Abstract
A series of methoxy and deoxy derivatives of mannopyranose-1-phosphate (Manp-1P) were chemically synthesized, and their ability to be converted into the corresponding guanosine diphosphate mannopyranose (GDP-Manp) analogues by a pyrophosphorylase (GDP-ManPP) from Salmonella enterica was studied. Evaluation of methoxy analogues demonstrated that GDP-ManPP is intolerant of bulky substituents at the C-2, C-3, and C-4 positions, in turn suggesting that these positions are buried inside the enzyme active site. Additionally, both the 6-methoxy and 6-deoxy Manp-1P derivatives are good or moderate substrates for GDP-ManPP, thus indicating that the C-6 hydroxy group of the Manp-1P substrate is not required for binding to the enzyme. When taken into consideration with other previously published work, it appears that this enzyme has potential utility for the chemoenzymatic synthesis of GDP-Manp analogues, which are useful probes for studying enzymes that employ this sugar nucleotide as a substrate.
Graphical Abstract
Introduction
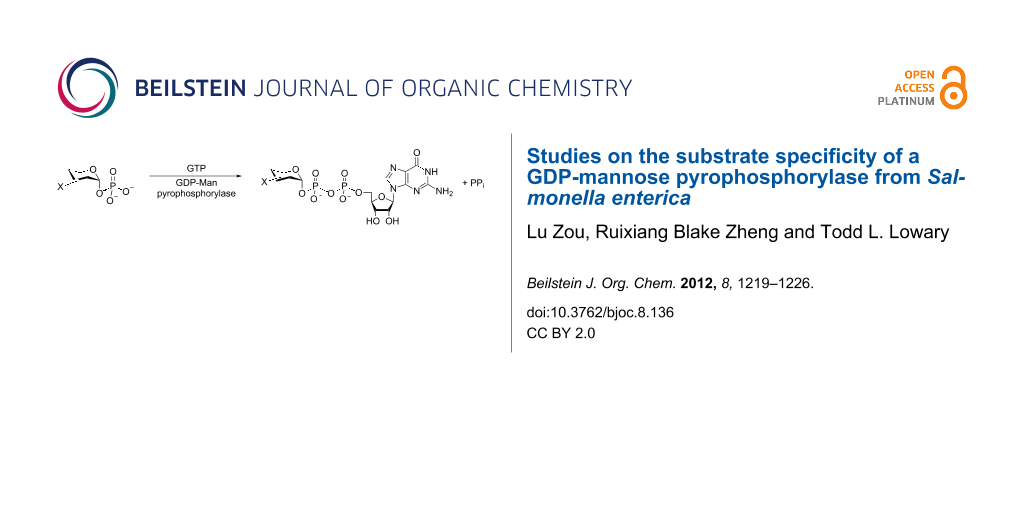
Modified sugar nucleotide analogues are valuable probes to study glycosyltransferases and other enzymes that use these activated glycosylating agents as substrates [1-5]. The synthesis of natural and non-natural sugar nucleotides is therefore a topic of continuing interest [6]. The classical method for chemically synthesizing sugar nucleotides involves the preparation of a sugar 1-phosphate derivative followed by its coupling to an activated nucleoside monophosphate to form the key pyrophosphate moiety (Figure 1A) [7]. In general, the yield of this process is low, and the purification of the product can be tedious; hence, the development of new methods to prepare sugar nucleotides remains an area of active research [6]. Although improved chemical methods have been developed [8-13], another attractive strategy is to employ a chemoenzymatic approach, in which a synthetic sugar 1-phosphate derivative is converted to the sugar nucleotide by a pyrophosphorylase (Figure 1B) [14,15]. This approach is increasingly used for the synthesis of sugar nucleotides, but a limitation is that the specificity of the pyrophosphorylase must be sufficiently broad to recognize the synthetic sugar 1-phosphate derivative. However, some of these enzymes have been demonstrated to have broad specificity, or can be engineered to have broad specificity, with regard to both the sugar 1-phosphate and nucleotide substrates [16-19].
Figure 1: (A) Conventional approach for the chemical synthesis of sugar nucleotides from sugar 1-phosphates; (B) enzymatic conversion of sugar 1-phosphates into sugar nucleotides.
Figure 1: (A) Conventional approach for the chemical synthesis of sugar nucleotides from sugar 1-phosphates; ...
As part of a larger study on the specificity of mannosyltransferases involved in mycobacterial glycan biosynthesis [20-22], we had the need for a panel of singly deoxygenated and methylated guanosine diphosphosphate mannopyranose (GDP-Man) derivatives. In developing a strategy for the synthesis of these compounds, we chose to take advantage of a GDP-mannose pyrophosphorylase (GDP-ManPP) from Salmonella enterica [23], which had previously been shown to have a relaxed specificity for the sugar 1-phosphate moiety [24,25]. In particular, it has been shown that the enzyme will accept mannopyranosyl 1-phosphate (Manp-1P) derivatives deoxygenated at C-2, C-3 and C-4 (1–3, Figure 2), as well as a substrate lacking the hydroxymethyl group at C-5 (4) [24]. A series monoazido derivatives (5–8) were also shown to be substrates [25]. To further probe the potential of this enzyme for the chemoenzymatic synthesis of modified GDP-Manp derivatives, we describe here the preparation of all four singly methylated Manp-1P analogues 9–12, as well as the 6-deoxy-Manp-1P derivative 13, and an initial evaluation of their ability to serve as a substrate for S. enterica GDP-ManPP.
Figure 2: Structures of the Manp-1P derivatives (1–8) previously shown [24,25] to be substrates for S. enterica GDP-ManPP and analogues 9–13 studied in this paper.
Figure 2: Structures of the Manp-1P derivatives (1–8) previously shown [24,25] to be substrates for S. enterica GDP-...
Results and Discussion
Synthesis of 2-methoxy derivative 9
The synthesis of sugar 1-phosphate 9 containing a methyl group at O-2 commenced from 3-O-benzyl-4,6-O-benzylidene-α-D-mannopyranoside 14 [26] as illustrated in Scheme 1. Methylation of the alcohol under standard conditions proceeded in 80% yield affording 15. The benzylidene protecting group was cleaved, together with the methyl glycoside, by acetolysis giving the tetra-O-acetylated compound 16 in 81% yield. This glycosyl acetate was converted to the corresponding thioglycoside (17), which was, in turn, coupled with dibenzyl phosphate under NIS–AgOTf activation conditions, providing compound 18 in 55% yield over two steps from 16. The anomeric stereochemistry in 18 was confirmed by the magnitude of the 1JC1,H1, which was 177.9 Hz, consistent with α-stereochemistry as described earlier by Timmons and Jakeman for rhamnopyranosyl phosphates [27]. In the other phosphorylation reactions reported in this paper, the anomeric stereochemistry was determined in an analogous manner. Compound 18 was then deprotected in two steps, namely catalytic hydrogenolysis and then, without further purification, treatment with a mixture of CH3OH–H2O–Et3N 5:2:1 to remove the acetyl groups. This series of reactions gave 2-methoxy Manp-1P analogue 9 in 92% overall yield from 18.
Scheme 1: Reagents and conditions: (a) CH3I, NaH, DMF, 80%; (b) Ac2O–HOAc–H2SO4, 35:15:1, 81%; (c) EtSH, BF3·OEt2, CH2Cl2, 65%; (d) HO-P(O)(OBn)2, NIS, AgOTf, CH2Cl2, 84%; (e) (i) H2, Pd(OH)2–C, toluene, Et3N, pyridine; (ii) CH3OH–H2O–Et3N, 5:2:1, 92%.
Scheme 1: Reagents and conditions: (a) CH3I, NaH, DMF, 80%; (b) Ac2O–HOAc–H2SO4, 35:15:1, 81%; (c) EtSH, BF3·...
Synthesis of 3-methoxy derivative 10
The preparation of the 3-methoxy Manp-1P analogue 10 followed a route similar to that used for the synthesis of 9 (Scheme 2). Methyl 2-O-benzyl-4,6-O-benzylidene-α-D-mannopyranoside (19) [26] was first methylated giving 20 and then converted into glycosyl acetate 21 in 49% yield over the two steps. Subsequent thioglycosylation provided a 52% yield of 22. The protected dibenzyl phosphate 23 was next formed by the NIS–AgOTf promoted glycosylation of dibenzyl phosphate with 22, which afforded the desired compound, 23, in 75% yield. Hydrogenolysis of the benzyl groups and deacylation led to the formation, in 67% yield, of Manp-1P derivative 10.
Scheme 2: Reagents and conditions: (a) CH3I, NaH, DMF, 76%; (b) Ac2O–HOAc–H2SO4, 35:15:1, 65%; (c) EtSH, BF3·OEt2, CH2Cl2, 52%; (d) HO-P(O)(OBn)2, NIS, AgOTf, CH2Cl2, 75%; (e) (i) H2, Pd(OH)2–C, toluene, Et3N, pyridine; (ii) CH3OH–H2O–Et3N, 5:2:1, 67%.
Scheme 2: Reagents and conditions: (a) CH3I, NaH, DMF, 76%; (b) Ac2O–HOAc–H2SO4, 35:15:1, 65%; (c) EtSH, BF3·...
Synthesis of 4-methoxy derivative 11
As illustrated in Scheme 3, the synthesis of the 4-methoxy Manp-1P analogue 11 started by treatment of methyl α-D-mannopyranoside (24) with trityl chloride in pyridine. The product, 25, was then converted to the isopropylidene acetal 26 in 65% overall yield from 24. The hydroxy group in 26 was methylated under standard conditions (CH3I, NaH) to give the 4-methoxy analogue 27 in 91% yield. Acetolysis of 27 to the corresponding glycosyl acetate 28, followed by reaction with ethanethiol and BF3·OEt2, yielded thioglycoside 29, in a modest 39% yield from 27 over two steps. This compound was then converted to 11, in 56% yield, as outlined above, by successive phosphorylation and deprotection.
Scheme 3: Reagents and conditions: (a) TrCl, DMAP, pyridine, 85%; (b) DMP, p-TsOH, 76%; (c) CH3I, NaH, DMF, 91%; (d) Ac2O–HOAc–H2SO4, 35:15:1, 55%; (e) EtSH, BF3·OEt2, CH2Cl2, 70%; (f) HO-P(O)(OBn)2, NIS, AgOTf, CH2Cl2, 80%; (g) (i) H2, Pd(OH)2–C, toluene, Et3N, pyridine; (ii) CH3OH–H2O–Et3N, 5:2:1, 70%.
Scheme 3: Reagents and conditions: (a) TrCl, DMAP, pyridine, 85%; (b) DMP, p-TsOH, 76%; (c) CH3I, NaH, DMF, 9...
Synthesis of 6-methoxy derivative 12
Two routes, differing in the choice of protecting groups, were explored to produce the 6-methoxy Manp-1P derivative 12 (Scheme 4 and Scheme 5). In one route, the C-2, C-3, and C-4 hydroxy groups of the mannose residues were protected with benzyl ethers and in the other they were protected with benzoyl esters. The overall yields of these two methods were 30% and 17%, respectively. In the first method (Scheme 4), the initial step was the conversion, in 78% yield, of the fully acetylated thioglycoside 31 [28] into silyl ether 32 by treatment with sodium methoxide and then tert-butyldiphenylchlorosilane in DMF. Benzylation of 32 using benzyl bromide and sodium hydride gave 33 in 84% yield. The TBDPS group was then cleaved and replaced with a methyl group to give the 6-methoxy compound 35 in 72% yield over two steps. The protected dibenzyl phosphate 36 was formed in 70% yield by phosphorylation as described for the synthesis of 9–11. Catalytic hydrogenolysis in the presence of NaHCO3 was used to cleave all the benzyl groups, which gave the 6-methoxy Manp-1P derivative 12 in 91% yield.
Scheme 4: Reagents and conditions: (a) (i) NaOCH3, CH3OH; (ii) TBDPSCl, imidazole, DMF, 78%; (b) BnBr, NaH, TBAI, 84%; (c) TBAF, THF, 83%; (d) CH3I, NaH, DMF, 87%; (e) HO-P(O)(OBn)2, NIS, AgOTf, CH2Cl2, 70%; (f) H2, Pd(OH)2–C, NaHCO3, CH3OH, 91%.
Scheme 4: Reagents and conditions: (a) (i) NaOCH3, CH3OH; (ii) TBDPSCl, imidazole, DMF, 78%; (b) BnBr, NaH, T...
Scheme 5: Reagents and conditions: (a) Ag2O, CaSO4, CH3I, 52%; (b) Ac2O–HOAc–H2SO4, 70:30:1, 96%; (c) EtSH, BF3·OEt2, CH2Cl2, 75%; (d) HO-P(O)(OBn)2, NIS, AgOTf, CH2Cl2, 89%; (e) (i) H2, Pd(OH)2–C, toluene, Et3N, pyridine; (ii) CH3OH–H2O–Et3N, 5:2:1, 85%.
Scheme 5: Reagents and conditions: (a) Ag2O, CaSO4, CH3I, 52%; (b) Ac2O–HOAc–H2SO4, 70:30:1, 96%; (c) EtSH, BF...
The second route to 12 began with methyl 2,3,4-tri-O-benzoyl-α-D-mannopyranoside (37) [29] and is illustrated in Scheme 5. Methylation of the free OH, even under mildly basic conditions (e.g., Ag2O–CaSO4), led to significant amounts of acyl group migration, and the desired product was obtained in only 52% yield. Nevertheless, enough material was produced to move forward. Acetolysis conditions were used to replace the methyl group at the anomeric center in 38 with an acetyl group, resulting in a 96% yield of 39. Thioglycosylation, followed by coupling of the resulting thioglycoside donor 40 (obtained in 75% yield) with dibenzyl phosphate, gave phosphate 41 in a yield of 67% over the two steps. The 6-methoxy Manp-1P analogue 12 was obtained by catalytic hydrogenolysis of the benzyl ethers followed by treatment with CH3OH–H2O–Et3N 5:2:1 providing 12 in 85% yield over two steps.
Synthesis of 6-deoxy derivative 13
The synthesis of the 6-deoxy Manp-1P analogue 13 used an intermediate (37) prepared in the course of the synthesis of the 6-methoxy analogue (Scheme 6). First, the hydroxy group of 37 was converted to the corresponding iodide in 65% yield, by using triphenylphospine and iodine. The product, 42, was then subjected to acetolysis and catalytic hydrogenation, which gave 6-deoxy glycosyl acetate derivative 43 in 72% yield. The subsequent thioglycosylation, phosphorylation and deprotection steps proceeded, as outlined above, to give the 6-deoxy Manp-1P 13 in 43% yield over four steps.
Scheme 6:
Reagents and conditions: (a) PPh3, imidazole, I2, 65%; (b) (i) Ac2O–HOAc–H2SO4, 35:15:1; (ii) Pd–C, H2, Et3N, EtOAc, 72%; (c) EtSH, BF3·OEt2, CH2Cl2, 89%, α/β 4:1; (d) HO-P(O)(OBn)2, NIS, AgOTf, CH2Cl2, 67%; (e) (i) H2, Pd(OH)2–C, toluene, Et3N, pyridine;
(ii) CH3OH–H2O–Et3N, 5:2:1, 72%.
Scheme 6: Reagents and conditions: (a) PPh3, imidazole, I2, 65%; (b) (i) Ac2O–HOAc–H2SO4, 35:15:1; (ii) Pd–C,...
Evaluation of 9–13 as substrates for GDP-Man pyrophosphorylase
With 9–13 in hand, each was evaluated as a substrate for the S. enterica GDP-ManPP. Before doing that, the recombinant protein was produced and the natural substrate for the enzyme, Manp-1P (46, Figure 3), was evaluated by incubation with the enzyme and GTP. The reaction was monitored by HPLC (Figure S1 in Supporting Information File 1) and stopped when the complete consumption of GTP was observed. Simultaneous with the loss of the GTP was the appearance of the signal for a new product, which was found to elute at a retention time similar to that for an authentic sample of GDP-Manp. The product was isolated, and analysis by high-resolution electrospray ionization mass spectrometry revealed an ion with m/z = 604.0691, which corresponds to the [M − H]− ion (calcd m/z = 604.0699) of GDP-Manp.
Figure 3: Reaction catalyzed by GDP-ManPP.
Figure 3: Reaction catalyzed by GDP-ManPP.
Having established that the enzyme GDP-ManPP was active, we carried out the same incubations for 9–13, and in all cases the corresponding GDP-Manp analogue peaks could be observed (Figure S2 in Supporting Information File 1). However, in the case of 11 and 9, a peak corresponding to GDP, resulting from hydrolysis of the GDP-sugar, was also observed, and, in the case of 9, a much smaller amount of the GDP-Manp analogue was produced. To confirm the identity of each GDP-Manp analogue, the product peaks were isolated and analysed by electrospray ionization mass spectrometry. For the reactions involving 9–12 a signal at m/z ≈ 618 was observed, as would be expected for the [M − H]− ion of the methylated GDP-Man derivatives (48–51, Figure 4). Similarly, for the reaction with 13, a signal at m/z ≈ 588 was observed in the mass spectrum consistent with the 6-deoxy GDP-Man derivative 52.
Figure 4: Structure of modified GDP-Man derivatives 48–52 produced from 9–13.
Figure 4: Structure of modified GDP-Man derivatives 48–52 produced from 9–13.
Relative activity of Manp-1P analogues with GDP-ManPP
After it was established that all five Manp-1P analogues could serve as substrates for GDP-ManPP, the relative activity with each was assessed. This was done by using an established colorimetric activity assay, which relies on the detection of the pyrophosphate (PPi, Figure 3) formed as a byproduct of the enzymatic reaction [30]. As illustrated in Figure 5, all five synthetic derivatives 9–13 were active as substrates, although at lower levels than the parent compound 46. The 6-methoxy (12) and 6-deoxy (13) analogues, demonstrated moderate to good relative activities, while the 2-methoxy (9), 3-methoxy (10), and 4-methoxy (11) compounds showed much lower activities. For example, the 2-methoxy, 3-methoxy, and 4-methoxy analogues displayed a 6-, 14-, and 17-fold decrease relative to 46, respectively. Because both the 6-deoxy and 6-methoxy analogues (12 and 13) showed relatively good activity it is likely that this hydroxy group does not interact significantly with the enzyme. On the other hand, because the 2-methoxy, 3-methoxy, and 4-methoxy compounds all showed a large decrease in activity, it is likely that these positions are bound tightly in the active site of the enzyme. A graphical summary of the substrate specificity for GDP-ManPP is shown in Figure 6.
![[1860-5397-8-136-5]](/bjoc/content/figures/1860-5397-8-136-5.png?scale=1.8&max-width=1024&background=FFFFFF)
Figure 5: Comparison of the relative activity of synthetic Manp-1P analogues 9–13 for GDP-ManPP, with that of the parent compound 46. Error bars represent the standard deviation of duplicate reactions.
Figure 5: Comparison of the relative activity of synthetic Manp-1P analogues 9–13 for GDP-ManPP, with that of...
Figure 6: Summary of the substrate specificity of GDP-ManPP. Data from previous studies on the enzyme are also included as indicated [24,25].
Figure 6: Summary of the substrate specificity of GDP-ManPP. Data from previous studies on the enzyme are als...
Kinetic analysis of Manp-1P analogues with GDP-ManPP
To better understand how these 9–13 interact with GDP-ManPP, kinetic analyses were performed by using the colorimetric activity assay mentioned above (Table 1). Both the 6-methoxy Manp-1P (12) and 6-deoxy Manp-1P (13) derivatives bind relatively well to the enzyme, showing only a two- or three-fold increase in KM, respectively, compared to the native Manp-1P donor 46. The turnover rate of 6-methoxy analogue 12 is, however, much lower than the 6-deoxy counterpart (13) and the natural substrate 46, as substantiated by a greater than 10-fold decrease in kcat. Taken together, these results suggest that the C-6 hydroxy group does not engage in any critical hydrogen-bonding interactions and that a bulky substituent interferes with the rate of substrate turnover. The binding of the 2-methoxy (9) and 4-methoxy (11) analogues is very weak compared to the native substrate, as seen by the greater then 100-fold increase in KM; consequently, the turnover rates are also low. The binding between 3-methoxy analogue 10 is moderate, with only a five-fold increase in the observed KM, but it shows an extremely low turnover rate. These results all suggest that GDP-ManPP is not tolerant of bulky substituents at the C-2, C-3, and C-4 positions, which is consistent with the results obtained from their relative activity. It should be noted that these trends are consistent with earlier studies of the enzyme using deoxygenated or azido analogues [24,25].
Table 1: KM, kcat, and kcat/KM of GDP-ManPP kinetic studies.
| compound | KM (μM) | kcat (min−1) | kcat/KM (min−1·μM−1) |
|---|---|---|---|
| 9 (2-methoxy analogue) | 4000 ± 1100 | 70 ± 11 | (2 ± 1) × 10−2 |
| 10 (3-methoxy analogue) | 200 ± 72 | 5.2 ± 0.7 | (2.6 ± 0.1) × 10−2 |
| 11 (4-methoxy analogue) | 3400 ± 870 | 31 ± 4.7 | (9 ± 5) × 10−3 |
| 12 (6-methoxy analogue) | 120 ± 18 | 27 ± 1 | 0.23 ± 0.06 |
| 13 (6-deoxy analogue) | 70 ± 13 | 300 ± 13 | 4 ± 1 |
| 46 (Man-1P) | 40 ± 6 | 360 ± 16 | 9 ± 3 |
Conclusion
In this paper, we report the synthesis of a panel of methoxy and deoxy analogues of Manp-1P. Five analogues, 9–13, in which one of the hydroxy groups was methylated or deoxygenated were generated by chemical synthesis, and the ability of these compounds to be converted to the corresponding GDP-Manp analogues by GDP-ManPP from S. enterica was evaluated. All the derivatives acted as substrates for GDP-ManPP, but with uniformly lower activity than the natural substrate Man-1P. The results suggest that the C-2, C-3, and C-4 hydroxy groups of Manp-1P are bound within the active site of GDP-ManPP and the addition of a methyl group at these positions is tolerated very poorly. Conversely, the addition of a methyl group to, or deoxygenation of, O-6 had a much smaller effect, suggesting that this position protrudes from the active site, or is accommodated in a pocket that can tolerate either of these modifications. These results are consistent with earlier studies of this enzyme, which were focused on deoxygenated and azido derivatives [24,25]. Considered together, our studies and those published previously suggest that this enzyme can be used to access deoxy and azido derivatives of GDP-Man on a preparative scale, but that the synthesis of analogues containing more sterically demanding groups is likely to be only possible when the modifications are present on O-6.
Supporting Information
| Supporting Information File 1: Detailed experimental procedures. | ||
| Format: PDF | Size: 854.9 KB | Download |
Acknowledgements
This work was supported by the Alberta Glycomics Centre, the University of Alberta and the Natural Sciences and Engineering Research Council of Canada. We thank Dr. Warren Wakarchuk at the National Research Council of Canada for providing the plasmid containing the GDP-ManPP gene, and Mr. Myles B. Poulin for technical assistance on the enzyme assay.
References
-
Errey, J. C.; Mann, M. C.; Fairhurst, S. A.; Hill, L.; McNeil, M. R.; Naismith, J. H.; Percy, J. M.; Whitfield, C.; Field, R. A. Org. Biomol. Chem. 2009, 7, 1009–1016. doi:10.1039/b815549f
Return to citation in text: [1] -
Peltier, P.; Beláňová, M.; Dianišková, P.; Zhou, R.; Zheng, R. B.; Pearcey, J. A.; Joe, M.; Brennan, P. J.; Nugier-Chauvin, C.; Ferrières, V.; Lowary, T. L.; Daniellou, R.; Mikušová, K. Chem. Biol. 2010, 17, 1356–1366. doi:10.1016/j.chembiol.2010.10.014
Return to citation in text: [1] -
Poulin, M. B.; Zhou, R.; Lowary, T. L. Org. Biomol. Chem. 2012, 10, 4074–4087. doi:10.1039/c2ob25159k
Return to citation in text: [1] -
Brown, C. D.; Rusek, M. S.; Kiessling, L. L. J. Am. Chem. Soc. 2012, 134, 6552–6555. doi:10.1021/ja301723p
Return to citation in text: [1] -
Zhang, Q.; Liu, H.-w. J. Am. Chem. Soc. 2001, 123, 6756–6766. doi:10.1021/ja010473l
Return to citation in text: [1] -
Wagner, G. K.; Pesnot, T.; Field, R. A. Nat. Prod. Rep. 2009, 26, 1172–1194. doi:10.1039/b909621n
Return to citation in text: [1] [2] -
Roseman, S.; Distler, J. J.; Moffatt, J. G.; Khorana, H. G. J. Am. Chem. Soc. 1961, 83, 659–663. doi:10.1021/ja01464a035
Return to citation in text: [1] -
Arlt, M.; Hindsgaul, O. J. Org. Chem. 1995, 60, 14–15. doi:10.1021/jo00106a007
Return to citation in text: [1] -
Timmons, S. C.; Jakeman, D. L. Org. Lett. 2007, 9, 1227–1230. doi:10.1021/ol063068d
Return to citation in text: [1] -
Wolf, S.; Zismann, T.; Lunau, N.; Meier, C. Chem.–Eur. J. 2009, 15, 7656–7664. doi:10.1002/chem.200900572
Return to citation in text: [1] -
Gold, H.; van Delft, P.; Meeuwenoord, N.; Codée, J. D. C.; Filippov, D. V.; Eggink, G.; Overkleeft, H. S.; van der Marel, G. A. J. Org. Chem. 2008, 73, 9458–9460. doi:10.1021/jo802021t
Return to citation in text: [1] -
Warnecke, S.; Meier, C. J. Org. Chem. 2009, 74, 3024–3030. doi:10.1021/jo802348h
Return to citation in text: [1] -
Mohamady, S.; Taylor, S. D. J. Org. Chem. 2011, 76, 6344–6349. doi:10.1021/jo200540e
Return to citation in text: [1] -
Timmons, S. C.; Hui, J. P. M.; Pearson, J. L.; Peltier, P.; Daniellou, R.; Nugier-Chauvin, C.; Soo, E. C.; Syvitski, R. T.; Ferrières, V.; Jakeman, D. L. Org. Lett. 2008, 10, 161–163. doi:10.1021/ol7023949
Return to citation in text: [1] -
Errey, J. C.; Mukhopadhyay, B.; Kartha, K. P. R.; Field, R. A. Chem. Commun. 2004, 2706–2707. doi:10.1039/b410184g
Return to citation in text: [1] -
Mizanur, R. M.; Pohl, N. L. B. Org. Biomol. Chem. 2009, 7, 2135–2139. doi:10.1039/b822794b
Return to citation in text: [1] -
Barton, W. A.; Biggins, J. B.; Jiang, J.; Thorson, J. S.; Nikolov, D. B. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 13397–13402. doi:10.1073/pnas.192468299
Return to citation in text: [1] -
Jiang, J.; Biggins, J. B.; Thorson, J. S. J. Am. Chem. Soc. 2000, 122, 6803–6804. doi:10.1021/ja001444y
Return to citation in text: [1] -
Moretti, R.; Chang, A.; Peltier-Pain, P.; Bingman, C. A.; Phillips, G. N., Jr.; Thorson, J. S. J. Biol. Chem. 2011, 286, 13235–13243. doi:10.1074/jbc.M110.206433
Return to citation in text: [1] -
Xia, L.; Zheng, R. B.; Lowary, T. L. ChemBioChem 2012, 13, 1139–1151. doi:10.1002/cbic.201200121
Return to citation in text: [1] -
Tam, P.-H.; Lowary, T. L. Org. Biomol. Chem. 2010, 8, 181–192. doi:10.1039/b916580k
Return to citation in text: [1] -
Tam, P.-H.; Besra, G. S.; Lowary, T. L. ChemBioChem 2008, 9, 267–278. doi:10.1002/cbic.200700391
Return to citation in text: [1] -
Elling, L.; Ritter, J. E.; Verseck, S. Glycobiology 1996, 6, 591–597. doi:10.1093/glycob/6.6.591
Return to citation in text: [1] -
Watt, G. M.; Flitsch, S. L.; Fey, S.; Elling, L.; Kragl, U. Tetrahedron: Asymmetry 2000, 11, 621–628. doi:10.1016/S0957-4166(99)00556-X
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Marchesan, S.; Macmillan, D. Chem. Commun. 2008, 4321–4323. doi:10.1039/b807016d
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Tam, P.-H.; Lowary, T. L. Carbohydr. Res. 2007, 342, 1741–1772. doi:10.1016/j.carres.2007.05.001
Return to citation in text: [1] [2] -
Timmons, S. C.; Jakeman, D. L. Carbohydr. Res. 2008, 343, 865–874. doi:10.1016/j.carres.2008.01.046
Return to citation in text: [1] -
Zhong, W.; Kuntz, D. A.; Ernber, B.; Singh, H.; Moremen, K. W.; Rose, D. R.; Boons, G.-J. J. Am. Chem. Soc. 2008, 130, 8975–8983. doi:10.1021/ja711248y
Return to citation in text: [1] -
Esmurziev, A. M.; Simic, N.; Hoff, B. H.; Sundby, E. J. Carbohydr. Chem. 2010, 29, 348–367. doi:10.1080/07328303.2010.540055
Return to citation in text: [1] -
Davis, A. J.; Perugini, M. A.; Smith, B. J.; Stewart, J. D.; Ilg, T.; Hodder, A. N.; Handman, E. J. Biol. Chem. 2004, 279, 12462–12468. doi:10.1074/jbc.M312365200
Return to citation in text: [1]
| 30. | Davis, A. J.; Perugini, M. A.; Smith, B. J.; Stewart, J. D.; Ilg, T.; Hodder, A. N.; Handman, E. J. Biol. Chem. 2004, 279, 12462–12468. doi:10.1074/jbc.M312365200 |
| 28. | Zhong, W.; Kuntz, D. A.; Ernber, B.; Singh, H.; Moremen, K. W.; Rose, D. R.; Boons, G.-J. J. Am. Chem. Soc. 2008, 130, 8975–8983. doi:10.1021/ja711248y |
| 29. | Esmurziev, A. M.; Simic, N.; Hoff, B. H.; Sundby, E. J. Carbohydr. Chem. 2010, 29, 348–367. doi:10.1080/07328303.2010.540055 |
| 1. | Errey, J. C.; Mann, M. C.; Fairhurst, S. A.; Hill, L.; McNeil, M. R.; Naismith, J. H.; Percy, J. M.; Whitfield, C.; Field, R. A. Org. Biomol. Chem. 2009, 7, 1009–1016. doi:10.1039/b815549f |
| 2. | Peltier, P.; Beláňová, M.; Dianišková, P.; Zhou, R.; Zheng, R. B.; Pearcey, J. A.; Joe, M.; Brennan, P. J.; Nugier-Chauvin, C.; Ferrières, V.; Lowary, T. L.; Daniellou, R.; Mikušová, K. Chem. Biol. 2010, 17, 1356–1366. doi:10.1016/j.chembiol.2010.10.014 |
| 3. | Poulin, M. B.; Zhou, R.; Lowary, T. L. Org. Biomol. Chem. 2012, 10, 4074–4087. doi:10.1039/c2ob25159k |
| 4. | Brown, C. D.; Rusek, M. S.; Kiessling, L. L. J. Am. Chem. Soc. 2012, 134, 6552–6555. doi:10.1021/ja301723p |
| 5. | Zhang, Q.; Liu, H.-w. J. Am. Chem. Soc. 2001, 123, 6756–6766. doi:10.1021/ja010473l |
| 8. | Arlt, M.; Hindsgaul, O. J. Org. Chem. 1995, 60, 14–15. doi:10.1021/jo00106a007 |
| 9. | Timmons, S. C.; Jakeman, D. L. Org. Lett. 2007, 9, 1227–1230. doi:10.1021/ol063068d |
| 10. | Wolf, S.; Zismann, T.; Lunau, N.; Meier, C. Chem.–Eur. J. 2009, 15, 7656–7664. doi:10.1002/chem.200900572 |
| 11. | Gold, H.; van Delft, P.; Meeuwenoord, N.; Codée, J. D. C.; Filippov, D. V.; Eggink, G.; Overkleeft, H. S.; van der Marel, G. A. J. Org. Chem. 2008, 73, 9458–9460. doi:10.1021/jo802021t |
| 12. | Warnecke, S.; Meier, C. J. Org. Chem. 2009, 74, 3024–3030. doi:10.1021/jo802348h |
| 13. | Mohamady, S.; Taylor, S. D. J. Org. Chem. 2011, 76, 6344–6349. doi:10.1021/jo200540e |
| 27. | Timmons, S. C.; Jakeman, D. L. Carbohydr. Res. 2008, 343, 865–874. doi:10.1016/j.carres.2008.01.046 |
| 6. | Wagner, G. K.; Pesnot, T.; Field, R. A. Nat. Prod. Rep. 2009, 26, 1172–1194. doi:10.1039/b909621n |
| 26. | Tam, P.-H.; Lowary, T. L. Carbohydr. Res. 2007, 342, 1741–1772. doi:10.1016/j.carres.2007.05.001 |
| 7. | Roseman, S.; Distler, J. J.; Moffatt, J. G.; Khorana, H. G. J. Am. Chem. Soc. 1961, 83, 659–663. doi:10.1021/ja01464a035 |
| 24. | Watt, G. M.; Flitsch, S. L.; Fey, S.; Elling, L.; Kragl, U. Tetrahedron: Asymmetry 2000, 11, 621–628. doi:10.1016/S0957-4166(99)00556-X |
| 25. | Marchesan, S.; Macmillan, D. Chem. Commun. 2008, 4321–4323. doi:10.1039/b807016d |
| 6. | Wagner, G. K.; Pesnot, T.; Field, R. A. Nat. Prod. Rep. 2009, 26, 1172–1194. doi:10.1039/b909621n |
| 26. | Tam, P.-H.; Lowary, T. L. Carbohydr. Res. 2007, 342, 1741–1772. doi:10.1016/j.carres.2007.05.001 |
| 23. | Elling, L.; Ritter, J. E.; Verseck, S. Glycobiology 1996, 6, 591–597. doi:10.1093/glycob/6.6.591 |
| 24. | Watt, G. M.; Flitsch, S. L.; Fey, S.; Elling, L.; Kragl, U. Tetrahedron: Asymmetry 2000, 11, 621–628. doi:10.1016/S0957-4166(99)00556-X |
| 24. | Watt, G. M.; Flitsch, S. L.; Fey, S.; Elling, L.; Kragl, U. Tetrahedron: Asymmetry 2000, 11, 621–628. doi:10.1016/S0957-4166(99)00556-X |
| 25. | Marchesan, S.; Macmillan, D. Chem. Commun. 2008, 4321–4323. doi:10.1039/b807016d |
| 20. | Xia, L.; Zheng, R. B.; Lowary, T. L. ChemBioChem 2012, 13, 1139–1151. doi:10.1002/cbic.201200121 |
| 21. | Tam, P.-H.; Lowary, T. L. Org. Biomol. Chem. 2010, 8, 181–192. doi:10.1039/b916580k |
| 22. | Tam, P.-H.; Besra, G. S.; Lowary, T. L. ChemBioChem 2008, 9, 267–278. doi:10.1002/cbic.200700391 |
| 25. | Marchesan, S.; Macmillan, D. Chem. Commun. 2008, 4321–4323. doi:10.1039/b807016d |
| 16. | Mizanur, R. M.; Pohl, N. L. B. Org. Biomol. Chem. 2009, 7, 2135–2139. doi:10.1039/b822794b |
| 17. | Barton, W. A.; Biggins, J. B.; Jiang, J.; Thorson, J. S.; Nikolov, D. B. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 13397–13402. doi:10.1073/pnas.192468299 |
| 18. | Jiang, J.; Biggins, J. B.; Thorson, J. S. J. Am. Chem. Soc. 2000, 122, 6803–6804. doi:10.1021/ja001444y |
| 19. | Moretti, R.; Chang, A.; Peltier-Pain, P.; Bingman, C. A.; Phillips, G. N., Jr.; Thorson, J. S. J. Biol. Chem. 2011, 286, 13235–13243. doi:10.1074/jbc.M110.206433 |
| 24. | Watt, G. M.; Flitsch, S. L.; Fey, S.; Elling, L.; Kragl, U. Tetrahedron: Asymmetry 2000, 11, 621–628. doi:10.1016/S0957-4166(99)00556-X |
| 25. | Marchesan, S.; Macmillan, D. Chem. Commun. 2008, 4321–4323. doi:10.1039/b807016d |
| 14. | Timmons, S. C.; Hui, J. P. M.; Pearson, J. L.; Peltier, P.; Daniellou, R.; Nugier-Chauvin, C.; Soo, E. C.; Syvitski, R. T.; Ferrières, V.; Jakeman, D. L. Org. Lett. 2008, 10, 161–163. doi:10.1021/ol7023949 |
| 15. | Errey, J. C.; Mukhopadhyay, B.; Kartha, K. P. R.; Field, R. A. Chem. Commun. 2004, 2706–2707. doi:10.1039/b410184g |
| 24. | Watt, G. M.; Flitsch, S. L.; Fey, S.; Elling, L.; Kragl, U. Tetrahedron: Asymmetry 2000, 11, 621–628. doi:10.1016/S0957-4166(99)00556-X |
| 25. | Marchesan, S.; Macmillan, D. Chem. Commun. 2008, 4321–4323. doi:10.1039/b807016d |
| 24. | Watt, G. M.; Flitsch, S. L.; Fey, S.; Elling, L.; Kragl, U. Tetrahedron: Asymmetry 2000, 11, 621–628. doi:10.1016/S0957-4166(99)00556-X |
| 25. | Marchesan, S.; Macmillan, D. Chem. Commun. 2008, 4321–4323. doi:10.1039/b807016d |
© 2012 Zou et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)