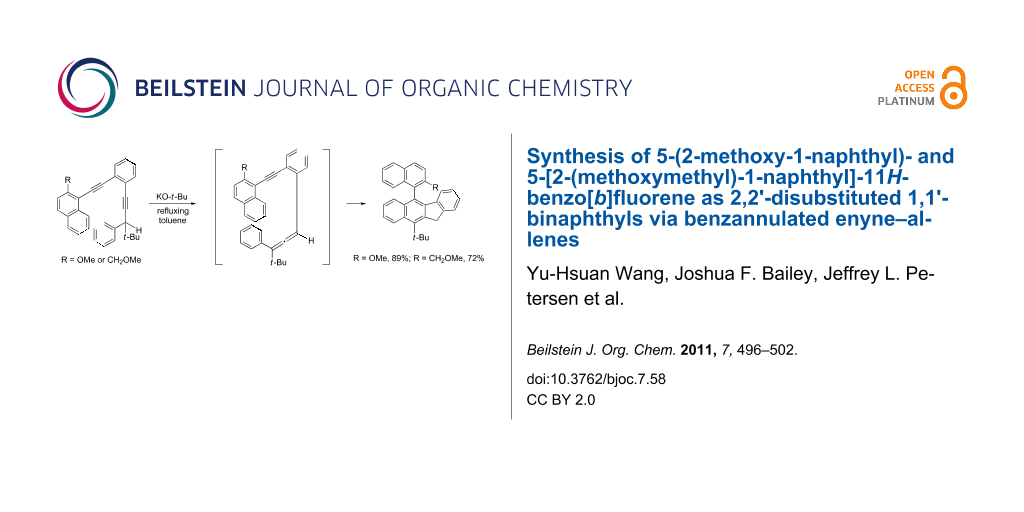
Abstract
5-(2-Methoxy-1-naphthyl)- and 5-[2-(methoxymethyl)-1-naphthyl]-11H-benzo[b]fluorene were synthesized by treatment of the corresponding benzannulated enediynes with potassium tert-butoxide in refluxing toluene to give benzannulated enyne–allenes for the subsequent Schmittel cascade cyclization reactions. The structures of these two 5-(1-naphthyl)-11H-benzo[b]fluorenes could be regarded as 2,2'-disubstituted 1,1'-binaphthyls with the newly constructed benzofluorenyl group serving as a naphthyl moiety.
Graphical Abstract
Introduction
Benzannulated enyne–allenes bearing an aryl substituent at the alkynyl terminus are excellent precursors of 5-aryl-11H-benzo[b]fluorenes [1-5]. Several synthetic pathways to the benzannulated enyne–allene systems have been reported, including generation in situ from the corresponding benzannulated enediynes. Specifically, treatment of the benzannulated enediyne 1a with potassium tert-butoxide in refluxing toluene for six hours promoted a 1,3-prototropic rearrangement to produce, in situ, the benzannulated enyne–allene 2a, which in turn underwent a sequence of Schmittel cascade cyclization reactions to form 5-phenyl-11H-benzo[b]fluorene (3a) in a single operation (Scheme 1) [5]. It is interesting to note that the newly formed benzo[b]fluorenyl moiety in 3a could also be regarded as a 1-arylnaphthyl derivative with three additional substituents at the 2, 3, and 4 positions.
Scheme 1: Synthesis of 5-aryl-11H-benzo[b]fluorenes via benzannulated enyne–allenes.
Scheme 1: Synthesis of 5-aryl-11H-benzo[b]fluorenes via benzannulated enyne–allenes.
The reaction is not sensitive to the steric requirement of the substituent at the alkynyl terminus. The benzannulated enediyne 1b with a sterically demanding 2,6-dibromophenyl substituent was also smoothly converted to 3b [6]. With 1c having a 1,1'-binaphthyl substituent, a 1:1 mixture of the syn and the anti atropisomers of 3c was likewise obtained (Scheme 2) [7].
Scheme 2: Synthesis of 1,1'-binaphthyl-substituted 11H-benzo[b]fluorene 3c.
Scheme 2: Synthesis of 1,1'-binaphthyl-substituted 11H-benzo[b]fluorene 3c.
We now have successfully extended the cascade sequence to the synthesis of other sterically congested analogues with an ortho-methoxy or an ortho-methoxymethyl group on the phenyl substituent. In addition, by placing a 2-methoxy-1-naphthyl or a 2-(methoxymethyl)-1-naphthyl group at one of the alkynyl termini of the benzannulated enediyne system, the resulting naphthyl-substituted benzo[b]fluorenes could be regarded as 2,2'-disubstituted 1,1'-binaphthyls with two additional substituents at the 3 and 4 positions. The versatility of 1,1'-binaphthyl-2,2'-diol (BINOL) and BINOL derivatives as reagents in asymmetric synthesis has stimulated the development of new synthetic methods for 2,2'-disubstituted 1,1'-binaphthyls [8-16]. However, the great majority of the reported methods involved coupling of two properly substituted 1-naphthyl derivatives. Construction of a new 1-naphthyl ring as an essential step toward 1,1'-binaphthyls is rare [17-19].
Results and Discussion
The Sonogashira reaction between 1-ethynyl-2-methoxybenzene (4a) and 1-iodo-2-[2-(trimethylsilyl)ethynyl]benzene produced 5a, which was desilylated to give 6a (Scheme 3). Condensation between 6a and pivalophenone (7) then furnished the benzannulated enediynyl alcohol 8a. Subsequent reduction with triethylsilane in the presence of trifluoroacetic acid afforded the benzannulated enediyne 9a. Similarly, the benzannulated enediyne 9b was synthesized from 1-ethynyl-2-(methoxymethyl)benzene (4b). On exposure to potassium tert-butoxide in refluxing toluene for five hours, 9a was transformed to 5-(2-methoxyphenyl)-11H-benzo[b]fluorene 13a along with a small amount (ca. 2%) of 14a in a single operation. Presumably, the cascade sequence involved an initial 1,3-protropic rearrangement to form the corresponding benzannulated enyne–allene 10a. A Schmittel cyclization reaction [1-4] generates biradical 11a, which then undergoes an intramolecular radical–radical coupling to afford 12a. This is followed by a second prototropic rearrangement to restore the aromaticity to furnish 13a as proposed previously [5]. An intramolecular [2 + 2] cycloaddition reaction of 10a or a direct radical–radical coupling of 11a could account for the formation of 14a [5]. From 9b, 5-[2-(methoxymethyl)phenyl]-11H-benzo[b]fluorene 13b and the [2 + 2] cycloaddition adduct 14b were produced in a 5:1 ratio. The presence of the carbon–carbon double bonds in 14b allows easy removal of 14b by treatment of the resulting mixture with BH3·THF followed by silica gel column chromatography. The presence of a benzofluorenyl moiety and a methoxy or a methoxymethyl group in 13a and 13b could allow these compounds to serve as hetero-bidentate ligands for complex formation with transition metals [20].
Scheme 3: Synthesis of 5-(2-methoxyphenyl)- and 5-[2-(methoxymethyl)phenyl]-11H-benzo[b]fluorene 13a and 13b.
Scheme 3: Synthesis of 5-(2-methoxyphenyl)- and 5-[2-(methoxymethyl)phenyl]-11H-benzo[b]fluorene 13a and 13b.
We also investigated the possibility of using the benzannulated enediynes bearing a 1-naphthyl, a 2-methoxy-1-naphthyl, or a 2-(methoxymethyl)-1-naphthyl substituent at one of the alkynyl termini for the cascade cyclization reaction. Using a slightly different synthetic sequence from that shown in Scheme 3 but reminiscent of a sequence developed for the synthesis of 4,5-diheteroarylphenanthrenes [21], the benzannulated enediynes 19a and 19b were obtained (Scheme 4). It was gratifying to observe that on treatment with potassium tert-butoxide, 19a and 19b were smoothly converted to 20a and 20b, respectively. Similarly 20c was obtained via the synthetic sequence outlined in Scheme 5. It is worth noting that the synthetic sequences outlined in Scheme 4 and Scheme 5 represent new routes to 2,2'-disubstituted 1,1'-binaphthyls with the newly constructed benzofluorenyl serving as one of the naphthyl groups.
Scheme 4: Synthesis of 5-(1-naphthyl)- and 5-(2-methoxy-1-naphthyl)-11H-benzo[b]fluorene 20a and 20b.
Scheme 4: Synthesis of 5-(1-naphthyl)- and 5-(2-methoxy-1-naphthyl)-11H-benzo[b]fluorene 20a and 20b.
Scheme 5: Synthesis of 5-[2-(methoxymethyl)-1-naphthyl]-11H-benzo[b]fluorene 20c.
Scheme 5: Synthesis of 5-[2-(methoxymethyl)-1-naphthyl]-11H-benzo[b]fluorene 20c.
The 1H NMR spectrum of 13a in C6D6 recorded on a 600 MHz NMR spectrometer exhibited an AB system with signals at δ 4.21 (J = 21.0 Hz) and 4.13 (J = 21.0 Hz), attributable to the methylene hydrogens on the five-membered ring. AB systems from the methylene hydrogens were also observed in other similar 11H-benzo[b]fluorenyl structures [7,22-24]. The AB pattern remained unchanged at 70 °C, suggesting a relatively slow rate of rotation, on the NMR time scale, around the carbon–carbon single bond connecting the 2-methoxyphenyl substituent to the C5 of the benzofluorenyl moiety. The rotational barrier was calculated to be at least 16.7 kcal/mol at 70 °C on the basis of the lack of coalescence of signals at this temperature. This lowest possible rotational barrier is significantly higher than that of 1-phenylnaphthalene, which was calculated by MM2' to be 12.4 kcal/mol [25,26]. Similarly, the 1H NMR spectrum of 13b taken in CDCl3 showed a clear AB system at δ 4.07 (J = 13.8 Hz) and 4.04 (J = 13.8 Hz), attributable to the methylene hydrogens on the carbon attached to the methoxy group. The signals of the methylene hydrogens on the five-membered ring could barely be discerned as an AB system with the two inner signals overlapped at δ 4.51 and two small outer signals at δ 4.55 and 4.47.
The rotational barrier of the parent 1,1'-binaphthyl in N,N-dimethylformamide was determined to be 23.5 kcal/mol at 50 °C, corresponding to a half-life of 14.5 minutes for racemization [27,28]. Because the structure of 20a could be regarded as a 2,3,4-trisubstituted 1,1'-binaphthyl, the rate of rotation can be expected to be even slower. Again, in C6D6 recorded on a 600 MHz NMR spectrometer, the signals of the methylene hydrogens on the five-membered ring could be discerned as an AB system at δ 4.27 (J = 21 Hz) and 4.25 (J = 21 Hz).
The rotational barrier of BINOL as a member of the 2,2'-disubstituted 1,1'-binaphthyls was determined to be 37.2 kcal/mol at 195 °C in naphthalene, corresponding to a half-life of 4.5 hours for racemization [28]. The high stability of the configuration even at such an elevated temperature allows BINOL to be used in a variety of synthetic applications. The configurational stability of 20b and 20c, which could be regarded as 2,2'-disubstituted 1,1'-binaphthyls with two additional substituents at the 3 and 4 positions, could also be expected to be high. AB patterns were observed for the methylene hydrogens on the five-membered ring of 20b and on the carbon bearing the methoxy group of 20c.
Treatment of 20b with boron tribromide converted the methoxy group to the hydroxy group, providing a handle for resolution of 24 with (1S)-(−)-camphanoyl chloride (Scheme 6) [29]. It was possible to achieve partial separation of a small fraction of the two diastereomeric (1S)-camphanates in a 5:1 ratio by silica gel column chromatography.
Scheme 6: Demethylation of 22b to form 5-(2-hydroxy-1-naphthyl)-11H-benzo[b]fluorene 24.
Scheme 6: Demethylation of 22b to form 5-(2-hydroxy-1-naphthyl)-11H-benzo[b]fluorene 24.
Conclusion
In conclusion, the use of benzannulated enediynes as precursors to 2,2'-disubstituted 1,1'-binaphthyls represents a new synthetic approach to these sterically hindered molecules. The assembly of the enediynyl precursors from three separate aromatic fragments allows the possibility of placing a variety of functional groups at various positions of the 1,1'-binaphthyl system. Transformation of the methoxy group in 20b to a hydroxy group provides a handle for resolution with optically active reagents.
Experimental
General information
All organometallic reactions were conducted in oven-dried (120 °C) glassware under a nitrogen atmosphere. Diethyl ether and tetrahydrofuran (THF) were distilled from benzophenone ketyl prior to use. 1-Ethynyl-2-methoxybenzene (4a), pivalophenone (7), n-butyllithium (1.6 M) in hexanes, 1-bromo-2-[2-(trimethylsilyl)ethynyl]benzene, triethylsilane, trifluoroacetic acid, potassium tert-butoxide (1.0 M) in 2-methyl-2-propanol, lithium diisopropylamide (LDA, 2.0 M) in heptane/THF/ethylbenzene, Pd(PPh3)2Cl2, Pd(PPh3)4, copper(I) iodide, triethylamine, 1.0 M BH3·THF solution in THF, 1-ethynylnaphthalene (18a), 2-methoxy-1-naphthaldehyde, 1-bromo-2-(methoxymethyl)naphthalene, (trimethylsilyl)acetylene, boron tribromide, and (1S)-(−)-camphanoyl chloride were purchased from chemical suppliers and were used as received. 1-Iodo-2-[2-(trimethylsilyl)ethynyl]benzene was prepared by treatment of 1-bromo-2-[2-(trimethylsilyl)ethynyl]benzene in THF with n-butyllithium at −78 °C followed by treatment with iodine [7]. 1-Ethynyl-2-iodo-benzene (15) [30] was prepared in quantitative yield by desilylation of 1-iodo-2-[2-(trimethylsilyl)ethynyl]benzene with sodium hydroxide in methanol. 1-Ethynyl-2-(methoxymethyl)benzene (4b) [31] and dimethyl (1-diazo-2-oxopropyl)phosphonate [32] were prepared according to the reported procedures.
1-Iodo-2-(4,4-dimethyl-3-phenyl-1-pentynyl)benzene (17). To 1.032 g (4.528 mmol) of 15 in 10 mL THF under a nitrogen atmosphere at 0 °C, was added 3.77 mL of a 2.0 M solution of LDA (7.55 mmol) in THF. After stirring for 30 min, a solution of 0.616 g of 7 (3.774 mmol) in 10 mL of THF was introduced via cannula, and the reaction mixture allowed to warm to room temperature. After an additional 3 h, 20 mL of water was introduced, and the reaction mixture extracted with diethyl ether. The combined organic extracts were washed successively with brine and water, dried over sodium sulfate, and concentrated. The residue was purified by flash chromatography (silica gel/10% diethyl ether in hexanes) to afford 1.476 g of crude 16 as a light yellow liquid. Crude 16 without any further purification was treated with 0.810 g of triethylsilane (6.983 mmol) and 2.1 g of trifluoroacetic acid (18.4 mmol) to afford 1.382 g (3.699 mmol, 86% for 2 steps) of 17 as a colorless liquid: IR 2966, 1463 cm−1; 1H NMR (CDCl3, 270 MHz) δ 7.83 (1H, dd, J = 7.9, 1.2 Hz), 7.47–7.41 (3H, m), 7.36–7.23 (4H, m), 6.96 (1H, td, J = 7.7, 1.7 Hz), 3.69 (1H, s), 1.09 (9H, s); 13C NMR (CDCl3, 67.9 MHz) δ 138.9, 138.6, 132.9, 130.5, 129.9, 128.8, 127.6, 126.7, 100.5, 95.5, 85.5, 50.6, 35.7, 27.9.
Benzannulated enediyne 19b. To a mixture of 0.307 g of 17 (0.822 mmol), Pd(PPh3)4 (0.040 g, 0.035 mmol), and copper(I) iodide (0.015 g, 0.080 mmol) in 10 mL of toluene, was added via cannula a solution of 0.150 g of 18b (0.824 mmol) in 5 mL of triethylamine. After stirring at 120 °C for 12 h, 15 mL of a saturated ammonium chloride solution and 15 mL of diethyl ether were added. The organic layer was separated and the aqueous layer back extracted with diethyl ether. The combined organic layers were washed successively with brine and water, dried over sodium sulfate, and concentrated. Purification of the residue by flash column chromatography (silica gel/30% methylene chloride in hexanes) afforded 0.331 g of 19b (0.773 mmol, 94%) as a colorless liquid: IR 2207, 1271, 1078 cm−1; 1H NMR (CDCl3, 270 MHz) δ 8.43–8.39 (1H, m), 7.85 (1H, d, J = 9.2 Hz), 7.82–7.77 (1H, m), 7.70–7.65 (1H, m), 7.56–7.52 (1H, m), 7.43–7.25 (7H, m), 7.10–7.00 (3H, m), 4.00 (3H, s), 3.66 (1H, s), 0.95 (9H, s); 13C NMR (CDCl3, 67.9 MHz) δ 158.9, 139.0, 134.5, 132.3, 130.2, 129.7, 128.4, 127.9, 127.8, 127.3, 127.2, 126.4, 126.3, 126.0, 125.7, 124.1, 112.6, 106.5, 97.8, 95.5, 87.3, 82.6, 56.6, 50.5, 35.5, 27.7; HRMS m/z [M + H]+ calcd for C32H29O, 429.2218; found, 429.2217.
Benzannulated enediyne 19c. To a mixture of 0.242 g of 23 (0.812 mmol), Pd(PPh3)2Cl2 (0.030 g, 0.043 mmol), and copper(I) iodide (0.015 g, 0.080 mmol) in 6 mL of triethylamine, was added via cannula a solution of 0.265 g of 22 (0.974 mmol) in 2 mL of triethylamine. After stirring at 60 °C for 12 h, 15 mL of a saturated ammonium chloride solution and 15 mL of diethyl ether were added. The organic layer was separated and the aqueous layer back extracted with diethyl ether. The combined organic layers were washed successively with brine and water, dried over sodium sulfate, and concentrated. Purification of the residue by flash column chromatography (silica gel/5% methylene chloride in hexanes) afforded 0.255 g of 19c (0.577 mmol, 71%) as a colorless liquid: 1H NMR (CDCl3, 270 MHz) δ 8.51 (1H, d, J = 7.7 Hz), 7.88–7.83 (2H, m), 7.67–7.57 (3H, m), 7.53–7.42 (2H, m), 7.38–7.32 (4H, m), 7.12–7.05 (3H, m), 4.92 (1H, d, J = 13.1 Hz), 4.83 (1H, d, J = 12.9 Hz), 3.69 (2H, s), 3.41 (3H, s), 0.97 (9H, s); 13C NMR (CDCl3, 67.9 MHz) δ 139.1, 138.9, 133.2, 132.5, 132.1, 129.6, 128.7, 128.1, 128.0, 127.4, 126.8, 126.6, 126.3, 126.2, 125.5, 125.0, 119.0, 98.3, 96.0, 88.4, 82.7, 72.8, 58.3, 50.5, 35.5, 27.7.
5-(2-Methoxy-1-naphthyl)-10-(1,1-dimethylethyl)-11H-benzo[b]fluorene (20b). To 0.295 g of 19b (0.689 mmol) in 10 mL of anhydrous toluene under a nitrogen atmosphere, was added 0.77 mL of a 1.0 M solution of potassium tert-butoxide (0.77 mmol) in 2-methyl-2-propanol. The reaction mixture was then heated under reflux for 6 h. After the reaction mixture was allowed to cool to room temperature, 10 mL of water and 40 mL of methylene chloride were introduced. The organic layer was separated, dried over sodium sulfate and concentrated. The residue was purified by flash column chromatography (silica gel/5% methylene chloride in hexanes) to provide 0.263 g of 20b (0.614 mmol, 89%) as a light yellow liquid: IR 1267, 1250, 766 cm−1; 1H NMR (CDCl3, 600 MHz) δ 8.66 (1H, d, J = 9.0 Hz), 8.11 (1H, d, J = 9.6 Hz), 7.92 (1H, d, J = 8.4 Hz), 7.53 (1H, d, J = 9.0 Hz), 7.44 (1H, d, J = 7.2 Hz), 7.40 (1H, ddd, J = 8.4, 6.6, 1.8 Hz), 7.35 (1H, d, J = 9.0 Hz), 7.31 (1H, td, J = 6.6, 1.2 Hz), 7.18 (1H, t, J = 7.8 Hz), 7.13–7.05 (3H, m), 6.77 (1H, t, J = 7.8 Hz), 6.08 (1H, d, J = 7.8 Hz), 4.55 (2H, s), 3.68 (3H, s), 1.97 (9H, s); 1H NMR (C6D6, 600 MHz) δ 8.67 (1H, d, J = 9.0 Hz), 7.90 (1H, d, J = 9.6 Hz), 7.79 (1H, d, J = 8.4 Hz), 7.70 (1H, d, J = 8.4 Hz), 7.42 (1H, d, J = 8.4 Hz), 7.29 (1H, t, J = 7.8 Hz), 7.23 (1H, d, J = 7.2 Hz), 7.17 (1H, d, J = 6.6 Hz), 7.12 (1H, t, J = 7.5 Hz), 7.06 (1H, t, J = 7.5 Hz), 6.98 (1H, t, J = 7.2 Hz), 6.89 (1H, t, J = 7.8 Hz), 6.72 (1H, t, J = 7.5 Hz), 6.53 (1H, d, J = 7.8 Hz), 4.25 (1H, d, J = 21.6 Hz), 4.19 (1H, d, J = 21.6 Hz), 3.13 (3H, s), 1.77 (9H, s); 13C NMR (CDCl3, 150 MHz) δ 154.8, 144.2, 141.0, 140.3, 139.3, 137.9, 134.7, 133.9, 131.8, 129.8, 129.5, 128.1, 127.8, 127.2, 127.0, 126.7, 126.6, 126.4, 125.2, 124.1, 123.9, 123.7, 123.3, 122.8, 122.2, 114.4, 56.9, 40.3, 38.9, 34.5; MS m/z 428 (M+), 413, 400, 371; HRMS m/z calcd for C32H28O, 428.2140; found, 428.2126.
Recrystallization from a mixture of isopropyl alcohol and methylene chloride produced a crystal for X-ray structure analysis. Although the weakly diffracting crystal limited the amount of observed data, the analysis of these data supports the structural assignment of 20b.
5-[2-(Methoxymethyl)-1-naphthyl]-10-(1,1-dimethylethyl)-11H-benzo[b]fluorene (20c). The same procedure as described for 20b was repeated except that 0.142 g of 19c (0.321 mmol) was treated with 0.48 mL of a 1.0 M solution of potassium tert-butoxide (0.48 mmol) in 2-methyl-2-propanol to afford 0.102 g of 20c (0.231 mmol, 72%) as a light yellow liquid: IR 2943, 1273, 1248, 774 cm−1; 1H NMR (CDCl3, 270 MHz) δ 8.67 (1H, d, J = 8.9 Hz), 8.12 (1H, d, J = 8.7 Hz), 7.97 (1H, d, J = 8.2 Hz), 7.91 (1H, d, J = 8.6 Hz), 7.47–7.39 (3H, m), 7.28–7.08 (5H, m), 6.75 (1H, t, J = 7.7 Hz), 5.89 (1H, d, J = 7.9 Hz), 4.56 (2H, s), 4.16 (1H, d, J = 13.4 Hz), 4.10 (1H, d, J = 13.3 Hz), 3.09 (3H, s), 1.98 (9H, s); 13C NMR (CDCl3, 67.9 MHz) δ 144.1, 141.5, 139.7, 139.2, 137.7, 135.3, 134.3, 134.0, 133.2, 132.7, 131.6, 128.4, 128.1, 127.9, 126.95, 126.91, 126.6, 126.4, 126.0, 125.9, 124.9, 124.5, 123.8, 123.6, 122.9, 71.9, 58.4, 40.3, 39.0, 34.5; MS m/z 442 (M+), 427, 395; HRMS m/z calcd for C33H30O, 442.2297; found, 442.2283.
Supporting Information
| Supporting Information File 1: Experimental procedures, spectroscopic data, and 1H and/or 13C NMR spectra of 5a,b, 6a,b, 8a,b, 9a,b, 13a,b, 17, 18b, 19a–c, 20a–c, 21–24, and the (1S)-camphanates of 24. | ||
| Format: PDF | Size: 5.6 MB | Download |
Acknowledgements
K.K.W. thanks the National Science Foundation (Grant No. CHE-0909613) for financial support. J.L.P. acknowledges the support (Grant No. CHE-9120098) provided by the National Science Foundation for the acquisition of a Siemens P4 X-ray diffractometer. The financial support of the NSF-EPSCoR (Grant No.1002165R) for the purchase of a 600 MHz NMR spectrometer is also gratefully acknowledged.
References
-
Schmittel, M.; Strittmatter, M.; Vollmann, K.; Kiau, S. Tetrahedron Lett. 1996, 37, 999–1002. doi:10.1016/0040-4039(95)02369-0
Return to citation in text: [1] [2] -
Schmittel, M.; Strittmatter, M.; Kiau, S. Angew. Chem., Int. Ed. Engl. 1996, 35, 1843–1845. doi:10.1002/anie.199618431
Return to citation in text: [1] [2] -
Schmittel, M.; Vavilala, C. J. Org. Chem. 2005, 70, 4865–4868. doi:10.1021/jo0504971
Return to citation in text: [1] [2] -
Wang, K. K. Enyne–Allenes. In Modern Allene Chemistry; Krause, N.; Hashmi, A. S. K., Eds.; Wiley-VCH: Weinheim, Germany, 2004; Vol. 2, pp 1091–1126.
Return to citation in text: [1] [2] -
Li, H.; Zhang, H.-R.; Petersen, J. L.; Wang, K. K. J. Org. Chem. 2001, 66, 6662–6668. doi:10.1021/jo0104577
Return to citation in text: [1] [2] [3] [4] -
Kim, D.; Petersen, J. L.; Wang, K. K. Org. Lett. 2006, 8, 2313–2316. doi:10.1021/ol0605676
Return to citation in text: [1] -
Yang, H.; Petersen, J. L.; Wang, K. K. Tetrahedron 2006, 62, 8133–8141. doi:10.1016/j.tet.2006.06.004
Return to citation in text: [1] [2] [3] -
Brunel, J. M. Chem. Rev. 2005, 105, 857–898. doi:10.1021/cr040079g
Return to citation in text: [1] -
Noyori, R.; Tomino, I.; Tanimoto, Y. J. Am. Chem. Soc. 1979, 101, 3129–3131. doi:10.1021/ja00505a056
Return to citation in text: [1] -
Noyori, R.; Tomino, I.; Nishizawa, M. J. Am. Chem. Soc. 1979, 101, 5843–5844. doi:10.1021/ja00513a072
Return to citation in text: [1] -
Nishizawa, M.; Noyori, R. Tetrahedron Lett. 1980, 21, 2821–2824. doi:10.1016/S0040-4039(00)78616-3
Return to citation in text: [1] -
Noyori, R. Chem. Soc. Rev. 1989, 18, 187–208. doi:10.1039/CS9891800187
Return to citation in text: [1] -
Chen, Y.; Yekta, S.; Yudin, A. K. Chem. Rev. 2003, 103, 3155–3212. doi:10.1021/cr020025b
Return to citation in text: [1] -
Pu, L. Chem. Rev. 1998, 98, 2405–2494. doi:10.1021/cr970463w
Return to citation in text: [1] -
Kočovský, P.; Vyskočil, Š.; Smrčina, M. Chem. Rev. 2003, 103, 3213–3246. doi:10.1021/cr9900230
Return to citation in text: [1] -
Berthod, M.; Mignani, G.; Woodward, G.; Lemaire, M. Chem. Rev. 2005, 105, 1801–1836. doi:10.1021/cr040652w
Return to citation in text: [1] -
Einhorn, C.; Einhorn, J.; Marcadal-Abbadi, C.; Pierre, J.-L. J. Org. Chem. 1999, 64, 4542–4546. doi:10.1021/jo982527o
Return to citation in text: [1] -
Dufková, L.; Kotora, M.; Císařová, I. Eur. J. Org. Chem. 2005, 2491–2499. doi:10.1002/ejoc.200400881
Return to citation in text: [1] -
Hapke, M.; Kral, K.; Fischer, C.; Spannenberg, A.; Gutnov, A.; Redkin, D.; Heller, B. J. Org. Chem. 2010, 75, 3993–4003. doi:10.1021/jo100122d
Return to citation in text: [1] -
Alt, H. G.; Köppl, A. Chem. Rev. 2000, 100, 1205–1222. doi:10.1021/cr9804700
Return to citation in text: [1] -
Wen, B.; Petersen, J. L.; Wang, K. K. Chem. Commun. 2010, 46, 1938–1940. doi:10.1039/b921667g
Return to citation in text: [1] -
Li, H.; Petersen, J. L.; Wang, K. K. J. Org. Chem. 2001, 66, 7804–7810. doi:10.1021/jo010687l
Return to citation in text: [1] -
Dai, W.; Petersen, J. L.; Wang, K. K. Org. Lett. 2004, 6, 4355–4357. doi:10.1021/ol0481434
Return to citation in text: [1] -
Yang, H.; Petersen, J. L.; Wang, K. K. Tetrahedron 2006, 62, 1231–1238. doi:10.1016/j.tet.2005.10.062
Return to citation in text: [1] -
Tsuzuki, S.; Tanabe, K.; Nagawa, Y.; Nakanishi, H. J. Mol. Struct. 1990, 216, 279–295. doi:10.1016/0022-2860(90)80335-H
Return to citation in text: [1] -
Nori-shargh, D.; Asadzadeh, S.; Ghanizadeh, F.-R.; Deyhimi, F.; Amini, M. M.; Jameh-Bozorghi, S. J. Mol. Struct.: THEOCHEM 2005, 717, 41–51. doi:10.1016/j.theochem.2004.11.022
Return to citation in text: [1] -
Cooke, A. S.; Harris, M. M. J. Chem. Soc. 1963, 2365–2373. doi:10.1039/JR9630002365
Return to citation in text: [1] -
Meca, L.; Řeha, D.; Havlas, Z. J. Org. Chem. 2003, 68, 5677–5680. doi:10.1021/jo034344u
Return to citation in text: [1] [2] -
Thongpanchang, T.; Paruch, K.; Katz, T. J.; Rheingold, A. L.; Lam, K.-C.; Liable-Sands, L. J. Org. Chem. 2000, 65, 1850–1856. doi:10.1021/jo9919411
Return to citation in text: [1] -
Li, Y.; Zhang, J.; Wang, W.; Miao, Q.; She, X.; Pan, X. J. Org. Chem. 2005, 70, 3285–3287. doi:10.1021/jo047836v
Return to citation in text: [1] -
Li, H.; Yang, H.; Petersen, J. L.; Wang, K. K. J. Org. Chem. 2004, 69, 4500–4508. doi:10.1021/jo049716t
Return to citation in text: [1] -
Müller, S.; Liepold, B.; Roth, G. J.; Bestmann, H. J. Synlett 1996, 521–522. doi:10.1055/s-1996-5474
Return to citation in text: [1]
| 31. | Li, H.; Yang, H.; Petersen, J. L.; Wang, K. K. J. Org. Chem. 2004, 69, 4500–4508. doi:10.1021/jo049716t |
| 7. | Yang, H.; Petersen, J. L.; Wang, K. K. Tetrahedron 2006, 62, 8133–8141. doi:10.1016/j.tet.2006.06.004 |
| 30. | Li, Y.; Zhang, J.; Wang, W.; Miao, Q.; She, X.; Pan, X. J. Org. Chem. 2005, 70, 3285–3287. doi:10.1021/jo047836v |
| 1. | Schmittel, M.; Strittmatter, M.; Vollmann, K.; Kiau, S. Tetrahedron Lett. 1996, 37, 999–1002. doi:10.1016/0040-4039(95)02369-0 |
| 2. | Schmittel, M.; Strittmatter, M.; Kiau, S. Angew. Chem., Int. Ed. Engl. 1996, 35, 1843–1845. doi:10.1002/anie.199618431 |
| 3. | Schmittel, M.; Vavilala, C. J. Org. Chem. 2005, 70, 4865–4868. doi:10.1021/jo0504971 |
| 4. | Wang, K. K. Enyne–Allenes. In Modern Allene Chemistry; Krause, N.; Hashmi, A. S. K., Eds.; Wiley-VCH: Weinheim, Germany, 2004; Vol. 2, pp 1091–1126. |
| 5. | Li, H.; Zhang, H.-R.; Petersen, J. L.; Wang, K. K. J. Org. Chem. 2001, 66, 6662–6668. doi:10.1021/jo0104577 |
| 8. | Brunel, J. M. Chem. Rev. 2005, 105, 857–898. doi:10.1021/cr040079g |
| 9. | Noyori, R.; Tomino, I.; Tanimoto, Y. J. Am. Chem. Soc. 1979, 101, 3129–3131. doi:10.1021/ja00505a056 |
| 10. | Noyori, R.; Tomino, I.; Nishizawa, M. J. Am. Chem. Soc. 1979, 101, 5843–5844. doi:10.1021/ja00513a072 |
| 11. | Nishizawa, M.; Noyori, R. Tetrahedron Lett. 1980, 21, 2821–2824. doi:10.1016/S0040-4039(00)78616-3 |
| 12. | Noyori, R. Chem. Soc. Rev. 1989, 18, 187–208. doi:10.1039/CS9891800187 |
| 13. | Chen, Y.; Yekta, S.; Yudin, A. K. Chem. Rev. 2003, 103, 3155–3212. doi:10.1021/cr020025b |
| 14. | Pu, L. Chem. Rev. 1998, 98, 2405–2494. doi:10.1021/cr970463w |
| 15. | Kočovský, P.; Vyskočil, Š.; Smrčina, M. Chem. Rev. 2003, 103, 3213–3246. doi:10.1021/cr9900230 |
| 16. | Berthod, M.; Mignani, G.; Woodward, G.; Lemaire, M. Chem. Rev. 2005, 105, 1801–1836. doi:10.1021/cr040652w |
| 28. | Meca, L.; Řeha, D.; Havlas, Z. J. Org. Chem. 2003, 68, 5677–5680. doi:10.1021/jo034344u |
| 7. | Yang, H.; Petersen, J. L.; Wang, K. K. Tetrahedron 2006, 62, 8133–8141. doi:10.1016/j.tet.2006.06.004 |
| 29. | Thongpanchang, T.; Paruch, K.; Katz, T. J.; Rheingold, A. L.; Lam, K.-C.; Liable-Sands, L. J. Org. Chem. 2000, 65, 1850–1856. doi:10.1021/jo9919411 |
| 6. | Kim, D.; Petersen, J. L.; Wang, K. K. Org. Lett. 2006, 8, 2313–2316. doi:10.1021/ol0605676 |
| 25. | Tsuzuki, S.; Tanabe, K.; Nagawa, Y.; Nakanishi, H. J. Mol. Struct. 1990, 216, 279–295. doi:10.1016/0022-2860(90)80335-H |
| 26. | Nori-shargh, D.; Asadzadeh, S.; Ghanizadeh, F.-R.; Deyhimi, F.; Amini, M. M.; Jameh-Bozorghi, S. J. Mol. Struct.: THEOCHEM 2005, 717, 41–51. doi:10.1016/j.theochem.2004.11.022 |
| 5. | Li, H.; Zhang, H.-R.; Petersen, J. L.; Wang, K. K. J. Org. Chem. 2001, 66, 6662–6668. doi:10.1021/jo0104577 |
| 27. | Cooke, A. S.; Harris, M. M. J. Chem. Soc. 1963, 2365–2373. doi:10.1039/JR9630002365 |
| 28. | Meca, L.; Řeha, D.; Havlas, Z. J. Org. Chem. 2003, 68, 5677–5680. doi:10.1021/jo034344u |
| 5. | Li, H.; Zhang, H.-R.; Petersen, J. L.; Wang, K. K. J. Org. Chem. 2001, 66, 6662–6668. doi:10.1021/jo0104577 |
| 21. | Wen, B.; Petersen, J. L.; Wang, K. K. Chem. Commun. 2010, 46, 1938–1940. doi:10.1039/b921667g |
| 5. | Li, H.; Zhang, H.-R.; Petersen, J. L.; Wang, K. K. J. Org. Chem. 2001, 66, 6662–6668. doi:10.1021/jo0104577 |
| 7. | Yang, H.; Petersen, J. L.; Wang, K. K. Tetrahedron 2006, 62, 8133–8141. doi:10.1016/j.tet.2006.06.004 |
| 22. | Li, H.; Petersen, J. L.; Wang, K. K. J. Org. Chem. 2001, 66, 7804–7810. doi:10.1021/jo010687l |
| 23. | Dai, W.; Petersen, J. L.; Wang, K. K. Org. Lett. 2004, 6, 4355–4357. doi:10.1021/ol0481434 |
| 24. | Yang, H.; Petersen, J. L.; Wang, K. K. Tetrahedron 2006, 62, 1231–1238. doi:10.1016/j.tet.2005.10.062 |
| 1. | Schmittel, M.; Strittmatter, M.; Vollmann, K.; Kiau, S. Tetrahedron Lett. 1996, 37, 999–1002. doi:10.1016/0040-4039(95)02369-0 |
| 2. | Schmittel, M.; Strittmatter, M.; Kiau, S. Angew. Chem., Int. Ed. Engl. 1996, 35, 1843–1845. doi:10.1002/anie.199618431 |
| 3. | Schmittel, M.; Vavilala, C. J. Org. Chem. 2005, 70, 4865–4868. doi:10.1021/jo0504971 |
| 4. | Wang, K. K. Enyne–Allenes. In Modern Allene Chemistry; Krause, N.; Hashmi, A. S. K., Eds.; Wiley-VCH: Weinheim, Germany, 2004; Vol. 2, pp 1091–1126. |
| 32. | Müller, S.; Liepold, B.; Roth, G. J.; Bestmann, H. J. Synlett 1996, 521–522. doi:10.1055/s-1996-5474 |
| 17. | Einhorn, C.; Einhorn, J.; Marcadal-Abbadi, C.; Pierre, J.-L. J. Org. Chem. 1999, 64, 4542–4546. doi:10.1021/jo982527o |
| 18. | Dufková, L.; Kotora, M.; Císařová, I. Eur. J. Org. Chem. 2005, 2491–2499. doi:10.1002/ejoc.200400881 |
| 19. | Hapke, M.; Kral, K.; Fischer, C.; Spannenberg, A.; Gutnov, A.; Redkin, D.; Heller, B. J. Org. Chem. 2010, 75, 3993–4003. doi:10.1021/jo100122d |
© 2011 Wang et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)