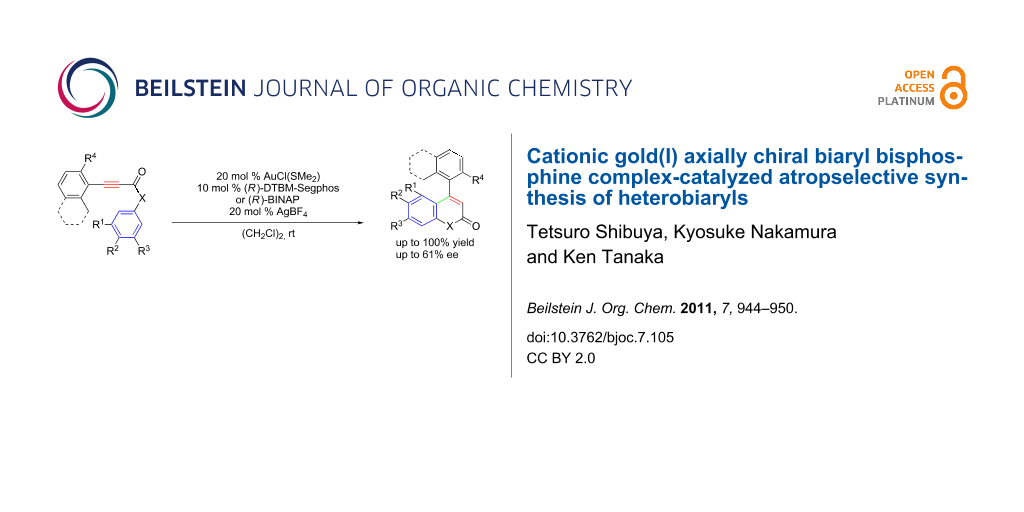
Abstract
It has been established that a cationic gold(I)/(R)-DTBM-Segphos or (R)-BINAP complex catalyzes the atropselective intramolecular hydroarylation of alkynes leading to enantioenriched axially chiral 4-aryl-2-quinolinones and 4-arylcoumarins with up to 61% ee.
Graphical Abstract
Introduction
Atropselective biaryl synthesis [1-4] has attracted significant interest due to its great utility in asymmetric catalysis and natural product synthesis. In 2004, three research groups, including ours, independently reported transition-metal catalyzed asymmetric [2 + 2 + 2] cycloaddition reactions to produce axially chiral biaryls [5-7]. These reports clearly demonstrated the utility of the asymmetric annulation strategy for the atropselective biaryl synthesis [8]. As an alternative asymmetric annulation method for the atropselective biaryl synthesis, we turned our attention to transition-metal catalyzed hydroalkenylation and hydroarylation reactions [9-15]. In this context, our research group developed the cationic gold(I)/PPh3-complex catalyzed intramolecular hydroalkenylation of N-alkenyl-arylethynylamides leading to 4-aryl-2-pyridones (Scheme 1) [16,17].
Scheme 1: Cationic gold(I)/PPh3-complex catalyzed intramolecular hydroalkenylation of alkynes.
Scheme 1: Cationic gold(I)/PPh3-complex catalyzed intramolecular hydroalkenylation of alkynes.
The atropselective synthesis of 6-aryl-2-pyridones has already been achieved by rhodium catalyzed [2 + 2 + 2] cycloaddition [18], while the atropselective synthesis of 4-aryl-2-pyridones has not yet been realized. The application of this intramolecular hydroalkenylation reaction to the atropselective synthesis of 4-aryl-2-pyridones from N-alkenyl-arylethynylamides was thus investigated. Although cationic gold(I)/axially chiral biaryl bisphosphine complexes [19-31] have been frequently employed in asymmetric variants of cationic gold(I) catalyses [32-38], including 6-endo-dig and 6-exo-dig cyclizations [39-41], the use of these gold(I) complexes gave almost racemic products [42]. Fortunately, cationic palladium(II)/axially chiral biaryl bisphosphine complexes were found to be effective catalysts, and a cationic palladium(II)/(S)-xyl-Segphos complex showed the highest enantioselectivity (Scheme 2) [42].
Scheme 2: Cationic palladium(II)/(S)-xyl-Segphos-complex catalyzed atropselective intramolecular hydroalkenylation of alkynes.
Scheme 2: Cationic palladium(II)/(S)-xyl-Segphos-complex catalyzed atropselective intramolecular hydroalkenyl...
In addition, the cationic palladium(II)/axially chiral biaryl bisphosphine complexes were able to catalyze the asymmetric intramolecular hydroarylation of N-aryl-arylethynylamides leading to axially chiral 4-aryl-2-quinolinones, and the cationic palladium(II)/(S)-xyl-H8-BINAP complex showed the highest enantioselectivity (Scheme 3) [43,44].
Scheme 3: Cationic palladium(II)/(S)-xyl-H8-BINAP complex-catalyzed atropselective intramolecular hydroarylation of alkynes.
Scheme 3: Cationic palladium(II)/(S)-xyl-H8-BINAP complex-catalyzed atropselective intramolecular hydroarylat...
In this paper, we report the use of the cationic gold(I)/axially chiral biaryl bisphosphine complexes in the catalytic asymmetric intramolecular hydroarylation for the synthesis of axially chiral 4-aryl-2-quinolinones and 4-arylcoumarins.
Results and Discussion
The reaction of N-benzyl-N-(2-naphthyl)propiolamide 1a, bearing a 2-methoxynaphthyl group at an alkyne terminus, was first investigated in the presence of a cationic gold(I)/(R)-BINAP complex (20 mol % Au). Although the reaction proceeded at room temperature in good yield, enantioselectivity was low (Table 1, entry 1). The effect of axially chiral biaryl bisphosphine ligands (Figure 1) on the yield and the enantioselectivity was then investigated. Among the bis(diphenylphosphine) ligands examined (Table 1, entries 1–3), the use of (R)-H8-BINAP furnished 2a with the highest enantiomeric excess (Table 1, entry 3). An increase in the steric bulk of the aryl group on the phosphorus atom of H8-BINAP lead to a decrease in the ee (Table 1, entry 4). The use of sterically more demanding (R)-DTBM-Segphos as a ligand furnished 2a in high yield with the highest ee (Table 1, entry 5). Unfortunately, a reduction in the amount of gold to 10 mol % significantly decreased both product yield and enantioselectivity (Table 1, entry 6).
Table 1: Screening of axially chiral biaryl bisphosphine ligands for the cationic gold(I)-complex catalyzed atropselective intramolecular hydroarylation of 1a.a
|
|
||||
| Entry | Ligand | Convn (%)b | Yield (%)c | ee (%) |
|---|---|---|---|---|
| 1 | (R)-BINAP | 81 | 75 | 7 (S) |
| 2 | (R)-Segphos | 90 | 81 | 8 (R) |
| 3 | (R)-H8-BINAP | 94 | 93 | 17 (S) |
| 4 | (S)-xyl-H8-BINAP | 100 | 96 | 13 (R) |
| 5 | (R)-DTBM-Segphos | 100 | 96 | 59 (R) |
| 6d | (R)-DTBM-Segphos | 71 | 49 | 31 (R) |
aAuCl(SMe2) (0.010 mmol, 20 mol %), AgBF4 (0.010 mmol, 20 mol %), ligand (0.0050 mmol, 10 mol %), 1a (0.050 mmol), and (CH2Cl)2 (1.5 mL) were used. bDetermined by 1H NMR. cIsolated yield. dAuCl(SMe2) (0.010 mmol, 10 mol %), AgBF4 (0.010 mmol, 10 mol %), ligand (0.0050 mmol, 5 mol %), 1a (0.10 mmol), and (CH2Cl)2 (1.5 mL) were used. Reaction time: 72 h.
Figure 1: Structures of axially chiral biaryl bisphosphine ligands.
Figure 1: Structures of axially chiral biaryl bisphosphine ligands.
Thus, the scope of the cationic gold(I)-complex catalyzed atropselective intramolecular hydroarylation of alkynes was explored at room temperature, as shown in Table 2. The 2-methoxynaphthalene derivative 1a (Table 2, entry 1) and the 2-methoxymethoxynaphthalene derivative 1b (Table 2, entry 2) furnished the desired benzoquinolinones 2a and 2b, respectively, in high yields and high ee, using (R)-DTBM-Segphos as a ligand. In addition, benzocoumarin 2c (Table 2, entry 3) was obtained in moderate ee, although the yield was low due to partial deprotection of the methoxymethoxynaphthalene moiety (Table 2, entry 3). The reactions of carbazole and dialkoxybenzene derivatives 1d–g, using (R)-DTBM-Segphos as a ligand, furnished the corresponding quinolinone and coumarin derivatives 2d–g in high yields with perfect regioselectivity, while the observed ee values were very low (<10% ee). However, interestingly, the use of (R)-BINAP as a ligand improved the enantoselectivity (14–32% ee, Table 2, entries 4–7).
Table 2: Cationic gold(I)-complex catalyzed atropselective intramolecular hydroarylation of 1a–g leading to heterobiaryls 2a–g.a
| Entry | 1 | Ligand (time) | 2 | % yieldb (% ee) | ||
|---|---|---|---|---|---|---|
| 1 |
|
1a |
(R)-DTBM-Segphos
(40 h) |
|
(R)-(−)-2a | 96 (59) |
| 2 |
|
1b |
(R)-DTBM-Segphos
(72 h) |
|
(−)-2b | 87 (61) |
| 3 |
|
1c |
(R)-DTBM-Segphos
(40 h) |
|
(−)-2c | 33 (49) |
| 4 |
|
1d |
(R)-BINAP
(72 h) |
|
(+)-2d | 82 (28) |
| 5 |
|
1e |
(R)-BINAP
(40 h) |
|
(−)-2e | 100 (32) |
| 6 |
|
1f |
(R)-BINAP
(40 h) |
|
(+)-2f | 88 (27) |
| 7 |
|
1g |
(R)-BINAP
(40 h) |
|
(+)-2g | 93 (14) |
aReactions were conducted using AuCl(SMe2) (0.010 mmol), AgBF4 (0.010 mmol), (R)-DTBM-Segphos or (R)-BINAP (0.0050 mmol), 1a–g (0.050 mmol), and (CH2Cl)2 (1.5 mL) at rt. In all entries, 100% convn of substrates 1a–g was observed.
bIsolated yield.
In the previously reported cationic palladium(II)/(S)-xyl-H8-BINAP-complex catalyzed atropselective intramolecular hydroarylation of alkynes, the presence of the 2-methoxy-substituted aryl group at the alkyne terminus was important for the realization of both high reactivity and enantioselectivity [40]. Similarly, the reaction of 2-methylnaphthalene derivative 1h in the presence of the cationic gold(I)/(R)-DTBM-Segphos complex furnished the corresponding benzoquinolinone 2h with lower yield and ee than those of 2a (Scheme 4).
Scheme 4: Cationic gold(I)/(R)-DTBM-Segphos-complex catalyzed atropselective intramolecular hydroarylation of 2-methylnaphthalene derivative 1h.
Scheme 4: Cationic gold(I)/(R)-DTBM-Segphos-complex catalyzed atropselective intramolecular hydroarylation of...
Conclusion
In conclusion, it has been established that a cationic gold(I)/(R)-DTBM-Segphos or (R)-BINAP complex catalyzes the atropselective intramolecular hydroarylation of alkynes leading to enantioenriched axially chiral 4-aryl-2-quinolinones and 4-arylcoumarins in up to 61% ee. Although there clearly remains room for improvement in enantioselectivity, the present asymmetric catalysis is a rare example of the utilization of gold(I)/chiral phosphine catalysts for the construction of noncentrochirality [45-47].
Experimental
General: 1H NMR spectra were recorded at 300 MHz (JEOL AL 300). 13C NMR spectra were obtained with complete proton decoupling at 75 MHz (JEOL AL 300). HRMS data were obtained on a Bruker micrOTOF Focus II. Infrared spectra were obtained on a JASCO FT/IR-4100. Optical rotations were obtained on a JASCO DIP-1000. Melting points were obtained on a METTLER MP50. Anhydrous (CH2Cl)2 (No. 28,450-5) was purchased from Aldrich and used as received. Solvents for the synthesis of substrates were dried over molecular sieves (4 Å, Wako) prior to use. Substrates 1a, 1b, 1d, 1e, 1f, and 1h were prepared according to the literature [43]. Products 2a, 2b, 2d, 2e, 2f, and 2h were already reported [43]. All other reagents were obtained from commercial sources and used as received. All reactions were carried out under an atmosphere of argon or nitrogen in oven-dried glassware with magnetic stirring.
(2-Methoxymethoxynaphthalen-1-yl)propynoic acid naphthalen-2-yl ester (1c): To a stirred solution of 3-[2-(methoxymethoxy)-1-naphthalenyl]-2-propynoic acid [48] (0.256 g, 1.00 mmol), 2-naphthol (0.159 g, 1.10 mmol), and 4-dimethylaminopyridine (12.2 mg, 0.100 mmol) in CH2Cl2 (10 mL) was added a solution of dicyclohexylcarbodiimide (0.248 g, 1.20 mmol) in CH2Cl2 (3 mL) at 0 °C, and the mixture was stirred at 0 °C for 2 h and at room temperature for 18 h. The crude mixture was filtered with CH2Cl2. The filtrate was washed with brine, dried over Na2SO4, and concentrated. The residue was purified by a silica gel column chromatography (hexane/EtOAc = 10:1) to give 1c (0.222 g, 0.580 mmol, 58% yield). Yellow solid; mp 97.3–99.3 °C; IR (KBr): 2203, 1717, 1229, 1149, 1005 cm−1; 1H NMR (CDCl3, 300 MHz) δ 8.15–8.01 (m, 1H), 7.97–7.70 (m, 6H), 7.61–7.31 (m, 6H), 5.36 (s, 2H), 3.56 (s, 3H); 13C NMR (CDCl3, 125 MHz) δ 160.0, 152.8, 148.1, 134.7, 133.7, 133.4, 131.7, 129.7, 128.8, 128.3, 127.82, 127.79, 126.7, 126.0, 125.1, 124.8, 121.0, 118.8, 115.5, 103.8, 95.1, 89.1, 85.2, 56.6; HRMS–ESI (m/z): [M + Na]+ calcd for C25H18O4Na, 405.1097; found, 405.1107.
(2-Methoxynaphthalen-1-yl)propynoic acid 3,5-dimethoxyphenyl ester (1g): The title compound was prepared from (2-methoxynaphthalen-1-yl)propynoic acid [49] and 3,5-dimethoxyphenol in 70% yield by the procedure used for 1c. Yellow solid; mp 102.9–104.7 °C; IR (KBr): 2211, 1714, 1621, 1269, 1156 cm−1; 1H NMR (CDCl3, 300 MHz) δ 8.16 (d, J = 8.4 Hz, 1H), 7.96 (d, J = 9.2 Hz, 1H), 7.80 (d, J = 8.1 Hz, 1H), 7.59 (dd, J = 8.1, 6.5 Hz, 1H), 7.42 (dd, J = 8.4, 6.5 Hz, 1H), 7.26 (d, J = 9.2 Hz, 1H), 6.43 (d, J = 2.1 Hz, 2H), 6.40 (t, J = 2.1 Hz, 1H), 4.06 (s, 3H), 3.80 (s, 6H); 13C NMR (CDCl3, 125 MHz) δ 162.1, 161.1, 152.5, 151.8, 135.0, 133.6, 128.4, 128.3, 128.1, 124.7, 112.1, 101.8, 100.2, 98.7, 89.3, 85.0, 56.5, 55.5; HRMS–ESI (m/z): [M + Na]+ calcd for C22H18O5Na, 385.1046; found, 385.1047.
General procedure for cationic gold(I)/axially chiral biaryl bisphosphine complex-catalyzed atropselective intramolecular hydroarylation of N-aryl-arylethynylamides 1: To AuCl(SMe2) (0.010 mmol) was added a solution of axially chiral biaryl bisphosphine ligand (0.0050 mmol) in (CH2Cl)2 (0.5 mL), and the mixture was stirred at room temperature for 1 h. To this solution was added AgBF4 (0.010 mmol) in (CH2Cl)2 (0.5 mL) at room temperature, and the mixture was stirred at room temperature for 0.5 h. To this mixture was added a solution of 1 (0.050 mmol) in (CH2Cl)2 (0.5 mL) at room temperature. After stirring at room temperature for 40–72 h, the mixture was directly purified on a preparative TLC to afford 2.
(−)-1-(2-Methoxymethoxynaphthalen-1-yl)benzo[f]chromen-3-one [(−)-2c]: Colorless solid; mp 169.4–170.8 °C; [α]25D −86.1 (c 0.28, CHCl3, 49% ee); IR (KBr): 1738, 1510, 1244, 1050, 1011 cm−1; 1H NMR (CDCl3, 500 MHz) δ 8.05 (d, J = 8.7 Hz, 1H), 8.02 (d, J = 9.2 Hz, 1H), 7.92 (d, J = 8.4 Hz, 1H), 7.81 (d, J = 8.0 Hz, 1H), 7.62 (d, J = 8.7 Hz, 1H), 7.57 (d, J = 9.2 Hz, 1H), 7.53 (d, J = 8.4 Hz, 1H), 7.42 (ddd, J = 8.4, 7.0, 1.4 Hz, 1H), 7.36 (ddd, J = 8.4, 6.9, 1.5 Hz, 1H), 7.32 (ddd, J = 8.0, 7.0, 1.0 Hz, 1H), 7.17 (d, J = 8.3 Hz, 1H), 6.97 (ddd, J = 8.3, 6.9, 1.4 Hz, 1H), 6.45 (s, 1H), 5.04 (dd, J = 22.4, 6.9 Hz, 2H), 3.05 (s, 3H); 13C NMR (CDCl3, 125 MHz) δ 160.6, 154.8, 152.5, 150.6, 133.8, 131.6, 131.0, 130.9, 129.8, 129.5, 129.0, 128.2, 127.7, 127.6, 125.4, 124.8, 124.4, 123.8, 122.8, 118.6, 117.8, 115.7, 114.2, 94.2, 56.0; HRMS–ESI (m/z): [M + Na]+ calcd for C25H18O4Na, 405.1097; found, 405.1085; CHIRALPAK OD-H, hexane/iPrOH = 80:20, 1.0 mL/min, retention times: 14.3 min (major isomer) and 19.0 min (minor isomer).
(+)-5,7-Dimethoxy-4-(2-methoxynaphthalen-1-yl)chromen-2-one [(+)-2g]: Colorless solid; mp 148.8–150.4 °C; [α]25D +44.9 (c 0.24, CHCl3, 14% ee); IR (KBr): 1718, 1618, 1598, 1351, 1114 cm−1; 1H NMR (CDCl3, 300 MHz) δ 7.88 (d, J = 9.0 Hz, 1H), 7.85–7.77 (m, 1H), 7.48–7.39 (m, 1H), 7.38–7.27 (m, 3H), 6.57 (d, J = 2.4 Hz, 1H), 6.12 (d, J = 2.4 Hz, 1H), 6.07 (s, 1H), 3.85 (s, 3H), 3.83 (s, 3H) 3.07 (s, 3H); 13C NMR (CDCl3, 125 MHz) δ 163.1, 161.2, 158.5, 157.1, 152.4, 151.0, 131.9, 129.3, 128.5, 127.9, 126.6, 124.2, 123.6, 122.9, 114.0, 113.1, 105.0, 95.8, 93.6, 56.7, 55.75, 55.71; HRMS–ESI (m/z): [M + Na]+ calcd for C22H18O5Na, 385.1046; found, 385.1036; CHIRALPAK AD-H, hexane/iPrOH = 80:20, 1.0 mL/min, retention times: 8.8 min (minor isomer) and 10.5 min (major isomer).
Supporting Information
| Supporting Information File 1: 1H and 13C NMR spectra for new compounds 1c, 1g, 2c, and 2g. | ||
| Format: PDF | Size: 50.8 KB | Download |
References
-
Kozlowski, M. C.; Morgan, B. J.; Linton, E. C. Chem. Soc. Rev. 2009, 38, 3193–3207. doi:10.1039/b821092f
Return to citation in text: [1] -
Wallace, T. W. Org. Biomol. Chem. 2006, 4, 3197–3210. doi:10.1039/b608470m
Return to citation in text: [1] -
Bringmann, G.; Price Mortimer, A. J.; Keller, P. A.; Gresser, M. J.; Garner, J.; Breuning, M. Angew. Chem., Int. Ed. 2005, 44, 5384–5427. doi:10.1002/anie.200462661
Return to citation in text: [1] -
Baudoin, O. Eur. J. Org. Chem. 2005, 4223–4229. doi:10.1002/ejoc.200500394
Return to citation in text: [1] -
Gutnov, A.; Heller, B.; Fischer, C.; Drexler, H.-J.; Spannenberg, A.; Sundermann, B.; Sundermann, C. Angew. Chem., Int. Ed. 2004, 43, 3795–3797. doi:10.1002/anie.200454164
Return to citation in text: [1] -
Shibata, T.; Fujimoto, T.; Yokota, K.; Takagi, K. J. Am. Chem. Soc. 2004, 126, 8382–8383. doi:10.1021/ja048131d
Return to citation in text: [1] -
Tanaka, K.; Nishida, G.; Wada, A.; Noguchi, K. Angew. Chem., Int. Ed. 2004, 43, 6510–6512. doi:10.1002/anie.200461533
Return to citation in text: [1] -
Tanaka, K. Chem.–Asian J. 2009, 4, 508–518. doi:10.1002/asia.200800378
Return to citation in text: [1] -
Kitamura, T. Eur. J. Org. Chem. 2009, 1111–1125. doi:10.1002/ejoc.200801054
Return to citation in text: [1] -
Skouta, R.; Li, C.-J. Tetrahedron 2008, 64, 4917–4938. doi:10.1016/j.tet.2008.03.083
Return to citation in text: [1] -
Shen, H. C. Tetrahedron 2008, 64, 3885–3903. doi:10.1016/j.tet.2008.01.081
Return to citation in text: [1] -
Bandini, M.; Emer, E.; Tommasi, S.; Umani-Ronchi, A. Eur. J. Org. Chem. 2006, 3527–3544. doi:10.1002/ejoc.200500995
Return to citation in text: [1] -
Nevado, C.; Echavarren, A. M. Synthesis 2005, 167–182. doi:10.1055/s-2005-861781
Return to citation in text: [1] -
Kakiuchi, F.; Chatani, N. Adv. Synth. Catal. 2003, 345, 1077–1101. doi:10.1002/adsc.200303094
Return to citation in text: [1] -
Jia, C.; Kitamura, T.; Fujiwara, Y. Acc. Chem. Res. 2001, 34, 633–639. doi:10.1021/ar000209h
Return to citation in text: [1] -
Imase, H.; Noguchi, K.; Hirano, M.; Tanaka, K. Org. Lett. 2008, 10, 3563–3566. doi:10.1021/ol801466f
Return to citation in text: [1] -
Imase, H.; Tanaka, K. Chem. Lett. 2009, 38, 1152–1153. doi:10.1246/cl.2009.1152
Return to citation in text: [1] -
Tanaka, K.; Wada, A.; Noguchi, K. Org. Lett. 2005, 7, 4737–4739. doi:10.1021/ol052041b
Return to citation in text: [1] -
Melhado, A. D.; Amarante, G. W.; Wang, Z. J.; Luparia, M.; Toste, F. D. J. Am. Chem. Soc. 2011, 133, 3517–3527. doi:10.1021/ja1095045
Return to citation in text: [1] -
LaLonde, R. L.; Wang, Z. J.; Mba, M.; Lackner, A. D.; Toste, F. D. Angew. Chem., Int. Ed. 2010, 49, 598–601. doi:10.1002/anie.200905000
Return to citation in text: [1] -
Kleinbeck, F.; Toste, F. D. J. Am. Chem. Soc. 2009, 131, 9178–9179. doi:10.1021/ja904055z
Return to citation in text: [1] -
Zhang, Z.; Lee, S. D.; Widenhoefer, R. A. J. Am. Chem. Soc. 2009, 131, 5372–5373. doi:10.1021/ja9001162
Return to citation in text: [1] -
Uemura, M.; Watson, I. D. G.; Katsukawa, M.; Toste, F. D. J. Am. Chem. Soc. 2009, 131, 3464–3465. doi:10.1021/ja900155x
Return to citation in text: [1] -
Watson, I. D. G.; Ritter, S.; Toste, F. D. J. Am. Chem. Soc. 2009, 131, 2056–2057. doi:10.1021/ja8085005
Return to citation in text: [1] -
Chao, C.-M.; Vitale, M. R.; Toullec, P. Y.; Genêt, J.-P.; Michelet, V. Chem.–Eur. J. 2009, 15, 1319–1323. doi:10.1002/chem.200802341
Return to citation in text: [1] -
Luzung, M. R.; Mauleón, P.; Toste, F. D. J. Am. Chem. Soc. 2007, 129, 12402–12403. doi:10.1021/ja075412n
Return to citation in text: [1] -
Tarselli, M. A.; Chianese, A. R.; Lee, S. J.; Gagné, M. R. Angew. Chem., Int. Ed. 2007, 46, 6670–6673. doi:10.1002/anie.200701959
Return to citation in text: [1] -
Liu, C.; Widenhoefer, R. A. Org. Lett. 2007, 9, 1935–1938. doi:10.1021/ol070483c
Return to citation in text: [1] -
LaLonde, R. L.; Sherry, B. D.; Kang, E. J.; Toste, F. D. J. Am. Chem. Soc. 2007, 129, 2452–2453. doi:10.1021/ja068819l
Return to citation in text: [1] -
Zhang, Z.; Widenhoefer, R. A. Angew. Chem., Int. Ed. 2007, 46, 283–285. doi:10.1002/anie.200603260
Return to citation in text: [1] -
Johansson, M. J.; Gorin, D. J.; Staben, S. T.; Toste, F. D. J. Am. Chem. Soc. 2005, 127, 18002–18003. doi:10.1021/ja0552500
Return to citation in text: [1] -
Fürstner, A. Chem. Soc. Rev. 2009, 38, 3208–3221. doi:10.1039/b816696j
Return to citation in text: [1] -
Widenhoefer, R. A. Chem.–Eur. J. 2008, 14, 5382–5391. doi:10.1002/chem.200800219
Return to citation in text: [1] -
Shen, H. C. Tetrahedron 2008, 64, 7847–7870. doi:10.1016/j.tet.2008.05.082
Return to citation in text: [1] -
Gorin, D. J.; Sherry, B. D.; Toste, F. D. Chem. Rev. 2008, 108, 3351–3378. doi:10.1021/cr068430g
Return to citation in text: [1] -
Li, Z.; Brouwer, C.; He, C. Chem. Rev. 2008, 108, 3239–3265. doi:10.1021/cr068434l
Return to citation in text: [1] -
Gorin, D. J.; Toste, F. D. Nature 2007, 446, 395–403. doi:10.1038/nature05592
Return to citation in text: [1] -
Hashmi, A. S. K.; Hutchings, G. J. Angew. Chem., Int. Ed. 2006, 45, 7896–7936. doi:10.1002/anie.200602454
Return to citation in text: [1] -
Chao, C.-M.; Beltrami, D.; Toullec, P. Y.; Michelet, V. Chem. Commun. 2009, 6988–6990. doi:10.1039/b913554e
Return to citation in text: [1] -
Sethofer, S. G.; Staben, S. T.; Hung, O. Y.; Toste, F. D. Org. Lett. 2008, 10, 4315–4318. doi:10.1021/ol801760w
Return to citation in text: [1] [2] -
Sethofer, S. G.; Mayer, T.; Toste, F. D. J. Am. Chem. Soc. 2010, 132, 8276–8277. doi:10.1021/ja103544p
Return to citation in text: [1] -
Imase, H.; Suda, T.; Shibata, Y.; Noguchi, K.; Hirano, M.; Tanaka, K. Org. Lett. 2009, 11, 1805–1808. doi:10.1021/ol900373z
Return to citation in text: [1] [2] -
Shibuya, T.; Shibata, Y.; Noguchi, K.; Tanaka, K. Angew. Chem., Int. Ed. 2011, 50, 3963–3967. doi:10.1002/anie.201100152
Return to citation in text: [1] [2] [3] -
Ototake, N.; Morimoto, Y.; Mokuya, A.; Fukaya, H.; Shida, Y.; Kitagawa, O. Chem.–Eur. J. 2010, 16, 6752–6755. doi:10.1002/chem.201000243
Return to citation in text: [1] -
Ogasawara, M.; Watanabe, S. Synthesis 2009, 1761–1785. doi:10.1055/s-0029-1216818
Return to citation in text: [1] -
Michon, C.; Liu, S.; Hiragushi, S.; Uenishi, J.; Uemura, M. Synlett 2008, 1321–1324. doi:10.1055/s-2008-1072614
Return to citation in text: [1] -
Murai, M.; Uenishi, J.; Uemura, M. Org. Lett. 2010, 12, 4788–4791. doi:10.1021/ol1019376
Return to citation in text: [1] -
Sato, Y.; Tamura, T.; Kinbara, A.; Mori, M. Adv. Synth. Catal. 2007, 349, 647–661. doi:10.1002/adsc.200600587
Return to citation in text: [1] -
Wessig, P.; Müller, G. Chem. Commun. 2006, 4524–4526. doi:10.1039/B609374D
Return to citation in text: [1]
| 48. | Sato, Y.; Tamura, T.; Kinbara, A.; Mori, M. Adv. Synth. Catal. 2007, 349, 647–661. doi:10.1002/adsc.200600587 |
| 1. | Kozlowski, M. C.; Morgan, B. J.; Linton, E. C. Chem. Soc. Rev. 2009, 38, 3193–3207. doi:10.1039/b821092f |
| 2. | Wallace, T. W. Org. Biomol. Chem. 2006, 4, 3197–3210. doi:10.1039/b608470m |
| 3. | Bringmann, G.; Price Mortimer, A. J.; Keller, P. A.; Gresser, M. J.; Garner, J.; Breuning, M. Angew. Chem., Int. Ed. 2005, 44, 5384–5427. doi:10.1002/anie.200462661 |
| 4. | Baudoin, O. Eur. J. Org. Chem. 2005, 4223–4229. doi:10.1002/ejoc.200500394 |
| 16. | Imase, H.; Noguchi, K.; Hirano, M.; Tanaka, K. Org. Lett. 2008, 10, 3563–3566. doi:10.1021/ol801466f |
| 17. | Imase, H.; Tanaka, K. Chem. Lett. 2009, 38, 1152–1153. doi:10.1246/cl.2009.1152 |
| 43. | Shibuya, T.; Shibata, Y.; Noguchi, K.; Tanaka, K. Angew. Chem., Int. Ed. 2011, 50, 3963–3967. doi:10.1002/anie.201100152 |
| 9. | Kitamura, T. Eur. J. Org. Chem. 2009, 1111–1125. doi:10.1002/ejoc.200801054 |
| 10. | Skouta, R.; Li, C.-J. Tetrahedron 2008, 64, 4917–4938. doi:10.1016/j.tet.2008.03.083 |
| 11. | Shen, H. C. Tetrahedron 2008, 64, 3885–3903. doi:10.1016/j.tet.2008.01.081 |
| 12. | Bandini, M.; Emer, E.; Tommasi, S.; Umani-Ronchi, A. Eur. J. Org. Chem. 2006, 3527–3544. doi:10.1002/ejoc.200500995 |
| 13. | Nevado, C.; Echavarren, A. M. Synthesis 2005, 167–182. doi:10.1055/s-2005-861781 |
| 14. | Kakiuchi, F.; Chatani, N. Adv. Synth. Catal. 2003, 345, 1077–1101. doi:10.1002/adsc.200303094 |
| 15. | Jia, C.; Kitamura, T.; Fujiwara, Y. Acc. Chem. Res. 2001, 34, 633–639. doi:10.1021/ar000209h |
| 43. | Shibuya, T.; Shibata, Y.; Noguchi, K.; Tanaka, K. Angew. Chem., Int. Ed. 2011, 50, 3963–3967. doi:10.1002/anie.201100152 |
| 40. | Sethofer, S. G.; Staben, S. T.; Hung, O. Y.; Toste, F. D. Org. Lett. 2008, 10, 4315–4318. doi:10.1021/ol801760w |
| 5. | Gutnov, A.; Heller, B.; Fischer, C.; Drexler, H.-J.; Spannenberg, A.; Sundermann, B.; Sundermann, C. Angew. Chem., Int. Ed. 2004, 43, 3795–3797. doi:10.1002/anie.200454164 |
| 6. | Shibata, T.; Fujimoto, T.; Yokota, K.; Takagi, K. J. Am. Chem. Soc. 2004, 126, 8382–8383. doi:10.1021/ja048131d |
| 7. | Tanaka, K.; Nishida, G.; Wada, A.; Noguchi, K. Angew. Chem., Int. Ed. 2004, 43, 6510–6512. doi:10.1002/anie.200461533 |
| 45. | Ogasawara, M.; Watanabe, S. Synthesis 2009, 1761–1785. doi:10.1055/s-0029-1216818 |
| 46. | Michon, C.; Liu, S.; Hiragushi, S.; Uenishi, J.; Uemura, M. Synlett 2008, 1321–1324. doi:10.1055/s-2008-1072614 |
| 47. | Murai, M.; Uenishi, J.; Uemura, M. Org. Lett. 2010, 12, 4788–4791. doi:10.1021/ol1019376 |
| 39. | Chao, C.-M.; Beltrami, D.; Toullec, P. Y.; Michelet, V. Chem. Commun. 2009, 6988–6990. doi:10.1039/b913554e |
| 40. | Sethofer, S. G.; Staben, S. T.; Hung, O. Y.; Toste, F. D. Org. Lett. 2008, 10, 4315–4318. doi:10.1021/ol801760w |
| 41. | Sethofer, S. G.; Mayer, T.; Toste, F. D. J. Am. Chem. Soc. 2010, 132, 8276–8277. doi:10.1021/ja103544p |
| 42. | Imase, H.; Suda, T.; Shibata, Y.; Noguchi, K.; Hirano, M.; Tanaka, K. Org. Lett. 2009, 11, 1805–1808. doi:10.1021/ol900373z |
| 32. | Fürstner, A. Chem. Soc. Rev. 2009, 38, 3208–3221. doi:10.1039/b816696j |
| 33. | Widenhoefer, R. A. Chem.–Eur. J. 2008, 14, 5382–5391. doi:10.1002/chem.200800219 |
| 34. | Shen, H. C. Tetrahedron 2008, 64, 7847–7870. doi:10.1016/j.tet.2008.05.082 |
| 35. | Gorin, D. J.; Sherry, B. D.; Toste, F. D. Chem. Rev. 2008, 108, 3351–3378. doi:10.1021/cr068430g |
| 36. | Li, Z.; Brouwer, C.; He, C. Chem. Rev. 2008, 108, 3239–3265. doi:10.1021/cr068434l |
| 37. | Gorin, D. J.; Toste, F. D. Nature 2007, 446, 395–403. doi:10.1038/nature05592 |
| 38. | Hashmi, A. S. K.; Hutchings, G. J. Angew. Chem., Int. Ed. 2006, 45, 7896–7936. doi:10.1002/anie.200602454 |
| 43. | Shibuya, T.; Shibata, Y.; Noguchi, K.; Tanaka, K. Angew. Chem., Int. Ed. 2011, 50, 3963–3967. doi:10.1002/anie.201100152 |
| 44. | Ototake, N.; Morimoto, Y.; Mokuya, A.; Fukaya, H.; Shida, Y.; Kitagawa, O. Chem.–Eur. J. 2010, 16, 6752–6755. doi:10.1002/chem.201000243 |
| 19. | Melhado, A. D.; Amarante, G. W.; Wang, Z. J.; Luparia, M.; Toste, F. D. J. Am. Chem. Soc. 2011, 133, 3517–3527. doi:10.1021/ja1095045 |
| 20. | LaLonde, R. L.; Wang, Z. J.; Mba, M.; Lackner, A. D.; Toste, F. D. Angew. Chem., Int. Ed. 2010, 49, 598–601. doi:10.1002/anie.200905000 |
| 21. | Kleinbeck, F.; Toste, F. D. J. Am. Chem. Soc. 2009, 131, 9178–9179. doi:10.1021/ja904055z |
| 22. | Zhang, Z.; Lee, S. D.; Widenhoefer, R. A. J. Am. Chem. Soc. 2009, 131, 5372–5373. doi:10.1021/ja9001162 |
| 23. | Uemura, M.; Watson, I. D. G.; Katsukawa, M.; Toste, F. D. J. Am. Chem. Soc. 2009, 131, 3464–3465. doi:10.1021/ja900155x |
| 24. | Watson, I. D. G.; Ritter, S.; Toste, F. D. J. Am. Chem. Soc. 2009, 131, 2056–2057. doi:10.1021/ja8085005 |
| 25. | Chao, C.-M.; Vitale, M. R.; Toullec, P. Y.; Genêt, J.-P.; Michelet, V. Chem.–Eur. J. 2009, 15, 1319–1323. doi:10.1002/chem.200802341 |
| 26. | Luzung, M. R.; Mauleón, P.; Toste, F. D. J. Am. Chem. Soc. 2007, 129, 12402–12403. doi:10.1021/ja075412n |
| 27. | Tarselli, M. A.; Chianese, A. R.; Lee, S. J.; Gagné, M. R. Angew. Chem., Int. Ed. 2007, 46, 6670–6673. doi:10.1002/anie.200701959 |
| 28. | Liu, C.; Widenhoefer, R. A. Org. Lett. 2007, 9, 1935–1938. doi:10.1021/ol070483c |
| 29. | LaLonde, R. L.; Sherry, B. D.; Kang, E. J.; Toste, F. D. J. Am. Chem. Soc. 2007, 129, 2452–2453. doi:10.1021/ja068819l |
| 30. | Zhang, Z.; Widenhoefer, R. A. Angew. Chem., Int. Ed. 2007, 46, 283–285. doi:10.1002/anie.200603260 |
| 31. | Johansson, M. J.; Gorin, D. J.; Staben, S. T.; Toste, F. D. J. Am. Chem. Soc. 2005, 127, 18002–18003. doi:10.1021/ja0552500 |
| 18. | Tanaka, K.; Wada, A.; Noguchi, K. Org. Lett. 2005, 7, 4737–4739. doi:10.1021/ol052041b |
| 42. | Imase, H.; Suda, T.; Shibata, Y.; Noguchi, K.; Hirano, M.; Tanaka, K. Org. Lett. 2009, 11, 1805–1808. doi:10.1021/ol900373z |
© 2011 Shibuya et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)