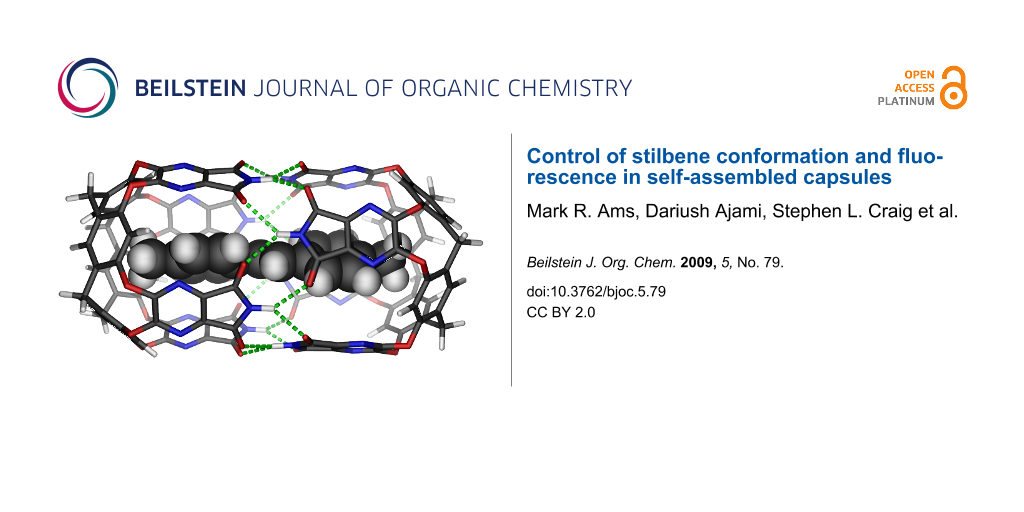
Abstract
The extensively studied trans-stilbene molecule is known to give only weak fluorescence in solution and inside loosely-fitting synthetic capsules. However, trans-stilbene has been recently studied in the context of antibody interiors, where binding results in strong blue fluorescence. The present research was undertaken to understand the spatial factors that influence stilbene fluorescence. trans-Stilbene was encapsulated in the snug, self-assembled complex 1.1 and exhibited fluorescence quenching due to the distortion of its ground-state geometry. When the complex is elongated by incorporating glycouril spacers, trans-stilbene is allowed to adapt a fully coplanar arrangement and fluorescence returns.
Graphical Abstract
Introduction
The fluorescence of trans-stilbene has been extensively researched [1], and weak fluorescence occurs in aqueous solutions or typical organic solvents. In a highly structured environment such as an antibody interior [2-4], recent studies show that nearby tryptophans can transfer electrons to the stilbene excited state and an intense blue fluorescence develops. Inside the tight-fitting capsule 1.1 [5,6] (Figure 1) where it is surrounded by 16 aromatic panels, trans-stilbene’s fluorescence is reduced to only 2% of what is observed in bulk solution. In contrast, normal fluorescence is observed in a loose-fitting capsule [7] although the photostationary trans-/cis-isomerization equilibria are altered in the limited space [8]. Isomerization of trans- to cis-stilbene is not possible in 1.1 but little else is known about the photophysics of guests in this capsule. This research was undertaken to understand what controls the behavior of stilbenes in this and related constrained environments.
Figure 1: (Top) Tetraimide cavitand 1, the dimeric capsule 1.1 and its cartoon representation. (Bottom) The shape of the space inside (A), a schematic view along the central axis with two aromatic guests (B) and an energy-minimized (AM1) complex of stilbene in 1.1 (C).
Figure 1: (Top) Tetraimide cavitand 1, the dimeric capsule 1.1 and its cartoon representation. (Bottom) The s...
Results and Discussion
The space inside capsule 1.1 is defined by two pyramids comprising resorcinarenes at the ends and square prisms of the heterocyclic walls near the middle. The quenching of stilbene fluorescence may be an effect of the fixed aromatics of the resorcinarene or the heterocyclic walls, but we surmised that there was a subtler cause. As seen in the skeletal model 1.1 of the space within depicted in Figure 1A, the square prisms are twisted by about 45° along their long axes. A typical aromatic guest such as benzene fits best when it is nestled diagonally in a prism’s space. Accordingly, the aromatic rings of longer molecules such as biphenyls and stilbenes cannot be coplanar in their lowest energy conformations inside 1.1. Rather, they must be twisted by 45° or so along their rotatable internal bonds.
Among guests of 1.1, 4,4′-dimethylstilbene (2) provides an excellent fit; it fills about 53% of the capsule’s space and the methyl groups of the guest can access the tapered ends of the host. The homologue, 4-ethyl-4′-methylstilbene (3), is also encapsulated (see Supporting Information File 1 for NMR spectrum), but the slightly longer 4,4′-diethyl derivative 4 simply does not fit. Figure 2 illustrates the effect of encapsulation on 3 (assembly 6). The fluorescence is 96% quenched when λexc = 318 nm. For comparison, the emission of the permanently twisted, o-substituted stilbene 5 (λexc = 300 nm) is also shown [9].
Figure 2: Room temperature fluorescence spectra at λexc = 318 nm for 10 µM mesitylene solutions of 4,4′-dimethylstilbene (2); 4-ethyl-4′-methylstilbene (3); 4,4′-diethylstilbene (4); 2,4,4′,6-tetramethylstilbene (5) and assembly 6 (λexc = 300 nm).
Figure 2: Room temperature fluorescence spectra at λexc = 318 nm for 10 µM mesitylene solutions of 4,4′-dimet...
Can the fluorescence of the encapsulated stilbene be restored? When suitable guests are present, addition of glycolurils such as 7 to solutions of 1.1 generates extended capsule 1.74.1 (Figure 3) [10]. The glycolurils are arranged in a chiral manner, in either cycloenantiomer of the extended capsule. The glycolurils force the two square prisms of 1.74.1 into registry (Figure 3A). That is, the square prisms are now aligned and stilbene as well as related guests may be found either in the fully coplanar arrangement favored by extended resonance stabilization (Figure 3B) or at another minimum with a 90° dihedral angle between their aromatic rings. 4-Ethyl-4′-methylstilbene (3) is a guest for 1.74.1 when CD2Cl2 is a co-guest (see Supporting Information File 1 for NMR spectrum). In the fluorescence experiments at λexc = 318 nm, the fluorescence of 4-ethyl-4′-methylstilbene 3 and assembly 8 is restored, as shown in Figure 4. As a control experiment to test for the possibility of any fluorescence contribution from assembly 1.74.1, the fluorescence of the complex of 1.74.1 with alkyl chain C17H36 was also investigated. No additional fluorescence was observed when C17H36 was a guest (see Supporting Information File 1 for fluorescence and NMR spectra).
Figure 3: (Top) Glycouril 7, the extended capsule 1.74.1, (only one enantiomeric arrangement is shown) and its cartoon representation. The shape of the space inside (A) a schematic view along the central axis with two aromatic guests (B) and an end-on view of an energy-minimized complex of stilbene inside (C).
Figure 3: (Top) Glycouril 7, the extended capsule 1.74.1, (only one enantiomeric arrangement is shown) and it...
Figure 4: Room temperature emission spectra for 10 µM solutions of 4-ethyl-4′-ethylstilbene (3) in the capsule 1.1 (6) and 1.74.1 (8). λexc = 318 nm.
Figure 4: Room temperature emission spectra for 10 µM solutions of 4-ethyl-4′-ethylstilbene (3) in the capsul...
Earlier we showed that it is possible to reversibly interconvert capsules 1.1 and 1.74.1. The weakly basic glycoluril spacer is protonated by addition of gaseous HCl and precipitates in typical organic solutions. The remaining components reassemble to the original capsule 1.1 [11]. Subsequent addition of NEt3 releases the glycoluril into solution and restores the extended capsule 1.74.1. This was shown previously to be a fully reversible process with long chain alkane guests. We are currently pursuing this application with stilbenes and studying the exchange of subunits in the process [12,13].
Many fluorescent sensors have been reported in the literature [14-17], however they usually respond to chemical changes rather than purely geometrical ones. Here, self-assembly of an external host system is responsible for turning on and off stilbene fluorescence through geometrical control of the stilbene’s surroundings.
Supporting Information
| Supporting Information File 1: Control of stilbene conformation and fluorescence in self-assembled capsules. | ||
| Format: PDF | Size: 578.3 KB | Download |
References
-
Waldeck, D. H. Chem. Rev. 1991, 91, 415–436. doi:10.1021/cr00003a007
Return to citation in text: [1] -
Debler, E. W.; Kaufmann, F. G.; Meijler, M. M.; Heine, A.; Mee, J. M.; Plevaljčić, G.; Di Bilio, A. J.; Schultz, P. G.; Millar, D. P.; Janda, K. D.; Wilson, I. A.; Gray, H. B.; Lerner, R. A. Science 2008, 319, 1232–1235. doi:10.1126/science.1153445
Return to citation in text: [1] -
Armitage, B. A.; Berget, P. B. Science 2008, 319, 1195–1196. doi:10.1126/science.1155093
Return to citation in text: [1] -
Matsushita, M.; Meijler, M. M.; Wirsching, P.; Lerner, R. A.; Janda, K. D. Org. Lett. 2005, 7, 4943–4946. doi:10.1021/ol051919w
Return to citation in text: [1] -
Heinz, T.; Rudkevich, D. M.; Rebek, J., Jr. Angew. Chem., Int. Ed. 1999, 38, 1136–1139. doi:10.1002/(SICI)1521-3773(19990419)38:8<1136::AID-ANIE1136>3.0.CO;2-I
Return to citation in text: [1] -
Heinz, T.; Rudkevich, D. M.; Rebek, J., Jr. Nature 1998, 394, 764–766. doi:10.1038/29501
Return to citation in text: [1] -
Natarajan, A.; Kaanumalle, L. S.; Jockusch, S.; Gibb, C. L. D.; Gibb, B. C.; Turro, N. J.; Ramamurthy, V. J. Am. Chem. Soc. 2007, 129, 4132–4133. doi:10.1021/ja070086x
Return to citation in text: [1] -
Parthasarathy, A.; Kaanumalle, L. S.; Ramamurthy, V. Org. Lett. 2007, 9, 5059–5062. doi:10.1021/ol702322u
Return to citation in text: [1] -
Gegiou, D.; Muszkat, K. A.; Fischer, E. J. Am. Chem. Soc. 1968, 90, 3907–3918. doi:10.1021/ja01017a002
Return to citation in text: [1] -
Ajami, D.; Rebek, J., Jr. J. Am. Chem. Soc. 2006, 128, 5314–5315. doi:10.1021/ja060095q
Return to citation in text: [1] -
Ajami, D.; Rebek, J., Jr. J. Am. Chem. Soc. 2006, 128, 15038–15039. doi:10.1021/ja064233n
Return to citation in text: [1] -
Barrett, E. S.; Dale, T. J.; Rebek, J., Jr. J. Am. Chem. Soc. 2007, 129, 8818–8824. doi:10.1021/ja071774j
Return to citation in text: [1] -
Castellano, R. K.; Craig, S. L.; Nuckolls, C.; Rebek, J., Jr. J. Am. Chem. Soc. 2000, 122, 7876–7882. doi:10.1021/ja994397m
Return to citation in text: [1] -
Thomas, S. W., III; Joly, G. D.; Swager, T. M. Chem. Rev. 2007, 107, 1339–1386. doi:10.1021/cr0501339
Return to citation in text: [1] -
Suksai, C.; Tuntulani, T. Top. Curr. Chem. 2005, 255, 163–198. doi:10.1007/b101166
Return to citation in text: [1] -
Martínez-Máñez, R.; Sancenón, F. Chem. Rev. 2003, 103, 4419–4476. doi:10.1021/cr010421e
Return to citation in text: [1] -
McQuade, D. T.; Pullen, A. E.; Swager, T. M. Chem. Rev. 2000, 100, 2537–2574. doi:10.1021/cr9801014
Return to citation in text: [1]
| 8. | Parthasarathy, A.; Kaanumalle, L. S.; Ramamurthy, V. Org. Lett. 2007, 9, 5059–5062. doi:10.1021/ol702322u |
| 7. | Natarajan, A.; Kaanumalle, L. S.; Jockusch, S.; Gibb, C. L. D.; Gibb, B. C.; Turro, N. J.; Ramamurthy, V. J. Am. Chem. Soc. 2007, 129, 4132–4133. doi:10.1021/ja070086x |
| 5. | Heinz, T.; Rudkevich, D. M.; Rebek, J., Jr. Angew. Chem., Int. Ed. 1999, 38, 1136–1139. doi:10.1002/(SICI)1521-3773(19990419)38:8<1136::AID-ANIE1136>3.0.CO;2-I |
| 6. | Heinz, T.; Rudkevich, D. M.; Rebek, J., Jr. Nature 1998, 394, 764–766. doi:10.1038/29501 |
| 2. | Debler, E. W.; Kaufmann, F. G.; Meijler, M. M.; Heine, A.; Mee, J. M.; Plevaljčić, G.; Di Bilio, A. J.; Schultz, P. G.; Millar, D. P.; Janda, K. D.; Wilson, I. A.; Gray, H. B.; Lerner, R. A. Science 2008, 319, 1232–1235. doi:10.1126/science.1153445 |
| 3. | Armitage, B. A.; Berget, P. B. Science 2008, 319, 1195–1196. doi:10.1126/science.1155093 |
| 4. | Matsushita, M.; Meijler, M. M.; Wirsching, P.; Lerner, R. A.; Janda, K. D. Org. Lett. 2005, 7, 4943–4946. doi:10.1021/ol051919w |
| 12. | Barrett, E. S.; Dale, T. J.; Rebek, J., Jr. J. Am. Chem. Soc. 2007, 129, 8818–8824. doi:10.1021/ja071774j |
| 13. | Castellano, R. K.; Craig, S. L.; Nuckolls, C.; Rebek, J., Jr. J. Am. Chem. Soc. 2000, 122, 7876–7882. doi:10.1021/ja994397m |
| 11. | Ajami, D.; Rebek, J., Jr. J. Am. Chem. Soc. 2006, 128, 15038–15039. doi:10.1021/ja064233n |
| 10. | Ajami, D.; Rebek, J., Jr. J. Am. Chem. Soc. 2006, 128, 5314–5315. doi:10.1021/ja060095q |
| 9. | Gegiou, D.; Muszkat, K. A.; Fischer, E. J. Am. Chem. Soc. 1968, 90, 3907–3918. doi:10.1021/ja01017a002 |
| 14. | Thomas, S. W., III; Joly, G. D.; Swager, T. M. Chem. Rev. 2007, 107, 1339–1386. doi:10.1021/cr0501339 |
| 15. | Suksai, C.; Tuntulani, T. Top. Curr. Chem. 2005, 255, 163–198. doi:10.1007/b101166 |
| 16. | Martínez-Máñez, R.; Sancenón, F. Chem. Rev. 2003, 103, 4419–4476. doi:10.1021/cr010421e |
| 17. | McQuade, D. T.; Pullen, A. E.; Swager, T. M. Chem. Rev. 2000, 100, 2537–2574. doi:10.1021/cr9801014 |
© 2009 Ams et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)