022, 13, 6445. doi:10.1038/s41467-022-34223-7 |
| 132. | Yao, L.; Hu, Q.; Lei, Y.; Bao, L.; Hu, Y. Org. Chem. Front. 2020, 7, 3633–3637. doi:10.1039/d0qo00967a |
| 149. | Kardile, R. D.; Liu, R.-S. Org. Lett. 2019, 21, 6452–6456. doi:10.1021/acs.orglett.9b02343 |
| 146. | Liu, X.; Werner, T. Adv. Synth. Catal. 2021, 363, 1096–1104. doi:10.1002/adsc.202001209 |
| 145. | Yan, K.; He, M.; Li, J.; He, H.; Lai, R.; Luo, Y.; Guo, L.; Wu, Y. Chem. Commun. 2020, 56, 14287–14290. doi:10.1039/d0cc06236g |
| 148. | Ramavath, V.; Rupanawar, B. D.; More, S. G.; Bansode, A. H.; Suryavanshi, G. New J. Chem. 2021, 45, 8806–8813. doi:10.1039/d1nj01170g |
| 147. | Longwitz, L.; Spannenberg, A.; Werner, T. ACS Catal. 2019, 9, 9237–9244. doi:10.1021/acscatal.9b02456 |
| 142. | Seifert, F.; Drikermann, D.; Steinmetzer, J.; Zi, Y.; Kupfer, S.; Vilotijevic, I. Org. Biomol. Chem. 2021, 19, 6092–6097. doi:10.1039/d1ob00909e |
| 141. | Verma, A.; Grams, R. J.; Rastatter, B. P.; Santos, W. L. Tetrahedron 2019, 75, 2113–2117. doi:10.1016/j.tet.2019.02.030 |
| 144. | Merad, J.; Grant, P. S.; Stopka, T.; Sabbatani, J.; Meyrelles, R.; Preinfalk, A.; Matyasovsky, J.; Maryasin, B.; González, L.; Maulide, N. J. Am. Chem. Soc. 2022, 144, 12536–12543. doi:10.1021/jacs.2c05637 |
| 143. | Volchkov, I.; Powell, B. V.; Zatolochnaya, O. V.; Leung, J. C.; Pennino, S.; Wu, L.; Gonnella, N. C.; Bhaskararao, B.; Kozlowski, M. C.; Reeves, J. T. J. Org. Chem. 2023, 88, 10881–10904. doi:10.1021/acs.joc.3c00917 |
| 140. | Wu, Y.; Ao, Y.; Li, Z.; Liu, C.; Zhao, J.; Gao, W.; Li, X.; Wang, H.; Liu, Y.; Liu, Y. Nat. Commun. 2023, 14, 1655. doi:10.1038/s41467-023-37022-w |
| 96. | Wang, G.; Wei, C.; Hong, X.; Fu, Z.; Huang, W. Green Chem. 2020, 22, 6819–6826. doi:10.1039/d0gc02555k |
| 97. | Xin, H.-L.; Pang, B.; Choi, J.; Akkad, W.; Morimoto, H.; Ohshima, T. J. Org. Chem. 2020, 85, 11592–11606. doi:10.1021/acs.joc.0c01458 |
| 95. | Wu, Z.; Jiang, D.; Wang, J. Org. Chem. Front. 2019, 6, 688–693. doi:10.1039/c8qo01420e |
| 92. | Yu, H.; Wang, J.; Wu, Z.; Zhao, Q.; Dan, D.; Han, S.; Tang, J.; Wei, Y. Green Chem. 2019, 21, 4550–4554. doi:10.1039/c9gc02053e |
| 157. | Akkarasamiyo, S.; Chitsomkhuan, S.; Buakaew, S.; Samec, J. S. M.; Songsri, C.; Kuntiyong, P. Asian J. Org. Chem. 2024, 13, e202400009. doi:10.1002/ajoc.202400009 |
| 93. | Mali, G.; Tiwari, G.; Kamboj, N.; Metre, R. K.; Erande, R. D. Tetrahedron Lett. 2023, 124, 154608. doi:10.1016/j.tetlet.2023.154608 |
| 156. | Cruché, C.; Neiderer, W.; Collins, S. K. ACS Catal. 2021, 11, 8829–8836. doi:10.1021/acscatal.1c01983 |
| 159. | Bakthadoss, M.; Agarwal, V.; Tadiparthi, T. R.; Mohammad, M. Mol. Diversity 2021, 25, 2467–2478. doi:10.1007/s11030-020-10105-2 |
| 26. | Jiang, J.; Li, X.; Du, S.; Shi, L.; Jiang, P.; Zhang, P.; Dong, Y.; Leng, Y. New J. Chem. 2020, 44, 7780–7785. doi:10.1039/d0nj00172d |
| 158. | Ozeki, M.; Hachino, A.; Shigeta, T.; Niki, A.; Kobayashi, N.; Mizutani, H.; Nakamura, A.; Horie, A.; Arimitsu, K.; Kajimoto, T.; Hosoi, S.; Iwasaki, H.; Kojima, N.; Yamashita, M.; Kawasaki, I. Chem. Pharm. Bull. 2019, 67, 71–74. doi:10.1248/cpb.c18-00666 |
| 153. | Tran, U. P. N.; Oss, G.; Breugst, M.; Detmar, E.; Pace, D. P.; Liyanto, K.; Nguyen, T. V. ACS Catal. 2019, 9, 912–919. doi:10.1021/acscatal.8b03769 |
| 152. | Chérif, S. E.; Ghosh, A.; Chelli, S.; Dixon, I. M.; Kraiem, J.; Lakhdar, S. Chem. Sci. 2022, 13, 12065–12070. doi:10.1039/d2sc03961c |
| 155. | Brégent, T.; Bouillon, J.-P.; Poisson, T. Org. Lett. 2020, 22, 7688–7693. doi:10.1021/acs.orglett.0c02894 |
| 14. | Yang, H. H.; Oh, K.-E.; Jo, Y. H.; Ahn, J. H.; Liu, Q.; Turk, A.; Jang, J. Y.; Hwang, B. Y.; Lee, K. Y.; Lee, M. K. Bioorg. Med. Chem. 2018, 26, 509–515. doi:10.1016/j.bmc.2017.12.011 |
| 154. | Steenackers, W.; El Houari, I.; Baekelandt, A.; Witvrouw, K.; Dhondt, S.; Leroux, O.; Gonzalez, N.; Corneillie, S.; Cesarino, I.; Inzé, D.; Boerjan, W.; Vanholme, B. J. Exp. Bot. 2019, 70, 6293–6304. doi:10.1093/jxb/erz392 |
| 100. | Shamnad, A.; Nayak, K. H.; Babu, B. P. J. Org. Chem. 2024, 89, 6545–6554. doi:10.1021/acs.joc.4c00156 |
| 98. | Bacaicoa, S.; Stenkvist, S.; Sundén, H. Org. Lett. 2024, 26, 3114–3118. doi:10.1021/acs.orglett.4c00731 |
| 151. | Shang, W.; Duan, D.; Liu, Y.; Lv, J. Org. Lett. 2019, 21, 8013–8017. doi:10.1021/acs.orglett.9b03005 |
| 99. | Smeyne, D.; Verboom, K.; Bryan, M.; LoBue, J.; Shaikh, A. Tetrahedron Lett. 2021, 68, 152898. doi:10.1016/j.tetlet.2021.152898 |
| 150. | Golubev, A. A.; Smetanin, I. A.; Agafonova, A. V.; Rostovskii, N. V.; Khlebnikov, A. F.; Starova, G. L.; Novikov, M. S. Org. Biomol. Chem. 2019, 17, 6821–6830. doi:10.1039/c9ob01301f |
| 169. | Zhou, M.-J.; Zhang, L.; Liu, G.; Xu, C.; Huang, Z. J. Am. Chem. Soc. 2021, 143, 16470–16485. doi:10.1021/jacs.1c05479 |
| 168. | Huang, D.; Szewczyk, S. M.; Zhang, P.; Newhouse, T. R. J. Am. Chem. Soc. 2019, 141, 5669–5674. doi:10.1021/jacs.9b02552 |
| 170. | Han, J.; Xu, Y.-W.; Wang, X.-M.; Zhang, L.; Hu, X.-P. Tetrahedron Lett. 2020, 61, 151409. doi:10.1016/j.tetlet.2019.151409 |
| 164. | Pandia, B. K.; Gunanathan, C. J. Org. Chem. 2021, 86, 9994–10005. doi:10.1021/acs.joc.1c00685 |
| 163. | Zhao, T.-T.; Song, J.-L.; Hong, F.-Q.; Xia, J.-S.; Li, J.-J. Chem. Pap. 2019, 73, 2857–2868. doi:10.1007/s11696-019-00838-2 |
| 166. | Chen, Y.; Turlik, A.; Newhouse, T. R. J. Am. Chem. Soc. 2016, 138, 1166–1169. doi:10.1021/jacs.5b12924 |
| 167. | Chen, Y.; Romaire, J. P.; Newhouse, T. R. J. Am. Chem. Soc. 2015, 137, 5875–5878. doi:10.1021/jacs.5b02243 |
| 165. | Surya Prakash Rao, H.; Padder, A. H. New J. Chem. 2020, 44, 9010–9017. doi:10.1039/d0nj00231c |
| 160. | Wang, C.; Dam, P.; Elghobashy, M.; Brückner, A.; Rabeah, J.; Azofra, L. M.; El-Sepelgy, O. ACS Catal. 2023, 13, 14205–14212. doi:10.1021/acscatal.3c04305 |
| 162. | Dutta, S.; Kumar, P.; Sharma, S.; Yadav, S.; Priyanka; Dixit, R.; Srivastava, A.; Sharma, R. K. RSC Sustainability 2023, 1, 975–986. doi:10.1039/d3su00073g |
| 161. | Chen, K.-Q.; Shen, J.; Wang, Z.-X.; Chen, X.-Y. Chem. Sci. 2021, 12, 6684–6690. doi:10.1039/d1sc01024g |
| 212. | Mishra, S.; Halpani, C. G.; Patel, S. ChemistrySelect 2019, 4, 6913–6916. doi:10.1002/slct.201901131 |
| 211. | Li, B.; Ju, C.-W.; Wang, W.; Gu, Y.; Chen, S.; Luo, Y.; Zhang, H.; Yang, J.; Liang, H.-w.; Bonn, M.; Müllen, K.; Goddard, W. A., III; Zhou, Y. J. Am. Chem. Soc. 2023, 145, 24126–24135. doi:10.1021/jacs.3c07851 |
| 218. | Lin, Y.; Ma, W.-Y.; Xu, Z.; Zheng, Z.-J.; Cao, J.; Yang, K.-F.; Cui, Y.-M.; Xu, L.-W. Chem. – Asian J. 2019, 14, 2082–2085. doi:10.1002/asia.201900408 |
| 217. | Chen, J.; Xu, J.; Zhou, Y.; Xie, S.; Gao, F.; Xu, X.; Xu, X.; Jin, Z. Org. Lett. 2019, 21, 7928–7932. doi:10.1021/acs.orglett.9b02910 |
| 220. | Baghel, A. S.; Aghi, A.; Kumar, A. J. Org. Chem. 2021, 86, 9744–9754. doi:10.1021/acs.joc.1c01090 |
| 219. | Yang, X.; Sun, R.; Zhang, C.; Zheng, X.; Yuan, M.; Fu, H.; Li, R.; Chen, H. Org. Lett. 2019, 21, 1002–1006. doi:10.1021/acs.orglett.8b04005 |
| 214. | Sruthi, P. R.; Anjali, S.; Varghese, N.; Anas, S. J. Organomet. Chem. 2020, 921, 121354. doi:10.1016/j.jorganchem.2020.121354 |
| 213. | Sobhani, S.; Hosseini Moghadam, H.; Skibsted, J.; Sansano, J. M. Green Chem. 2020, 22, 1353–1365. doi:10.1039/c9gc03455b |
| 216. | Arghan, M.; Koukabi, N.; Kolvari, E. Appl. Organomet. Chem. 2019, 33, e4823. doi:10.1002/aoc.4823 |
| 215. | Alavi G., S. A.; Nasseri, M. A.; Kazemnejadi, M.; Allahresani, A.; HussainZadeh, M. New J. Chem. 2021, 45, 7741–7757. doi:10.1039/d0nj06208a |
| 21. | Zuo, M.; Li, Z.; Fu, W.; Guo, R.; Hou, C.; Guo, W.; Sun, Z.; Chu, W. Catal. Lett. 2019, 149, 3217–3223. doi:10.1007/s10562-019-02885-6 |
| 221. | Wang, H.; Li, Z.; Chen, X.; Wong, J. J.; Bi, T.; Tong, X.; Xu, Z.; Zhen, M.; Wan, Y.; Tang, L.; Liu, B.; Zong, X.; Xu, D.; Zuo, J.; Yang, L.; Huang, W.; Houk, K. N.; Yang, W. Chem 2023, 9, 607–623. doi:10.1016/j.chempr.2022.10.019 |
| 107. | Yan, S.-S.; Wu, D.-S.; Ye, J.-H.; Gong, L.; Zeng, X.; Ran, C.-K.; Gui, Y.-Y.; Li, J.; Yu, D.-G. ACS Catal. 2019, 9, 6987–6992. doi:10.1021/acscatal.9b02351 |
| 229. | Cai, L.; Li, S.; Zhou, C.; Li, G. Org. Lett. 2020, 22, 7791–7796. doi:10.1021/acs.orglett.0c02528 |
| 106. | Xu, J.-D.; Su, X.-B.; Wang, C.; Yao, L.-W.; Liu, J.-H.; Hu, G.-Q. Synlett 2021, 32, 833–837. doi:10.1055/a-1377-7369 |
| 228. | Bag, S.; Jana, S.; Pradhan, S.; Bhowmick, S.; Goswami, N.; Sinha, S. K.; Maiti, D. Nat. Commun. 2021, 12, 1393. doi:10.1038/s41467-021-21633-2 |
| 109. | Zhu, C.; Zhang, Y.-F.; Liu, Z.-Y.; Zhou, L.; Liu, H.; Feng, C. Chem. Sci. 2019, 10, 6721–6726. doi:10.1039/c9sc01336a |
| 231. | Panja, S.; Ahsan, S.; Pal, T.; Kolb, S.; Ali, W.; Sharma, S.; Das, C.; Grover, J.; Dutta, A.; Werz, D. B.; Paul, A.; Maiti, D. Chem. Sci. 2022, 13, 9432–9439. doi:10.1039/d2sc03288k |
| 108. | Xie, S.-L.; Gao, X.-T.; Wu, H.-H.; Zhou, F.; Zhou, J. Org. Lett. 2020, 22, 8424–8429. doi:10.1021/acs.orglett.0c03051 |
| 230. | Dutta, U.; Maiti, S.; Pimparkar, S.; Maiti, S.; Gahan, L. R.; Krenske, E. H.; Lupton, D. W.; Maiti, D. Chem. Sci. 2019, 10, 7426–7432. doi:10.1039/c9sc01824g |
| 103. | Saitou, T.; Jin, Y.; Isobe, K.; Suga, T.; Takaya, J.; Iwasawa, N. Chem. – Asian J. 2020, 15, 1941–1944. doi:10.1002/asia.202000476 |
| 225. | Luo, J.; Zhang, T.; Wang, L.; Liao, G.; Yao, Q.-J.; Wu, Y.-J.; Zhan, B.-B.; Lan, Y.; Lin, X.-F.; Shi, B.-F. Angew. Chem., Int. Ed. 2019, 58, 6708–6712. doi:10.1002/anie.201902126 |
| 102. | Tang, W.-Y.; Chen, L.; Zheng, M.; Zhan, L.-W.; Hou, J.; Li, B.-D. Org. Lett. 2021, 23, 3939–3943. doi:10.1021/acs.orglett.1c01096 |
| 224. | Sk, M. R.; Bera, S. S.; Maji, M. S. Adv. Synth. Catal. 2019, 361, 585–590. doi:10.1002/adsc.201801385 |
| 105. | Cao, Y.-F.; Li, L.-J.; Liu, M.; Xu, H.; Dai, H.-X. J. Org. Chem. 2020, 85, 4475–4481. doi:10.1021/acs.joc.0c00198 |
| 227. | Xu, J.; Chen, J.; Gao, F.; Xie, S.; Xu, X.; Jin, Z.; Yu, J.-Q. J. Am. Chem. Soc. 2019, 141, 1903–1907. doi:10.1021/jacs.8b13403 |
| 104. | Sahoo, H.; Zhang, L.; Cheng, J.; Nishiura, M.; Hou, Z. J. Am. Chem. Soc. 2022, 144, 23585–23594. doi:10.1021/jacs.2c10754 |
| 226. | Song, H.; Li, Y.; Yao, Q.-J.; Jin, L.; Liu, L.; Liu, Y.-H.; Shi, B.-F. Angew. Chem., Int. Ed. 2020, 59, 6576–6580. doi:10.1002/anie.201915949 |
| 101. | Xie, C.; Lin, L.; Huang, L.; Wang, Z.; Jiang, Z.; Zhang, Z.; Han, B. Nat. Commun. 2021, 12, 4823. doi:10.1038/s41467-021-25118-0 |
| 223. | Shinde, V. N.; Rangan, K.; Kumar, D.; Kumar, A. J. Org. Chem. 2021, 86, 9755–9770. doi:10.1021/acs.joc.1c01097 |
| 222. | Bakthadoss, M.; Reddy, T. T.; Sharada, D. S. RSC Adv. 2020, 10, 31570–31574. doi:10.1039/d0ra06580c |
| 110. | Sun, C.; Zhou, Q.; Li, C.-Y.; Hou, Z.-W.; Wang, L. Org. Lett. 2024, 26, 883–888. doi:10.1021/acs.orglett.3c04071 |
| 117. | Lv, J.; Zong, L.; Zhang, J.; Song, J.; Zhao, J.; Zhang, K.; Zhou, Z.; Gao, M.; Xie, C.; Jia, X.; Ren, X. Catal. Sci. Technol. 2021, 11, 4708–4713. doi:10.1039/d1cy00699a |
| 116. | Luo, Y.; Wang, X.; Liu, Q.; He, Y.; Li, J.; Luo, S.; Zhu, Q. Green Chem. 2023, 25, 1120–1127. doi:10.1039/d2gc04689j |
| 119. | Xiong, W.; Shi, F.; Cheng, R.; Zhu, B.; Wang, L.; Chen, P.; Lou, H.; Wu, W.; Qi, C.; Lei, M.; Jiang, H. ACS Catal. 2020, 10, 7968–7978. doi:10.1021/acscatal.0c01687 |
| 118. | Huang, Z.; Dong, Y.; Li, Y.; Makha, M.; Li, Y. ChemCatChem 2019, 11, 5236–5240. doi:10.1002/cctc.201900995 |
| 1. | Wang, S.; Chen, J.; Shi, J.; Wang, Z.; Hu, D.; Song, B. J. Agric. Food Chem. 2021, 69, 11804–11815. doi:10.1021/acs.jafc.1c03087 |
| 113. | Xu, J.-X.; Zhao, F.; Wu, X.-F. Org. Biomol. Chem. 2020, 18, 9796–9799. doi:10.1039/d0ob02043e |
| 235. | Hu, Y.; Wang, Z.; Luo, H.; Jin, H.; Liu, Y.; Zhou, B. Org. Biomol. Chem. 2021, 19, 3834–3837. doi:10.1039/d1ob00443c |
| 112. | Lee, H.-J.; Yonekura, Y.; Kim, N.; Yoshida, J.-i.; Kim, H. Org. Lett. 2021, 23, 2904–2910. doi:10.1021/acs.orglett.1c00538 |
| 234. | Wang, Y.; Tang, Y.; Zong, Y.; Tsui, G. C. Org. Lett. 2022, 24, 4087–4092. doi:10.1021/acs.orglett.2c01639 |
| 115. | Yan, Q.; Yuan, Q.-J.; Shatskiy, A.; Alvey, G. R.; Stepanova, E. V.; Liu, J.-Q.; Kärkäs, M. D.; Wang, X.-S. Org. Lett. 2024, 26, 3380–3385. doi:10.1021/acs.orglett.4c00872 |
| 114. | Yang, Z.-X.; Lai, L.; Chen, J.; Yan, H.; Ye, K.-Y.; Chen, F.-E. Chin. Chem. Lett. 2023, 34, 107956. doi:10.1016/j.cclet.2022.107956 |
| 236. | Li, X.; Cai, W.; Huang, Y. Adv. Synth. Catal. 2022, 364, 1879–1883. doi:10.1002/adsc.202200071 |
| 7. | Zhang, W.-X.; Wang, H.; Cui, H.-R.; Guo, W.-B.; Zhou, F.; Cai, D.-S.; Xu, B.; Jia, X.-H.; Huang, X.-M.; Yang, Y.-Q.; Chen, H.-S.; Qi, J.-C.; Wang, P.-L.; Lei, H.-M. Eur. J. Med. Chem. 2019, 183, 111695. doi:10.1016/j.ejmech.2019.111695 |
| 232. | Wu, X.; Chen, W.; Holmberg-Douglas, N.; Bida, G. T.; Tu, X.; Ma, X.; Wu, Z.; Nicewicz, D. A.; Li, Z. Chem 2023, 9, 343–362. doi:10.1016/j.chempr.2022.12.007 |
| 6. | Lima, G. D. d. A.; Rodrigues, M. P.; Mendes, T. A. d. O.; Moreira, G. A.; Siqueira, R. P.; da Silva, A. M.; Vaz, B. G.; Fietto, J. L. R.; Bressan, G. C.; Machado-Neves, M.; Teixeira, R. R. Toxicol. In Vitro 2018, 53, 1–9. doi:10.1016/j.tiv.2018.07.015 |
| 5. | Bezerra França, S.; Carine Barros de Lima, L.; Rychard da Silva Cunha, C.; Santos Anunciação, D.; Ferreira da Silva-Júnior, E.; Ester de Sá Barreto Barros, M.; José da Paz Lima, D. Bioorg. Med. Chem. 2021, 44, 116299. doi:10.1016/j.bmc.2021.116299 |
| 31. | Osako, T.; Kaiser, R.; Torii, K.; Uozumi, Y. Synlett 2019, 30, 961–966. doi:10.1055/s-0037-1611769 |
| 184. | Bakthadoss, M.; Reddy, T. T. Chem. Sci. 2023, 14, 5880–5886. doi:10.1039/d2sc06206b |
| 1. | Wang, S.; Chen, J.; Shi, J.; Wang, Z.; Hu, D.; Song, B. J. Agric. Food Chem. 2021, 69, 11804–11815. doi:10.1021/acs.jafc.1c03087 |
| 2. | Wiedeman, P. E.; Djuric, S. W.; Pilushchev, M.; Sciotti, R. J.; Madar, D. J.; Kopecka, H. Oxazolidinone Chemotherapeutic Agents. U.S. Pat. Appl. US2002/0115669A1, Aug 22, 2002. |
| 3. | Zhou, Y.; Zhou, X. E.; Gong, Y.; Zhu, Y.; Cao, X.; Brunzelle, J. S.; Xu, H. E.; Zhou, M.; Melcher, K.; Zhang, F. PLoS Pathog. 2020, 16, e1008323. doi:10.1371/journal.ppat.1008323 |
| 4. | Yap, P. S. X.; Krishnan, T.; Chan, K.-G.; Lim, S. H. E. J. Microbiol. Biotechnol. 2015, 25, 1299–1306. doi:10.4014/jmb.1407.07054 |
| 111. | Yang, L.; Guan, A.-L.; Xu, Z.-Y.; Yao, Z.-J. Inorg. Chem. 2023, 62, 18198–18208. doi:10.1021/acs.inorgchem.3c02663 |
| 233. | Timelthaler, D.; Schöfberger, W.; Topf, C. Eur. J. Org. Chem. 2021, 2114–2120. doi:10.1002/ejoc.202100073 |
| 11. | Vivier, D.; Soussia, I. B.; Rodrigues, N.; Lolignier, S.; Devilliers, M.; Chatelain, F. C.; Prival, L.; Chapuy, E.; Bourdier, G.; Bennis, K.; Lesage, F.; Eschalier, A.; Busserolles, J.; Ducki, S. J. Med. Chem. 2017, 60, 1076–1088. doi:10.1021/acs.jmedchem.6b01285 |
| 10. | Uchida, Y.; Murao, S. Jpn. Heart J. 1981, 22, 971–975. doi:10.1536/ihj.22.971 |
| 9. | Scott, J. S.; Bailey, A.; Buttar, D.; Carbajo, R. J.; Curwen, J.; Davey, P. R. J.; Davies, R. D. M.; Degorce, S. L.; Donald, C.; Gangl, E.; Greenwood, R.; Groombridge, S. D.; Johnson, T.; Lamont, S.; Lawson, M.; Lister, A.; Morrow, C. J.; Moss, T. A.; Pink, J. H.; Polanski, R. J. Med. Chem. 2019, 62, 1593–1608. doi:10.1021/acs.jmedchem.8b01837 |
| 8. | Rodrigues, M. P.; Tomaz, D. C.; Ângelo de Souza, L.; Onofre, T. S.; Aquiles de Menezes, W.; Almeida-Silva, J.; Suarez-Fontes, A. M.; Rogéria de Almeida, M.; Manoel da Silva, A.; Bressan, G. C.; Vannier-Santos, M. A.; Rangel Fietto, J. L.; Teixeira, R. R. Eur. J. Med. Chem. 2019, 183, 111688. doi:10.1016/j.ejmech.2019.111688 |
| 128. | Yang, Y.-F.; Huang, X.-B.; Gao, W.-X.; Zhou, Y.-B.; Liu, M.-C.; Wu, H.-Y. Org. Biomol. Chem. 2020, 18, 5822–5825. doi:10.1039/d0ob00601g |
| 127. | Xu, J.-L.; Tian, H.; Kang, J.-H.; Kang, W.-X.; Sun, W.; Sun, R.; Li, Y.-M.; Sun, M. Org. Lett. 2020, 22, 6739–6743. doi:10.1021/acs.orglett.0c02099 |
| 129. | Greco, R.; Tiburcio, E.; Palomar-De Lucas, B.; Ferrando-Soria, J.; Armentano, D.; Pardo, E.; Leyva-Pérez, A. Mol. Catal. 2022, 522, 112228. doi:10.1016/j.mcat.2022.112228 |
| 124. | Sun, X.; Duan, X.; Zheng, N.; Song, W. Org. Lett. 2023, 25, 2798–2805. doi:10.1021/acs.orglett.3c00682 |
| 123. | Wang, C.-S.; Di Monaco, S.; Thai, A. N.; Rahman, M. S.; Pang, B. P.; Wang, C.; Yoshikai, N. J. Am. Chem. Soc. 2020, 142, 12878–12889. doi:10.1021/jacs.0c06412 |
| 126. | Lu, L.-G.; Chen, J.-H.; Huang, X.-B.; Liu, M.-C.; Zhou, Y.-B.; Wu, H.-Y. J. Org. Chem. 2022, 87, 16851–16859. doi:10.1021/acs.joc.2c01976 |
| 125. | Nanda, T.; Biswal, P.; Pati, B. V.; Banjare, S. K.; Ravikumar, P. C. J. Org. Chem. 2021, 86, 2682–2695. doi:10.1021/acs.joc.0c02700 |
| 120. | Taniguchi, T.; Saito, N.; Doi, R.; Kimoto, A.; Hoshiya, N.; Fujiki, K.; Shuto, S.; Fujioka, H.; Arisawa, M.; Sato, Y. Chem. Lett. 2019, 48, 1406–1409. doi:10.1246/cl.190650 |
| 122. | Xia, S.-M.; Yang, Z.-W.; Yao, X.-Y.; Chen, K.-H.; Qiu, L.-Q.; He, L.-N. Green Chem. 2021, 23, 8089–8095. doi:10.1039/d1gc02735b |
| 121. | Xia, S.-M.; Yang, Z.-W.; Chen, K.-H.; Wang, N.; He, L.-N. Chin. J. Catal. 2022, 43, 1642–1651. doi:10.1016/s1872-2067(21)63848-2 |
| 37. | Liu, J.; Fujita, H.; Kitamura, M.; Shimada, D.; Kunishima, M. Org. Biomol. Chem. 2021, 19, 4712–4719. doi:10.1039/d1ob00450f |
| 30. | Atapalkar, R. S.; Kulkarni, A. A. Chem. Commun. 2023, 59, 9231–9234. doi:10.1039/d3cc02402d |
| 38. | Marx, D.; Wingen, L. M.; Schnakenburg, G.; Müller, C. E.; Scholz, M. S. Front. Chem. (Lausanne, Switz.) 2019, 7, 56. doi:10.3389/fchem.2019.00056 |
| 45. | Wang, S.-P.; Cheung, C. W.; Ma, J.-A. J. Org. Chem. 2019, 84, 13922–13934. doi:10.1021/acs.joc.9b02068 |
| 46. | Wu, X.; Zhou, L.; Li, F.; Xiao, J. J. Chem. Res. 2021, 45, 491–497. doi:10.1177/1747519820987530 |
| 43. | Ramachandran, P. V.; Hamann, H. J.; Choudhary, S. Org. Lett. 2020, 22, 8593–8597. doi:10.1021/acs.orglett.0c03184 |
| 44. | Phakhodee, W.; Yamano, D.; Pattarawarapan, M. Synlett 2020, 31, 703–707. doi:10.1055/s-0039-1691583 |
| 41. | Chetankumar, E.; Bharamawadeyar, S.; Srinivasulu, C.; Sureshbabu, V. V. Org. Biomol. Chem. 2023, 21, 8875–8882. doi:10.1039/d3ob01351k |
| 42. | Braddock, D. C.; Davies, J. J.; Lickiss, P. D. Org. Lett. 2022, 24, 1175–1179. doi:10.1021/acs.orglett.1c04265 |
| 39. | Wang, Z.; Wang, X.; Wang, P.; Zhao, J. J. Am. Chem. Soc. 2021, 143, 10374–10381. doi:10.1021/jacs.1c04614 |
| 40. | Xu, X.; Feng, H.; Li, H.; Huang, L. Eur. J. Org. Chem. 2019, 3921–3928. doi:10.1002/ejoc.201900137 |
| 47. | Brittain, W. D. G.; Cobb, S. L. Org. Lett. 2021, 23, 5793–5798. doi:10.1021/acs.orglett.1c01953 |
| 48. | Lee, H.-J.; Huang, X.; Sakaki, S.; Maruoka, K. Green Chem. 2021, 23, 848–855. doi:10.1039/d0gc03912h |
| 49. | Zhao, Z.; Ikawa, S.; Mori, S.; Sumii, Y.; Adachi, H.; Kagawa, T.; Shibata, N. ACS Sustainable Chem. Eng. 2024, 12, 3565–3574. doi:10.1021/acssuschemeng.3c06417 |
| 56. | Takeuchi, H.; Fujimori, Y.; Ueda, Y.; Shibayama, H.; Nagaishi, M.; Yoshimura, T.; Sasamori, T.; Tokitoh, N.; Furuta, T.; Kawabata, T. Org. Lett. 2020, 22, 4754–4759. doi:10.1021/acs.orglett.0c01549 |
| 57. | Wang, K.-K.; Li, Y.-L.; Zhao, Y.-C.; Zhang, S.-S.; Chen, R.; Sun, A. RSC Adv. 2021, 11, 40193–40196. doi:10.1039/d1ra06860a |
| 54. | Shen, Z.; Zhang, S.; Geng, H.; Wang, J.; Zhang, X.; Zhou, A.; Yao, C.; Chen, X.; Wang, W. Org. Lett. 2019, 21, 448–452. doi:10.1021/acs.orglett.8b03641 |
| 55. | Mahajani, N. S.; Meador, R. I. L.; Smith, T. J.; Canarelli, S. E.; Adhikari, A. A.; Shah, J. P.; Russo, C. M.; Wallach, D. R.; Howard, K. T.; Millimaci, A. M.; Chisholm, J. D. J. Org. Chem. 2019, 84, 7871–7882. doi:10.1021/acs.joc.9b00745 |
| 52. | Bi, X.; Li, J.; Shi, E.; Li, Y.; Liu, Y.; Wang, H.; Xiao, J. Phosphorus, Sulfur Silicon Relat. Elem. 2019, 194, 236–240. doi:10.1080/10426507.2018.1539991 |
| 53. | Norcott, P. L.; Hammill, C. L.; Noble, B. B.; Robertson, J. C.; Olding, A.; Bissember, A. C.; Coote, M. L. J. Am. Chem. Soc. 2019, 141, 15450–15455. doi:10.1021/jacs.9b08634 |
| 50. | Wang, X.; Wang, Z.; Ishida, T.; Nishihara, Y. J. Org. Chem. 2020, 85, 7526–7533. doi:10.1021/acs.joc.0c00640 |
| 51. | Liu, J.; Parker, M. F. L.; Wang, S.; Flavell, R. R.; Toste, F. D.; Wilson, D. M. Chem 2021, 7, 2245–2255. doi:10.1016/j.chempr.2021.07.005 |
| 17. | Thiyam, U.; Stöckmann, H.; Zum Felde, T.; Schwarz, K. Eur. J. Lipid Sci. Technol. 2006, 108, 239–248. doi:10.1002/ejlt.200500292 |
| 18. | Li, C.-Y.; Tsai, W.-J.; Damu, A. G.; Lee, E.-J.; Wu, T.-S.; Dung, N. X.; Thang, T. D.; Thanh, L. J. Agric. Food Chem. 2007, 55, 9436–9442. doi:10.1021/jf071963l |
| 15. | Abe, M.; Nishikawa, K.; Fukuda, H.; Nakanishi, K.; Tazawa, Y.; Taniguchi, T.; Park, S.-y.; Hiradate, S.; Fujii, Y.; Okuda, K.; Shindo, M. Phytochemistry 2012, 84, 56–67. doi:10.1016/j.phytochem.2012.08.001 |
| 16. | Dagang, Y.; Jing, L.; Sishun, Y.; Dongshan, W.; Jianheng, Y.; Li, G.; Xin, Z.; Chuankun, R.; Yongyuan, G. Fluorine-Containing Carboxylic Acid Compound and Preparation Method Thereof. Chin. Pat. Appl. CN111592519A, Aug 28, 2020. |
| 13. | Xu, X.-T.; Deng, X.-Y.; Chen, J.; Liang, Q.-M.; Zhang, K.; Li, D.-L.; Wu, P.-P.; Zheng, X.; Zhou, R.-P.; Jiang, Z.-Y.; Ma, A.-J.; Chen, W.-H.; Wang, S.-H. Eur. J. Med. Chem. 2020, 189, 112013. doi:10.1016/j.ejmech.2019.112013 |
| 14. | Yang, H. H.; Oh, K.-E.; Jo, Y. H.; Ahn, J. H.; Liu, Q.; Turk, A.; Jang, J. Y.; Hwang, B. Y.; Lee, K. Y.; Lee, M. K. Bioorg. Med. Chem. 2018, 26, 509–515. doi:10.1016/j.bmc.2017.12.011 |
| 12. | Marquard, L.; Petersen, K. D.; Persson, M.; Hoff, K. D.; Jensen, P. B.; Sehested, M. APMIS 2008, 116, 382–392. doi:10.1111/j.1600-0463.2008.00957.x |
| 21. | Zuo, M.; Li, Z.; Fu, W.; Guo, R.; Hou, C.; Guo, W.; Sun, Z.; Chu, W. Catal. Lett. 2019, 149, 3217–3223. doi:10.1007/s10562-019-02885-6 |
| 22. | Li, Y.; Li, J.; Bao, G.; Yu, C.; Liu, Y.; He, Z.; Wang, P.; Ma, W.; Xie, J.; Sun, W.; Wang, R. Org. Lett. 2022, 24, 1169–1174. doi:10.1021/acs.orglett.1c04258 |
| 23. | Du, Y.; Barber, T.; Lim, S. E.; Rzepa, H. S.; Baxendale, I. R.; Whiting, A. Chem. Commun. 2019, 55, 2916–2919. doi:10.1039/c8cc09913h |
| 19. | Henrique, T.; Zanon, C. d. F.; Girol, A. P.; Stefanini, A. C. B.; Contessoto, N. S. d. A.; da Silveira, N. J. F.; Bezerra, D. P.; Silveira, E. R.; Barbosa-Filho, J. M.; Cornélio, M. L.; Oliani, S. M.; Tajara, E. H. Sci. Rep. 2020, 10, 22283. doi:10.1038/s41598-020-78220-6 |
| 20. | Bonin, A. M.; Arlauskas, A. P.; Angus, D. S..; Baker, R. S. U.; Gallagher, C. H.; Greenoak, G.; Brown, M. M. L.; Meher-Homji, K. M.; Reeve, V. Mutat. Res. Lett. 1982, 105, 303–308. doi:10.1016/0165-7992(82)90097-5 |
| 208. | Wang, J.; Qin, C.; Lumb, J.-P.; Luan, X. Chem 2020, 6, 2097–2109. doi:10.1016/j.chempr.2020.06.021 |
| 207. | Lv, W.; Chen, Y.; Wen, S.; Ba, D.; Cheng, G. J. Am. Chem. Soc. 2020, 142, 14864–14870. doi:10.1021/jacs.0c07634 |
| 210. | Yao, T.; Hui, J.; Liu, T.; Li, T. J. Org. Chem. 2021, 86, 5477–5488. doi:10.1021/acs.joc.0c02994 |
| 209. | Niedballa, J.; Reiss, G. J.; Müller, T. J. J. Eur. J. Org. Chem. 2020, 5019–5024. doi:10.1002/ejoc.202000823 |
| 201. | Bagherzadeh, M.; Kaveh, R.; Mahmoudi, H. J. Mater. Chem. A 2019, 7, 16257–16266. doi:10.1039/c9ta02882j |
| 204. | Lei, L. Appl. Organomet. Chem. 2019, 33, e5158. doi:10.1002/aoc.5158 |
| 205. | Dohendou, M.; Dekamin, M. G.; Namaki, D. Nanoscale Adv. 2023, 5, 3463–3484. doi:10.1039/d3na00157a |
| 206. | Eslahi, H.; Sardarian, A. R.; Esmaeilpour, M. Appl. Organomet. Chem. 2021, 35, e6260. doi:10.1002/aoc.6260 |
| 203. | Sobhani, S.; Zarei, H.; Sansano, J. M. Sci. Rep. 2021, 11, 17025. doi:10.1038/s41598-021-95931-6 |
| 22. | Li, Y.; Li, J.; Bao, G.; Yu, C.; Liu, Y.; He, Z.; Wang, P.; Ma, W.; Xie, J.; Sun, W.; Wang, R. Org. Lett. 2022, 24, 1169–1174. doi:10.1021/acs.orglett.1c04258 |
| 36. | Salimiyan, K.; Saberi, D. ChemistrySelect 2019, 4, 3985–3989. doi:10.1002/slct.201804066 |
| 33. | Samuel Rajan, I. A. P.; Rajendran, S. Org. Biomol. Chem. 2023, 21, 4760–4765. doi:10.1039/d3ob00418j |
| 34. | Samuel Rajan, I. A. P.; Rajendran, S. New J. Chem. 2023, 47, 10480–10483. doi:10.1039/d3nj01490h |
| 35. | Mao, F.; Jin, C.; Wang, J.; Yang, H.; Yan, X.; Li, X.; Xu, X. Org. Biomol. Chem. 2023, 21, 3825–3828. doi:10.1039/d3ob00452j |
| 30. | Atapalkar, R. S.; Kulkarni, A. A. Chem. Commun. 2023, 59, 9231–9234. doi:10.1039/d3cc02402d |
| 31. | Osako, T.; Kaiser, R.; Torii, K.; Uozumi, Y. Synlett 2019, 30, 961–966. doi:10.1055/s-0037-1611769 |
| 32. | DellaGreca, M.; Longobardo, L. ChemistrySelect 2020, 5, 4588–4591. doi:10.1002/slct.202000176 |
| 24. | Gopi, E.; Gravel, E.; Doris, E. Nanoscale Adv. 2019, 1, 1181–1185. doi:10.1039/c8na00192h |
| 25. | Zhang, R.; Yang, X.; Tao, Z.; Wang, X.; Wang, H.; Wang, L.; Lv, B. ACS Appl. Mater. Interfaces 2019, 11, 46678–46687. doi:10.1021/acsami.9b14460 |
| 26. | Jiang, J.; Li, X.; Du, S.; Shi, L.; Jiang, P.; Zhang, P.; Dong, Y.; Leng, Y. New J. Chem. 2020, 44, 7780–7785. doi:10.1039/d0nj00172d |
| 27. | Soika, J.; McLaughlin, C.; Neveselý, T.; Daniliuc, C. G.; Molloy, J. J.; Gilmour, R. ACS Catal. 2022, 12, 10047–10056. doi:10.1021/acscatal.2c02991 |
| 28. | Bourboula, A.; Mountanea, O. G.; Krasakis, G.; Mantzourani, C.; Kokotou, M. G.; Kokotos, C. G.; Kokotos, G. Eur. J. Org. Chem. 2023, 26, e202300008. doi:10.1002/ejoc.202300008 |
| 29. | Zhu, K.; Cao, M.; Zhao, G.; Zhao, J.; Li, P. Org. Lett. 2022, 24, 5855–5859. doi:10.1021/acs.orglett.2c02426 |
© 2025 Kustiana et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.