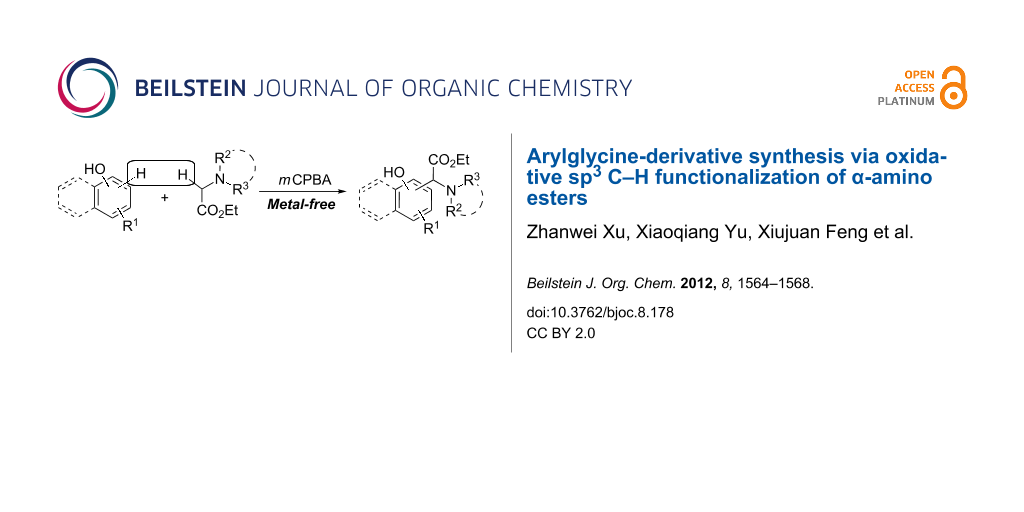
Abstract
An efficient method for the synthesis of arylglycine derivatives is described. The oxidative coupling reactions of naphthols and phenols with α-amino esters proceeded smoothly in the presence of meta-chloroperoxybenzoic acid as an oxidant under ambient conditions, to produce arylglycine derivatives in satisfactory yields.
Graphical Abstract
Findings
Arylglycine derivatives represent important synthetic intermediates or building blocks for drug development and natural-product synthesis [1,2]. The arylglycine moiety also occurs in several bioactive natural products [3]. Consequently, the development of convenient and efficient methods for the preparation of arylglycine derivatives has attracted considerable attention. Over the past years, many methods have been developed for the preparation of arylglycine derivatives [3]. Among these, the addition reaction of a carbon nucleophile to imines or iminium ions through Mannich-type reaction appears more useful (Scheme 1, reactions 1–3). However, these reactions need expensive arylboronic acids (Petasis reaction) [4-9] and suitable leaving groups [10-12] as well as a metal catalyst (Polonovsky reaction; this route requires the preparation of amine N-oxide in advance) [13,14].
Scheme 1: Synthesis of arylglycine derivatives.
Scheme 1: Synthesis of arylglycine derivatives.
We have recently reported the copper-catalyzed oxidative coupling reaction of alkynes with tertiary amine N-oxides [15]. This new strategy for the direct functionalization of sp3 C–H bonds adjacent to a nitrogen atom, via tertiary amine N-oxide intermediates, was successfully applied to the coupling reaction of ethyl 2-(disubstituted amino)acetates with indoles to achieve indolylglycine derivatives (Scheme 2, reaction 1) [16]. In the course of our continuous research on the direct functionalization of sp3 C–H bonds, we found that this new strategy could also be applied to the coupling reaction of naphthols and phenols with ethyl 2-(disubstituted amino)acetates. The results are reported in the current work (Scheme 2, reaction 2).
Scheme 2: Oxidative sp3 C–H functionalization of α-amino esters.
Scheme 2: Oxidative sp3 C–H functionalization of α-amino esters.
In our initial studies, the reaction of 2-naphthol (1a) with ethyl 2-morpholinoacetate (2a) was chosen as a model for optimizing the reaction conditions. The results are shown in Table 1. The proportions of substrate 2a and oxidant meta-chloroperoxybenzoic acid (mCPBA) were initially screened with CH3CN as the solvent (Table 1, entries 1–3). The yield of 3a was increased to 77% when 1.2 equiv of 2a and mCPBA were used (Table 1, entry 2). Further increasing the amounts of 2a and mCPBA or adding a copper catalyst could not improve the yield of 3a (Table 1, entries 3 and 4). The solvents were then screened (Table 1, entries 5–10). The best result was observed when CH2Cl2 was used as the solvent (79%, Table 1, entry 5). Therefore, the subsequent reactions of naphthols and phenols with ethyl 2-(disubstituted amino)acetates were performed in the presence of mCPBA (1.2 equiv) in CH2Cl2 under ambient conditions.
Table 1: Optimization of reaction conditions.a
|
|
|||||
| Entry | 2a (equiv) | mCPBA (equiv) | Time (h) | Solvent | Yield of 3a (%)b |
|---|---|---|---|---|---|
| 1 | 1.0 | 1.0 | 40 | CH3CN | 63 |
| 2 | 1.2 | 1.2 | 40 | CH3CN | 77 |
| 3 | 1.5 | 1.5 | 40 | CH3CN | 77 |
| 4c | 1.2 | 1.2 | 40 | CH3CN | 75 |
| 5 | 1.2 | 1.2 | 24 | CH2Cl2 | 79 |
| 6 | 1.2 | 1.2 | 40 | THF | 65 |
| 7 | 1.2 | 1.2 | 48 | dioxane | 16 |
| 8 | 1.2 | 1.2 | 48 | CH3CH2OH | 14 |
| 9 | 1.2 | 1.2 | 48 | toluene | 70 |
| 10 | 1.2 | 1.2 | 48 | DMF | trace |
aReaction conditions: 2-naphthol (1a, 72.1 mg, 0.5 mmol), ethyl 2-morpholinoacetate (2a, 1.0 equiv to 1.5 equiv), and mCPBA (1.0 equiv to 1.5 equiv) in solvent (3.0 mL) under air at 25 °C. bIsolated yield. c10 mol % Cu(OTf)2 was used as a catalyst.
The substrate scope was determined under the optimized reaction conditions, and the results are shown in Table 2. As expected, the reactions of ethyl 2-morpholinoacetate (2a), ethyl 2-(piperidin-1-yl)acetate (2b), and ethyl 2-(benzyl(methyl)amino)acetate (2c) proceeded smoothly to give the corresponding products 3a–3c in good yields (Table 2, entries 1–3, 64–79%). These results indicated that both α-cyclic and acyclic amino esters could be employed in this type oxidative coupling reaction. The desired products 3d–3f were obtained in yields of 66–79% from the reactions of naphthols 1b–1d with 2a (Table 2, entries 4–6). However, relatively low yields were observed from the reactions of phenols 1e–1h with 2a (Table 2, entries 7–10, 30–55%). The poor reactivity of phenols 1e–1h was considered to be due to their lower electron density compared to naphthols 1b–1d. No reaction was observed from the mixture of phenol 1i, bearing an electron-withdrawing Br substituent on para-position, and 2a (Table 2, entry 11).
Table 2: Oxidative coupling reaction of naphthols and phenols with α-amino esters.a
|
|
|||||
| Entry | Phenol 1 | Amine 2 | Time (h) | Product 3 | Yield (%)b |
|---|---|---|---|---|---|
| 1 |
1a |
2a |
24 |
3a |
79 |
| 2 |
1a |
2b |
24 |
3b |
64 |
| 3 |
1a |
2c |
36 |
3c |
64 |
| 4 |
1b |
2a |
20 |
3d |
79 |
| 5 |
1c |
2a |
20 |
3e |
75 |
| 6 |
1d |
2a |
18 |
3f |
66 |
| 7 |
1e |
2a |
48 |
3g |
30 |
| 8 |
1f |
2a |
36 |
3h |
30 |
| 9 |
1g |
2a |
24 |
3i |
35 |
| 10 |
1h |
2a |
16 |
3j |
55 |
| 11 |
1i |
2a |
48 |
3k |
0 |
aReaction conditions: naphthols or phenols (1, 0.5 mmol), α-amino esters (2, 0.6 mmol, 1.2 equiv), and mCPBA (121.8 mg, 0.6 mmol, 85% purity) in CH2Cl2 (3.0 mL) under air at 25 °C. bIsolated yield.
The plausible mechanism for the coupling reaction of naphthols and phenols with ethyl 2-aminoacetate derivatives is shown in Scheme 3 [16-19]. mCPBA oxidized 2a to amine N-oxide 4 before being transformed into 3-chlorobenzoic acid. The interaction of 4 with 3-chlorobenzoic acid led to the generation of the iminium ion 5 and 3-chlorobenzoate anion. The Mannich-type reaction of 5 with 2-naphthol may have occurred to generate the coupling product 3a. The generated 3-chlorobenzoate anion acted as a proton acceptor.
In conclusion, a new strategy for the functionalization of sp3 C–H bonds of amino esters was successfully applied to the coupling reaction of ethyl 2-(disubstituted amino)acetates with naphthols and phenols. The proposed coupling reaction proceeded smoothly in the presence of mCPBA as an oxidant under ambient conditions to provide arylglycine derivatives in satisfactory yields.
Supporting Information
| Supporting Information File 1: General methods, characterization data and NMR spectra of all synthesized compounds. | ||
| Format: PDF | Size: 1.3 MB | Download |
References
-
Gröger, H. Adv. Synth. Catal. 2001, 343, 547–558. doi:10.1002/1615-4169(200108)343:6/7<547::AID-ADSC547>3.0.CO;2-A
Return to citation in text: [1] -
Nicolaou, K. C.; Boddy, C. N. C.; Bräse, S.; Winssinger, N. Angew. Chem., Int. Ed. 1999, 38, 2096–2152. doi:10.1002/(SICI)1521-3773(19990802)38:15<2096::AID-ANIE2096>3.0.CO;2-F
And references therein.
Return to citation in text: [1] -
Williams, R. M.; Hendrix, J. A. Chem. Rev. 1992, 92, 889–917. doi:10.1021/cr00013a007
Return to citation in text: [1] [2] -
Nanda, K. K.; Trotter, B. W. Tetrahedron Lett. 2005, 46, 2025–2028. doi:10.1016/j.tetlet.2005.01.151
Return to citation in text: [1] -
Petasis, N. A.; Zavialov, I. A. J. Am. Chem. Soc. 1998, 120, 11798–11799. doi:10.1021/ja981075u
Return to citation in text: [1] -
Calí, P.; Begtrup, M. Tetrahedron 2002, 58, 1595–1605. doi:10.1016/S0040-4020(02)00021-2
Return to citation in text: [1] -
Beenen, M. A.; Weix, D. J.; Ellman, J. A. J. Am. Chem. Soc. 2006, 128, 6304–6305. doi:10.1021/ja060529h
Return to citation in text: [1] -
Candeias, N. R.; Montalbano, F.; Cal, P. M. S. D.; Gois, P. M. P. Chem. Rev. 2010, 110, 6169–6193. doi:10.1021/cr100108k
Return to citation in text: [1] -
Frauenlob, R.; García, C.; Bradshaw, G. A.; Burke, H. M.; Bergin, E. J. Org. Chem. 2012, 77, 4445–4449. doi:10.1021/jo3003503
Return to citation in text: [1] -
Grundmann, P.; Fessner, W.-D. Adv. Synth. Catal. 2008, 350, 1729–1735. doi:10.1002/adsc.200800203
Return to citation in text: [1] -
Sakai, N.; Asano, J.; Shimano, Y.; Konakahara, T. Tetrahedron 2008, 64, 9208–9215. doi:10.1016/j.tet.2008.07.052
Return to citation in text: [1] -
Grumbach, H.-J.; Merla, B.; Risch, N. Synthesis 1999, 1027–1033. doi:10.1055/s-1999-3497
Return to citation in text: [1] -
Royer, J.; Bonin, M.; Micouin, L. Chem. Rev. 2004, 104, 2311–2352. doi:10.1021/cr020083x
Return to citation in text: [1] -
Hwang, D.-R.; Uang, B.-J. Org. Lett. 2002, 4, 463–466. doi:10.1021/ol017229j
Return to citation in text: [1] -
Xu, Z.; Yu, X.; Feng, X.; Bao, M. J. Org. Chem. 2011, 76, 6901–6905. doi:10.1021/jo201059h
Return to citation in text: [1] -
Xu, Z.; Yu, X.; Feng, X.; Bao, M. J. Org. Chem. 2012, 77, 7114–7118. doi:10.1021/jo301035h
Return to citation in text: [1] [2] -
Rosenau, T.; Potthast, A.; Kosma, P.; Chen, C.-L.; Gratzl, J. S. J. Org. Chem. 1999, 64, 2166–2167. doi:10.1021/jo982350y
Return to citation in text: [1] -
Grierson, D. Org. React. 1990, 39, 85–295.
Return to citation in text: [1] -
Li, Z.; Bohle, D. S.; Li, C.-J. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 8928–8933. doi:10.1073/pnas.0601687103
Return to citation in text: [1]
| 1. | Gröger, H. Adv. Synth. Catal. 2001, 343, 547–558. doi:10.1002/1615-4169(200108)343:6/7<547::AID-ADSC547>3.0.CO;2-A |
| 2. |
Nicolaou, K. C.; Boddy, C. N. C.; Bräse, S.; Winssinger, N. Angew. Chem., Int. Ed. 1999, 38, 2096–2152. doi:10.1002/(SICI)1521-3773(19990802)38:15<2096::AID-ANIE2096>3.0.CO;2-F
And references therein. |
| 10. | Grundmann, P.; Fessner, W.-D. Adv. Synth. Catal. 2008, 350, 1729–1735. doi:10.1002/adsc.200800203 |
| 11. | Sakai, N.; Asano, J.; Shimano, Y.; Konakahara, T. Tetrahedron 2008, 64, 9208–9215. doi:10.1016/j.tet.2008.07.052 |
| 12. | Grumbach, H.-J.; Merla, B.; Risch, N. Synthesis 1999, 1027–1033. doi:10.1055/s-1999-3497 |
| 4. | Nanda, K. K.; Trotter, B. W. Tetrahedron Lett. 2005, 46, 2025–2028. doi:10.1016/j.tetlet.2005.01.151 |
| 5. | Petasis, N. A.; Zavialov, I. A. J. Am. Chem. Soc. 1998, 120, 11798–11799. doi:10.1021/ja981075u |
| 6. | Calí, P.; Begtrup, M. Tetrahedron 2002, 58, 1595–1605. doi:10.1016/S0040-4020(02)00021-2 |
| 7. | Beenen, M. A.; Weix, D. J.; Ellman, J. A. J. Am. Chem. Soc. 2006, 128, 6304–6305. doi:10.1021/ja060529h |
| 8. | Candeias, N. R.; Montalbano, F.; Cal, P. M. S. D.; Gois, P. M. P. Chem. Rev. 2010, 110, 6169–6193. doi:10.1021/cr100108k |
| 9. | Frauenlob, R.; García, C.; Bradshaw, G. A.; Burke, H. M.; Bergin, E. J. Org. Chem. 2012, 77, 4445–4449. doi:10.1021/jo3003503 |
| 3. | Williams, R. M.; Hendrix, J. A. Chem. Rev. 1992, 92, 889–917. doi:10.1021/cr00013a007 |
| 3. | Williams, R. M.; Hendrix, J. A. Chem. Rev. 1992, 92, 889–917. doi:10.1021/cr00013a007 |
| 16. | Xu, Z.; Yu, X.; Feng, X.; Bao, M. J. Org. Chem. 2012, 77, 7114–7118. doi:10.1021/jo301035h |
| 17. | Rosenau, T.; Potthast, A.; Kosma, P.; Chen, C.-L.; Gratzl, J. S. J. Org. Chem. 1999, 64, 2166–2167. doi:10.1021/jo982350y |
| 18. | Grierson, D. Org. React. 1990, 39, 85–295. |
| 19. | Li, Z.; Bohle, D. S.; Li, C.-J. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 8928–8933. doi:10.1073/pnas.0601687103 |
| 16. | Xu, Z.; Yu, X.; Feng, X.; Bao, M. J. Org. Chem. 2012, 77, 7114–7118. doi:10.1021/jo301035h |
| 15. | Xu, Z.; Yu, X.; Feng, X.; Bao, M. J. Org. Chem. 2011, 76, 6901–6905. doi:10.1021/jo201059h |
| 13. | Royer, J.; Bonin, M.; Micouin, L. Chem. Rev. 2004, 104, 2311–2352. doi:10.1021/cr020083x |
| 14. | Hwang, D.-R.; Uang, B.-J. Org. Lett. 2002, 4, 463–466. doi:10.1021/ol017229j |
© 2012 Xu et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)