Abstract
Regioselective bromine–lithium exchange reactions on polybrominated biaryls enable the modular synthesis of various polysubstituted biphenyls such as bis(dialkylphosphino)-, bis(diarylphosphino)- and dialkyl(diaryl)phosphinobiphenyls. All permutations of substituents at the ortho positions of the biphenyls are possible. In a similar manner, one can gain access to monophosphine analogues. So far, such a process, based on the effective discrimination between bromine atoms as a function of their chemical environment, has been observed only sporadically.
Graphical Abstract
Introduction
Atropisomeric biaryls are important compounds in various fields. In particular, pharmaceuticals and agrochemicals with biaryl substructures are of general interest [1]. In addition, they have widespread applications as ligands in catalysis, or in materials sciences [2]. The atropisomeric C2-symmetric binaphthyl- or biphenyl-bridged diphosphine ligands BINAP [3], H8-BINAP [4], BIPHEMP [5], MeO-BIPHEP [6], SEGPHOS [7-9], P-Phos [10], SYNPHOS [11,12], Cn-TUNAPHOS [13] and DIFLUORPHOS [14-16] and their analogues are well known as highly efficient chiral ligands for a variety of transition metal-catalyzed asymmetric transformations. The biphenyl backbone has the advantage that substituents at the 6- and 6'-positions can affect the dihedral angle of the biphenyl backbone, one of the key factors for ligand efficiency. Both the aryl phosphorus substituents and the biphenyl backbone can be tailored in order to modify the stereoelectronic profile of a ligand.
Most frequently, biaryls are prepared through transition metal-catalyzed reactions of suitable functionalized starting materials [17-22]. Although these methods are well established, alternatives are investigated in order to avoid expensive transition metals or ligands, which are especially required for the coupling of deactivated or sterically hindered substrates.
Our group is developing new methods towards the synthesis of highly functionalized atropisomeric biphenyls [23-32]. We seek to perform their synthesis (a) in a modular way starting from a few common and easily available precursors; (b) with a high degree of structural diversity; (c) in a straightforward, short, reproducible manner; (d) in high yield and on multigram scale; and, last but not least, (e) with a restricted use of transition metals and ligands.
Polar organometallic chemistry [33-35] allows the performance of highly selective reactions. Therefore, it seemed to us the ideal tool to reaching this goal. In this context, we recently developed a novel transition metal-free aryl–aryl coupling protocol, the "ARYNE-coupling", which allows the preparation of di-, tri-, and even tetra-substituted ortho,ortho'-dibromobiphenyls [25,28,31,36-38]. These have the advantage that, by means of successive or simultaneous bromine–lithium exchanges, a huge panel of substituents can be introduced into the biphenyl backbone.
The bromine–lithium exchange reaction is certainly one of the most fundamental synthetic transformations [39]. Although this reaction was reported by C. S. Marvel in 1927 [40], G. Wittig [41] and H. Gilman [42-44] were the first to apply it in organic synthesis in the late thirties. Since then, this reaction has been considered as a mature method lacking both appeal and surprise [33], and only new applications of this reaction or mechanistic studies have been reported [30,32,33,45-55]. However, in the last few years, the halogen–metal permutation has recaptured its former role as one of the most important and versatile methods in organic synthesis. New exchange reagents, such as isopropylmagnesium chloride, its LiCl complex [53-61] and lithium tributylmagnesate [62,63], have been developed and allow reactions under noncryogenic conditions [64-66]. New access routes to synthetically challenging aryl halide precursors have been devised.
A. Alexakis et al. recently achieved a significant breakthrough. They succeeded in the desymmetrization of prochiral polybrominated [32,51] compounds by an asymmetric bromine–lithium exchange in the presence of a stoichiometric amount of chiral diamines. An enantiomeric excess of up to 63% was obtained [67]. H. Kagan et al. reported the desymmetrization of prochiral aromatic or vinylic dihalide substrates by halogen–metal exchange in the presence of a stoichiometric amount of diamines, with enantiomeric excess up to 26% [68]. Very recently, the Alexakis group achieved the catalytic bromine–lithium exchange allowing the preparation of biarylatropisomers in quantitative yields and enantiomeric excesses up to 82% [69].
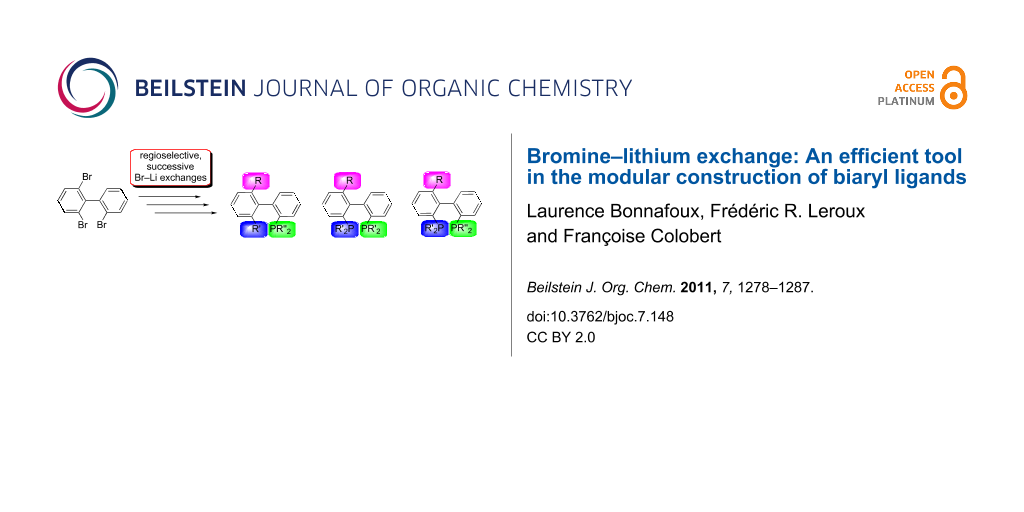
Herein, we report on the preparation of C1 analogues of the most efficient and popular C2-symmetric biphenyl ligands. We will show that by means of regioselective bromine–lithium exchanges all possible permutations of bis(diaryldiphosphino)-, bis(dialkylphosphino)- and dialkyl(diaryl)phosphinobiphenyls become feasible. In a similar way, biphenyl-based monophosphine ligands were also obtained (Figure 1).
Figure 1: Modular synthesis of bis(diarylphosphino)-, bis(dialkylphosphino)- and dialkyl(diaryl)phosphinobiphenyls as well as monophosphinobiphenyls by means of polar organometallic chemistry.
Figure 1: Modular synthesis of bis(diarylphosphino)-, bis(dialkylphosphino)- and dialkyl(diaryl)phosphinobiph...
Results and Discussion
Regioselective bromine–lithium exchange on polybrominated biphenyls
Our group recently reported the efficient coupling of organolithium intermediates with arynes, the so-called "ARYNE coupling" [25,31,36]. This protocol is based on the formation of a thermodynamically stable aryllithium intermediate and its subsequent reaction with a 1,2-dibromobenzene derivative. The transient benzyne adds the aryllithium derivative, followed by in situ transfer of bromine between the resulting 2-biaryllithium intermediate and another molecule of 1,2-dibromobenzene. Mono-, di- or even tetra-substituted ortho-bromobiaryls can be obtained on a gram scale (Figure 2).
The intriguing question is whether one bromine would be exchanged preferentially on the substrate when the bromine atoms are not activated by adjacent heteroatoms. An effective discrimination between two bromine atoms as a function of their chemical environment has so far been observed only sporadically in such processes [30,34,35,51,52,70-72].
Fortunately, the reaction occurred exclusively on the doubly halogenated ring when 2,2',6-tribromobiphenyl [28,38], obtained almost quantitatively by the ARYNE coupling protocol, was submitted to the bromine–lithium exchange reaction. When 2,2',6-tribromobiphenyl (1a) [28] was treated at −78 °C with BuLi and the intermediate aryllithium trapped with iodomethane, 2,2'-dibromo-6-methylbiphenyl (1b) was obtained in an excellent yield of 96%. Analogously, when benzenesulfonylazide was used as an electrophile, 2-azido-2',6-dibromobiphenyl was obtained. The use of lithium aluminium hydride in ether at reflux for 4.5 h gave exclusively 2-amino-2',6-dibromobiphenyl, which was submitted to a reductive methylation by means of formaldehyde and sodium cyanoborohydride. 2-N,N-Dimethylamino-2',6-dibromobiphenyl (1c) was obtained in an overall yield of 79% in 3 steps (Scheme 1). To introduce the methoxy group, 2,2',6-tribromobiphenyl (1a) was successively subjected to lithiation, borylation with fluorodimethoxyborane·diethyl ether, followed by oxidation with hydrogen peroxide and O-methylation with iodomethane in acetone. 2,2'-Dibromo-6-methoxybiphenyl (1d) was finally obtained in a very good global yield of 68% in 3 steps. Finally, we proposed to introduce the phenyl ring (1f, 95%) by a regioselective Suzuki–Miyaura coupling via the iodo derivative 1e, the latter being obtained in 83% yield after trapping with iodine.
Scheme 1: Functionalization of 2,2',6-tribromobiphenyl (1a) by regioselective bromine–lithium exchange.
Scheme 1: Functionalization of 2,2',6-tribromobiphenyl (1a) by regioselective bromine–lithium exchange.
Similarly, as shown for 2,2',6-tribromobiphenyl (1a), when 6-substituted 2,2'-dibromobiphenyls 1b–e were treated with just one equivalent of butyllithium in tetrahydrofuran at −78 °C, another regioselective bromine–lithium exchange occurs on the functionalized ring (Scheme 2). Trapping with iodomethane afforded the biphenyls 2 in high yield and perfect regioselectivity, except for R = Ph (1f) and the benzodioxole derivative (1i), where the regioselectivity was slightly lower (91:9 and 92:8, respectively).
Scheme 2: Functionalization of 2,2'-dibromobiphenyls (1b–e) by regioselective bromine–lithium exchange.
Scheme 2: Functionalization of 2,2'-dibromobiphenyls (1b–e) by regioselective bromine–lithium exchange.
M. Schlosser and J. Gorecka-Kobylinska recently reported on the relative basicities of aryllithiums bearing methoxy, chlorine, fluorine, trifluoromethyl and trifluoromethoxy substituents at the ortho, meta, and para positions. Equilibration studies of two aryllithiums of comparable basicity with the corresponding bromo- or iodoarenes allowed them to determine the "basicity" (protodelithiation) increments ∆∆G, derived from the equilibrium constants. The authors showed that the basicity increments are linearly correlated with the relative protonation enthalpies of the corresponding aryl anions in the gas phase. Compared with "naked" aryl anions, the basicity of aryllithiums mirrors the effects of ortho, meta, and para substituents to the extent of 36%, 30%, and 25%, respectively [73].
These results explain the difference in regioselectivity of the bromine–lithium exchange, between a bromine atom residing on a phenyl ring that bears a "stabilizing" substituent at a remote meta position and a bromine atom on an "unstabilized" phenyl ring.
Biaryl mono- and diphosphines
In the following section we will show how a large family of biaryl mono- and diphosphines becomes readily accessible through these common building-blocks. The general access is depicted in Figure 3.
Figure 3: General access to biaryl mono- and diphosphine ligands; (Cy = cyclohexyl).
Figure 3: General access to biaryl mono- and diphosphine ligands; (Cy = cyclohexyl).
Biarylmonophosphines: Path A
From the methylated intermediates 2a–d, new monophosphines became accessible in one additional step (Scheme 3). The bromine–lithium exchange was either performed with just one equivalent of butyllithium in tetrahydrofuran at −78 °C (conditions a) or in toluene at 0 °C (conditions b). After cooling to −78 °C, the lithiated intermediate was then allowed to react with a solution of ClPCy2, or ClPPh2 in toluene. In these cases, the monophosphines 3 were obtained in good yields (Scheme 3).
Scheme 3: Synthesis of monophosphines 3; (Cy = cyclohexyl).
Scheme 3: Synthesis of monophosphines 3; (Cy = cyclohexyl).
Single crystal X-ray analyses [74] of one ligand of each family (R1 = NMe2 (3a, Figure 4), OMe (3b, see Supporting Information File 3), Ph (3d, see Supporting Information File 4), OCH2O (3f, see Supporting Information File 5)) were performed in order to confirm their structure. Thus, we confirmed the selectivity of the different halogen–metal exchange reactions performed throughout the synthesis.
![[1860-5397-7-148-4]](/bjoc/content/figures/1860-5397-7-148-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Molecular structure of compound 3a (crystallized from ethyl acetate/hexane) [74].
Figure 4: Molecular structure of compound 3a (crystallized from ethyl acetate/hexane) [74].
Mixed dialkyl(diaryl)phosphinobiphenyls: Path B
In order to synthesize mixed diphosphines, we took advantage of the non-equivalence of the two phenyl rings towards lithiation. We started from the same dibromobiaryls 1 as before, but submitted them now to just one equivalent of butyllithium in THF. The aryllithium intermediate was then trapped with one equivalent of ClPCy2. The resulting monophosphine 4 was isolated and then submitted to a second bromine–lithium exchange in toluene followed by addition of ClPPh2 (Scheme 4). The lithiation–phosphination sequence was not substrate-dependent and reaction conditions were the same for all the different ortho substituents. In this way, pure ligands were obtained on a gram scale.
Scheme 4: Preparation of mixed dialkyl(diaryl)phosphinobiphenyls 5 via successive bromine–lithium exchange.
Scheme 4: Preparation of mixed dialkyl(diaryl)phosphinobiphenyls 5 via successive bromine–lithium exchange.
Bis(dialkyl)phosphinobiphenyls: Path C
In order to gain access to bis(dicyclohexylphosphino)biphenyls 3, a double bromine–lithium exchange was performed on the 2,2'-dibromobiaryls 1. A screening of the reaction conditions revealed higher yields for the double phosphination when the bromine–lithium exchange and trapping with ClPCy2 were carried out in toluene rather than in THF and at higher temperature. For example, with R1 = Me, OCF2O, and OCF2CF2O, the corresponding diphosphines were obtained in a yield of 54% (in toluene) versus 12% (in THF), 53% versus 36% and 73% versus 35%, respectively (Table 1, entries 1, 2, 9, 10, 11 and 12). A series of bis(dicyclohexylphosphino)biphenyls 6 was obtained in good yield. However, we noticed lower yields for biaryls carrying α-fluorinated ether substituents, such as OCF2O (53%; Table 1, entry 9) or OCF3 (33%; Table 1, entry 14), in comparison with their nonfluorinated counterparts, OCH2O (70%; Table 1, entry 8) and OMe [30] (74%; Table 1, entry 5).
Table 1: Synthesis of bis(dicyclohexylphosphino)biphenyls 6.
|
|
|||||||||||
| Entry | R1 | X | RLi | n | Solvent | T [° C] | t | T' [°C] | t' | Ligand | Yield |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Me | Br | BuLi | 2 | toluene | 110 | 1 h | 50 | 2 h | 6b | 54% |
| 2 | Me | Br | BuLi | 2 | THF | −78 | 1 h | −78 | 1 h | 6b | 12% |
| 3 | NMe2 | Br | t-BuLi | 4 | THF | −78 | 1 h | −78 | 1 h | 6c | 22% |
| 4 | NMe2 | Br | BuLi | 2 | toluene | 0 | 15 min | −78 | 1 h | 6c | 30% |
| 5 | OMe [30] | Br | BuLi | 2 | THF | −78 | 1 h | −78 | 1 h | 6d | 74% |
| 6 | Ph | Br | t-BuLi | 4 | THF | −78 | 1 h | −78 | 1 h | 6f | 66% |
| 7 | Cl | Br | BuLi | 2 | THF | −78 | 1 h | −78 | 1 h | 6g | 49% |
| 8 | OCH2O | Br | BuLi | 2 | toluene | 110 | 1 h | 50 | 2 h | 6h | 70% |
| 9 | OCF2O | I | BuLi | 3 | toluene | 25 | 1 h | 25 | 2 h | 6i | 53% |
| 10 | OCF2O | I | BuLi | 3 | THF | −78 | 1 h | −78 | 1 h | 6i | 36% |
| 11 | O(CF2)2O | I | BuLi | 3 | toluene | 25 | 1 h | 25 | 2 h | 6j | 73% |
| 12 | O(CF2)2O | I | BuLi | 3 | THF | −78 | 1 h | −78 | 1 h | 6j | 35% |
| 13 | F | Br | BuLi | 2 | THF | −78 | 15 min | −78 | 1 h | 6k | 79% |
| 14 | OCF3 | Br | BuLi | 2 | toluene | 110 | 1 h | 50 | 2 h | 6l | 33% |
In the case of 2-N,N-dimethylamino-2',6-dibromobiphenyl (1c), higher yields were obtained when the double bromine–lithium exchange was realized successively instead of simultaneously. Indeed, the double phosphination in THF or toluene gave the corresponding ligand in a very poor yield (Table 1, entries 3 and 4). When the first Br–Li exchange was carried out in THF at −78 °C, followed by trapping with ClPCy2, the corresponding monophosphine 7 was obtained in a good yield of 63%. The second Br–Li exchange was performed in toluene at 0 °C, affording ligand 6c in a moderate yield of 44% after trapping with ClPCy2 (Scheme 5).
Scheme 5: Stepwise bromine–lithium exchange on 1c.
Scheme 5: Stepwise bromine–lithium exchange on 1c.
Single crystal X-ray analysis of 6c was performed (see Supporting Information File 6) [74].
Phosphafluorenes and bis(diarylphosphino)biphenyls: Path D and E
When the ortho,ortho'-dibromobiphenyls 1 were submitted to a double bromine–lithium exchange in THF followed by trapping with two equivalents of ClPPh2, whatever the nature of the ortho substituent (R = Me, OMe, NMe2, Cl, OCF3, Ph, OCH2O, OCF2O, OCF2CF2O, H, F), the formation of an intramolecular cyclization product, a dibenzophosphole, was exclusively observed. This is consistent with the observations of O. Desponds et al. [75]. However, when THF was replaced by toluene, the outcome of the reaction could be modified in favor of the 2,2'-bis(diphenylphosphino)biphenyls 8 [27] which were still contaminated with varying amounts of phosphafluorenes 9 and triphenylphosphine (Table 2).
Table 2: Synthesis of bis(diphenylphosphino)biphenyls 8.
|
|
||||||
| Cpd. | R | X' | n [equiv] | Ta [°C] | T' b [°C] | Yield 8c |
|---|---|---|---|---|---|---|
| 8b | Me | Br | 2 | 110 | 50 | 30% |
| 8c | NMe2 | Br | 2 | 110 | 50 | 44% |
| 8d | OMe | Br | 2 | 110 | 50 | 17% |
| 8f | Ph | Br | 2 | 110 | 50 | 24% |
| 8g | Cl | Br | 2 | 110 | 50 | 23% |
| 8h | OCH2O | Br | 2 | 110 | 50 | 18% |
| 8i | OCF2O | I | 3 | 25 | 25 | 47% |
| 8j | OCF2CF2O | I | 3 | 25 | 25 | 34% |
| 8l | OCF3 | Br | 2 | 110 | 50 | 10% |
aTemperature for the halogen/metal exchange; bTrapping temperature; cIsolated yield of 8 after flash-chromatography on silica gel.
Conclusion
In the present work, we showed how completely regioselective bromine–lithium exchange reactions on polybrominated biphenyls allow the construction of a new family of di- and monophosphine ligands. The required polybrominated biphenyls can be easily obtained through an efficient transition metal-free aryl–aryl coupling protocol developed by our group. The regioselectivity can be explained by recent equilibration studies of M. Schlosser et al. which allowed the determination of ∆∆G increments for substituents at the ortho, meta, and para positions.
Overall, the methodology presented in this study offers the possibility to consider new pathways for the synthesis of more sophisticated biaryl scaffolds. Catalytic studies as well as the control of biaryl axial chirality are currently underway and will be reported in due course.
Supporting Information
| Supporting Information File 1: Experimental details and spectroscopic data for new compounds. | ||
| Format: PDF | Size: 145.1 KB | Download |
| Supporting Information File 2: Crystal structure data for 3a. | ||
| Format: PDF | Size: 513.8 KB | Download |
| Supporting Information File 3: Crystal structure data for 3b. | ||
| Format: PDF | Size: 243.0 KB | Download |
| Supporting Information File 4: Crystal structure data for 3d. | ||
| Format: PDF | Size: 275.4 KB | Download |
| Supporting Information File 5: Crystal structure data for 3f. | ||
| Format: PDF | Size: 510.0 KB | Download |
| Supporting Information File 6: Crystal structure data for 6c. | ||
| Format: PDF | Size: 627.7 KB | Download |
References
-
Patchett, A. A.; Nargund, R. P. Annu. Rep. Med. Chem. 2000, 35, 289–298. doi:10.1016/S0065-7743(00)35027-8
Return to citation in text: [1] -
Bringmann, G.; Breuning, M.; Tasler, S. Synthesis 1999, 525–558. doi:10.1055/s-1999-3435
Return to citation in text: [1] -
Miyashita, A.; Yasuda, A.; Takaya, H.; Toriumi, K.; Ito, T.; Souchi, T.; Noyori, R. J. Am. Chem. Soc. 1980, 102, 7932–7934. doi:10.1021/ja00547a020
Return to citation in text: [1] -
Zhang, X.; Mashima, K.; Koyano, K.; Sayo, N.; Kumobayashi, H.; Akutagawa, S.; Takaya, H. Tetrahedron Lett. 1991, 32, 7283–7286. doi:10.1016/0040-4039(91)80499-V
Return to citation in text: [1] -
Schmid, R.; Cereghetti, M.; Heiser, B.; Schönholzer, P.; Hansen, H.-J. Helv. Chim. Acta 1988, 71, 897–929. doi:10.1002/hlca.19880710427
Return to citation in text: [1] -
Schmid, R.; Foricher, J.; Cereghetti, M.; Schönholzer, P. Helv. Chim. Acta 1991, 74, 370–389. doi:10.1002/hlca.19910740215
Return to citation in text: [1] -
Saito, T.; Yokozawa, T.; Zhang, X.; Sayo, N. Chiral diphosphine compound, intermediate for preparing the same transition metal complex having the same diphosphine compound as ligand and asymmetric hydrogenation catalyst. Eur. Pat. EP 0850945 A1, July 1, 1998.
Return to citation in text: [1] -
Sayo, N.; Saito, T.; Yokozawa, T. Ruthenium-phosphine complex and method for producing the same. Eur. Pat. EP 0945457 A2, Sept 29, 1999.
Return to citation in text: [1] -
Saito, T.; Yokozawa, T.; Ishizaki, T.; Moroi, T.; Sayo, N.; Miura, T.; Kumobayashi, H. Adv. Synth. Catal. 2001, 343, 264–267. doi:10.1002/1615-4169(20010330)343:3<264::AID-ADSC264>3.0.CO;2-T
Return to citation in text: [1] -
Pai, C.-C.; Lin, C.-W.; Lin, C.-C.; Chen, C.-C.; Chan, A. S. C.; Wong, W. T. J. Am. Chem. Soc. 2000, 122, 11513–11514. doi:10.1021/ja000163n
Return to citation in text: [1] -
Pai, C.-C.; Li, Y.-M.; Zhou, Z.-Y.; Chan, A. S. C. Tetrahedron Lett. 2002, 43, 2789–2792. doi:10.1016/S0040-4039(02)00383-0
Return to citation in text: [1] -
Duprat de Paule, S.; Jeulin, S.; Ratovelomanana-Vidal, V.; Genêt, J.-P.; Champion, N.; Dellis, P. Tetrahedron Lett. 2003, 44, 823–826. doi:10.1016/S0040-4039(02)02637-0
Return to citation in text: [1] -
Zhang, Z.; Qian, H.; Longmire, J.; Zhang, X. J. Org. Chem. 2000, 65, 6223–6226. doi:10.1021/jo000462v
Return to citation in text: [1] -
Jeulin, S.; Duprat de Paule, S.; Ratovelomanana-Vidal, V.; Genêt, J.-P.; Champion, N.; Dellis, P. Angew. Chem., Int. Ed. 2004, 43, 320–325. doi:10.1002/anie.200352453
Return to citation in text: [1] -
Leroux, F.; Gorecka, J.; Schlosser, M. Synthesis 2004, 326–328. doi:10.1055/s-2004-815926
Return to citation in text: [1] -
Mettler, H.; Leroux, F. Process for the preparation of (S)- or (R)-4-halo-3-hydroxybutyrates. WO Pat. Appl. WO 2005049545 A1, June 2, 2005.
Return to citation in text: [1] -
Trost, B. M.; Verhoeven, T. R. 57 - Organopalladium Compounds in Organic Synthesis and in Catalysis. In Comprehensive Organometallic Chemistry; Wilkinson, G.; Stone, F. G. A.; Abel, E. W., Eds.; Pergamon: Oxford, UK, 1982; Vol. 8, pp 799–938. doi:10.1016/B978-008046518-0.00121-5
Return to citation in text: [1] -
Tsuji, J. Palladium Reagents and Catalysts; John Wiley and Sons: Chichester, UK, 1995.
Return to citation in text: [1] -
Farina, V. 3.4 - Transition Metal Alkyl Complexes: Oxidative Addition and Transmetallation. In Transition Metal Organometallics in Organic Synthesis; Abel, E.; Stone, F. G. A.; Wilkinson, G., Eds.; Comprehensive Organometallic Chemistry II, Vol. 12; Pergamon: Oxford, UK, 1995; pp 161–240.
Return to citation in text: [1] -
Diederich, F.; Stang, P. J. Metal-catalyzed Cross-coupling Reactions; Wiley-VCH: Weinheim, Germany, 1998. doi:10.1002/9783527612222
Return to citation in text: [1] -
Suzuki, A. J. Organomet. Chem. 1999, 576, 147–168. doi:10.1016/S0022-328X(98)01055-9
Return to citation in text: [1] -
Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457–2483. doi:10.1021/cr00039a007
Return to citation in text: [1] -
Diemer, V.; Leroux, F. R.; Colobert, F. Eur. J. Org. Chem. 2011, 327–340. doi:10.1002/ejoc.201001217
Return to citation in text: [1] -
Diemer, V.; Garcia, J. S.; Leroux, F. R.; Colobert, F. J. Fluorine Chem. 2011, in press. doi:10.1016/j.jfluchem.2011.02.017
Return to citation in text: [1] -
Diemer, V.; Begaud, M.; Leroux, F. R.; Colobert, F. Eur. J. Org. Chem. 2011, 341–354. doi:10.1002/ejoc.201001283
Return to citation in text: [1] [2] [3] -
Bonnafoux, L.; Gramage-Doria, R.; Colobert, F.; Leroux, F. R. Chem.–Eur. J. 2011, in press. doi:10.1002/chem.201101529
Return to citation in text: [1] -
Bonnafoux, L.; Ernst, L.; Leroux, F. R.; Colobert, F. Eur. J. Inorg. Chem. 2011, 3387–3397. doi:10.1002/ejic.201100428
Return to citation in text: [1] [2] -
Bonnafoux, L.; Colobert, F.; Leroux, F. R. Synlett 2010, 2953–2955. doi:10.1055/s-0030-1259025
Return to citation in text: [1] [2] [3] [4] -
Colobert, F.; Valdivia, V.; Choppin, S.; Leroux, F. R.; Fernandez, I.; Alvarez, E.; Khiar, N. Org. Lett. 2009, 11, 5130–5133. doi:10.1021/ol9020755
Return to citation in text: [1] -
Leroux, F. R.; Mettler, H. Adv. Synth. Catal. 2007, 349, 323–336. doi:10.1002/adsc.200600300
Return to citation in text: [1] [2] [3] [4] [5] -
Leroux, F. R.; Bonnafoux, L.; Heiss, C.; Colobert, F.; Lanfranchi, D. A. Adv. Synth. Catal. 2007, 349, 2705–2713. doi:10.1002/adsc.200700211
Return to citation in text: [1] [2] [3] -
Leroux, F. R.; Simon, R.; Nicod, N. Lett. Org. Chem. 2006, 3, 948–954. doi:10.2174/157017806779467979
Return to citation in text: [1] [2] [3] -
Schlosser, M. Angew. Chem., Int. Ed. 2005, 44, 376–393. doi:10.1002/anie.200300645
Return to citation in text: [1] [2] [3] -
Schlosser, M. Organoalkali Chemistry. In Organometallic in Synthesis: A Manual, 2nd ed.; Schlosser, M., Ed.; John Wiley and Sons: Chichester, UK, 2002; pp 1–352.
Return to citation in text: [1] [2] -
Leroux, F.; Schlosser, M.; Zohar, E.; Marek, I. The preparation of organolithium reagents and intermediates. In The Chemistry of Organolithium Compounds; Rappoport, Z.; Marek, I., Eds.; Wiley: Chichester, UK, 2004; Vol. 1, pp 435–493.
Return to citation in text: [1] [2] -
Leroux, F.; Schlosser, M. Angew. Chem., Int. Ed. 2002, 41, 4272–4274. doi:10.1002/1521-3773(20021115)41:22<4272::AID-ANIE4272>3.0.CO;2-B
Return to citation in text: [1] [2] -
Bonnafoux, L. Modular Synthesis of New C1-Biaryl Ligands and Application in Catalytic Hydrogenation and Coupling Reactions. Ph.D. Thesis, Université Louis Pasteur, Strasbourg, France, 2008.
Return to citation in text: [1] -
Leroux, F. R.; Bonnafoux, F.; Colobert, F. Process for the synthesis of 2,2',6-tribromobiphenyl. WO Pat. Appl. WO 2008/037440 A1, April 3, 2008.
Return to citation in text: [1] [2] -
Clayden, J. Organolithiums: Selectivity for Synthesis; Pergamon: Amsterdam, The Netherlands, 2002.
Return to citation in text: [1] -
Marvel, C. S.; Hager, F. D.; Coffman, D. D. J. Am. Chem. Soc. 1927, 49, 2323–2328. doi:10.1021/ja01408a030
Return to citation in text: [1] -
Wittig, G.; Pockels, U.; Droge, H. Ber. Dtsch. Chem. Ges. 1938, 71B, 1903–1912.
Return to citation in text: [1] -
Gilman, H.; Langham, W.; Jacoby, A. L. J. Am. Chem. Soc. 1939, 61, 106–109. doi:10.1021/ja01870a036
Return to citation in text: [1] -
Gilman, H.; Moore, F. W. J. Am. Chem. Soc. 1940, 62, 1843–1846. doi:10.1021/ja01864a058
Return to citation in text: [1] -
Langham, W.; Brewster, R. Q.; Gilman, H. J. Am. Chem. Soc. 1941, 63, 545–549. doi:10.1021/ja01847a053
Return to citation in text: [1] -
Jones, R. G.; Gilman, H. Chem. Rev. 1954, 54, 853–890. doi:10.1021/cr60171a004
Return to citation in text: [1] -
Bailey, W. F.; Patricia, J. J. J. Organomet. Chem. 1988, 352, 1–46. doi:10.1016/0022-328X(88)83017-1
Return to citation in text: [1] -
Beak, P.; Musick, T. J.; Chen, C. W. J. Am. Chem. Soc. 1988, 110, 3538–3542. doi:10.1021/ja00219a031
Return to citation in text: [1] -
Beak, P.; Allen, D. J. J. Am. Chem. Soc. 1992, 114, 3420–3425. doi:10.1021/ja00035a039
Return to citation in text: [1] -
Eisch, J. J. Organometallics 2002, 21, 5439–5463. doi:10.1021/om0109408
Return to citation in text: [1] -
Nájera, C.; Sansano, J. M.; Yus, M. Tetrahedron 2003, 59, 9255–9303. doi:10.1016/j.tet.2003.09.065
Return to citation in text: [1] -
Leroux, F.; Nicod, N.; Bonnafoux, L.; Quissac, B.; Colobert, F. Lett. Org. Chem. 2006, 3, 165–169.
Return to citation in text: [1] [2] [3] -
Leroux, F.; Mettler, H. Synlett 2006, 766–770. doi:10.1055/s-2006-933107
Return to citation in text: [1] [2] -
Shi, L.; Chu, Y.; Knochel, P.; Mayr, H. Org. Lett. 2009, 11, 3502–3505. doi:10.1021/ol9013393
Return to citation in text: [1] [2] -
Shi, L.; Chu, Y.; Knochel, P.; Mayr, H. J. Org. Chem. 2009, 74, 2760–2764. doi:10.1021/jo802770h
Return to citation in text: [1] [2] -
Shi, L.; Chu, Y.; Knochel, P.; Mayr, H. Angew. Chem., Int. Ed. 2008, 47, 202–204. doi:10.1002/anie.200704100
Return to citation in text: [1] [2] -
Krasovskiy, A.; Straub, B. F.; Knochel, P. Angew. Chem., Int. Ed. 2006, 45, 159–162. doi:10.1002/anie.200502220
Return to citation in text: [1] -
Krasovskiy, A.; Knochel, P. Angew. Chem., Int. Ed. 2004, 43, 3333–3336. doi:10.1002/anie.200454084
Return to citation in text: [1] -
Knochel, P.; Dohle, W.; Gommermann, N.; Kneisel, F. F.; Kopp, F.; Korn, T.; Sapountzis, I.; Vu, V. A. Angew. Chem., Int. Ed. 2003, 42, 4302–4320. doi:10.1002/anie.200300579
Return to citation in text: [1] -
Knochel, P.; Krasovskiy, A.; Sapountzis, I. Polyfunctional Magnesium Organometallics for Organic Synthesis. In Handbook of Functionalized Organometallics; Knochel, P., Ed.; Wiley-VCH: Weinheim, Germany, 2005; pp 109–172. doi:10.1002/9783527619467
Return to citation in text: [1] -
Boudier, A.; Bromm, L. O.; Lotz, M.; Knochel, P. Angew. Chem., Int. Ed. 2000, 39, 4414–4435. doi:10.1002/1521-3773(20001215)39:24<4414::AID-ANIE4414>3.0.CO;2-C
Return to citation in text: [1] -
Abarbri, M.; Dehmel, F.; Knochel, P. Tetrahedron Lett. 1999, 40, 7449–7453. doi:10.1016/S0040-4039(99)01404-5
Return to citation in text: [1] -
Iida, T.; Wada, T.; Tomimoto, K.; Mase, T. Tetrahedron Lett. 2001, 42, 4841–4844. doi:10.1016/S0040-4039(01)00861-9
Return to citation in text: [1] -
Kondo, J.; Inoue, A.; Shinokubo, H.; Oshima, K. Angew. Chem., Int. Ed. 2001, 40, 2085–2087. doi:10.1002/1521-3773(20010601)40:11<2085::AID-ANIE2085>3.0.CO;2-K
Return to citation in text: [1] -
Gallou, F.; Haenggi, R.; Hirt, H.; Marterer, W.; Schaefer, F.; Seeger-Weibel, M. Tetrahedron Lett. 2008, 49, 5024–5027. doi:10.1016/j.tetlet.2008.06.046
Return to citation in text: [1] -
Choe, J.; Seo, J. H.; Kwon, Y.; Song, K. H. Chem. Eng. J. 2008, 135, S17–S20. doi:10.1016/j.cej.2007.07.015
Return to citation in text: [1] -
Kato, S.; Nonoyama, N.; Tomimoto, K.; Mase, T. Tetrahedron Lett. 2002, 43, 7315–7317. doi:10.1016/S0040-4039(02)01747-1
Return to citation in text: [1] -
Perron, Q.; Praz, J.; Alexakis, A. Tetrahedron: Asymmetry 2009, 20, 1004–1007. doi:10.1016/j.tetasy.2009.02.027
Return to citation in text: [1] -
Fan, C.-A.; Ferber, B.; Kagan, H. B.; Lafon, O.; Lesot, P. Tetrahedron: Asymmetry 2008, 19, 2666–2677. doi:10.1016/j.tetasy.2008.12.003
Return to citation in text: [1] -
Perron, Q.; Alexakis, A. Adv. Synth. Catal. 2010, 352, 2611–2620. doi:10.1002/adsc.201000517
Return to citation in text: [1] -
Mettler, H.; Leroux, F.; Schlosser, M. Process for the preparation of asymmetrically substituted biaryldiphosphines. WO Pat. Appl. WO 2006002729 A1, Jan 12, 2006.
Return to citation in text: [1] -
Mettler, H.; Leroux, F.; Schlosser, M. Process for the preparation of asymmetrically substituted biaryldiphosphines. WO Pat. Appl. WO 2006002731 A1, Jan 12, 2006.
Return to citation in text: [1] -
Mettler, H.; Leroux, F.; Schlosser, M. Process for the preparation of asymmetrically substituted biaryldiphosphines. WO Pat. Appl. WO 2006002730 A1, Jan 12, 2006.
Return to citation in text: [1] -
Gorecka-Kobylinska, J.; Schlosser, M. J. Org. Chem. 2009, 74, 222–229. doi:10.1021/jo8020083
Return to citation in text: [1] -
CCDC 827188 (3a), CCDC 827186 (3b), CCDC 827187 (3d), CCDC 827189 (3f) and CCDC 827190 (6c) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
Return to citation in text: [1] [2] [3] -
Desponds, O.; Schlosser, M. J. Organomet. Chem. 1996, 507, 257–261. doi:10.1016/0022-328X(95)05767-J
Return to citation in text: [1]
| 69. | Perron, Q.; Alexakis, A. Adv. Synth. Catal. 2010, 352, 2611–2620. doi:10.1002/adsc.201000517 |
| 25. | Diemer, V.; Begaud, M.; Leroux, F. R.; Colobert, F. Eur. J. Org. Chem. 2011, 341–354. doi:10.1002/ejoc.201001283 |
| 31. | Leroux, F. R.; Bonnafoux, L.; Heiss, C.; Colobert, F.; Lanfranchi, D. A. Adv. Synth. Catal. 2007, 349, 2705–2713. doi:10.1002/adsc.200700211 |
| 36. | Leroux, F.; Schlosser, M. Angew. Chem., Int. Ed. 2002, 41, 4272–4274. doi:10.1002/1521-3773(20021115)41:22<4272::AID-ANIE4272>3.0.CO;2-B |
| 30. | Leroux, F. R.; Mettler, H. Adv. Synth. Catal. 2007, 349, 323–336. doi:10.1002/adsc.200600300 |
| 34. | Schlosser, M. Organoalkali Chemistry. In Organometallic in Synthesis: A Manual, 2nd ed.; Schlosser, M., Ed.; John Wiley and Sons: Chichester, UK, 2002; pp 1–352. |
| 35. | Leroux, F.; Schlosser, M.; Zohar, E.; Marek, I. The preparation of organolithium reagents and intermediates. In The Chemistry of Organolithium Compounds; Rappoport, Z.; Marek, I., Eds.; Wiley: Chichester, UK, 2004; Vol. 1, pp 435–493. |
| 51. | Leroux, F.; Nicod, N.; Bonnafoux, L.; Quissac, B.; Colobert, F. Lett. Org. Chem. 2006, 3, 165–169. |
| 52. | Leroux, F.; Mettler, H. Synlett 2006, 766–770. doi:10.1055/s-2006-933107 |
| 70. | Mettler, H.; Leroux, F.; Schlosser, M. Process for the preparation of asymmetrically substituted biaryldiphosphines. WO Pat. Appl. WO 2006002729 A1, Jan 12, 2006. |
| 71. | Mettler, H.; Leroux, F.; Schlosser, M. Process for the preparation of asymmetrically substituted biaryldiphosphines. WO Pat. Appl. WO 2006002731 A1, Jan 12, 2006. |
| 72. | Mettler, H.; Leroux, F.; Schlosser, M. Process for the preparation of asymmetrically substituted biaryldiphosphines. WO Pat. Appl. WO 2006002730 A1, Jan 12, 2006. |
| 1. | Patchett, A. A.; Nargund, R. P. Annu. Rep. Med. Chem. 2000, 35, 289–298. doi:10.1016/S0065-7743(00)35027-8 |
| 5. | Schmid, R.; Cereghetti, M.; Heiser, B.; Schönholzer, P.; Hansen, H.-J. Helv. Chim. Acta 1988, 71, 897–929. doi:10.1002/hlca.19880710427 |
| 25. | Diemer, V.; Begaud, M.; Leroux, F. R.; Colobert, F. Eur. J. Org. Chem. 2011, 341–354. doi:10.1002/ejoc.201001283 |
| 28. | Bonnafoux, L.; Colobert, F.; Leroux, F. R. Synlett 2010, 2953–2955. doi:10.1055/s-0030-1259025 |
| 31. | Leroux, F. R.; Bonnafoux, L.; Heiss, C.; Colobert, F.; Lanfranchi, D. A. Adv. Synth. Catal. 2007, 349, 2705–2713. doi:10.1002/adsc.200700211 |
| 36. | Leroux, F.; Schlosser, M. Angew. Chem., Int. Ed. 2002, 41, 4272–4274. doi:10.1002/1521-3773(20021115)41:22<4272::AID-ANIE4272>3.0.CO;2-B |
| 37. | Bonnafoux, L. Modular Synthesis of New C1-Biaryl Ligands and Application in Catalytic Hydrogenation and Coupling Reactions. Ph.D. Thesis, Université Louis Pasteur, Strasbourg, France, 2008. |
| 38. | Leroux, F. R.; Bonnafoux, F.; Colobert, F. Process for the synthesis of 2,2',6-tribromobiphenyl. WO Pat. Appl. WO 2008/037440 A1, April 3, 2008. |
| 30. | Leroux, F. R.; Mettler, H. Adv. Synth. Catal. 2007, 349, 323–336. doi:10.1002/adsc.200600300 |
| 4. | Zhang, X.; Mashima, K.; Koyano, K.; Sayo, N.; Kumobayashi, H.; Akutagawa, S.; Takaya, H. Tetrahedron Lett. 1991, 32, 7283–7286. doi:10.1016/0040-4039(91)80499-V |
| 39. | Clayden, J. Organolithiums: Selectivity for Synthesis; Pergamon: Amsterdam, The Netherlands, 2002. |
| 74. | CCDC 827188 (3a), CCDC 827186 (3b), CCDC 827187 (3d), CCDC 827189 (3f) and CCDC 827190 (6c) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. |
| 3. | Miyashita, A.; Yasuda, A.; Takaya, H.; Toriumi, K.; Ito, T.; Souchi, T.; Noyori, R. J. Am. Chem. Soc. 1980, 102, 7932–7934. doi:10.1021/ja00547a020 |
| 23. | Diemer, V.; Leroux, F. R.; Colobert, F. Eur. J. Org. Chem. 2011, 327–340. doi:10.1002/ejoc.201001217 |
| 24. | Diemer, V.; Garcia, J. S.; Leroux, F. R.; Colobert, F. J. Fluorine Chem. 2011, in press. doi:10.1016/j.jfluchem.2011.02.017 |
| 25. | Diemer, V.; Begaud, M.; Leroux, F. R.; Colobert, F. Eur. J. Org. Chem. 2011, 341–354. doi:10.1002/ejoc.201001283 |
| 26. | Bonnafoux, L.; Gramage-Doria, R.; Colobert, F.; Leroux, F. R. Chem.–Eur. J. 2011, in press. doi:10.1002/chem.201101529 |
| 27. | Bonnafoux, L.; Ernst, L.; Leroux, F. R.; Colobert, F. Eur. J. Inorg. Chem. 2011, 3387–3397. doi:10.1002/ejic.201100428 |
| 28. | Bonnafoux, L.; Colobert, F.; Leroux, F. R. Synlett 2010, 2953–2955. doi:10.1055/s-0030-1259025 |
| 29. | Colobert, F.; Valdivia, V.; Choppin, S.; Leroux, F. R.; Fernandez, I.; Alvarez, E.; Khiar, N. Org. Lett. 2009, 11, 5130–5133. doi:10.1021/ol9020755 |
| 30. | Leroux, F. R.; Mettler, H. Adv. Synth. Catal. 2007, 349, 323–336. doi:10.1002/adsc.200600300 |
| 31. | Leroux, F. R.; Bonnafoux, L.; Heiss, C.; Colobert, F.; Lanfranchi, D. A. Adv. Synth. Catal. 2007, 349, 2705–2713. doi:10.1002/adsc.200700211 |
| 32. | Leroux, F. R.; Simon, R.; Nicod, N. Lett. Org. Chem. 2006, 3, 948–954. doi:10.2174/157017806779467979 |
| 74. | CCDC 827188 (3a), CCDC 827186 (3b), CCDC 827187 (3d), CCDC 827189 (3f) and CCDC 827190 (6c) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. |
| 2. | Bringmann, G.; Breuning, M.; Tasler, S. Synthesis 1999, 525–558. doi:10.1055/s-1999-3435 |
| 33. | Schlosser, M. Angew. Chem., Int. Ed. 2005, 44, 376–393. doi:10.1002/anie.200300645 |
| 34. | Schlosser, M. Organoalkali Chemistry. In Organometallic in Synthesis: A Manual, 2nd ed.; Schlosser, M., Ed.; John Wiley and Sons: Chichester, UK, 2002; pp 1–352. |
| 35. | Leroux, F.; Schlosser, M.; Zohar, E.; Marek, I. The preparation of organolithium reagents and intermediates. In The Chemistry of Organolithium Compounds; Rappoport, Z.; Marek, I., Eds.; Wiley: Chichester, UK, 2004; Vol. 1, pp 435–493. |
| 30. | Leroux, F. R.; Mettler, H. Adv. Synth. Catal. 2007, 349, 323–336. doi:10.1002/adsc.200600300 |
| 11. | Pai, C.-C.; Li, Y.-M.; Zhou, Z.-Y.; Chan, A. S. C. Tetrahedron Lett. 2002, 43, 2789–2792. doi:10.1016/S0040-4039(02)00383-0 |
| 12. | Duprat de Paule, S.; Jeulin, S.; Ratovelomanana-Vidal, V.; Genêt, J.-P.; Champion, N.; Dellis, P. Tetrahedron Lett. 2003, 44, 823–826. doi:10.1016/S0040-4039(02)02637-0 |
| 14. | Jeulin, S.; Duprat de Paule, S.; Ratovelomanana-Vidal, V.; Genêt, J.-P.; Champion, N.; Dellis, P. Angew. Chem., Int. Ed. 2004, 43, 320–325. doi:10.1002/anie.200352453 |
| 15. | Leroux, F.; Gorecka, J.; Schlosser, M. Synthesis 2004, 326–328. doi:10.1055/s-2004-815926 |
| 16. | Mettler, H.; Leroux, F. Process for the preparation of (S)- or (R)-4-halo-3-hydroxybutyrates. WO Pat. Appl. WO 2005049545 A1, June 2, 2005. |
| 73. | Gorecka-Kobylinska, J.; Schlosser, M. J. Org. Chem. 2009, 74, 222–229. doi:10.1021/jo8020083 |
| 10. | Pai, C.-C.; Lin, C.-W.; Lin, C.-C.; Chen, C.-C.; Chan, A. S. C.; Wong, W. T. J. Am. Chem. Soc. 2000, 122, 11513–11514. doi:10.1021/ja000163n |
| 17. | Trost, B. M.; Verhoeven, T. R. 57 - Organopalladium Compounds in Organic Synthesis and in Catalysis. In Comprehensive Organometallic Chemistry; Wilkinson, G.; Stone, F. G. A.; Abel, E. W., Eds.; Pergamon: Oxford, UK, 1982; Vol. 8, pp 799–938. doi:10.1016/B978-008046518-0.00121-5 |
| 18. | Tsuji, J. Palladium Reagents and Catalysts; John Wiley and Sons: Chichester, UK, 1995. |
| 19. | Farina, V. 3.4 - Transition Metal Alkyl Complexes: Oxidative Addition and Transmetallation. In Transition Metal Organometallics in Organic Synthesis; Abel, E.; Stone, F. G. A.; Wilkinson, G., Eds.; Comprehensive Organometallic Chemistry II, Vol. 12; Pergamon: Oxford, UK, 1995; pp 161–240. |
| 20. | Diederich, F.; Stang, P. J. Metal-catalyzed Cross-coupling Reactions; Wiley-VCH: Weinheim, Germany, 1998. doi:10.1002/9783527612222 |
| 21. | Suzuki, A. J. Organomet. Chem. 1999, 576, 147–168. doi:10.1016/S0022-328X(98)01055-9 |
| 22. | Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457–2483. doi:10.1021/cr00039a007 |
| 74. | CCDC 827188 (3a), CCDC 827186 (3b), CCDC 827187 (3d), CCDC 827189 (3f) and CCDC 827190 (6c) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. |
| 7. | Saito, T.; Yokozawa, T.; Zhang, X.; Sayo, N. Chiral diphosphine compound, intermediate for preparing the same transition metal complex having the same diphosphine compound as ligand and asymmetric hydrogenation catalyst. Eur. Pat. EP 0850945 A1, July 1, 1998. |
| 8. | Sayo, N.; Saito, T.; Yokozawa, T. Ruthenium-phosphine complex and method for producing the same. Eur. Pat. EP 0945457 A2, Sept 29, 1999. |
| 9. | Saito, T.; Yokozawa, T.; Ishizaki, T.; Moroi, T.; Sayo, N.; Miura, T.; Kumobayashi, H. Adv. Synth. Catal. 2001, 343, 264–267. doi:10.1002/1615-4169(20010330)343:3<264::AID-ADSC264>3.0.CO;2-T |
| 28. | Bonnafoux, L.; Colobert, F.; Leroux, F. R. Synlett 2010, 2953–2955. doi:10.1055/s-0030-1259025 |
| 38. | Leroux, F. R.; Bonnafoux, F.; Colobert, F. Process for the synthesis of 2,2',6-tribromobiphenyl. WO Pat. Appl. WO 2008/037440 A1, April 3, 2008. |
| 6. | Schmid, R.; Foricher, J.; Cereghetti, M.; Schönholzer, P. Helv. Chim. Acta 1991, 74, 370–389. doi:10.1002/hlca.19910740215 |
| 13. | Zhang, Z.; Qian, H.; Longmire, J.; Zhang, X. J. Org. Chem. 2000, 65, 6223–6226. doi:10.1021/jo000462v |
| 28. | Bonnafoux, L.; Colobert, F.; Leroux, F. R. Synlett 2010, 2953–2955. doi:10.1055/s-0030-1259025 |
| 42. | Gilman, H.; Langham, W.; Jacoby, A. L. J. Am. Chem. Soc. 1939, 61, 106–109. doi:10.1021/ja01870a036 |
| 43. | Gilman, H.; Moore, F. W. J. Am. Chem. Soc. 1940, 62, 1843–1846. doi:10.1021/ja01864a058 |
| 44. | Langham, W.; Brewster, R. Q.; Gilman, H. J. Am. Chem. Soc. 1941, 63, 545–549. doi:10.1021/ja01847a053 |
| 40. | Marvel, C. S.; Hager, F. D.; Coffman, D. D. J. Am. Chem. Soc. 1927, 49, 2323–2328. doi:10.1021/ja01408a030 |
| 75. | Desponds, O.; Schlosser, M. J. Organomet. Chem. 1996, 507, 257–261. doi:10.1016/0022-328X(95)05767-J |
| 41. | Wittig, G.; Pockels, U.; Droge, H. Ber. Dtsch. Chem. Ges. 1938, 71B, 1903–1912. |
| 27. | Bonnafoux, L.; Ernst, L.; Leroux, F. R.; Colobert, F. Eur. J. Inorg. Chem. 2011, 3387–3397. doi:10.1002/ejic.201100428 |
| 67. | Perron, Q.; Praz, J.; Alexakis, A. Tetrahedron: Asymmetry 2009, 20, 1004–1007. doi:10.1016/j.tetasy.2009.02.027 |
| 68. | Fan, C.-A.; Ferber, B.; Kagan, H. B.; Lafon, O.; Lesot, P. Tetrahedron: Asymmetry 2008, 19, 2666–2677. doi:10.1016/j.tetasy.2008.12.003 |
| 64. | Gallou, F.; Haenggi, R.; Hirt, H.; Marterer, W.; Schaefer, F.; Seeger-Weibel, M. Tetrahedron Lett. 2008, 49, 5024–5027. doi:10.1016/j.tetlet.2008.06.046 |
| 65. | Choe, J.; Seo, J. H.; Kwon, Y.; Song, K. H. Chem. Eng. J. 2008, 135, S17–S20. doi:10.1016/j.cej.2007.07.015 |
| 66. | Kato, S.; Nonoyama, N.; Tomimoto, K.; Mase, T. Tetrahedron Lett. 2002, 43, 7315–7317. doi:10.1016/S0040-4039(02)01747-1 |
| 32. | Leroux, F. R.; Simon, R.; Nicod, N. Lett. Org. Chem. 2006, 3, 948–954. doi:10.2174/157017806779467979 |
| 51. | Leroux, F.; Nicod, N.; Bonnafoux, L.; Quissac, B.; Colobert, F. Lett. Org. Chem. 2006, 3, 165–169. |
| 53. | Shi, L.; Chu, Y.; Knochel, P.; Mayr, H. Org. Lett. 2009, 11, 3502–3505. doi:10.1021/ol9013393 |
| 54. | Shi, L.; Chu, Y.; Knochel, P.; Mayr, H. J. Org. Chem. 2009, 74, 2760–2764. doi:10.1021/jo802770h |
| 55. | Shi, L.; Chu, Y.; Knochel, P.; Mayr, H. Angew. Chem., Int. Ed. 2008, 47, 202–204. doi:10.1002/anie.200704100 |
| 56. | Krasovskiy, A.; Straub, B. F.; Knochel, P. Angew. Chem., Int. Ed. 2006, 45, 159–162. doi:10.1002/anie.200502220 |
| 57. | Krasovskiy, A.; Knochel, P. Angew. Chem., Int. Ed. 2004, 43, 3333–3336. doi:10.1002/anie.200454084 |
| 58. | Knochel, P.; Dohle, W.; Gommermann, N.; Kneisel, F. F.; Kopp, F.; Korn, T.; Sapountzis, I.; Vu, V. A. Angew. Chem., Int. Ed. 2003, 42, 4302–4320. doi:10.1002/anie.200300579 |
| 59. | Knochel, P.; Krasovskiy, A.; Sapountzis, I. Polyfunctional Magnesium Organometallics for Organic Synthesis. In Handbook of Functionalized Organometallics; Knochel, P., Ed.; Wiley-VCH: Weinheim, Germany, 2005; pp 109–172. doi:10.1002/9783527619467 |
| 60. | Boudier, A.; Bromm, L. O.; Lotz, M.; Knochel, P. Angew. Chem., Int. Ed. 2000, 39, 4414–4435. doi:10.1002/1521-3773(20001215)39:24<4414::AID-ANIE4414>3.0.CO;2-C |
| 61. | Abarbri, M.; Dehmel, F.; Knochel, P. Tetrahedron Lett. 1999, 40, 7449–7453. doi:10.1016/S0040-4039(99)01404-5 |
| 62. | Iida, T.; Wada, T.; Tomimoto, K.; Mase, T. Tetrahedron Lett. 2001, 42, 4841–4844. doi:10.1016/S0040-4039(01)00861-9 |
| 63. | Kondo, J.; Inoue, A.; Shinokubo, H.; Oshima, K. Angew. Chem., Int. Ed. 2001, 40, 2085–2087. doi:10.1002/1521-3773(20010601)40:11<2085::AID-ANIE2085>3.0.CO;2-K |
| 33. | Schlosser, M. Angew. Chem., Int. Ed. 2005, 44, 376–393. doi:10.1002/anie.200300645 |
| 30. | Leroux, F. R.; Mettler, H. Adv. Synth. Catal. 2007, 349, 323–336. doi:10.1002/adsc.200600300 |
| 32. | Leroux, F. R.; Simon, R.; Nicod, N. Lett. Org. Chem. 2006, 3, 948–954. doi:10.2174/157017806779467979 |
| 33. | Schlosser, M. Angew. Chem., Int. Ed. 2005, 44, 376–393. doi:10.1002/anie.200300645 |
| 45. | Jones, R. G.; Gilman, H. Chem. Rev. 1954, 54, 853–890. doi:10.1021/cr60171a004 |
| 46. | Bailey, W. F.; Patricia, J. J. J. Organomet. Chem. 1988, 352, 1–46. doi:10.1016/0022-328X(88)83017-1 |
| 47. | Beak, P.; Musick, T. J.; Chen, C. W. J. Am. Chem. Soc. 1988, 110, 3538–3542. doi:10.1021/ja00219a031 |
| 48. | Beak, P.; Allen, D. J. J. Am. Chem. Soc. 1992, 114, 3420–3425. doi:10.1021/ja00035a039 |
| 49. | Eisch, J. J. Organometallics 2002, 21, 5439–5463. doi:10.1021/om0109408 |
| 50. | Nájera, C.; Sansano, J. M.; Yus, M. Tetrahedron 2003, 59, 9255–9303. doi:10.1016/j.tet.2003.09.065 |
| 51. | Leroux, F.; Nicod, N.; Bonnafoux, L.; Quissac, B.; Colobert, F. Lett. Org. Chem. 2006, 3, 165–169. |
| 52. | Leroux, F.; Mettler, H. Synlett 2006, 766–770. doi:10.1055/s-2006-933107 |
| 53. | Shi, L.; Chu, Y.; Knochel, P.; Mayr, H. Org. Lett. 2009, 11, 3502–3505. doi:10.1021/ol9013393 |
| 54. | Shi, L.; Chu, Y.; Knochel, P.; Mayr, H. J. Org. Chem. 2009, 74, 2760–2764. doi:10.1021/jo802770h |
| 55. | Shi, L.; Chu, Y.; Knochel, P.; Mayr, H. Angew. Chem., Int. Ed. 2008, 47, 202–204. doi:10.1002/anie.200704100 |
© 2011 Bonnafoux et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)