Abstract
gem-Difluoro-1,7-enyne amides are suitable building blocks for the synthesis of difluorodihydropyridinones via a ring-closing metathesis reaction, and of 4,4-difluoro-3-oxoisoquinolines through a ring-closing metathesis–enyne metathesis tandem reaction. These products, in turn, undergo a Diels–Alder reaction to yield heterotricyclic systems in moderate to good yields.
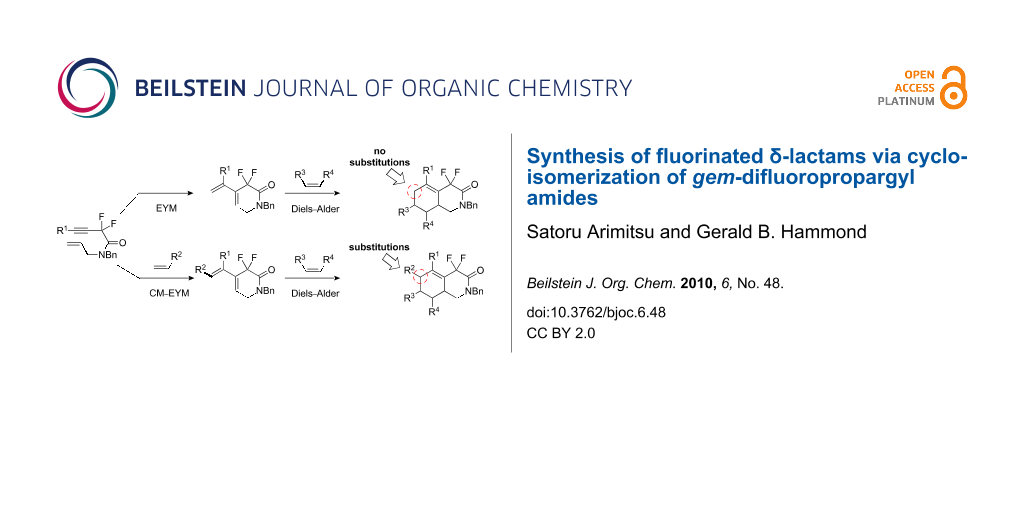
Graphical Abstract
Introduction
It has been estimated that as many as 25% of all synthetic pharmaceutical drugs contain an amide bond [1]. Commonly, β- and γ-lactams are present in many natural products and pharmaceuticals, and the introduction of a gem-difluoromethylene moiety has been reported to improve their biological activities. For example, a gem-difluoro-γ-lactam can inhibit γ-lactamase, which is responsible for bacterial resistance to γ-lactam antibiotics [2-4]. Additionally, α,α-difluoro lactams are precursors of some biologically active compounds [5-8]. Our group’s entry in this arena started as a collaboration with Professor Fustero and resulted in the syntheses of fluorinated β- and γ-lactams [9-13]. This sparked our interest in the synthesis of larger-ring lactams, with six to eight members, because nitrogen-containing medium-size heterocyclics are found in many natural products as part of fused cyclic structures. In their pioneering work on middle-range lactams bearing fluorine(s), Fustero et al. developed a ring-closing metathesis of α,α-difluoro-1,n-dienyl amides to furnish the corresponding α,α-difluorinated lactams [14]. The synthesis of medium-size heterocycles by a metathesis reaction is quite relevant, as demonstrated by its extensive application to multi-fused heterocyclics [15-19]. We postulated that functionalized fluorinated enyne amides could be used for the synthesis of a chemically diverse suite of δ-lactams because enynes are suitable partners in ring-closing metathesis reactions or cycloisomerizations. An additional benefit of using enynes in metathesis reactions is that the resulting diene product could be further elaborated using a Diels–Alder reaction to construct bi- or tricyclic ring systems [20].
Results and Discussion
Initially, we investigated the enyne metathesis reaction of fluorinated enyne 1a with commercially available ruthenium carbene complexes, the Hoveyda–Grubbs second-generation catalyst being the most reactive (entries 1–3, Table 1). The reaction at 110 °C gave 2a-iso as the major compound, probably through the isomerization of 2a (entry 3, Table 1) [14]. The latter (2a) was isolated when the reaction was carried out at 70 °C in toluene (entry 4, Table 1). Other solvents did not give good yields or selectivities (entries 5 and 6, Table 1). From experimentation, it became clear that ethylene gas was crucial for driving this reaction forward (compare entry 4 with 7, Table 1) [21]. 2,6-Dichloro-1,4-benzoquinone, which has been reported to prevent isomerization [22], gave disappointing results (entry 8, Table 1). When our optimized conditions were applied to other fluorinated 1,7-enynes we isolated the desired lactams (entries 1–3, Table 2). Higher temperatures were required with internal alkynes (entries 2–5, Table 2), where isomerization occurs and the enyne ester 1d did not yield satisfactory results. Interestingly, although enyne ketone 1e gave a good 19F NMR yield (97%) of the desired diene 2e, we could only isolate the ortho-fluorophenol 3 in good yield after silica gel chromatography. This unexpected result could have positive synthetic repercussions, as ortho-fluorophenol is a moiety that has attracted attention because it is present in some bioactive compounds [23-25].
Table 1: Screening reaction conditions for the enyne metathesis of 1a.
|
|
|||||
| Entry | Solvent | Ru cat. | Gas | Temp. (°C) | Yield of products (%)a 1a/2a/2a-iso |
|---|---|---|---|---|---|
| 1 | Toluene | G-I | C2H4 | 110 | 53/0/0 |
| 2 | Toluene | G-II | C2H4 | 110 | 0/34/0 |
| 3 | Toluene | HG-II | C2H4 | 110 | 0/6/66 (60)b |
| 4 | Toluene | HG-II | C2H4 | 70 | 0/85 (70)/0 |
| 5 | 1,2-DCEc | HG-II | C2H4 | 70 | No rxn. |
| 6 | THF | HG-II | C2H4 | 70 | 30/25/0 |
| 7 | Toluene | HG-II | Argon | 70 | 28/34/0 |
| 8 | Toluene | HG-II | C2H4d | 110 | 0/20/11 |
aYield was determined by 19F NMR and the value in parentheses is the isolated yield.
b2a-iso was isolated as an E/Z mixture (E/Z = 3/1).
c1,2-Dichloroethane.
d20 mol % of 2,6-dichloro-1,4-benzoquinone was used.
Table 2: Metathesis reaction of fluorinated 1,7-enyne carbonyl compounds.
|
|
||||
| Entry | X | R | Temp. (°C) | Yield of 2 (%)a |
|---|---|---|---|---|
| 1 | NBn | H (1a) | 70 | 70 [85] (2a) |
| 2 | NBn | n-Hex (1b) | 110 | 52 [78] (2b) |
| 3 | NBn | Ph (1c) | 110 | 69 [95] (2c) |
| 4 | O | Ph (1d) | 110 | — [33]b (2d) |
| 5 | C | Ph (1e) | 110 | — [97]c (2e) |
aThe yields in brackets were determined by 19F NMR.
bIsolation of 2d was unsuccessful due to the complex mixture that had been formed.
cCompound 3 was isolated in 84% after silica gel chromatography.
Dienes 2a and 2b were used in Diels–Alder reactions with 4 and 6 to produce 5 and 4,4-difluoroisoquinolin-3-one derivatives 7, respectively, in excellent yield and good stereoselectivity (Scheme 1). Phenyl-substituted diene 2c gave no reaction, even after a longer reaction time. The stereochemistry of 7a and 7b was determined by COSY and NOESY experiments.
Scheme 1: Diels–Alder reaction of diene 2 with 4 and 6. aThe other isomers of 7a and 7b were isolated in 8% and 20% yield, respectively.
Scheme 1: Diels–Alder reaction of diene 2 with 4 and 6. aThe other isomers of 7a and 7b were isolated in 8% a...
Recently, various tandem reactions with ruthenium complexes have become popular in organic chemistry because Ru(II) complexes are capable of catalyzing additional reactions [26,27]. Since our enyne metathesis reaction of fluorinated 1,7-enynes does not permit substitution at the 6-position of the resultant gem-difluoroisoquinolinone (eq 1, Scheme 2), we examined a potential cross metathesis–enyne metathesis tandem-type reaction (CM–EYM reaction). In theory, if the terminal vinyl group of diene 2 can be modified by a tandem metathesis reaction, this would permit the synthesis of multi-substituted gem-difluoroisoquinolinones through a subsequent Diels–Alder reaction (eq 2, Scheme 2) [28].
Scheme 2: Synthetic concept toward multi-substituted gem-difluoroisoquinolinones.
Scheme 2: Synthetic concept toward multi-substituted gem-difluoroisoquinolinones.
In this regard, we screened various ruthenium carbene complexes using 1,7-enyne amide 1a and styrene 8a as a model reaction and found that the Hoveyda–Grubbs second-generation catalyst gave the best mass balance of products 2a and 9a (entry 3, Table 3). We obtained better results when the reaction was carried out in a sealed pressure reaction vessel (compare entries 3 and 4, Table 3). More interestingly, the choice of solvent had a tremendous effect on the selectivity between 2a (EYM product) and 9a (CM–EYM product) (entries 4–8, Table 3). Methylene chloride was found to be the best solvent (entry 5, Table 3). Other reaction factors were also examined carefully; higher concentrations reduced the yield and selectivity slightly (entries 9 and 10, Table 3). Lower reaction temperature (50 °C) resulted in no conversion (entry 11, Table 3), and the reaction produced a mixture of 2a and 9a in lower yield in the absence of ethylene gas (entry 12, Table 3).
Table 3: Screening of CM–EYM tandem reaction.
|
|
||||||
| Entry | Ru cat. | Solvent | Conc. (M) | Temp. (°C) | Timea (h) | Yield of products 2a/3a (%)b |
|---|---|---|---|---|---|---|
| 1c | G-I | Toluene | 0.02 | 110 | 1.5 | Complex |
| 2c | G-II | Toluene | 0.02 | 110 | 1.5 | 23/17 |
| 3c | HG-II | Toluene | 0.02 | 110 | 3 | 34/46 |
| 4 | HG-II | Toluene | 0.02 | 110 | 3 | 33/37 |
| 5 | HG-II | CH2Cl2 | 0.02 | 110 | 24 | 0/68 (67)d |
| 6 | HG-II | 1,2-DCE | 0.02 | 110 | 24 | 26/24 |
| 7 | HG-II | THF | 0.02 | 110 | 24 | 4/28 |
| 8 | HG-II | 1,4-Dioxane | 0.02 | 110 | 24 | 9/32 |
| 9 | HG-II | CH2Cl2 | 0.05 | 110 | 24 | 0/56 |
| 10 | HG-II | CH2Cl2 | 0.1 | 110 | 24 | 8/32 |
| 11 | HG-II | CH2Cl2 | 0.02 | 50 | 24 | No reaction |
| 12e | HG-II | CH2Cl2 | 0.02 | 110 | 24 | 18/30 |
aTime was determined by TLC and/or GC–MS.
bThe yield and ratio of products were determined by 19F NMR.
cThe reaction was carried out without a pressure vessel.
dThe value in parentheses is the isolated yield.
eThe reaction was carried out under argon.
These optimized reaction conditions were applied to other vinyl compounds 8 (Table 4). After 4-substituted aryl alkenes gave the desired product 9 in moderate yields with excellent selectivity (E-major) (entries 3 and 4, Table 4), it then became clear that steric hindrance and the electronic deficiency of alkenes 8 decrease the efficiency of the tandem reaction; the non-tandem product 2a being formed instead (entries 2 and 6, Table 4). Allyl acetate 8f gave the desired product only when toluene was employed as solvent (entry 6, Table 4).
Table 4: CM–EYM tandem reaction with fluorinated 1,7-enyne 1a and alkene 8.
|
|
|||
| Entry | R | Time (h)a | Isolated yields of 9 [E/Z]b + 2a (%) |
|---|---|---|---|
| 1 | Ph (8a) | 24 | 67 [1/0] (9a) + 0 |
| 2 | 3-MeO-C6H4 (8b) | 24 | 36 [1/0] (9b) + 15 |
| 3 | 4-MeO-C6H4 (8c) | 24 | 33 [1/0] (9c) + trace |
| 4 | 4-Cl-C6H4 (8d) | 24 | 43 [1/0] (9d) + trace |
| 5 | 4-F-C6H4 (8e) | 27 | 33 [1/0] (9e) + 19 |
| 6c | CH2OAc (8f) | 3 | 31 [1/0] (9f) + 31 |
aTime was determined by TLC and/or GC–MS.
bThe ratio of products was determined by 1H and/or 19F NMR.
cToluene was used instead of CH2Cl2.
The stereochemistry of the terminal double bond of 9 was determined by comparing coupling constants of vinyl protons of compound 2a. The coupling constants of trans-protons (Ha–Hc) and cis-protons (Ha–Hb) on a double bond are J = 17.5 Hz and J = 11.0 Hz, respectively (Figure 1).
Figure 1: Comparison of coupling constant of vinyl protons.
Figure 1: Comparison of coupling constant of vinyl protons.
As expected, the Diels–Alder reaction with N-phenylmaleimide 6 gave 6-substituted gem-difluoroisoquinolinones efficiently with slight stereoselectivity (Scheme 3).
Scheme 3: Diels–Alder reaction with 9 and 6. aCombined yield of two isomers.
Scheme 3: Diels–Alder reaction with 9 and 6. aCombined yield of two isomers.
In summary, gem-difluoro-1,7-enyne carbonyl derivatives are useful reaction partners in enyne metathesis cycloisomerization and CM–EYM tandem reactions catalyzed by ruthenium carbene complexes. The resulting diene products can be elaborated further using a Diels–Alder reaction.
Supporting Information
| Supporting Information File 1: Synthesis of fluorinated δ-lactams via cycloisomerization of gem-difluoropropargyl amides | ||
| Format: PDF | Size: 196.7 KB | Download |
References
-
Ghose, A. K.; Viswanadhan, V. N.; Wendoloski, J. J. J. Comb. Chem. 1999, 1, 55–68. doi:10.1021/cc9800071
Return to citation in text: [1] -
Wakselman, M.; Joyeau, R.; Kobaiter, R.; Boggetto, N.; Vergely, I.; Maillard, J.; Okochi, V.; Montagne, J.-J.; Reboud-Ravaux, M. FEBS Lett. 1991, 282, 377–381. doi:10.1016/0014-5793(91)80517-7
Return to citation in text: [1] -
Maillard, J.-L.; Favreau, C.; Reboud-Ravaux, M.; Kobaiter, R.; Joyeau, R.; Wakselman, M. Eur. J. Cell Biol. 1990, 52, 213–218.
Return to citation in text: [1] -
Joyeau, R.; Molines, H.; Labia, R.; Wakselman, M. J. Med. Chem. 1988, 31, 370–374. doi:10.1021/jm00397a018
Return to citation in text: [1] -
Angelastro, M. R.; Bey, P.; Mehdi, S.; Peet, N. P. Bioorg. Med. Chem. Lett. 1992, 2, 1235–1238. doi:10.1016/S0960-894X(00)80220-6
Return to citation in text: [1] -
Evans, G. B.; Furneaux, R. H.; Lewandowicz, A.; Schramm, V. L.; Tyler, P. C. J. Med. Chem. 2003, 46, 3412–3423. doi:10.1021/jm030145r
Return to citation in text: [1] -
Inagaki, H.; Miyauchi, S.; Miyauchi, R. N.; Kawato, H. C.; Ohki, H.; Matsuhashi, N.; Kawakami, K.; Takahashi, H.; Takemura, M. J. Med. Chem. 2003, 46, 1005–1015. doi:10.1021/jm020328y
Return to citation in text: [1] -
Thaisrivongs, S.; Pals, D. T.; Kati, W. M.; Turner, S. R.; Thomasco, L. M.; Watt, W. J. Med. Chem. 1986, 29, 2080–2087. doi:10.1021/jm00160a048
Return to citation in text: [1] -
Fustero, S.; Bello, P.; Fernández, B.; del Pozo, C.; Hammond, G. B. J. Org. Chem. 2009, 74, 7690–7696. doi:10.1021/jo9013436
Return to citation in text: [1] -
Hammond, G. B.; Arimitsu, S. Synthesis of gem-difluorinated heterocycles using a difluoropropargyl building block. In Fluorinated Heterocycles; Gakh, A. A.; Kirk, K. L., Eds.; ACS Symposium Series 1003; American Chemical Society: Washington, DC, 2009; pp 135–164.
Return to citation in text: [1] -
Arimitsu, S.; Bottom, R. L.; Hammond, G. B. J. Fluorine Chem. 2008, 129, 1047–1051. doi:10.1016/j.jfluchem.2008.05.010
Return to citation in text: [1] -
Arimitsu, S.; Fernández, B.; del Pozo, C.; Fustero, S.; Hammond, G. B. J. Org. Chem. 2008, 73, 2656–2661. doi:10.1021/jo7025965
Return to citation in text: [1] -
Fustero, S.; Fernández, B.; Bello, P.; del Pozo, C.; Arimitsu, S.; Hammond, G. B. Org. Lett. 2007, 9, 4251–4253. doi:10.1021/ol701811z
Return to citation in text: [1] -
Fustero, S.; Sánchez-Roselló, M.; Jiménez, D.; Sanz-Cervera, J. F.; del Pozo, C.; Aceña, J. L. J. Org. Chem. 2006, 71, 2706–2714. doi:10.1021/jo0525635
Return to citation in text: [1] [2] -
Deiters, A.; Martin, S. F. Chem. Rev. 2004, 104, 2199–2238. doi:10.1021/cr0200872
(see for a review).
Return to citation in text: [1] -
Cheng, Z.-L.; Chen, Q.-Y. J. Fluorine Chem. 2006, 127, 894–900. doi:10.1016/j.jfluchem.2006.03.020
Return to citation in text: [1] -
Wang, R.-W.; Qing, F.-L. Org. Lett. 2005, 7, 2189–2192. doi:10.1021/ol050558h
Return to citation in text: [1] -
Pan, Y.; Holmes, C. P.; Tumelty, D. J. Org. Chem. 2005, 70, 4897–4900. doi:10.1021/jo050599r
Return to citation in text: [1] -
Beeler, A. B.; Gadepalli, R. S. V. S.; Steyn, S.; Castagnoli, N., Jr.; Rimoldi, J. M. Bioorg. Med. Chem. 2003, 11, 5229–5234. doi:10.1016/j.bmc.2003.08.002
Return to citation in text: [1] -
Poulsen, C. S.; Madsen, R. Synthesis 2003, 1–18. doi:10.1055/s-2003-36243
Return to citation in text: [1] -
Mori, M.; Sakakibara, N.; Kinoshita, A. J. Org. Chem. 1998, 63, 6082–6083. doi:10.1021/jo980896e
Return to citation in text: [1] -
Hong, S. K.; Sanders, D. P.; Lee, C. W.; Grubbs, R. H. J. Am. Chem. Soc. 2005, 127, 17160–17161. doi:10.1021/ja052939w
Return to citation in text: [1] -
Dabideen, D. R.; Cheng, K. F.; Aljabari, B.; Miller, E. J.; Pavlov, V. A.; Al-Abed, Y. J. Med. Chem. 2007, 50, 1993–1997. doi:10.1021/jm061477+
Return to citation in text: [1] -
Mewshaw, R. E.; Bowen, S. M.; Harris, H. A.; Xu, Z. B.; Manas, E. S.; Cohn, S. T. Bioorg. Med. Chem. Lett. 2007, 17, 902–906. doi:10.1016/j.bmcl.2006.11.066
Return to citation in text: [1] -
Mewshaw, R. E.; Edsall, R. J., Jr.; Yang, C.; Manas, E. S.; Xu, Z. B.; Henderson, R. A.; Keith, J. C., Jr.; Harris, H. A. J. Med. Chem. 2005, 48, 3953–3979. doi:10.1021/jm058173s
Return to citation in text: [1] -
Trost, B. M.; Toste, F. D.; Pinkerton, A. B. Chem. Rev. 2001, 101, 2067–2096. doi:10.1021/cr000666b
Return to citation in text: [1] -
Naota, T.; Takaya, H.; Murahashi, S.-I. Chem. Rev. 1998, 98, 2599–2660. doi:10.1021/cr9403695
Return to citation in text: [1] -
Lee, H.-Y.; Kim, H. Y.; Tae, H.; Kim, B. G.; Lee, J. Org. Lett. 2003, 5, 3439–3442. doi:10.1021/ol035194c
(a similar approach with non-fluorinated building blocks has been reported).
Return to citation in text: [1]
| 1. | Ghose, A. K.; Viswanadhan, V. N.; Wendoloski, J. J. J. Comb. Chem. 1999, 1, 55–68. doi:10.1021/cc9800071 |
| 14. | Fustero, S.; Sánchez-Roselló, M.; Jiménez, D.; Sanz-Cervera, J. F.; del Pozo, C.; Aceña, J. L. J. Org. Chem. 2006, 71, 2706–2714. doi:10.1021/jo0525635 |
| 9. | Fustero, S.; Bello, P.; Fernández, B.; del Pozo, C.; Hammond, G. B. J. Org. Chem. 2009, 74, 7690–7696. doi:10.1021/jo9013436 |
| 10. | Hammond, G. B.; Arimitsu, S. Synthesis of gem-difluorinated heterocycles using a difluoropropargyl building block. In Fluorinated Heterocycles; Gakh, A. A.; Kirk, K. L., Eds.; ACS Symposium Series 1003; American Chemical Society: Washington, DC, 2009; pp 135–164. |
| 11. | Arimitsu, S.; Bottom, R. L.; Hammond, G. B. J. Fluorine Chem. 2008, 129, 1047–1051. doi:10.1016/j.jfluchem.2008.05.010 |
| 12. | Arimitsu, S.; Fernández, B.; del Pozo, C.; Fustero, S.; Hammond, G. B. J. Org. Chem. 2008, 73, 2656–2661. doi:10.1021/jo7025965 |
| 13. | Fustero, S.; Fernández, B.; Bello, P.; del Pozo, C.; Arimitsu, S.; Hammond, G. B. Org. Lett. 2007, 9, 4251–4253. doi:10.1021/ol701811z |
| 5. | Angelastro, M. R.; Bey, P.; Mehdi, S.; Peet, N. P. Bioorg. Med. Chem. Lett. 1992, 2, 1235–1238. doi:10.1016/S0960-894X(00)80220-6 |
| 6. | Evans, G. B.; Furneaux, R. H.; Lewandowicz, A.; Schramm, V. L.; Tyler, P. C. J. Med. Chem. 2003, 46, 3412–3423. doi:10.1021/jm030145r |
| 7. | Inagaki, H.; Miyauchi, S.; Miyauchi, R. N.; Kawato, H. C.; Ohki, H.; Matsuhashi, N.; Kawakami, K.; Takahashi, H.; Takemura, M. J. Med. Chem. 2003, 46, 1005–1015. doi:10.1021/jm020328y |
| 8. | Thaisrivongs, S.; Pals, D. T.; Kati, W. M.; Turner, S. R.; Thomasco, L. M.; Watt, W. J. Med. Chem. 1986, 29, 2080–2087. doi:10.1021/jm00160a048 |
| 28. |
Lee, H.-Y.; Kim, H. Y.; Tae, H.; Kim, B. G.; Lee, J. Org. Lett. 2003, 5, 3439–3442. doi:10.1021/ol035194c
(a similar approach with non-fluorinated building blocks has been reported). |
| 2. | Wakselman, M.; Joyeau, R.; Kobaiter, R.; Boggetto, N.; Vergely, I.; Maillard, J.; Okochi, V.; Montagne, J.-J.; Reboud-Ravaux, M. FEBS Lett. 1991, 282, 377–381. doi:10.1016/0014-5793(91)80517-7 |
| 3. | Maillard, J.-L.; Favreau, C.; Reboud-Ravaux, M.; Kobaiter, R.; Joyeau, R.; Wakselman, M. Eur. J. Cell Biol. 1990, 52, 213–218. |
| 4. | Joyeau, R.; Molines, H.; Labia, R.; Wakselman, M. J. Med. Chem. 1988, 31, 370–374. doi:10.1021/jm00397a018 |
| 21. | Mori, M.; Sakakibara, N.; Kinoshita, A. J. Org. Chem. 1998, 63, 6082–6083. doi:10.1021/jo980896e |
| 23. | Dabideen, D. R.; Cheng, K. F.; Aljabari, B.; Miller, E. J.; Pavlov, V. A.; Al-Abed, Y. J. Med. Chem. 2007, 50, 1993–1997. doi:10.1021/jm061477+ |
| 24. | Mewshaw, R. E.; Bowen, S. M.; Harris, H. A.; Xu, Z. B.; Manas, E. S.; Cohn, S. T. Bioorg. Med. Chem. Lett. 2007, 17, 902–906. doi:10.1016/j.bmcl.2006.11.066 |
| 25. | Mewshaw, R. E.; Edsall, R. J., Jr.; Yang, C.; Manas, E. S.; Xu, Z. B.; Henderson, R. A.; Keith, J. C., Jr.; Harris, H. A. J. Med. Chem. 2005, 48, 3953–3979. doi:10.1021/jm058173s |
| 14. | Fustero, S.; Sánchez-Roselló, M.; Jiménez, D.; Sanz-Cervera, J. F.; del Pozo, C.; Aceña, J. L. J. Org. Chem. 2006, 71, 2706–2714. doi:10.1021/jo0525635 |
| 26. | Trost, B. M.; Toste, F. D.; Pinkerton, A. B. Chem. Rev. 2001, 101, 2067–2096. doi:10.1021/cr000666b |
| 27. | Naota, T.; Takaya, H.; Murahashi, S.-I. Chem. Rev. 1998, 98, 2599–2660. doi:10.1021/cr9403695 |
| 15. |
Deiters, A.; Martin, S. F. Chem. Rev. 2004, 104, 2199–2238. doi:10.1021/cr0200872
(see for a review). |
| 16. | Cheng, Z.-L.; Chen, Q.-Y. J. Fluorine Chem. 2006, 127, 894–900. doi:10.1016/j.jfluchem.2006.03.020 |
| 17. | Wang, R.-W.; Qing, F.-L. Org. Lett. 2005, 7, 2189–2192. doi:10.1021/ol050558h |
| 18. | Pan, Y.; Holmes, C. P.; Tumelty, D. J. Org. Chem. 2005, 70, 4897–4900. doi:10.1021/jo050599r |
| 19. | Beeler, A. B.; Gadepalli, R. S. V. S.; Steyn, S.; Castagnoli, N., Jr.; Rimoldi, J. M. Bioorg. Med. Chem. 2003, 11, 5229–5234. doi:10.1016/j.bmc.2003.08.002 |
| 22. | Hong, S. K.; Sanders, D. P.; Lee, C. W.; Grubbs, R. H. J. Am. Chem. Soc. 2005, 127, 17160–17161. doi:10.1021/ja052939w |
© 2010 Arimitsu and Hammond; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)