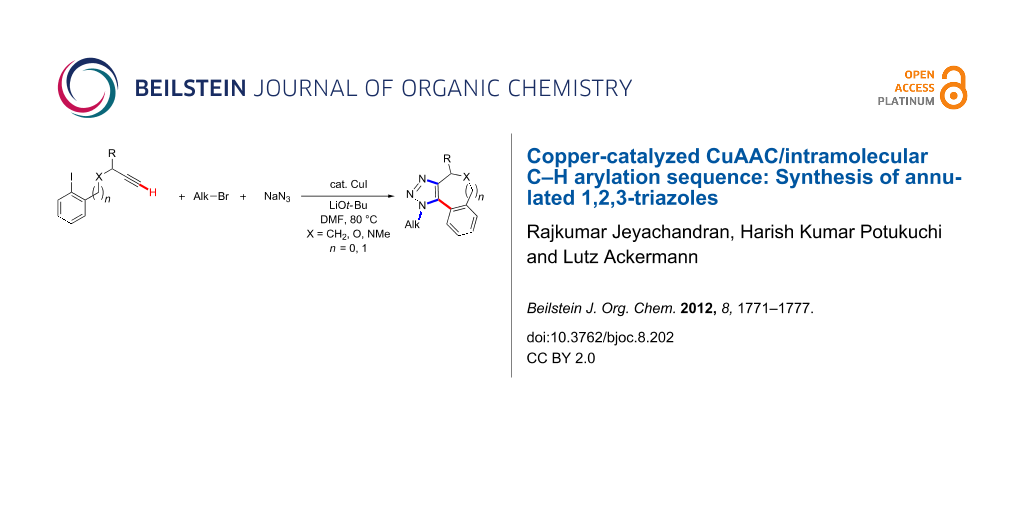
Abstract
Step-economical syntheses of annulated 1,2,3-triazoles were accomplished through copper-catalyzed intramolecular direct arylations in sustainable one-pot reactions. Thus, catalyzed cascade reactions involving [3 + 2]-azide–alkyne cycloadditions (CuAAC) and C–H bond functionalizations provided direct access to fully substituted 1,2,3-triazoles with excellent chemo- and regioselectivities. Likewise, the optimized catalytic system proved applicable to the direct preparation of 1,2-diarylated azoles through a one-pot C–H/N–H arylation reaction.
Graphical Abstract
Introduction
Transition-metal-catalyzed C–H bond functionalizations are increasingly viable tools for step-economical syntheses of various valuable bioactive compounds [1-3], which avoid the preparation and use of preactivated substrates [4-16]. This streamlining of organic synthesis has predominantly been accomplished with palladium [4-16], rhodium [17-19] or ruthenium [20-22] complexes [4-16]. However, less expensive nickel, cobalt, iron or copper catalysts bear great potential for the development of economically attractive transformations [23-50]. In this context, we previously reported on the use of cost-effective copper(I) catalysts for direct arylations of 1,2,3-triazoles. Thus, we showed that intermolecular copper-catalyzed C–H bond functionalizations could be combined with the Huisgen [51] copper(I)-catalyzed [52,53] [3 + 2]-azide–alkyne cycloaddition (CuAAC)[54], while C–H bond arylations of 1,2,3-triazoles were previously only accomplished with more expensive palladium [55-62] or ruthenium [63-66] catalysts. Notably, this strategy allowed for the atom-economical synthesis of fully substituted 1,2,3-triazoles in a highly regioselective fashion [54,67]. While the research groups of Rutjes [68] as well as Sharpless [69] elegantly devised alternative approaches exploiting 1-haloalkynes [70], we became interested in exploring a single [71-73] inexpensive copper catalyst for one-pot reaction sequences comprising a 1,3-dipolar cycloaddition along with an intramolecular C–H bond arylation; in particular, because of the notable biological activities exerted by fully substituted 1,2,3-triazoles [74-88]. As a consequence, we wish to present herein novel cascade reactions, in which cost-effective copper(I) compounds serve as the catalyst for two mechanistically distinct transformations for the synthesis of fully substituted annulated 1,2,3-triazoles as well as for twofold N–H/C–H bond arylations. Notable features of our strategy include (i) the development of a chemoselective C–H arylation-based three-component reaction, as well as (ii) the use of inexpensive CuI for the formation of up to one C–C and three C–N bonds in a site-selective fashion (Scheme 1).
Scheme 1: Copper-catalyzed step-economical C–H arylation-based cascade reaction.
Scheme 1: Copper-catalyzed step-economical C–H arylation-based cascade reaction.
Results and Discussion
We initiated our studies by exploring reaction conditions for the key copper-catalyzed intramolecular direct C–H bond arylation, employing substrate 3a (Table 1). Notably, the envisioned C–H bond functionalization occurred readily with the aryl iodide 3a when catalytic amounts of CuI were used, even at a reaction temperature as low as 60 °C, with optimal yields being obtained at 80 °C (Table 1, entries 1–6). While the transformation proceeded efficiently with LiOt-Bu as the stoichiometric base, K3PO4 only led to unsatisfactory results, even when additional stabilizing ligands were used (Table 1, entries 7–10).
Table 1: Optimization studies for the intramolecular direct arylation of triazole 3a.a
|
|
||||
| entry | base | ligand | T [°C] | isolated yield [%] |
|---|---|---|---|---|
| 1 | LiOt-Bu | – | 140 | 82 |
| 2 | LiOt-Bu | – | 120 | 97 |
| 3 | LiOt-Bu | – | 100 | 91 |
| 4 | LiOt-Bu | – | 80 | 93 |
| 5 | LiOt-Bu | – | 60 | 72 |
| 6 | LiOt-Bu | – | 20 | <2b |
| 7 | K3PO4 | DMEDA | 140 | 5b |
| 8 | K3PO4 | N,N-dimethylglycine | 140 | 5b |
| 9 | K3PO4 | 2,2-bipyridyl | 140 | 4b |
| 10 | K3PO4 | 1,10-phenanthroline | 140 | 11 |
aGeneral reaction conditions: 3a (1.00 mmol), CuI (10 mol %), ligand (10 mol %), DMF (3.0 mL).
bBy 1H NMR spectroscopy.
With optimized reaction conditions for the intramolecular direct arylation in hand, we tested the possibility of its implementation in a sequential synthesis of 1,4-dihydrochromeno[3,4-d][1,2,3]triazole (4b, Scheme 2). We were delighted to observe that the desired reaction sequence consisting of a copper-catalyzed 1,3-dipolar cycloaddition and an intramolecular C–H bond arylation converted alkyne 1a to the desired product 4b with high catalytic efficacy.
Scheme 2: Copper-catalyzed sequential catalysis with alkyne 1a.
Scheme 2: Copper-catalyzed sequential catalysis with alkyne 1a.
Subsequently, we explored the extension of this approach to the development of a chemoselective three-component one-pot reaction. Thus, we found that alkyl bromides 2 could be directly employed as user-friendly substrates for the in situ formation of the corresponding organic azides (Scheme 3). Notably, the catalytic system proved broadly applicable, and a variety of organic electrophiles 2, thereby, delivered differently decorated N-substituted 1,4-dihydrochromeno[3,4-d][1,2,3]triazoles 4.
Scheme 3: Copper-catalyzed reaction sequence using alkyl bromides 2. General reaction conditions: 1 (1.00 mmol), 2 (1.00 mmol), NaN3 (1.05 mmol), CuI (10 mol %), DMF (3.0 mL), LiOt-Bu (2.00 mmol); yields of isolated product. a60 °C in the first step.
Scheme 3: Copper-catalyzed reaction sequence using alkyl bromides 2. General reaction conditions: 1 (1.00 mmo...
Importantly, performing the one-pot reaction in a sequential fashion was not found to be mandatory. Indeed, our strategy turned out to be viable in a nonsequential manner by directly employing equimolar amounts of the three substrates. Hence, inexpensive CuI allowed the direct assembly of aryl iodides 1, alkyl bromides 2 and NaN3 with excellent chemo- and regioselectivities (Scheme 4). Thereby, a variety of annulated 1,2,3-triazoles 4 were obtained, featuring six- or seven-membered rings as key structural motifs. It is particularly noteworthy that the copper-catalyzed transformation enabled the formation of one C–C and three C–N bonds in a chemoselective manner, and thereby provided atom- and step-economical access to annulated carbo- as well as O- and N-heterocycles.
Scheme 4: Nonsequential cascade synthesis of fully substituted triazoles 4. General reaction conditions: 1 (1.00 mmol), 2 (1.00 mmol), NaN3 (1.05 mmol), CuI (10 mol %) DMF (3.0 mL), LiOt-Bu (2.00 mmol); yields of isolated product.
Scheme 4: Nonsequential cascade synthesis of fully substituted triazoles 4. General reaction conditions: 1 (1...
Finally, we found that the catalytic system also proved to be applicable to the one-pot copper-catalyzed direct arylation of various azoles 5 through N–H/C–H bond cleavages with aryl iodides 6 as the organic electrophiles (Scheme 5).
Scheme 5: Copper-catalyzed one-pot twofold C–H/N–H arylation with azoles 5. aReaction performed at 120 °C.
Scheme 5: Copper-catalyzed one-pot twofold C–H/N–H arylation with azoles 5. aReaction performed at 120 °C.
Conclusion
In summary, we have reported on the use of inexpensive copper(I) complexes for step- and atom-economical sequential catalytic transformations involving direct C–H bond arylations. Thus, CuI enabled the synthesis of fully substituted 1,2,3-triazoles through cascade reactions consisting of copper(I)-catalyzed [3 + 2]-azide–alkyne cycloadditions (CuAAC) and intramolecular C–H bond arylations. Notably, the optimized copper catalyst accelerated two mechanistically distinct transformations, which set the stage for the formation of up to one C–C and three C–N bonds in a chemo- and regioselective fashion, and also allowed for twofold C–H/N–H bond arylations on various azoles.
Experimental
General information
Catalytic reactions were carried out under an inert atmosphere of nitrogen using predried glassware. All chemicals were used as received without further purification unless otherwise specified. DMF was dried over CaH2. Alkynes 1 [89-92] and triazoles 3 [93] were synthesized according to previously described methods. CuI (99.999%) was purchased from ABCR with the following specifications: Ag <3 ppm, Ca = 2 ppm, Fe = 1 ppm, Mg <1 ppm, Zn <1 ppm. Yields refer to isolated compounds, estimated to be >95 % pure, as determined by 1H NMR. Thin-layer chromatography (TLC) was carried out on silica gel 60 F254 aluminum plates (Merck). Chromatography: Merck silica gel 60 (40–63 μm). NMR: Spectra were recorded on Varian Unity 300, Mercury 300 or Inova 500 in the solvent indicated; chemical shifts (δ) are given in parts per million (ppm). All IR spectra were taken on a Bruker FTIR Alpha device. MS: EIMS-spectra were recorded with Finnigan MAT 95, 70 eV; high-resolution mass spectrometry (HRMS) with APEX IV 7T FTICR, Bruker Daltonic. Melting points were determined with a Stuart melting-point apparatus SMP3, Barlworld Scientific; values are uncorrected.
General procedure for the synthesis of triazoles 4
NaN3 (1.05 equiv), CuI (10 mol %), LiOt-Bu (2.00 equiv), alkyne 1 (1.00 equiv) and alkyl bromide 2 (1.00 equiv) were dissolved in DMF (3.0 mL) and stirred at 80 °C for 20 h. Then, H2O (50 mL) was added at ambient temperature, and the resulting mixture was extracted with EtOAc (3 × 50 mL). The combined organic layers were washed with saturated aq NH4Cl (50 mL), H2O (50 mL) and brine (50 mL), dried over Na2SO4, filtered and concentrated in vacuo. The remaining residue was purified by column chromatography on silica gel (n-hexane/EtOAc).
Supporting Information
Supporting Information containing all experimental details and analytical data of new compounds as well as their 1H and 13C spectra are provided.
| Supporting Information File 1: Experimental procedures, characterization data, and NMR spectra for new compounds. | ||
| Format: PDF | Size: 2.0 MB | Download |
References
-
Seregin, I. V.; Gevorgyan, V. Chem. Soc. Rev. 2007, 36, 1173–1193. doi:10.1039/b606984n
Return to citation in text: [1] -
Nakao, Y. Synthesis 2011, 3209–3219. doi:10.1055/s-0030-1260212
Return to citation in text: [1] -
Zhao, D.; You, J.; Hu, C. Chem.–Eur. J. 2011, 17, 5466–5492. doi:10.1002/chem.201003039
Return to citation in text: [1] -
Special Issue 6 "C-H Functionalization". Acc. Chem. Res. 2012, 45. doi:10.1021/ar300096z
Return to citation in text: [1] [2] [3] -
Hickman, A. J.; Sanford, M. S. Nature 2012, 484, 177–185. doi:10.1038/nature11008
Return to citation in text: [1] [2] [3] -
Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215–1292. doi:10.1021/cr100280d
Return to citation in text: [1] [2] [3] -
Ackermann, L. Chem. Rev. 2011, 111, 1315–1345. doi:10.1021/cr100412j
Return to citation in text: [1] [2] [3] -
McMurray, L.; O'Hara, F.; Gaunt, M. J. Chem. Soc. Rev. 2011, 40, 1885–1898. doi:10.1039/c1cs15013h
Return to citation in text: [1] [2] [3] -
Wencel-Delord, J.; Dröge, T.; Liu, F.; Glorius, F. Chem. Soc. Rev. 2011, 40, 4740–4761. doi:10.1039/c1cs15083a
Return to citation in text: [1] [2] [3] -
Ackermann, L. Chem. Commun. 2010, 46, 4866–4877. doi:10.1039/c0cc00778a
Return to citation in text: [1] [2] [3] -
Sun, C.-L.; Li, B.-J.; Shi, Z.-J. Chem. Commun. 2010, 46, 677–685. doi:10.1039/b908581e
Return to citation in text: [1] [2] [3] -
Colby, D. A.; Bergman, R. G.; Ellman, J. A. Chem. Rev. 2010, 110, 624–655. doi:10.1021/cr900005n
Return to citation in text: [1] [2] [3] -
Fagnou, K. Top. Curr. Chem. 2010, 292, 35–56. doi:10.1007/128_2009_14
Return to citation in text: [1] [2] [3] -
Boutadla, Y.; Davies, D. L.; Macgregor, S. A.; Poblador-Bahamonde, A. I. Dalton Trans. 2009, 5820–5831. doi:10.1039/b904967c
Return to citation in text: [1] [2] [3] -
Ackermann, L.; Vicente, R.; Kapdi, A. Angew. Chem., Int. Ed. 2009, 48, 9792–9826. doi:10.1002/anie.200902996
Return to citation in text: [1] [2] [3] -
Thansandote, P.; Lautens, M. Chem.–Eur. J. 2009, 15, 5874–5883. doi:10.1002/chem.200900281
Return to citation in text: [1] [2] [3] -
Zhu, C.; Wang, R.; Falck, J. R. Chem.–Asian J. 2012, 7, 1502–1514. doi:10.1002/asia.201200035
Return to citation in text: [1] -
Song, G.; Wang, F.; Li, X. Chem. Soc. Rev. 2012, 41, 3651–3678. doi:10.1039/c2cs15281a
Return to citation in text: [1] -
Satoh, T.; Miura, M. Chem.–Eur. J. 2010, 16, 11212–11222. doi:10.1002/chem.201001363
Return to citation in text: [1] -
Ackermann, L. Pure Appl. Chem. 2010, 82, 1403–1413. doi:10.1351/PAC-CON-09-08-17
Return to citation in text: [1] -
Ackermann, L. Isr. J. Chem. 2010, 50, 652–663. doi:10.1002/ijch.201000043
Return to citation in text: [1] -
Ackermann, L.; Vicente, R. Top. Curr. Chem. 2010, 292, 211–229. doi:10.1007/128_2009_9
Return to citation in text: [1] -
Nakamura, E.; Yoshikai, N. J. Org. Chem. 2010, 75, 6061–6067. doi:10.1021/jo100693m
Return to citation in text: [1] -
Su, Y.; Jia, W.; Jiao, N. Synthesis 2011, 1678–1690. doi:10.1055/s-0030-1260028
Return to citation in text: [1] -
Daugulis, O. Top. Curr. Chem. 2010, 292, 57–84. doi:10.1007/128_2009_10
Return to citation in text: [1] -
Yoshikai, N. Synlett 2011, 1047–1051. doi:10.1055/s-0030-1259928
Return to citation in text: [1] -
Kulkarni, A.; Daugulis, O. Synthesis 2009, 4087–4109. doi:10.1055/s-0029-1217131
Return to citation in text: [1] -
Cao, H.; Zhan, H.; Lin, Y.; Lin, X.; Du, Z.; Jiang, H. Org. Lett. 2012, 14, 1688–1691. doi:10.1021/ol300232a
Return to citation in text: [1] -
Das, B.; Reddy, G. C.; Balasubramanyam, P.; Salvanna, N. Tetrahedron 2012, 68, 300–305. doi:10.1016/j.tet.2011.10.049
Return to citation in text: [1] -
Ciana, C.-L.; Phipps, R. J.; Brandt, J. R.; Meyer, F.-M.; Gaunt, M. J. Angew. Chem., Int. Ed. 2011, 50, 458–462. doi:10.1002/anie.201004703
Return to citation in text: [1] -
Duong, H. A.; Gilligan, R. E.; Cooke, M. L.; Phipps, R. J.; Gaunt, M. J. Angew. Chem., Int. Ed. 2011, 50, 463–466. doi:10.1002/anie.201004704
Return to citation in text: [1] -
Huang, G.; Sun, H.; Qiu, X.; Jin, C.; Lin, C.; Shen, Y.; Jiang, J.; Wang, L. Org. Lett. 2011, 13, 5224–5227. doi:10.1021/ol2021109
Return to citation in text: [1] -
Popov, I.; Lindeman, S.; Daugulis, O. J. Am. Chem. Soc. 2011, 133, 9286–9289. doi:10.1021/ja2041942
Return to citation in text: [1] -
Kawano, T.; Matsuyama, N.; Hirano, K.; Satoh, T.; Miura, M. J. Org. Chem. 2010, 75, 1764–1766. doi:10.1021/jo9025622
Return to citation in text: [1] -
Barbero, N.; San Martin, R.; Dominguez, E. Org. Biomol. Chem. 2010, 8, 841–845. doi:10.1039/b916549e
Return to citation in text: [1] -
Pacheco Berciano, B.; Lebrequier, S.; Besselievre, F.; Piguel, S. Org. Lett. 2010, 12, 4038–4041. doi:10.1021/ol1016433
Return to citation in text: [1] -
Besselievre, F.; Piguel, S. Angew. Chem., Int. Ed. 2009, 48, 9553–9555. doi:10.1002/anie.200904776
Return to citation in text: [1] -
Fukuzawa, S.-I.; Shimizu, E.; Ogata, K. Heterocycles 2009, 78, 645–655. doi:10.3987/COM-08-11546
Return to citation in text: [1] -
Kawano, T.; Yoshizumi, T.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2009, 11, 3072–3075. doi:10.1021/ol9011212
Return to citation in text: [1] -
Zhao, D.; Wang, W.; Yang, F.; Lan, J.; Yang, L.; Gao, G.; You, J. Angew. Chem., Int. Ed. 2009, 48, 3296–3300. doi:10.1002/anie.200900413
Return to citation in text: [1] -
Yotphan, S.; Bergman, R. G.; Ellman, J. A. Org. Lett. 2009, 11, 1511–1514. doi:10.1021/ol900103a
Return to citation in text: [1] -
Do, H.-Q.; Daugulis, O. J. Am. Chem. Soc. 2008, 130, 1128–1129. doi:10.1021/ja077862l
Return to citation in text: [1] -
Yoshizumi, T.; Tsurugi, H.; Satoh, T.; Miura, M. Tetrahedron Lett. 2008, 49, 1598–1600. doi:10.1016/j.tetlet.2008.01.042
Return to citation in text: [1] -
Do, H.-Q.; Daugulis, O. J. Am. Chem. Soc. 2007, 129, 12404–12405. doi:10.1021/ja075802+
Return to citation in text: [1] -
Nishino, M.; Hirano, K.; Satoh, T.; Miura, M. Angew. Chem., Int. Ed. 2012, 51, 6993–6997. doi:10.1002/anie.201201491
Return to citation in text: [1] -
Do, H.-Q.; Daugulis, O. J. Am. Chem. Soc. 2011, 133, 13577–13586. doi:10.1021/ja2047717
Return to citation in text: [1] -
Kitahara, M.; Umeda, N.; Hirano, K.; Satoh, T.; Miura, M. J. Am. Chem. Soc. 2011, 133, 2160–2162. doi:10.1021/ja111401h
Return to citation in text: [1] -
Zhu, M.; Fujita, K.-i.; Yamaguchi, R. Chem. Commun. 2011, 47, 12876–12878. doi:10.1039/c1cc15363c
Return to citation in text: [1] -
Song, W.; Ackermann, L. Angew. Chem., Int. Ed. 2012, 51, 8251–8254. doi:10.1002/anie.201202466
Return to citation in text: [1] -
Ackermann, L.; Punji, B.; Song, W. Adv. Synth. Catal. 2011, 353, 3325–3329. doi:10.1002/adsc.201100487
Return to citation in text: [1] -
Huisgen, R. Angew. Chem. 1963, 75, 604–637. doi:10.1002/ange.19630751304
Return to citation in text: [1] -
Tornoe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057–3064. doi:10.1021/jo011148j
Return to citation in text: [1] -
Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596–2599. doi:10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4
Return to citation in text: [1] -
Ackermann, L.; Potukuchi, H. K.; Landsberg, D.; Vicente, R. Org. Lett. 2008, 10, 3081–3084. doi:10.1021/ol801078r
Return to citation in text: [1] [2] -
Chuprakov, S.; Chernyak, N.; Dudnik, A. S.; Gevorgyan, V. Org. Lett. 2007, 9, 2333–2336. doi:10.1021/ol070697u
Return to citation in text: [1] -
Iwasaki, M.; Yorimitsu, H.; Oshima, K. Chem.–Asian J. 2007, 2, 1430–1435. doi:10.1002/asia.200700206
Return to citation in text: [1] -
Ackermann, L.; Vicente, R.; Born, R. Adv. Synth. Catal. 2008, 350, 741–748. doi:10.1002/adsc.200800016
Return to citation in text: [1] -
Ackermann, L.; Althammer, A.; Fenner, S. Angew. Chem., Int. Ed. 2009, 48, 201–204. doi:10.1002/anie.200804517
Return to citation in text: [1] -
Ackermann, L.; Vicente, R. Org. Lett. 2009, 11, 4922–4925. doi:10.1021/ol9020354
Return to citation in text: [1] -
Lapointe, D.; Fagnou, K. Org. Lett. 2009, 11, 4160–4163. doi:10.1021/ol901689q
Return to citation in text: [1] -
Schulman, J. M.; Friedman, A. A.; Panteleev, J.; Lautens, M. Chem. Commun. 2012, 48, 55–57. doi:10.1039/c1cc16110e
Return to citation in text: [1] -
Ackermann, L.; Jeyachandran, R.; Potukuchi, H. K.; Novak, P.; Büttner, L. Org. Lett. 2010, 12, 2056–2059. doi:10.1021/ol1005517
Return to citation in text: [1] -
Ackermann, L.; Vicente, R.; Althammer, A. Org. Lett. 2008, 10, 2299–2302. doi:10.1021/ol800773x
Return to citation in text: [1] -
Ackermann, L.; Born, R.; Vicente, R. ChemSusChem 2009, 2, 546–549. doi:10.1002/cssc.200900014
Return to citation in text: [1] -
Ackermann, L.; Vicente, R.; Potukuchi, H. K.; Pirovano, V. Org. Lett. 2010, 12, 5032–5035. doi:10.1021/ol102187e
Return to citation in text: [1] -
Ackermann, L.; Novák, P.; Vicente, R.; Pirovano, V.; Potukuchi, H. K. Synthesis 2010, 2245–2253. doi:10.1055/s-0029-1220010
Return to citation in text: [1] -
Ackermann, L.; Potukuchi, H. K. Org. Biomol. Chem. 2010, 8, 4503–4513. doi:10.1039/c0ob00212g
Return to citation in text: [1] -
Kuijpers, B. H. M.; Dijkmans, G. C. T.; Groothuys, S.; Quaedflieg, P. J. L. M.; Blaauw, R. H.; van Delft, F. L.; Rutjes, F. P. J. T. Synlett 2005, 3059–3062. doi:10.1055/s-2005-921919
Return to citation in text: [1] -
Hein, J. E.; Tripp, J. C.; Krasnova, L. B.; Sharpless, K. B.; Fokin, V. V. Angew. Chem., Int. Ed. 2009, 48, 8018–8021. doi:10.1002/anie.200903558
Return to citation in text: [1] -
Spiteri, C.; Moses, J. E. Angew. Chem., Int. Ed. 2010, 49, 31–33. doi:10.1002/anie.200905322
Return to citation in text: [1] -
Ackermann, L.; Born, R.; Álvarez-Bercedo, P. Angew. Chem., Int. Ed. 2007, 46, 6364–6367. doi:10.1002/anie.200701727
Return to citation in text: [1] -
Ackermann, L.; Althammer, A. Angew. Chem., Int. Ed. 2007, 46, 1627–1629. doi:10.1002/anie.200603833
Return to citation in text: [1] -
Ackermann, L.; Althammer, A.; Mayer, P. Synthesis 2009, 3493–3503. doi:10.1055/s-0029-1216977
Return to citation in text: [1] -
Astruc, D.; Liang, L.; Rapakousiou, A.; Ruiz, J. Acc. Chem. Res. 2012, 45, 630–640. doi:10.1021/ar200235m
Return to citation in text: [1] -
Agalave, S. G.; Maujan, S. R.; Pore, V. S. Chem.–Asian J. 2011, 6, 2696–2718. doi:10.1002/asia.201100432
Return to citation in text: [1] -
Pedersen, D. S.; Abell, A. Eur. J. Org. Chem. 2011, 2399–2411. doi:10.1002/ejoc.201100157
Return to citation in text: [1] -
Hänni, K. D.; Leigh, D. A. Chem. Soc. Rev. 2010, 39, 1240–1251. doi:10.1039/b901974j
Return to citation in text: [1] -
Kappe, C. O.; Van der Eycken, E. Chem. Soc. Rev. 2010, 39, 1280–1290. doi:10.1039/b901973c
Return to citation in text: [1] -
El-Sagheer, A. H.; Brown, T. Chem. Soc. Rev. 2010, 39, 1388–1405. doi:10.1039/b901971p
Return to citation in text: [1] -
Qin, A.; Lam, J. W. Y.; Tang, B. Z. Chem. Soc. Rev. 2010, 2522–2544. doi:10.1039/b909064a
Return to citation in text: [1] -
Meldal, M.; Tornoe, C. W. Chem. Rev. 2008, 108, 2952–3015. doi:10.1021/cr0783479
Return to citation in text: [1] -
Nandivada, H.; Jiang, X.; Lahann, J. Adv. Mater. 2007, 19, 2197–2208. doi:10.1002/adma.200602739
Return to citation in text: [1] -
Angell, Y. L.; Burgess, K. Chem. Soc. Rev. 2007, 36, 1674–1689. doi:10.1039/b701444a
Return to citation in text: [1] -
Fournier, D.; Hoogenboom, R.; Schubert, U. S. Chem. Soc. Rev. 2007, 36, 1369–1380. doi:10.1039/b700809k
Return to citation in text: [1] -
Moses, J. E.; Moorhouse, A. D. Chem. Soc. Rev. 2007, 36, 1249–1262. doi:10.1039/b613014n
Return to citation in text: [1] -
Lutz, J.-F. Angew. Chem., Int. Ed. 2007, 46, 1018–1025. doi:10.1002/anie.200604050
Return to citation in text: [1] -
Dondoni, A. Chem.–Asian J. 2007, 2, 700–708. doi:10.1002/asia.200700015
Return to citation in text: [1] -
Kolb, H. C.; Sharpless, K. B. Drug Discovery Today 2003, 8, 1128–1137. doi:10.1016/S1359-6446(03)02933-7
Return to citation in text: [1] -
Bowman, W. R.; Krintel, S. L.; Schilling, M. B. Org. Biomol. Chem. 2004, 2, 585–592. doi:10.1039/b310520b
Return to citation in text: [1] -
Pastine, S. J.; Youn, S. W.; Sames, D. Org. Lett. 2003, 5, 1055–1058. doi:10.1021/ol034177k
Return to citation in text: [1] -
Wang, R. T.; Chou, F. L.; Luo, F. T. J. Org. Chem. 1990, 55, 4846–4849. doi:10.1021/jo00303a017
Return to citation in text: [1] -
Shore, G.; Organ, M. G. Chem.–Eur. J. 2008, 14, 9641–9646. doi:10.1002/chem.200801610
Return to citation in text: [1] -
Kacprzak, K. Synlett 2005, 943–946. doi:10.1055/s-2005-864809
Return to citation in text: [1]
| 89. | Bowman, W. R.; Krintel, S. L.; Schilling, M. B. Org. Biomol. Chem. 2004, 2, 585–592. doi:10.1039/b310520b |
| 90. | Pastine, S. J.; Youn, S. W.; Sames, D. Org. Lett. 2003, 5, 1055–1058. doi:10.1021/ol034177k |
| 91. | Wang, R. T.; Chou, F. L.; Luo, F. T. J. Org. Chem. 1990, 55, 4846–4849. doi:10.1021/jo00303a017 |
| 92. | Shore, G.; Organ, M. G. Chem.–Eur. J. 2008, 14, 9641–9646. doi:10.1002/chem.200801610 |
| 71. | Ackermann, L.; Born, R.; Álvarez-Bercedo, P. Angew. Chem., Int. Ed. 2007, 46, 6364–6367. doi:10.1002/anie.200701727 |
| 72. | Ackermann, L.; Althammer, A. Angew. Chem., Int. Ed. 2007, 46, 1627–1629. doi:10.1002/anie.200603833 |
| 73. | Ackermann, L.; Althammer, A.; Mayer, P. Synthesis 2009, 3493–3503. doi:10.1055/s-0029-1216977 |
| 74. | Astruc, D.; Liang, L.; Rapakousiou, A.; Ruiz, J. Acc. Chem. Res. 2012, 45, 630–640. doi:10.1021/ar200235m |
| 75. | Agalave, S. G.; Maujan, S. R.; Pore, V. S. Chem.–Asian J. 2011, 6, 2696–2718. doi:10.1002/asia.201100432 |
| 76. | Pedersen, D. S.; Abell, A. Eur. J. Org. Chem. 2011, 2399–2411. doi:10.1002/ejoc.201100157 |
| 77. | Hänni, K. D.; Leigh, D. A. Chem. Soc. Rev. 2010, 39, 1240–1251. doi:10.1039/b901974j |
| 78. | Kappe, C. O.; Van der Eycken, E. Chem. Soc. Rev. 2010, 39, 1280–1290. doi:10.1039/b901973c |
| 79. | El-Sagheer, A. H.; Brown, T. Chem. Soc. Rev. 2010, 39, 1388–1405. doi:10.1039/b901971p |
| 80. | Qin, A.; Lam, J. W. Y.; Tang, B. Z. Chem. Soc. Rev. 2010, 2522–2544. doi:10.1039/b909064a |
| 81. | Meldal, M.; Tornoe, C. W. Chem. Rev. 2008, 108, 2952–3015. doi:10.1021/cr0783479 |
| 82. | Nandivada, H.; Jiang, X.; Lahann, J. Adv. Mater. 2007, 19, 2197–2208. doi:10.1002/adma.200602739 |
| 83. | Angell, Y. L.; Burgess, K. Chem. Soc. Rev. 2007, 36, 1674–1689. doi:10.1039/b701444a |
| 84. | Fournier, D.; Hoogenboom, R.; Schubert, U. S. Chem. Soc. Rev. 2007, 36, 1369–1380. doi:10.1039/b700809k |
| 85. | Moses, J. E.; Moorhouse, A. D. Chem. Soc. Rev. 2007, 36, 1249–1262. doi:10.1039/b613014n |
| 86. | Lutz, J.-F. Angew. Chem., Int. Ed. 2007, 46, 1018–1025. doi:10.1002/anie.200604050 |
| 87. | Dondoni, A. Chem.–Asian J. 2007, 2, 700–708. doi:10.1002/asia.200700015 |
| 88. | Kolb, H. C.; Sharpless, K. B. Drug Discovery Today 2003, 8, 1128–1137. doi:10.1016/S1359-6446(03)02933-7 |
| 1. | Seregin, I. V.; Gevorgyan, V. Chem. Soc. Rev. 2007, 36, 1173–1193. doi:10.1039/b606984n |
| 2. | Nakao, Y. Synthesis 2011, 3209–3219. doi:10.1055/s-0030-1260212 |
| 3. | Zhao, D.; You, J.; Hu, C. Chem.–Eur. J. 2011, 17, 5466–5492. doi:10.1002/chem.201003039 |
| 20. | Ackermann, L. Pure Appl. Chem. 2010, 82, 1403–1413. doi:10.1351/PAC-CON-09-08-17 |
| 21. | Ackermann, L. Isr. J. Chem. 2010, 50, 652–663. doi:10.1002/ijch.201000043 |
| 22. | Ackermann, L.; Vicente, R. Top. Curr. Chem. 2010, 292, 211–229. doi:10.1007/128_2009_9 |
| 69. | Hein, J. E.; Tripp, J. C.; Krasnova, L. B.; Sharpless, K. B.; Fokin, V. V. Angew. Chem., Int. Ed. 2009, 48, 8018–8021. doi:10.1002/anie.200903558 |
| 17. | Zhu, C.; Wang, R.; Falck, J. R. Chem.–Asian J. 2012, 7, 1502–1514. doi:10.1002/asia.201200035 |
| 18. | Song, G.; Wang, F.; Li, X. Chem. Soc. Rev. 2012, 41, 3651–3678. doi:10.1039/c2cs15281a |
| 19. | Satoh, T.; Miura, M. Chem.–Eur. J. 2010, 16, 11212–11222. doi:10.1002/chem.201001363 |
| 70. | Spiteri, C.; Moses, J. E. Angew. Chem., Int. Ed. 2010, 49, 31–33. doi:10.1002/anie.200905322 |
| 4. | Special Issue 6 "C-H Functionalization". Acc. Chem. Res. 2012, 45. doi:10.1021/ar300096z |
| 5. | Hickman, A. J.; Sanford, M. S. Nature 2012, 484, 177–185. doi:10.1038/nature11008 |
| 6. | Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215–1292. doi:10.1021/cr100280d |
| 7. | Ackermann, L. Chem. Rev. 2011, 111, 1315–1345. doi:10.1021/cr100412j |
| 8. | McMurray, L.; O'Hara, F.; Gaunt, M. J. Chem. Soc. Rev. 2011, 40, 1885–1898. doi:10.1039/c1cs15013h |
| 9. | Wencel-Delord, J.; Dröge, T.; Liu, F.; Glorius, F. Chem. Soc. Rev. 2011, 40, 4740–4761. doi:10.1039/c1cs15083a |
| 10. | Ackermann, L. Chem. Commun. 2010, 46, 4866–4877. doi:10.1039/c0cc00778a |
| 11. | Sun, C.-L.; Li, B.-J.; Shi, Z.-J. Chem. Commun. 2010, 46, 677–685. doi:10.1039/b908581e |
| 12. | Colby, D. A.; Bergman, R. G.; Ellman, J. A. Chem. Rev. 2010, 110, 624–655. doi:10.1021/cr900005n |
| 13. | Fagnou, K. Top. Curr. Chem. 2010, 292, 35–56. doi:10.1007/128_2009_14 |
| 14. | Boutadla, Y.; Davies, D. L.; Macgregor, S. A.; Poblador-Bahamonde, A. I. Dalton Trans. 2009, 5820–5831. doi:10.1039/b904967c |
| 15. | Ackermann, L.; Vicente, R.; Kapdi, A. Angew. Chem., Int. Ed. 2009, 48, 9792–9826. doi:10.1002/anie.200902996 |
| 16. | Thansandote, P.; Lautens, M. Chem.–Eur. J. 2009, 15, 5874–5883. doi:10.1002/chem.200900281 |
| 54. | Ackermann, L.; Potukuchi, H. K.; Landsberg, D.; Vicente, R. Org. Lett. 2008, 10, 3081–3084. doi:10.1021/ol801078r |
| 67. | Ackermann, L.; Potukuchi, H. K. Org. Biomol. Chem. 2010, 8, 4503–4513. doi:10.1039/c0ob00212g |
| 4. | Special Issue 6 "C-H Functionalization". Acc. Chem. Res. 2012, 45. doi:10.1021/ar300096z |
| 5. | Hickman, A. J.; Sanford, M. S. Nature 2012, 484, 177–185. doi:10.1038/nature11008 |
| 6. | Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215–1292. doi:10.1021/cr100280d |
| 7. | Ackermann, L. Chem. Rev. 2011, 111, 1315–1345. doi:10.1021/cr100412j |
| 8. | McMurray, L.; O'Hara, F.; Gaunt, M. J. Chem. Soc. Rev. 2011, 40, 1885–1898. doi:10.1039/c1cs15013h |
| 9. | Wencel-Delord, J.; Dröge, T.; Liu, F.; Glorius, F. Chem. Soc. Rev. 2011, 40, 4740–4761. doi:10.1039/c1cs15083a |
| 10. | Ackermann, L. Chem. Commun. 2010, 46, 4866–4877. doi:10.1039/c0cc00778a |
| 11. | Sun, C.-L.; Li, B.-J.; Shi, Z.-J. Chem. Commun. 2010, 46, 677–685. doi:10.1039/b908581e |
| 12. | Colby, D. A.; Bergman, R. G.; Ellman, J. A. Chem. Rev. 2010, 110, 624–655. doi:10.1021/cr900005n |
| 13. | Fagnou, K. Top. Curr. Chem. 2010, 292, 35–56. doi:10.1007/128_2009_14 |
| 14. | Boutadla, Y.; Davies, D. L.; Macgregor, S. A.; Poblador-Bahamonde, A. I. Dalton Trans. 2009, 5820–5831. doi:10.1039/b904967c |
| 15. | Ackermann, L.; Vicente, R.; Kapdi, A. Angew. Chem., Int. Ed. 2009, 48, 9792–9826. doi:10.1002/anie.200902996 |
| 16. | Thansandote, P.; Lautens, M. Chem.–Eur. J. 2009, 15, 5874–5883. doi:10.1002/chem.200900281 |
| 68. | Kuijpers, B. H. M.; Dijkmans, G. C. T.; Groothuys, S.; Quaedflieg, P. J. L. M.; Blaauw, R. H.; van Delft, F. L.; Rutjes, F. P. J. T. Synlett 2005, 3059–3062. doi:10.1055/s-2005-921919 |
| 52. | Tornoe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057–3064. doi:10.1021/jo011148j |
| 53. | Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596–2599. doi:10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 |
| 55. | Chuprakov, S.; Chernyak, N.; Dudnik, A. S.; Gevorgyan, V. Org. Lett. 2007, 9, 2333–2336. doi:10.1021/ol070697u |
| 56. | Iwasaki, M.; Yorimitsu, H.; Oshima, K. Chem.–Asian J. 2007, 2, 1430–1435. doi:10.1002/asia.200700206 |
| 57. | Ackermann, L.; Vicente, R.; Born, R. Adv. Synth. Catal. 2008, 350, 741–748. doi:10.1002/adsc.200800016 |
| 58. | Ackermann, L.; Althammer, A.; Fenner, S. Angew. Chem., Int. Ed. 2009, 48, 201–204. doi:10.1002/anie.200804517 |
| 59. | Ackermann, L.; Vicente, R. Org. Lett. 2009, 11, 4922–4925. doi:10.1021/ol9020354 |
| 60. | Lapointe, D.; Fagnou, K. Org. Lett. 2009, 11, 4160–4163. doi:10.1021/ol901689q |
| 61. | Schulman, J. M.; Friedman, A. A.; Panteleev, J.; Lautens, M. Chem. Commun. 2012, 48, 55–57. doi:10.1039/c1cc16110e |
| 62. | Ackermann, L.; Jeyachandran, R.; Potukuchi, H. K.; Novak, P.; Büttner, L. Org. Lett. 2010, 12, 2056–2059. doi:10.1021/ol1005517 |
| 63. | Ackermann, L.; Vicente, R.; Althammer, A. Org. Lett. 2008, 10, 2299–2302. doi:10.1021/ol800773x |
| 64. | Ackermann, L.; Born, R.; Vicente, R. ChemSusChem 2009, 2, 546–549. doi:10.1002/cssc.200900014 |
| 65. | Ackermann, L.; Vicente, R.; Potukuchi, H. K.; Pirovano, V. Org. Lett. 2010, 12, 5032–5035. doi:10.1021/ol102187e |
| 66. | Ackermann, L.; Novák, P.; Vicente, R.; Pirovano, V.; Potukuchi, H. K. Synthesis 2010, 2245–2253. doi:10.1055/s-0029-1220010 |
| 23. | Nakamura, E.; Yoshikai, N. J. Org. Chem. 2010, 75, 6061–6067. doi:10.1021/jo100693m |
| 24. | Su, Y.; Jia, W.; Jiao, N. Synthesis 2011, 1678–1690. doi:10.1055/s-0030-1260028 |
| 25. | Daugulis, O. Top. Curr. Chem. 2010, 292, 57–84. doi:10.1007/128_2009_10 |
| 26. | Yoshikai, N. Synlett 2011, 1047–1051. doi:10.1055/s-0030-1259928 |
| 27. | Kulkarni, A.; Daugulis, O. Synthesis 2009, 4087–4109. doi:10.1055/s-0029-1217131 |
| 28. | Cao, H.; Zhan, H.; Lin, Y.; Lin, X.; Du, Z.; Jiang, H. Org. Lett. 2012, 14, 1688–1691. doi:10.1021/ol300232a |
| 29. | Das, B.; Reddy, G. C.; Balasubramanyam, P.; Salvanna, N. Tetrahedron 2012, 68, 300–305. doi:10.1016/j.tet.2011.10.049 |
| 30. | Ciana, C.-L.; Phipps, R. J.; Brandt, J. R.; Meyer, F.-M.; Gaunt, M. J. Angew. Chem., Int. Ed. 2011, 50, 458–462. doi:10.1002/anie.201004703 |
| 31. | Duong, H. A.; Gilligan, R. E.; Cooke, M. L.; Phipps, R. J.; Gaunt, M. J. Angew. Chem., Int. Ed. 2011, 50, 463–466. doi:10.1002/anie.201004704 |
| 32. | Huang, G.; Sun, H.; Qiu, X.; Jin, C.; Lin, C.; Shen, Y.; Jiang, J.; Wang, L. Org. Lett. 2011, 13, 5224–5227. doi:10.1021/ol2021109 |
| 33. | Popov, I.; Lindeman, S.; Daugulis, O. J. Am. Chem. Soc. 2011, 133, 9286–9289. doi:10.1021/ja2041942 |
| 34. | Kawano, T.; Matsuyama, N.; Hirano, K.; Satoh, T.; Miura, M. J. Org. Chem. 2010, 75, 1764–1766. doi:10.1021/jo9025622 |
| 35. | Barbero, N.; San Martin, R.; Dominguez, E. Org. Biomol. Chem. 2010, 8, 841–845. doi:10.1039/b916549e |
| 36. | Pacheco Berciano, B.; Lebrequier, S.; Besselievre, F.; Piguel, S. Org. Lett. 2010, 12, 4038–4041. doi:10.1021/ol1016433 |
| 37. | Besselievre, F.; Piguel, S. Angew. Chem., Int. Ed. 2009, 48, 9553–9555. doi:10.1002/anie.200904776 |
| 38. | Fukuzawa, S.-I.; Shimizu, E.; Ogata, K. Heterocycles 2009, 78, 645–655. doi:10.3987/COM-08-11546 |
| 39. | Kawano, T.; Yoshizumi, T.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2009, 11, 3072–3075. doi:10.1021/ol9011212 |
| 40. | Zhao, D.; Wang, W.; Yang, F.; Lan, J.; Yang, L.; Gao, G.; You, J. Angew. Chem., Int. Ed. 2009, 48, 3296–3300. doi:10.1002/anie.200900413 |
| 41. | Yotphan, S.; Bergman, R. G.; Ellman, J. A. Org. Lett. 2009, 11, 1511–1514. doi:10.1021/ol900103a |
| 42. | Do, H.-Q.; Daugulis, O. J. Am. Chem. Soc. 2008, 130, 1128–1129. doi:10.1021/ja077862l |
| 43. | Yoshizumi, T.; Tsurugi, H.; Satoh, T.; Miura, M. Tetrahedron Lett. 2008, 49, 1598–1600. doi:10.1016/j.tetlet.2008.01.042 |
| 44. | Do, H.-Q.; Daugulis, O. J. Am. Chem. Soc. 2007, 129, 12404–12405. doi:10.1021/ja075802+ |
| 45. | Nishino, M.; Hirano, K.; Satoh, T.; Miura, M. Angew. Chem., Int. Ed. 2012, 51, 6993–6997. doi:10.1002/anie.201201491 |
| 46. | Do, H.-Q.; Daugulis, O. J. Am. Chem. Soc. 2011, 133, 13577–13586. doi:10.1021/ja2047717 |
| 47. | Kitahara, M.; Umeda, N.; Hirano, K.; Satoh, T.; Miura, M. J. Am. Chem. Soc. 2011, 133, 2160–2162. doi:10.1021/ja111401h |
| 48. | Zhu, M.; Fujita, K.-i.; Yamaguchi, R. Chem. Commun. 2011, 47, 12876–12878. doi:10.1039/c1cc15363c |
| 49. | Song, W.; Ackermann, L. Angew. Chem., Int. Ed. 2012, 51, 8251–8254. doi:10.1002/anie.201202466 |
| 50. | Ackermann, L.; Punji, B.; Song, W. Adv. Synth. Catal. 2011, 353, 3325–3329. doi:10.1002/adsc.201100487 |
| 4. | Special Issue 6 "C-H Functionalization". Acc. Chem. Res. 2012, 45. doi:10.1021/ar300096z |
| 5. | Hickman, A. J.; Sanford, M. S. Nature 2012, 484, 177–185. doi:10.1038/nature11008 |
| 6. | Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215–1292. doi:10.1021/cr100280d |
| 7. | Ackermann, L. Chem. Rev. 2011, 111, 1315–1345. doi:10.1021/cr100412j |
| 8. | McMurray, L.; O'Hara, F.; Gaunt, M. J. Chem. Soc. Rev. 2011, 40, 1885–1898. doi:10.1039/c1cs15013h |
| 9. | Wencel-Delord, J.; Dröge, T.; Liu, F.; Glorius, F. Chem. Soc. Rev. 2011, 40, 4740–4761. doi:10.1039/c1cs15083a |
| 10. | Ackermann, L. Chem. Commun. 2010, 46, 4866–4877. doi:10.1039/c0cc00778a |
| 11. | Sun, C.-L.; Li, B.-J.; Shi, Z.-J. Chem. Commun. 2010, 46, 677–685. doi:10.1039/b908581e |
| 12. | Colby, D. A.; Bergman, R. G.; Ellman, J. A. Chem. Rev. 2010, 110, 624–655. doi:10.1021/cr900005n |
| 13. | Fagnou, K. Top. Curr. Chem. 2010, 292, 35–56. doi:10.1007/128_2009_14 |
| 14. | Boutadla, Y.; Davies, D. L.; Macgregor, S. A.; Poblador-Bahamonde, A. I. Dalton Trans. 2009, 5820–5831. doi:10.1039/b904967c |
| 15. | Ackermann, L.; Vicente, R.; Kapdi, A. Angew. Chem., Int. Ed. 2009, 48, 9792–9826. doi:10.1002/anie.200902996 |
| 16. | Thansandote, P.; Lautens, M. Chem.–Eur. J. 2009, 15, 5874–5883. doi:10.1002/chem.200900281 |
| 54. | Ackermann, L.; Potukuchi, H. K.; Landsberg, D.; Vicente, R. Org. Lett. 2008, 10, 3081–3084. doi:10.1021/ol801078r |
© 2012 Jeyachandran et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)