Abstract
Background
Spiroketals and the corresponding aza-spiroketals are the structural features found in a number of bioactive natural products, and in compounds possessing photochromic properties for use in the area of photochemical erasable memory, self-development photography, actinometry, displays, filters, lenses of variable optical density, and photomechanical biomaterials etc. And (1R,8aS)-1-hydroxyindolizidine (3) has been postulated to be a biosynthetic precursor of hydroxylated indolizidines such as (+)-lentiginosine 1, (−)-2-epilentiginosine 2 and (−)-swainsonine, which are potentially useful antimetastasis drugs for the treatment of cancer. In continuation of a project aimed at the development of enantiomeric malimide-based synthetic methodology, we now report a divergent, concise and highly diastereoselective approach for the asymmetric syntheses of an aza-spiropyran derivative 7 and (1S,8aR)-1-hydroxyindolizidine (ent-3).
Results
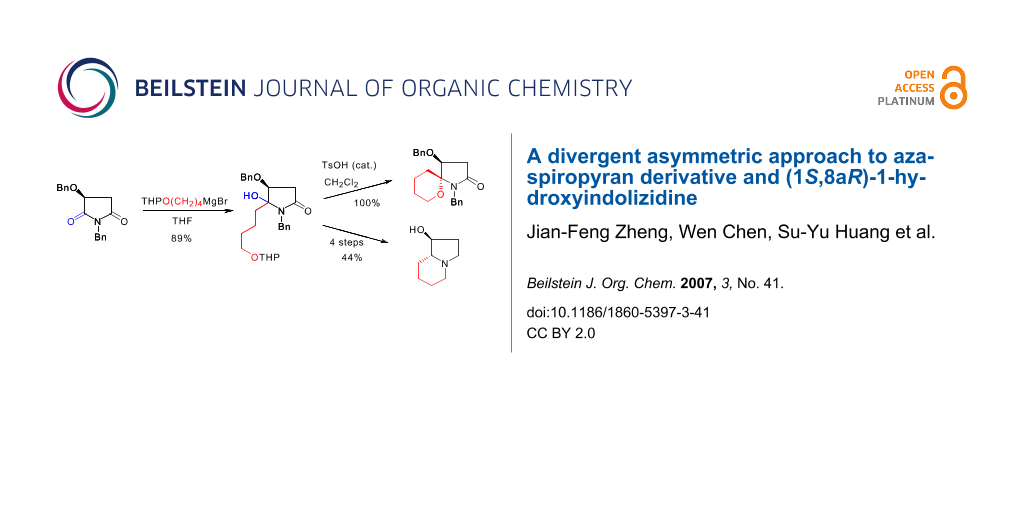
The synthesis of aza-spiropyran 7 started from the Grignard addition of malimide 4. Treatment of the THP-protected 4-hydroxybutyl magnesium bromide with malimide 4 at −20°C afforded N,O-acetal 5a as an epimeric mixture in a combined yield of 89%. Subjection of the diastereomeric mixture of N,O-acetal 5a to acidic conditions for 0.5 h resulted in the formation of the desired functionalized aza-spiropyran 7 as a single diastereomer in quantitative yield. The stereochemistry of the aza-spiropyran 7 was determined by NOESY experiment. For the synthesis of ent-3, aza-spiropyran 7, or more conveniently, N,O-acetal 5a, was converted to lactam 6a under standard reductive dehydroxylation conditions in 78% or 77% yield. Reduction of lactam 6a with borane-dimethylsulfide provided pyrrolidine 8 in 95% yield. Compound 8 was then converted to 1-hydroxyindolizidine ent-3 via a four-step procedure, namely, N-debenzylation/O-mesylation/Boc-cleavage/cyclization, and O-debenzylation. Alternatively, amino alcohol 8 was mesylated and the resultant mesylate 12 was subjected to hydrogenolytic conditions, which gave (1S,8aR)-1-hydroxyindolizidine (ent-3) in 60% overall yield from 8.
Conclusion
By the reaction of functionalized Grignard reagent with protected (S)-malimide, either aza-spiropyran or (1S,8aR)-1-hydroxyindolizidine skeleton could be constructed in a concise and selective manner. The results presented herein constitute an important extension of our malimide-based synthetic methodology.
Graphical Abstract
Background
Spiroketals of general structure A (Scheme 1) constitute key structural features of a number of bioactive natural products isolated from insects, microbes, fungi, plants or marine organisms. [1-3] The corresponding aza-spiroketal (cf: general structure B) containing natural products, while less common, are also found in plants, shellfish and microbes.[4,5] For example, pandamarilactone-1 and pandamarine were isolated from the leaves of Pandanus amaryllifolius;[6] solasodine and its derivatives were isolated from Solanum umbelliferum, which exhibited significant activity toward DNA repair-deficient yeast mutants;[7] azaspiracids are marine phycotoxins isolated from cultivated mussels in Killary harbor, Ireland;[8] and chlorofusin A is a novel fungal metabolite showing the potential as a lead in cancer therapy.[9] In addition, aza-spiropyrans C, being able to equilibrate with the corresponding non-spiro analogue D, is a well known class of compounds possessing photochromic properties for use in the area of photochemical erasable memory,[10] and also found applications as self-development photography, actinometry, displays, filters, lenses of variable optical density,[11] and photomechanical biomaterials etc.[12]
Scheme 1: The skeletons of useful aza-spiroketals and some naturally occurring hydroxylated indolizidines.
Scheme 1: The skeletons of useful aza-spiroketals and some naturally occurring hydroxylated indolizidines.
On the other hand, hydroxylated indolizidines [13-20] such as castanospermine, (−)-swainsonine, (+)-lentiginosine (1) [21-23] and (−)-2-epilentiginosine (2) [21-26] constitute a class of azasugars showing potent and selective glycosidase inhibitory activities. [13-20] (1R,8aS)-1-Hydroxyindolizidine 3 has been postulated as a biosynthetic precursor [21-26] of (+)-lentiginosine (1), (−)-2-epilentiginosine (2) and (−)-swainsonine, a potentially useful antimetastasis drug for the treatment of cancer.[15] In addition, these molecules serve as platforms for testing synthetic strategies, and several asymmetric syntheses of both enantiomers of 1-hydroxyindolizidine (3) have been reported. [27-34]In continuation of our efforts in the development of enantiomeric malimide-based synthetic methodologies, [35-38] we now report concise and highly diastereoselective syntheses of an aza-spiropyran derivative 7 and (1S,8aR)-1-hydroxyindolizidine (ent-3).
Results and discussion
Previously, we have shown that the addition of Grignard reagents to N,O-dibenzyl malimide (4) leads to N,O-acetals 5 in high regioselectivity (Scheme 2), and the subsequent reductive dehydroxylation gives 6 in high trans-diastereoselectivity.[35] On the other hand, treatment of N,O-acteals 5 with an acid furnished enamides E, which can be transformed stereoselectively to either hydroxylactams F or G under appropriate conditions. [36-38] It was envisioned that if a C4-bifunctional Grignard reagent was used, both aza-spiroketal H (such as aza-spiropyran, n = 1, path a) and indolizidine ring systems I (path b) could be obtained.
Scheme 2: Synthetic strategy based on N,O-dibenzylmalimide (4).
Scheme 2: Synthetic strategy based on N,O-dibenzylmalimide (4).
The synthesis of aza-spiropyran 7 started from the Grignard addition of malimide 4. Treatment of the THP-protected 4-hydroxybutyl magnesium bromide with malimide 4 at −20°C for 2.5 h afforded N,O-acetal 5a as an epimeric mixture in 7:1 ratio and with a combined yield of 89% (Scheme 3). If the reaction was allowed to stir at room temperature overnight, the diastereomeric ratio was inversed to 1: 1.8. Subjection of the diastereomeric mixture of the N,O-acetal 5a to acidic conditions [TsOH (cat.)/CH2Cl2, r.t.] for 0.5 h resulted in the formation of the desired functionalized aza-spiropyran derivative 7 as a single diastereomer in quantitative yield. The result means that a tandem dehydration-THP cleavage-intramolecular nucleophilic addition occurred. When the stirring was prolonged to 2 h, about 5% of another epimer (no shown) was also formed according to the 1H NMR analysis.
Scheme 3: Stereoselectivity synthesis of aza-spiropyran 7.
Scheme 3: Stereoselectivity synthesis of aza-spiropyran 7.
The stereochemistry of the aza-spiropyran 7 was determined on the basis of the NMR analysis. This was done firstly by a 1H-1H COSY experiment to confirm the proton assignments, and then by NOESY experiment. As shown in Figure 1, the strong NOE correlation of H-9a (δH 3.59) and H-4 (δH 4.22) indicates clearly O4/O5-trans relationship in compound 7.
Figure 1: The observed NOE correlations (in part) and the region expanded NOESY spectrum of compound 7.
Figure 1: The observed NOE correlations (in part) and the region expanded NOESY spectrum of compound 7.
These findings are surprising comparing with our recent observations. In our previous investigations, it was observed that the treatment of N,O-acetals 5 with an acid leads to the dehydration products E (Scheme 2), and the two diastereomers of 5 shows different reactivities towards the acid-promoted dehydration. [36-38] The trans-diastereomer reacts much more slower than the cis-diastereomer, and some un-reacted trans-epimer was always recovered even starting with a pure cis-diastereomer. In the present study, not only both two diastereomers have been completely converted to the aza-spiropyran 7, what is equally surprising is that no dehydration product was observed under acidic conditions!
For the synthesis of ent-3, aza-spiropyran 7, a cyclic N,O-acetal, was converted to lactam 6a under standard reductive dehydroxylation conditions (Et3SiH, BF3·OEt2, −78°C, 6 h; warm-up, yield: 78%) (Scheme 4). Under the same conditions, N,O-acetal 5a was converted to lactam 6a in 77% yield. It was observed that during the reaction of 5a, 7 was first formed as an intermediate after the addition of Et3SiH and BF3·OEt2, and stirring for 1 hour.
Scheme 4: Stereoselective synthesis of (1S,8aR)-1-hydroxyindolizidine (ent-3).
Scheme 4: Stereoselective synthesis of (1S,8aR)-1-hydroxyindolizidine (ent-3).
Reduction of lactam 6a with borane-dimethylsulfide provided pyrrolidine derivative 8 in 95% yield. Compound 8 was then converted to (1S,8aR)-1-hydroxyindolizidine (ent-3) {[α]D 27 +50 (c 0.90, EtOH); lit.[29] [α]D +51.0 (c 0.54, EtOH); lit.[32] −49.7 (c 0.95, EtOH) for the antipode} via a four-step procedure, namely, one-pot N-debenzylation-N-Boc formation/O-mesylation/Boc-cleavage/cyclication, and O-debenzylation.
In searching for a more concise method, amino alcohol 8 was mesylated (MsCl, NEt3, 0 °C) and the resultant labile mesylate 12 was subjected to catalytic hydrogenolysis (H2, l atm, 10% Pd/C, r.t.), which gave (1S,8aR)-1-hydroxyindolizidine (ent-3) in 60% overall yield from 8 (Scheme 5).[39,40] The one-pot N,O-bis-debenzylation and cyclization of mesylate 12 deserves comment. Because the N-debenzylation generally required longer reaction time,[41] or using of Pearlman's catalyst (cf. Scheme 4). The easy debenzylation of 12 allows assuming that an intramolecular substitution occurred firstly, and the formation of the quaternary ammonium salt K [40] then favors the reductive debenzylation. This mechanism is supported by the following observations. First, in a similar case, Thompson et al observed that the formation of a mesylate resulted in spontaneous quarternization leading to the bicyclic indolizidine.[40] Second, we have also observed that the tosylate of 8 is too labile to be isolated, and mesylate 12 decomposed upon flash column chromatography on silica gel, which are due to the spontaneous formation of a polar quaternary ammonium salt. In addition, the presence of the O-benzyl group in K is an assumption based on our previous observation on a similar case.[42]
Scheme 5: One-pot synthesis of ent-3 from amino alcohol 8.
Scheme 5: One-pot synthesis of ent-3 from amino alcohol 8.
Conclusion
In summary, we have demonstrated that by the reaction of functionalized Grignard reagent with the protected (S)-malimide 4, either aza-spiropyran derivative 7 or (1S,8aR)-1-hydroxyindolizidine skeleton (ent-3) can be constructed in a concise and selective manner. It is worthy of mention that in addition to the reductive dehydroxylation leading to 2-pyrrolidinones 6, and the acid-promoted dehydration leading to (E)-enamides E (and then F, G), acid treatment of the N,O-acetal 5a could provide, chemoselectively and quantitatively, the aza-spiropyran ring system 7. The results presented herein constitute a valuable extension of our malimides-based synthetic methodology.
See Supporting Information File 1 for full experimental procedures and characterization data of the synthesized compounds.
Supporting Information
| Supporting Information File 1: Experimental. Experimental procedures for the synthesis of all compounds described, and characterization data for the synthesized compounds. | ||
| Format: DOC | Size: 62.5 KB | Download |
Acknowledgements
The authors are grateful to the NSFC (20572088), NSF of Fujian Province and Xiamen City (2006J0268; 3502z20055019) and the program for Innovative Research Team in Science & Technology (University) in Fujian Province for financial support. We thank Professor Y. F. Zhao for the use of her Bruker Dalton Esquire 3000 plus LC-MS apparatus.
References
-
Perron, F.; Albizati, K. M. Chem. Rev. 1989, 89, 1617–1661. doi:10.1021/cr00097a015
Return to citation in text: [1] -
Boivin, T. L. B. Tetrahedron 1987, 43, 3309–3362. doi:10.1016/S0040-4020(01)81626-4
Return to citation in text: [1] -
Brimble, M. A.; Farès, F. A. Tetrahedron 1999, 55, 7661–7706. doi:10.1016/S0040-4020(99)00387-7
Return to citation in text: [1] -
Nonato, M. G.; Garson, M. J.; Truscott, R. J. W.; Carver, J. A. Phytochemistry 1993, 34, 1159–1163. doi:10.1016/S0031-9422(00)90735-0
Return to citation in text: [1] -
Byrne, L. T.; Guevara, B. Q.; Patalinghug, W. C.; Recio, B. V.; Ualat, C. R.; White, A. H. Aust. J. Chem. 1992, 45, 1903–1908.
Return to citation in text: [1] -
Pradhan, R.; Patra, M.; Behera, A. K.; Mishra, B. K.; Behera, R. K. Tetrahedron 2006, 62, 779–828. doi:10.1016/j.tet.2005.09.039
Return to citation in text: [1] -
Kim, Y. C.; Che, Q. M.; Gunatilake, A. A. L.; Kingston, D. G. I. J. Nat. Prod. 1996, 59, 283–285. doi:10.1021/np960125a
Return to citation in text: [1] -
Satake, M.; Ofuji, K.; Naoki, H.; James, K. J.; Furey, A.; McMahon, T.; Silke, J.; Yasumoto, T. J. Am. Chem. Soc. 1998, 120, 9967–9968. doi:10.1021/ja981413r
Return to citation in text: [1] -
Duncan, S. J.; Gruschow, S.; Williams, D. H.; McNicholas, C.; Purewal, R.; Hajek, M.; Gerlitz, M.; Martin, S.; Wrigley, S.; Moore, M. J. Am. Chem. Soc. 2001, 123, 554–560. doi:10.1021/ja002940p
Return to citation in text: [1] -
Fisher, E.; Hirshberg, Y. J. Chem. Soc. 1952, 4522–4524.
Return to citation in text: [1] -
Berkovic, G.; Krongauz, V.; Weiss, V. Chem. Rev. 2000, 100, 1741–1754. doi:10.1021/cr9800715
Return to citation in text: [1] -
McCoy, C. P.; Donnelly, L.; Jones, D. S.; Gorman, S. P. Tetrahedron Lett. 2007, 48, 657–661. doi:10.1016/j.tetlet.2006.11.110
Return to citation in text: [1] -
Asano, N.; Nash, R. J.; Molyneux, R. J.; Fleet, G. W. J. Tetrahedron: Asymmetry 2000, 11, 1645–1680. doi:10.1016/S0957-4166(00)00113-0
Return to citation in text: [1] [2] -
Ahmed, E. N. Tetrahedron 2000, 56, 8579–8629. doi:10.1016/S0040-4020(00)00178-2
Return to citation in text: [1] [2] -
Watson, A. A.; Fleet, G. W. J.; Asano, N.; Molyneux, R. J.; Nash, R. J. Phytochemistry 2001, 56, 265–295. doi:10.1016/S0031-9422(00)00451-9
Return to citation in text: [1] [2] [3] -
Michael, J. P. Nat. Prod. Rep. 2000, 17, 579–602. doi:10.1039/a904849i
Return to citation in text: [1] [2] -
Michael, J. P. Nat. Prod. Rep. 2001, 18, 520–542. doi:10.1039/b005384h
Return to citation in text: [1] [2] -
Michael, J. P. Nat. Prod. Rep. 2003, 20, 458–475. doi:10.1039/b208137g
Return to citation in text: [1] [2] -
Michael, J. P. Nat. Prod. Rep. 2004, 21, 625–649. doi:10.1039/b310689f
Return to citation in text: [1] [2] -
Michael, J. P. Nat. Prod. Rep. 2005, 22, 603–626. doi:10.1039/b413748p
Return to citation in text: [1] [2] -
Pastuszak, I.; Molyneux, R. J.; James, L. F.; Elbein, A. D. Biochemistry 1990, 29, 1886–1891. doi:10.1021/bi00459a032
Return to citation in text: [1] [2] [3] -
Rasmussen, M. O.; Delair, P.; Greene, A. E. J. Org. Chem. 2001, 66, 5438–5443. doi:10.1021/jo010298r
For recent asymmetric syntheses of lentiginosine, see [22,23]
Return to citation in text: [1] [2] [3] [4] -
Ha, D.-C.; Yun, C.-S.; Lee, Y. J. Org. Chem. 2000, 65, 621–623. doi:10.1021/jo9913762
Return to citation in text: [1] [2] [3] [4] -
Harris, T. M.; Harris, C. M.; Hill, J. E.; Ungemach, F. S.; Broquist, H. P.; Wickwire, B. M. J. Org. Chem. 1987, 52, 3094–3098. doi:10.1021/jo00390a024
Return to citation in text: [1] [2] -
Harris, C. M.; Campbell, B. C.; Molyneux, R. J.; Harris, T. M. Tetrahedron Lett. 1988, 29, 4815–4818. doi:10.1016/S0040-4039(00)80616-4
Return to citation in text: [1] [2] -
Harris, C. M.; Schneider, M. J.; Ungemach, F. S.; Hill, J. E.; Harris, T. M. J. Am. Chem. Soc. 1988, 110, 940–949. doi:10.1021/ja00211a039
Return to citation in text: [1] [2] -
Aaron, H. S.; Pader, C. P.; Wicks, G. E., Jr. J. Org. Chem. 1966, 31, 3502–3505. doi:10.1021/jo01349a008
For the synthesis of racemic 1-hydroxyindolizidine, see [27,28]
Return to citation in text: [1] [2] -
Clevenstine, E. C.; Walter, P.; Harris, T. M.; Broquist, H. P. Biochemistry 1979, 18, 3663–3667. doi:10.1021/bi00584a004
Return to citation in text: [1] [2] -
Harris, C. M.; Harris, T. M. Tetrahedron Lett. 1987, 28, 2559–2562. doi:10.1016/S0040-4039(00)96147-1
For the asymmetric synthesis of (1S,8aR)-1-hydroxyindolizidine, see [29,30]
Return to citation in text: [1] [2] [3] -
Klitzke, C. F.; Pilli, R. A. Tetrahedron Lett. 2001, 42, 5605–5608. doi:10.1016/S0040-4039(01)01084-X
Return to citation in text: [1] [2] -
Shono, T.; Kise, N.; Tanabe, T. J. Org. Chem. 1988, 53, 1364–1367. doi:10.1021/jo00242a004
For the asymmetric synthesis of (1R,8aS)-1-hydroxyindolizidine, see [29-34]
Return to citation in text: [1] [2] -
Takahata, H.; Banba, Y.; Momose, T. Tetrahedron: Asymmetry 1990, 1, 763–764. doi:10.1016/S0957-4166(00)80440-1
Return to citation in text: [1] [2] [3] -
Guerreiro, P.; Ratovelomanana-Vidal, V.; Genêt, J. P. Chirality 2000, 12, 408–410. doi:10.1002/(SICI)1520-636X(2000)12:5/6<408::AID-CHIR20>3.0.CO;2-G
Return to citation in text: [1] [2] -
Rasmussen, M. O.; Delair, P.; Greene, A. E. J. Org. Chem. 2001, 66, 5438–5443. doi:10.1021/jo010298r
Return to citation in text: [1] [2] -
Huang, P.-Q. Synlett 2006, 1133–1149. doi:10.1055/s-2006-941565
Return to citation in text: [1] [2] -
Zhou, X.; Huang, P.-Q. Synlett 2006, 1235–1239. doi:10.1055/s-2006-939695
Return to citation in text: [1] [2] [3] -
Zhou, X.; Zhang, P.-Y.; Ye, J.-L.; Huang, P.-Q. C. R. Chim. 2008, 11, 5–18. doi:10.1016/j.crci.2007.02.018
Return to citation in text: [1] [2] [3] -
Zhou, X.; Liu, W.-J.; Ye, J.-L.; Huang, P.-Q. J. Org. Chem. 2007, 72, 8904–8909. doi:10.1021/jo7018784
Return to citation in text: [1] [2] [3] -
Ikota, N.; Hanaki, A. Heterocycles 1987, 26, 2369–2370.
Return to citation in text: [1] -
Gren, D. L. C.; Kiddle, J. J.; Thompson, C. M. Tetrahedron 1995, 51, 2865–2874. doi:10.1016/0040-4020(95)00037-9
Return to citation in text: [1] [2] [3] -
Liu, L.-X.; Ruan, Y.-P.; Guo, Z.-Q.; Huang, P.-Q. J. Org. Chem. 2004, 69, 6001–6009. doi:10.1021/jo049166z
Return to citation in text: [1] -
Huang, P.-Q.; Meng, W.-H. Tetrahedron: Asymmetry 2004, 15, 3899–3910. doi:10.1016/j.tetasy.2004.05.037
Return to citation in text: [1]
| 22. |
Rasmussen, M. O.; Delair, P.; Greene, A. E. J. Org. Chem. 2001, 66, 5438–5443. doi:10.1021/jo010298r
For recent asymmetric syntheses of lentiginosine, see [22,23] |
| 23. | Ha, D.-C.; Yun, C.-S.; Lee, Y. J. Org. Chem. 2000, 65, 621–623. doi:10.1021/jo9913762 |
| 27. |
Aaron, H. S.; Pader, C. P.; Wicks, G. E., Jr. J. Org. Chem. 1966, 31, 3502–3505. doi:10.1021/jo01349a008
For the synthesis of racemic 1-hydroxyindolizidine, see [27,28] |
| 28. | Clevenstine, E. C.; Walter, P.; Harris, T. M.; Broquist, H. P. Biochemistry 1979, 18, 3663–3667. doi:10.1021/bi00584a004 |
| 29. |
Harris, C. M.; Harris, T. M. Tetrahedron Lett. 1987, 28, 2559–2562. doi:10.1016/S0040-4039(00)96147-1
For the asymmetric synthesis of (1S,8aR)-1-hydroxyindolizidine, see [29,30] |
| 30. | Klitzke, C. F.; Pilli, R. A. Tetrahedron Lett. 2001, 42, 5605–5608. doi:10.1016/S0040-4039(01)01084-X |
| 1. | Perron, F.; Albizati, K. M. Chem. Rev. 1989, 89, 1617–1661. doi:10.1021/cr00097a015 |
| 2. | Boivin, T. L. B. Tetrahedron 1987, 43, 3309–3362. doi:10.1016/S0040-4020(01)81626-4 |
| 3. | Brimble, M. A.; Farès, F. A. Tetrahedron 1999, 55, 7661–7706. doi:10.1016/S0040-4020(99)00387-7 |
| 8. | Satake, M.; Ofuji, K.; Naoki, H.; James, K. J.; Furey, A.; McMahon, T.; Silke, J.; Yasumoto, T. J. Am. Chem. Soc. 1998, 120, 9967–9968. doi:10.1021/ja981413r |
| 15. | Watson, A. A.; Fleet, G. W. J.; Asano, N.; Molyneux, R. J.; Nash, R. J. Phytochemistry 2001, 56, 265–295. doi:10.1016/S0031-9422(00)00451-9 |
| 7. | Kim, Y. C.; Che, Q. M.; Gunatilake, A. A. L.; Kingston, D. G. I. J. Nat. Prod. 1996, 59, 283–285. doi:10.1021/np960125a |
| 27. |
Aaron, H. S.; Pader, C. P.; Wicks, G. E., Jr. J. Org. Chem. 1966, 31, 3502–3505. doi:10.1021/jo01349a008
For the synthesis of racemic 1-hydroxyindolizidine, see [27,28] |
| 28. | Clevenstine, E. C.; Walter, P.; Harris, T. M.; Broquist, H. P. Biochemistry 1979, 18, 3663–3667. doi:10.1021/bi00584a004 |
| 29. |
Harris, C. M.; Harris, T. M. Tetrahedron Lett. 1987, 28, 2559–2562. doi:10.1016/S0040-4039(00)96147-1
For the asymmetric synthesis of (1S,8aR)-1-hydroxyindolizidine, see [29,30] |
| 30. | Klitzke, C. F.; Pilli, R. A. Tetrahedron Lett. 2001, 42, 5605–5608. doi:10.1016/S0040-4039(01)01084-X |
| 31. |
Shono, T.; Kise, N.; Tanabe, T. J. Org. Chem. 1988, 53, 1364–1367. doi:10.1021/jo00242a004
For the asymmetric synthesis of (1R,8aS)-1-hydroxyindolizidine, see [29-34] |
| 32. | Takahata, H.; Banba, Y.; Momose, T. Tetrahedron: Asymmetry 1990, 1, 763–764. doi:10.1016/S0957-4166(00)80440-1 |
| 33. | Guerreiro, P.; Ratovelomanana-Vidal, V.; Genêt, J. P. Chirality 2000, 12, 408–410. doi:10.1002/(SICI)1520-636X(2000)12:5/6<408::AID-CHIR20>3.0.CO;2-G |
| 34. | Rasmussen, M. O.; Delair, P.; Greene, A. E. J. Org. Chem. 2001, 66, 5438–5443. doi:10.1021/jo010298r |
| 6. | Pradhan, R.; Patra, M.; Behera, A. K.; Mishra, B. K.; Behera, R. K. Tetrahedron 2006, 62, 779–828. doi:10.1016/j.tet.2005.09.039 |
| 13. | Asano, N.; Nash, R. J.; Molyneux, R. J.; Fleet, G. W. J. Tetrahedron: Asymmetry 2000, 11, 1645–1680. doi:10.1016/S0957-4166(00)00113-0 |
| 14. | Ahmed, E. N. Tetrahedron 2000, 56, 8579–8629. doi:10.1016/S0040-4020(00)00178-2 |
| 15. | Watson, A. A.; Fleet, G. W. J.; Asano, N.; Molyneux, R. J.; Nash, R. J. Phytochemistry 2001, 56, 265–295. doi:10.1016/S0031-9422(00)00451-9 |
| 16. | Michael, J. P. Nat. Prod. Rep. 2000, 17, 579–602. doi:10.1039/a904849i |
| 17. | Michael, J. P. Nat. Prod. Rep. 2001, 18, 520–542. doi:10.1039/b005384h |
| 18. | Michael, J. P. Nat. Prod. Rep. 2003, 20, 458–475. doi:10.1039/b208137g |
| 19. | Michael, J. P. Nat. Prod. Rep. 2004, 21, 625–649. doi:10.1039/b310689f |
| 20. | Michael, J. P. Nat. Prod. Rep. 2005, 22, 603–626. doi:10.1039/b413748p |
| 4. | Nonato, M. G.; Garson, M. J.; Truscott, R. J. W.; Carver, J. A. Phytochemistry 1993, 34, 1159–1163. doi:10.1016/S0031-9422(00)90735-0 |
| 5. | Byrne, L. T.; Guevara, B. Q.; Patalinghug, W. C.; Recio, B. V.; Ualat, C. R.; White, A. H. Aust. J. Chem. 1992, 45, 1903–1908. |
| 21. | Pastuszak, I.; Molyneux, R. J.; James, L. F.; Elbein, A. D. Biochemistry 1990, 29, 1886–1891. doi:10.1021/bi00459a032 |
| 22. |
Rasmussen, M. O.; Delair, P.; Greene, A. E. J. Org. Chem. 2001, 66, 5438–5443. doi:10.1021/jo010298r
For recent asymmetric syntheses of lentiginosine, see [22,23] |
| 23. | Ha, D.-C.; Yun, C.-S.; Lee, Y. J. Org. Chem. 2000, 65, 621–623. doi:10.1021/jo9913762 |
| 24. | Harris, T. M.; Harris, C. M.; Hill, J. E.; Ungemach, F. S.; Broquist, H. P.; Wickwire, B. M. J. Org. Chem. 1987, 52, 3094–3098. doi:10.1021/jo00390a024 |
| 25. | Harris, C. M.; Campbell, B. C.; Molyneux, R. J.; Harris, T. M. Tetrahedron Lett. 1988, 29, 4815–4818. doi:10.1016/S0040-4039(00)80616-4 |
| 26. | Harris, C. M.; Schneider, M. J.; Ungemach, F. S.; Hill, J. E.; Harris, T. M. J. Am. Chem. Soc. 1988, 110, 940–949. doi:10.1021/ja00211a039 |
| 12. | McCoy, C. P.; Donnelly, L.; Jones, D. S.; Gorman, S. P. Tetrahedron Lett. 2007, 48, 657–661. doi:10.1016/j.tetlet.2006.11.110 |
| 21. | Pastuszak, I.; Molyneux, R. J.; James, L. F.; Elbein, A. D. Biochemistry 1990, 29, 1886–1891. doi:10.1021/bi00459a032 |
| 22. |
Rasmussen, M. O.; Delair, P.; Greene, A. E. J. Org. Chem. 2001, 66, 5438–5443. doi:10.1021/jo010298r
For recent asymmetric syntheses of lentiginosine, see [22,23] |
| 23. | Ha, D.-C.; Yun, C.-S.; Lee, Y. J. Org. Chem. 2000, 65, 621–623. doi:10.1021/jo9913762 |
| 11. | Berkovic, G.; Krongauz, V.; Weiss, V. Chem. Rev. 2000, 100, 1741–1754. doi:10.1021/cr9800715 |
| 21. | Pastuszak, I.; Molyneux, R. J.; James, L. F.; Elbein, A. D. Biochemistry 1990, 29, 1886–1891. doi:10.1021/bi00459a032 |
| 22. |
Rasmussen, M. O.; Delair, P.; Greene, A. E. J. Org. Chem. 2001, 66, 5438–5443. doi:10.1021/jo010298r
For recent asymmetric syntheses of lentiginosine, see [22,23] |
| 23. | Ha, D.-C.; Yun, C.-S.; Lee, Y. J. Org. Chem. 2000, 65, 621–623. doi:10.1021/jo9913762 |
| 24. | Harris, T. M.; Harris, C. M.; Hill, J. E.; Ungemach, F. S.; Broquist, H. P.; Wickwire, B. M. J. Org. Chem. 1987, 52, 3094–3098. doi:10.1021/jo00390a024 |
| 25. | Harris, C. M.; Campbell, B. C.; Molyneux, R. J.; Harris, T. M. Tetrahedron Lett. 1988, 29, 4815–4818. doi:10.1016/S0040-4039(00)80616-4 |
| 26. | Harris, C. M.; Schneider, M. J.; Ungemach, F. S.; Hill, J. E.; Harris, T. M. J. Am. Chem. Soc. 1988, 110, 940–949. doi:10.1021/ja00211a039 |
| 29. |
Harris, C. M.; Harris, T. M. Tetrahedron Lett. 1987, 28, 2559–2562. doi:10.1016/S0040-4039(00)96147-1
For the asymmetric synthesis of (1S,8aR)-1-hydroxyindolizidine, see [29,30] |
| 30. | Klitzke, C. F.; Pilli, R. A. Tetrahedron Lett. 2001, 42, 5605–5608. doi:10.1016/S0040-4039(01)01084-X |
| 31. |
Shono, T.; Kise, N.; Tanabe, T. J. Org. Chem. 1988, 53, 1364–1367. doi:10.1021/jo00242a004
For the asymmetric synthesis of (1R,8aS)-1-hydroxyindolizidine, see [29-34] |
| 32. | Takahata, H.; Banba, Y.; Momose, T. Tetrahedron: Asymmetry 1990, 1, 763–764. doi:10.1016/S0957-4166(00)80440-1 |
| 33. | Guerreiro, P.; Ratovelomanana-Vidal, V.; Genêt, J. P. Chirality 2000, 12, 408–410. doi:10.1002/(SICI)1520-636X(2000)12:5/6<408::AID-CHIR20>3.0.CO;2-G |
| 34. | Rasmussen, M. O.; Delair, P.; Greene, A. E. J. Org. Chem. 2001, 66, 5438–5443. doi:10.1021/jo010298r |
| 9. | Duncan, S. J.; Gruschow, S.; Williams, D. H.; McNicholas, C.; Purewal, R.; Hajek, M.; Gerlitz, M.; Martin, S.; Wrigley, S.; Moore, M. J. Am. Chem. Soc. 2001, 123, 554–560. doi:10.1021/ja002940p |
| 13. | Asano, N.; Nash, R. J.; Molyneux, R. J.; Fleet, G. W. J. Tetrahedron: Asymmetry 2000, 11, 1645–1680. doi:10.1016/S0957-4166(00)00113-0 |
| 14. | Ahmed, E. N. Tetrahedron 2000, 56, 8579–8629. doi:10.1016/S0040-4020(00)00178-2 |
| 15. | Watson, A. A.; Fleet, G. W. J.; Asano, N.; Molyneux, R. J.; Nash, R. J. Phytochemistry 2001, 56, 265–295. doi:10.1016/S0031-9422(00)00451-9 |
| 16. | Michael, J. P. Nat. Prod. Rep. 2000, 17, 579–602. doi:10.1039/a904849i |
| 17. | Michael, J. P. Nat. Prod. Rep. 2001, 18, 520–542. doi:10.1039/b005384h |
| 18. | Michael, J. P. Nat. Prod. Rep. 2003, 20, 458–475. doi:10.1039/b208137g |
| 19. | Michael, J. P. Nat. Prod. Rep. 2004, 21, 625–649. doi:10.1039/b310689f |
| 20. | Michael, J. P. Nat. Prod. Rep. 2005, 22, 603–626. doi:10.1039/b413748p |
| 36. | Zhou, X.; Huang, P.-Q. Synlett 2006, 1235–1239. doi:10.1055/s-2006-939695 |
| 37. | Zhou, X.; Zhang, P.-Y.; Ye, J.-L.; Huang, P.-Q. C. R. Chim. 2008, 11, 5–18. doi:10.1016/j.crci.2007.02.018 |
| 38. | Zhou, X.; Liu, W.-J.; Ye, J.-L.; Huang, P.-Q. J. Org. Chem. 2007, 72, 8904–8909. doi:10.1021/jo7018784 |
| 35. | Huang, P.-Q. Synlett 2006, 1133–1149. doi:10.1055/s-2006-941565 |
| 36. | Zhou, X.; Huang, P.-Q. Synlett 2006, 1235–1239. doi:10.1055/s-2006-939695 |
| 37. | Zhou, X.; Zhang, P.-Y.; Ye, J.-L.; Huang, P.-Q. C. R. Chim. 2008, 11, 5–18. doi:10.1016/j.crci.2007.02.018 |
| 38. | Zhou, X.; Liu, W.-J.; Ye, J.-L.; Huang, P.-Q. J. Org. Chem. 2007, 72, 8904–8909. doi:10.1021/jo7018784 |
| 40. | Gren, D. L. C.; Kiddle, J. J.; Thompson, C. M. Tetrahedron 1995, 51, 2865–2874. doi:10.1016/0040-4020(95)00037-9 |
| 42. | Huang, P.-Q.; Meng, W.-H. Tetrahedron: Asymmetry 2004, 15, 3899–3910. doi:10.1016/j.tetasy.2004.05.037 |
| 41. | Liu, L.-X.; Ruan, Y.-P.; Guo, Z.-Q.; Huang, P.-Q. J. Org. Chem. 2004, 69, 6001–6009. doi:10.1021/jo049166z |
| 40. | Gren, D. L. C.; Kiddle, J. J.; Thompson, C. M. Tetrahedron 1995, 51, 2865–2874. doi:10.1016/0040-4020(95)00037-9 |
| 32. | Takahata, H.; Banba, Y.; Momose, T. Tetrahedron: Asymmetry 1990, 1, 763–764. doi:10.1016/S0957-4166(00)80440-1 |
| 39. | Ikota, N.; Hanaki, A. Heterocycles 1987, 26, 2369–2370. |
| 40. | Gren, D. L. C.; Kiddle, J. J.; Thompson, C. M. Tetrahedron 1995, 51, 2865–2874. doi:10.1016/0040-4020(95)00037-9 |
| 36. | Zhou, X.; Huang, P.-Q. Synlett 2006, 1235–1239. doi:10.1055/s-2006-939695 |
| 37. | Zhou, X.; Zhang, P.-Y.; Ye, J.-L.; Huang, P.-Q. C. R. Chim. 2008, 11, 5–18. doi:10.1016/j.crci.2007.02.018 |
| 38. | Zhou, X.; Liu, W.-J.; Ye, J.-L.; Huang, P.-Q. J. Org. Chem. 2007, 72, 8904–8909. doi:10.1021/jo7018784 |
| 29. |
Harris, C. M.; Harris, T. M. Tetrahedron Lett. 1987, 28, 2559–2562. doi:10.1016/S0040-4039(00)96147-1
For the asymmetric synthesis of (1S,8aR)-1-hydroxyindolizidine, see [29,30] |
© 2007 Zheng et al; licensee Beilstein-Institut
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)