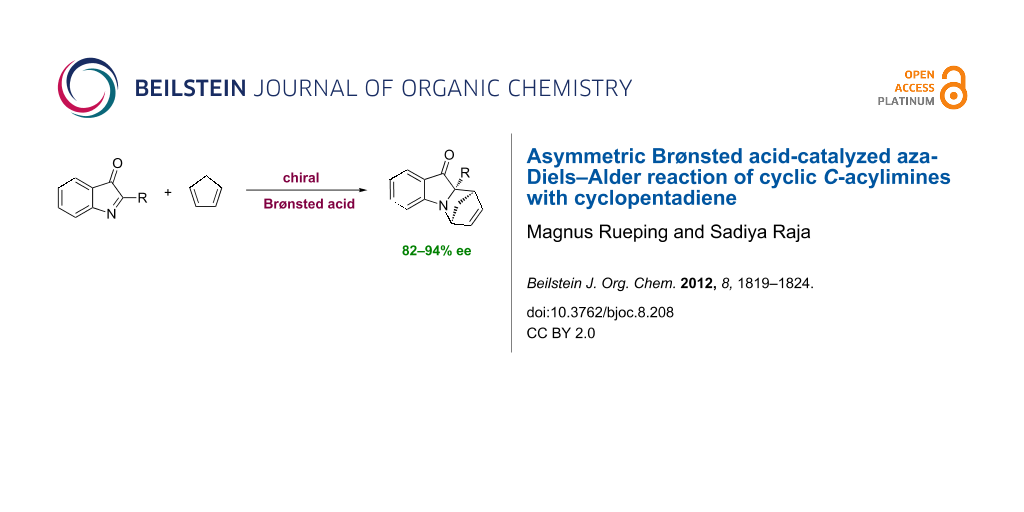
Abstract
A new chiral Brønsted acid-catalyzed aza-Diels–Alder reaction of cyclic C-acylimines with cyclopentadiene has been developed. The reaction provides optically active aza-tetracycles in good yields with high diastereo- and enantioselectivities under mild reaction conditions.
Graphical Abstract
Introduction
The enantioselective aza-Diels–Alder reaction is an important method for the construction of optically active, nitrogen-containing, six-membered rings, such as tetrahydroquinolines and piperidines. N-heterocycles are found in a wide range of natural products and many biologically active compounds [1-4]. To date, most aza-asymmetric Diels–Alder reactions have been catalyzed by chiral Lewis acids [5-16]. Recently, chiral Brønsted acids have attracted interest as effective catalysts for a variety of asymmetric transformations involving imine electrophiles [17-23]. Among others, the aza-Diels–Alder reaction of imino-dienophiles has been investigated and it was shown that the reaction between arylimines and dienes, catalyzed by chiral Brønsted acids, proceeds with high levels of enantioselectivity. However, these reactions are limited to electron-rich dienes including Brassard’s and Danishefsky dienes [24-32]. To the best of our knowledge the enantioselective Brønsted acid catalyzed aza-Diels–Alder reaction of imines with less-electron-rich dienes has not been reported. Thus, we decided to examine the unprecedented Brønsted acid catalyzed aza-Diels–Alder reaction of cyclic C-acylimines with cyclopentadiene providing optically active nitrogen-containing heterocycles (Scheme 1).
Scheme 1: Brønsted acid catalyzed aza-Diels–Alder reaction of cyclic C-acylimines with cyclopentadiene.
Scheme 1: Brønsted acid catalyzed aza-Diels–Alder reaction of cyclic C-acylimines with cyclopentadiene.
Results and Discussion
Our initial study began with the examination of the the aza-Diels–Alder reaction of cyclic C-acylimine 1 with cyclopentadiene (2) in the presence of BINOL-derived phosphoric acid diesters and N-triflylphosphoramides 4–6 (Table 1) [33-51] as the catalysts. We were delighted to see that the reaction proceeded smoothly at different temperatures and that the product could be obtained with an enantiomeric excess of 8% ee when the reaction was performed in toluene at −60 °C in the presence of catalyst 4a (Table 1, entry 1). A slight increase in enantioselectivity was observed when the reaction was conducted at −78 °C (Table 1, entry 2). Subsequently, different catalysts were applied in the Brønsted acid catalyzed hetero-Diels–Alder reaction. From the different catalysts tested, phosphoric acid diester 4b, with the 2,4,6-triisopropylphenyl substituent in the 3,3’-position of the BINOL backbone, proved to be the best catalyst, and the product was obtained with an encouraging enantiomeric excess of 74% (Table 1, entry 3). To optimize the reaction conditions further we evaluated the catalyst loading and solvent. However, the reduction of catalyst loading from 5 to 1 mol % resulted in a significant decrease in enantioselectivity (Table 1, entries 8 and 9).
Table 1: Optimization of reaction conditionsa.
|
|
|||||
| entry | catalyst | x mol % | solvent | t [min] | ee [%]b, c |
|---|---|---|---|---|---|
| 1d | 4a | 5 | toluene | 2 | 8 |
| 2 | 4a | 5 | toluene | 15 | 16 |
| 3 | 4b | 5 | toluene | 20 | 74 |
| 4 | 4c | 5 | toluene | 8h | 40 |
| 5 | 5 | 5 | toluene | 90 | 16 |
| 6 | 6a | 5 | toluene | 40 | 20 |
| 7 | 6b | 5 | toluene | 20 | 60 |
| 8 | 4b | 2 | toluene | 60 | 60 |
| 9 | 4b | 1 | toluene | 60 | 43 |
| 10 | 4b | 5 |
toluene:CHCl3
1:1 |
40 | 13 |
| 11 | 4b | 5 |
toluene:CH2Cl2
1:1 |
10 | 20 |
| 12 | 4b | 5 |
toluene:hexane
1:1 |
5 h | 74 |
| 13 | 4b | 5 |
toluene:hexane
1:2 |
6 h | 90 |
| 14 | 4b | 5 |
toluene:hexane
1:3 |
8 h | 94 |
| 15 | 4b | 5 |
toluene:hexane
1:4 |
16 h | 94 |
aReaction conditions: Imine 1, cyclopentadiene (2.0 equiv) and catalyst. bEnantiomeric excess was determined by HPLC on a chiral phase. cOnly one diastereomer is formed. dThe reaction was carried out at −60 °C.
In our previous studies in asymmetric Brønsted acid catalysis, we noticed that solvent mixtures can strongly influence both the reactivity and selectivity. Thus, we evaluated different solvent mixtures. When a 1:1 mixture of toluene and CHCl3 was used the enantioselectivity dropped considerably. The same effect was observed when a mixture of toluene and CH2Cl2 was used (Table 1, entries 10 and 11). Hence, the chlorinated solvents were replaced by hexane. Interestingly, use of a 1:1 mixture of toluene and hexane afforded the corresponding product without loss of selectivity, but, as anticipated, the reaction time was longer (Table 1, entry 12). Pleasingly, when the reaction was carried out in a 2:1 mixture of hexane/toluene the product exhibited excellent enantioselectivity (Table 1, entry 13). Further improvement of selectivity was obtained by increasing the hexane/toluene ratio to 3:1, which delivered the product with an excellent enantiomeric excess of 94% (Table 1, entry 14). With the optimal reaction conditions in hand, the substrate scope of the aza-Diels–Alder reaction was examined (Table 2). Various substituted cyclic C-acylimines 1a–i with electron-donating and electron-withdrawing groups, as well as different substitutions patterns, were applied. In all cases the corresponding tetracyclic products were obtained in high yields and with excellent diastereo- and enantioselectivities. However, the use of less reactive dienes including cyclohexadiene or linear 1,3-pentadienes resulted in reduced product formation or provided the desired products with low enantioselectivities [52-55].
Table 2: Scope of the aza-Diels–Alder reactiona.
|
|
||||
| entry | product | t [h] | yield [%]b | ee [%]c, d |
|---|---|---|---|---|
| 1 |
3a |
3 | 92 | 89 |
| 2 |
3b |
8 | 86 | 94 |
| 3 |
3c |
2 | 83 | 86 |
| 4 |
3d |
4 | 79 | 90 |
| 5 |
3e |
8 | 73 | 91 |
| 6 |
3f |
3 | 94 | 82 |
| 7 |
3g |
48 | 83 | 84 |
| 8 |
3h |
96 | 79 | 91 |
| 9 |
3i |
96 | 83 | 86 |
aReaction conditions: Imine 1, cyclopentadiene (2.0 equiv) and 5 mol % 4b. bYield of the isolated product after column chromatography. cThe enantiomeric excess was determined by HPLC on a chiral phase. dOnly one diastereomer is formed.
Conclusion
In conclusion, we have developed an enantioselective Brønsted acid catalyzed aza-Diels–Alder reaction of C-acylimines with cyclopentadiene. The corresponding aza-tetracycles were obtained in high yields and with excellent enantio- and diastereoselectivities under mild reaction conditions. The results reported not only show that chiral BINOL derived phosphoric acid diesters can be efficient catalysts for [4 + 2] cycloadditions involving less-electron-rich dienes but additionally demonstrate the high potential of these acidic Brønsted acids in asymmetric catalysis.
Experimental
The starting materials 1a–i were synthesized according to a known literature procedure [56].
General procedure for the aza-Diels–Alder reaction: In a typical experiment the imine and cyclopentadiene were suspended in a mixture of hexane/toluene (3:1) in a screw-capped test tube and stirred at −78 °C for 10 min. The catalyst (5 mol %) was added to the solution and the mixture was stirred until consumption of the imine. The crude reaction mixture was directly charged on silica gel and purified by column chromatography (hexane/ethyl acetate as eluent) to afford the desired products.
Supporting Information
| Supporting Information File 1: Experimental details and characterization of the synthesized compounds. | ||
| Format: PDF | Size: 2.1 MB | Download |
References
-
Kobayashi, S.; Jørgensen, K. A., Eds. Cycloaddition Reactions in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2002.
Return to citation in text: [1] -
Katritzky, A. R.; Rachwal, S.; Rachwal, B. Tetrahedron 1996, 52, 15031–15070. doi:10.1016/S0040-4020(96)00911-8
Return to citation in text: [1] -
Isambert, N.; Lavilla, R. Chem.–Eur. J. 2008, 14, 8444–8454. doi:10.1002/chem.200800473
Return to citation in text: [1] -
Sridharan, V.; Suryavanshi, P. A.; Menéndez, J. C. Chem. Rev. 2011, 111, 7157–7259. doi:10.1021/cr100307m
Return to citation in text: [1] -
Yamashita, Y.; Kobayashi, S. Catalytic Asymmetric Aza Diels–Alder Reactions. In Handbook of Cyclization Reactions; Ma, S., Ed.; Wiley-VCH: Weinheim, Germany, 2010; Vol. 1, pp 59–85.
Return to citation in text: [1] -
Hattori, K.; Yamamoto, H. Synlett 1993, 129–130. doi:10.1055/s-1993-22374
Return to citation in text: [1] -
Hattori, K.; Yamamoto, H. Tetrahedron 1993, 49, 1749–1760. doi:10.1016/S0040-4020(01)80532-9
Return to citation in text: [1] -
Ishihara, K.; Miyata, M.; Hattori, K.; Tada, T.; Yamamoto, H. J. Am. Chem. Soc. 1994, 116, 10520–10524. doi:10.1021/ja00102a019
Return to citation in text: [1] -
Ishitani, H.; Kobayashi, S. Tetrahedron Lett. 1996, 37, 7357–7360. doi:10.1016/0040-4039(96)01655-3
Return to citation in text: [1] -
Bromidge, S. W.; Whiting, P. C. Tetrahedron Lett. 1998, 39, 8905–8908. doi:10.1016/S0040-4039(98)01947-9
Return to citation in text: [1] -
Kobayashi, S.; Komiyama, S.; Ishitani, H. Angew. Chem. 1998, 110, 1026–1028. doi:10.1002/(SICI)1521-3757(19980403)110:7<1026::AID-ANGE1026>3.0.CO;2-G
Angew. Chem., Int. Ed. 1998, 110, 1026–1028. doi:10.1002/(SICI)1521-3773(19980420)37:7<979::AID-ANIE979>3.0.CO;2-5
Return to citation in text: [1] -
Kobayashi, S.; Kusakabe, K.-i.; Ishitani, H. Org. Lett. 2000, 2, 1225–1227. doi:10.1021/ol005656b
Return to citation in text: [1] -
Yamashita, Y.; Mizuki, Y.; Kobayashi, S. Tetrahedron Lett. 2005, 46, 1803–1806. doi:10.1016/j.tetlet.2005.01.111
Return to citation in text: [1] -
Josephsohn, N. S.; Snapper, M. L.; Hoveyda, A. H. J. Am. Chem. Soc. 2003, 125, 4018–4019. doi:10.1021/ja030033p
Return to citation in text: [1] -
Yao, S.; Saaby, S.; Hazell, R. G.; Jørgensen, K. A. Chem.–Eur. J. 2000, 6, 2435–2448. doi:10.1002/1521-3765(20000703)6:13<2435::AID-CHEM2435>3.0.CO;2-Z
Return to citation in text: [1] -
Mancheño, O. G.; Arrayás, R. G.; Carretero, J. C. J. Am. Chem. Soc. 2004, 126, 456–457. doi:10.1021/ja038494y
Return to citation in text: [1] -
Akiyama, T. Chem. Rev. 2007, 107, 5744–5758. doi:10.1021/cr068374j
Return to citation in text: [1] -
Akiyama, T.; Itoh, J.; Fuchibe, K. Adv. Synth. Catal. 2006, 348, 999–1010. doi:10.1002/adsc.200606074
Return to citation in text: [1] -
Taylor, M. S.; Jacobsen, E. N. Angew. Chem. 2006, 118, 1550–1573. doi:10.1002/ange.200503132
Angew. Chem., Int. Ed. 2006, 45, 1520–1543. doi:10.1002/anie.200503132
Return to citation in text: [1] -
Yamamoto, H.; Payette, N. Brønsted Acids, H-Bond Donors, and Combined Acid Systems in Asymmetric Catalysis. In Hydrogen Bonding in Organic Synthesis; Pihko, P. M., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp 73–140.
Return to citation in text: [1] -
Kampen, D.; Reisinger, C. M.; List, B. Top. Curr. Chem. 2009, 291, 395–456. doi:10.1007/128_2009_1
Return to citation in text: [1] -
Terada, M. Synthesis 2010, 1929–1982. doi:10.1055/s-0029-1218801
Return to citation in text: [1] -
Rueping, M.; Kuenkel, A.; Atodiresei, I. Chem. Soc. Rev. 2011, 40, 4539–4549. doi:10.1039/c1cs15087a
Return to citation in text: [1] -
Liu, H.; Cun, L.-F.; Mi, A.-Q.; Jiang, Y.-Z.; Gong, L.-Z. Org. Lett. 2006, 8, 6023–6026. doi:10.1021/ol062499t
Return to citation in text: [1] -
Rueping, M.; Azap, C. Angew. Chem. 2006, 118, 7996–7999. doi:10.1002/ange.200603199
Angew. Chem., Int. Ed. 2006, 45, 7832–7835. doi:10.1002/anie.200603199
Return to citation in text: [1] -
Itoh, J.; Fuchibe, K.; Akiyama, T. Angew. Chem. 2006, 118, 4914–4916. doi:10.1002/ange.200601345
Angew. Chem., Int. Ed. 2006, 45, 4796–4798. doi:10.1002/anie.200601345
Return to citation in text: [1] -
Akiyama, T.; Morita, H.; Fuchibe, K. J. Am. Chem. Soc. 2006, 128, 13070–13071. doi:10.1021/ja064676r
Return to citation in text: [1] -
Akiyama, T.; Tamura, Y.; Itoh, J.; Morita, H.; Fuchibe, K. Synlett 2006, 141–143. doi:10.1055/s-2005-922773
Return to citation in text: [1] -
Liu, H.; Dagousset, G.; Masson, G.; Retailleau, P.; Zhu, J. P. J. Am. Chem. Soc. 2009, 131, 4598–4599. doi:10.1021/ja900806q
Return to citation in text: [1] -
He, L.; Bekkaye, M.; Retailleau, P.; Masson, G. Org. Lett. 2012, 14, 3158–3161. doi:10.1021/ol301251h
Return to citation in text: [1] -
Huang, Y.; Unni, A. K.; Thadani, A. N.; Rawal, V. H. Nature 2003, 424, 146. doi:10.1038/424146a
Return to citation in text: [1] -
Unni, A. K.; Takenaka, N.; Yamamoto, H.; Rawal, V. H. J. Am. Chem. Soc. 2005, 127, 1336–1337. doi:10.1021/ja044076x
Return to citation in text: [1] -
Nakashima, D.; Yamamoto, H. J. Am. Chem. Soc. 2006, 128, 9626–9627. doi:10.1021/ja062508t
See for a pioneering work in the field of chiral BINOL-based N-triflylphosphoramides.
Return to citation in text: [1] -
Rueping, M.; Nachtsheim, B. J.; Ieawsuwan, W.; Atodiresei, I. Angew. Chem., Int. Ed. 2011, 50, 6706–6720. doi:10.1002/anie.201100169
Return to citation in text: [1] -
Rueping, M.; Uria, U.; Lin, M.-Y.; Atodiresei, I. J. Am. Chem. Soc. 2011, 133, 3732–3735. doi:10.1021/ja110213t
Return to citation in text: [1] -
Rueping, M.; Nachtsheim, B. J.; Koenigs, R. M.; Ieawsuwan, W. Chem.–Eur. J. 2010, 16, 13116–13126. doi:10.1002/chem.201001438
Return to citation in text: [1] -
Rueping, M.; Ieawsuwan, W.; Antonchick, A. P.; Nachtsheim, B. J. Angew. Chem., Int. Ed. 2007, 46, 2097–2100. doi:10.1002/anie.200604809
Return to citation in text: [1] -
Jiao, P.; Nakashima, D.; Yamamoto, H. Angew. Chem., Int. Ed. 2008, 47, 2411–2413. doi:10.1002/anie.200705314
Return to citation in text: [1] -
Rueping, M.; Nachtsheim, B. J.; Moreth, S. A.; Bolte, M. Angew. Chem., Int. Ed. 2008, 47, 593–596. doi:10.1002/anie.200703668
Return to citation in text: [1] -
Rueping, M.; Theissmann, T.; Kuenkel, A.; Koenigs, R. M. Angew. Chem., Int. Ed. 2008, 47, 6798–6801. doi:10.1002/anie.200802139
Return to citation in text: [1] -
Rueping, M.; Antonchick, A. P. Angew. Chem., Int. Ed. 2008, 47, 10090–10093. doi:10.1002/anie.200803610
Return to citation in text: [1] -
Enders, D.; Narine, A. A.; Toulgoat, F.; Bisschops, T. Angew. Chem., Int. Ed. 2008, 47, 5661–5665. doi:10.1002/anie.200801354
Return to citation in text: [1] -
Zeng, M.; Kang, Q.; He, Q.-L.; You, S.-L. Adv. Synth. Catal. 2008, 350, 2169–2173. doi:10.1002/adsc.200800523
Return to citation in text: [1] -
Rueping, M.; Ieawsuwan, W. Adv. Synth. Catal. 2009, 351, 78–84. doi:10.1002/adsc.200800623
Return to citation in text: [1] -
Rueping, M.; Lin, M.-Y. Chem.–Eur. J. 2010, 16, 4169–4172. doi:10.1002/chem.201000203
Return to citation in text: [1] -
Rueping, M.; Nachtsheim, B. J. Synlett 2010, 119–122. doi:10.1055/s-0029-1218539
Return to citation in text: [1] -
Rueping, M.; Merino, E.; Koenigs, R. M. Adv. Synth. Catal. 2010, 352, 2629–2634. doi:10.1002/adsc.201000547
Return to citation in text: [1] -
Cheon, C. H.; Yamamoto, H. Org. Lett. 2010, 12, 2476–2479. doi:10.1021/ol100233t
Return to citation in text: [1] -
Fleischmann, M.; Drettwann, D.; Sugiono, E.; Rueping, M.; Gschwind, R. M. Angew. Chem., Int. Ed. 2011, 50, 6364–6369. doi:10.1002/anie.201101385
Return to citation in text: [1] -
Hashimoto, T.; Nakatsu, H.; Yamamoto, K.; Maruoka, K. J. Am. Chem. Soc. 2011, 133, 9730–9733. doi:10.1021/ja203901h
Return to citation in text: [1] -
Rueping, M.; Ieawsuwan, W. Chem. Commun. 2011, 47, 11450–11452. doi:10.1039/c1cc15289k
Return to citation in text: [1] -
According to Mayr's nucleophilicity scale, which compares nucleophilictity relative to benzhydrylium ions, the nucleophilicity of the tested dienes decreases in the order: cyclopentadiene>1,3-pentadiene>2,3-dimethyl-1,3-butadiene>1,3-cyclohexadiene.
Return to citation in text: [1] -
Mayr, H.; Ofial, A. R. J. Phys. Org. Chem. 2008, 21, 584–595. doi:10.1002/poc.1325
Return to citation in text: [1] -
Mayr, H.; Ofial, A. R. Pure Appl. Chem. 2005, 77, 1807–1821. doi:10.1351/pac200577111807
Return to citation in text: [1] -
Mayr, H.; Kempf, B.; Ofial, A. R. Acc. Chem. Res. 2003, 36, 66–77. doi:10.1021/ar020094c
Return to citation in text: [1] -
Liu, Y.; McWhorter, W. W., Jr. J. Am. Chem. Soc. 2003, 125, 4240–4252. doi:10.1021/ja021380m
Return to citation in text: [1]
| 1. | Kobayashi, S.; Jørgensen, K. A., Eds. Cycloaddition Reactions in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2002. |
| 2. | Katritzky, A. R.; Rachwal, S.; Rachwal, B. Tetrahedron 1996, 52, 15031–15070. doi:10.1016/S0040-4020(96)00911-8 |
| 3. | Isambert, N.; Lavilla, R. Chem.–Eur. J. 2008, 14, 8444–8454. doi:10.1002/chem.200800473 |
| 4. | Sridharan, V.; Suryavanshi, P. A.; Menéndez, J. C. Chem. Rev. 2011, 111, 7157–7259. doi:10.1021/cr100307m |
| 33. |
Nakashima, D.; Yamamoto, H. J. Am. Chem. Soc. 2006, 128, 9626–9627. doi:10.1021/ja062508t
See for a pioneering work in the field of chiral BINOL-based N-triflylphosphoramides. |
| 34. | Rueping, M.; Nachtsheim, B. J.; Ieawsuwan, W.; Atodiresei, I. Angew. Chem., Int. Ed. 2011, 50, 6706–6720. doi:10.1002/anie.201100169 |
| 35. | Rueping, M.; Uria, U.; Lin, M.-Y.; Atodiresei, I. J. Am. Chem. Soc. 2011, 133, 3732–3735. doi:10.1021/ja110213t |
| 36. | Rueping, M.; Nachtsheim, B. J.; Koenigs, R. M.; Ieawsuwan, W. Chem.–Eur. J. 2010, 16, 13116–13126. doi:10.1002/chem.201001438 |
| 37. | Rueping, M.; Ieawsuwan, W.; Antonchick, A. P.; Nachtsheim, B. J. Angew. Chem., Int. Ed. 2007, 46, 2097–2100. doi:10.1002/anie.200604809 |
| 38. | Jiao, P.; Nakashima, D.; Yamamoto, H. Angew. Chem., Int. Ed. 2008, 47, 2411–2413. doi:10.1002/anie.200705314 |
| 39. | Rueping, M.; Nachtsheim, B. J.; Moreth, S. A.; Bolte, M. Angew. Chem., Int. Ed. 2008, 47, 593–596. doi:10.1002/anie.200703668 |
| 40. | Rueping, M.; Theissmann, T.; Kuenkel, A.; Koenigs, R. M. Angew. Chem., Int. Ed. 2008, 47, 6798–6801. doi:10.1002/anie.200802139 |
| 41. | Rueping, M.; Antonchick, A. P. Angew. Chem., Int. Ed. 2008, 47, 10090–10093. doi:10.1002/anie.200803610 |
| 42. | Enders, D.; Narine, A. A.; Toulgoat, F.; Bisschops, T. Angew. Chem., Int. Ed. 2008, 47, 5661–5665. doi:10.1002/anie.200801354 |
| 43. | Zeng, M.; Kang, Q.; He, Q.-L.; You, S.-L. Adv. Synth. Catal. 2008, 350, 2169–2173. doi:10.1002/adsc.200800523 |
| 44. | Rueping, M.; Ieawsuwan, W. Adv. Synth. Catal. 2009, 351, 78–84. doi:10.1002/adsc.200800623 |
| 45. | Rueping, M.; Lin, M.-Y. Chem.–Eur. J. 2010, 16, 4169–4172. doi:10.1002/chem.201000203 |
| 46. | Rueping, M.; Nachtsheim, B. J. Synlett 2010, 119–122. doi:10.1055/s-0029-1218539 |
| 47. | Rueping, M.; Merino, E.; Koenigs, R. M. Adv. Synth. Catal. 2010, 352, 2629–2634. doi:10.1002/adsc.201000547 |
| 48. | Cheon, C. H.; Yamamoto, H. Org. Lett. 2010, 12, 2476–2479. doi:10.1021/ol100233t |
| 49. | Fleischmann, M.; Drettwann, D.; Sugiono, E.; Rueping, M.; Gschwind, R. M. Angew. Chem., Int. Ed. 2011, 50, 6364–6369. doi:10.1002/anie.201101385 |
| 50. | Hashimoto, T.; Nakatsu, H.; Yamamoto, K.; Maruoka, K. J. Am. Chem. Soc. 2011, 133, 9730–9733. doi:10.1021/ja203901h |
| 51. | Rueping, M.; Ieawsuwan, W. Chem. Commun. 2011, 47, 11450–11452. doi:10.1039/c1cc15289k |
| 24. | Liu, H.; Cun, L.-F.; Mi, A.-Q.; Jiang, Y.-Z.; Gong, L.-Z. Org. Lett. 2006, 8, 6023–6026. doi:10.1021/ol062499t |
| 25. |
Rueping, M.; Azap, C. Angew. Chem. 2006, 118, 7996–7999. doi:10.1002/ange.200603199
Angew. Chem., Int. Ed. 2006, 45, 7832–7835. doi:10.1002/anie.200603199 |
| 26. |
Itoh, J.; Fuchibe, K.; Akiyama, T. Angew. Chem. 2006, 118, 4914–4916. doi:10.1002/ange.200601345
Angew. Chem., Int. Ed. 2006, 45, 4796–4798. doi:10.1002/anie.200601345 |
| 27. | Akiyama, T.; Morita, H.; Fuchibe, K. J. Am. Chem. Soc. 2006, 128, 13070–13071. doi:10.1021/ja064676r |
| 28. | Akiyama, T.; Tamura, Y.; Itoh, J.; Morita, H.; Fuchibe, K. Synlett 2006, 141–143. doi:10.1055/s-2005-922773 |
| 29. | Liu, H.; Dagousset, G.; Masson, G.; Retailleau, P.; Zhu, J. P. J. Am. Chem. Soc. 2009, 131, 4598–4599. doi:10.1021/ja900806q |
| 30. | He, L.; Bekkaye, M.; Retailleau, P.; Masson, G. Org. Lett. 2012, 14, 3158–3161. doi:10.1021/ol301251h |
| 31. | Huang, Y.; Unni, A. K.; Thadani, A. N.; Rawal, V. H. Nature 2003, 424, 146. doi:10.1038/424146a |
| 32. | Unni, A. K.; Takenaka, N.; Yamamoto, H.; Rawal, V. H. J. Am. Chem. Soc. 2005, 127, 1336–1337. doi:10.1021/ja044076x |
| 17. | Akiyama, T. Chem. Rev. 2007, 107, 5744–5758. doi:10.1021/cr068374j |
| 18. | Akiyama, T.; Itoh, J.; Fuchibe, K. Adv. Synth. Catal. 2006, 348, 999–1010. doi:10.1002/adsc.200606074 |
| 19. |
Taylor, M. S.; Jacobsen, E. N. Angew. Chem. 2006, 118, 1550–1573. doi:10.1002/ange.200503132
Angew. Chem., Int. Ed. 2006, 45, 1520–1543. doi:10.1002/anie.200503132 |
| 20. | Yamamoto, H.; Payette, N. Brønsted Acids, H-Bond Donors, and Combined Acid Systems in Asymmetric Catalysis. In Hydrogen Bonding in Organic Synthesis; Pihko, P. M., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp 73–140. |
| 21. | Kampen, D.; Reisinger, C. M.; List, B. Top. Curr. Chem. 2009, 291, 395–456. doi:10.1007/128_2009_1 |
| 22. | Terada, M. Synthesis 2010, 1929–1982. doi:10.1055/s-0029-1218801 |
| 23. | Rueping, M.; Kuenkel, A.; Atodiresei, I. Chem. Soc. Rev. 2011, 40, 4539–4549. doi:10.1039/c1cs15087a |
| 5. | Yamashita, Y.; Kobayashi, S. Catalytic Asymmetric Aza Diels–Alder Reactions. In Handbook of Cyclization Reactions; Ma, S., Ed.; Wiley-VCH: Weinheim, Germany, 2010; Vol. 1, pp 59–85. |
| 6. | Hattori, K.; Yamamoto, H. Synlett 1993, 129–130. doi:10.1055/s-1993-22374 |
| 7. | Hattori, K.; Yamamoto, H. Tetrahedron 1993, 49, 1749–1760. doi:10.1016/S0040-4020(01)80532-9 |
| 8. | Ishihara, K.; Miyata, M.; Hattori, K.; Tada, T.; Yamamoto, H. J. Am. Chem. Soc. 1994, 116, 10520–10524. doi:10.1021/ja00102a019 |
| 9. | Ishitani, H.; Kobayashi, S. Tetrahedron Lett. 1996, 37, 7357–7360. doi:10.1016/0040-4039(96)01655-3 |
| 10. | Bromidge, S. W.; Whiting, P. C. Tetrahedron Lett. 1998, 39, 8905–8908. doi:10.1016/S0040-4039(98)01947-9 |
| 11. |
Kobayashi, S.; Komiyama, S.; Ishitani, H. Angew. Chem. 1998, 110, 1026–1028. doi:10.1002/(SICI)1521-3757(19980403)110:7<1026::AID-ANGE1026>3.0.CO;2-G
Angew. Chem., Int. Ed. 1998, 110, 1026–1028. doi:10.1002/(SICI)1521-3773(19980420)37:7<979::AID-ANIE979>3.0.CO;2-5 |
| 12. | Kobayashi, S.; Kusakabe, K.-i.; Ishitani, H. Org. Lett. 2000, 2, 1225–1227. doi:10.1021/ol005656b |
| 13. | Yamashita, Y.; Mizuki, Y.; Kobayashi, S. Tetrahedron Lett. 2005, 46, 1803–1806. doi:10.1016/j.tetlet.2005.01.111 |
| 14. | Josephsohn, N. S.; Snapper, M. L.; Hoveyda, A. H. J. Am. Chem. Soc. 2003, 125, 4018–4019. doi:10.1021/ja030033p |
| 15. | Yao, S.; Saaby, S.; Hazell, R. G.; Jørgensen, K. A. Chem.–Eur. J. 2000, 6, 2435–2448. doi:10.1002/1521-3765(20000703)6:13<2435::AID-CHEM2435>3.0.CO;2-Z |
| 16. | Mancheño, O. G.; Arrayás, R. G.; Carretero, J. C. J. Am. Chem. Soc. 2004, 126, 456–457. doi:10.1021/ja038494y |
| 56. | Liu, Y.; McWhorter, W. W., Jr. J. Am. Chem. Soc. 2003, 125, 4240–4252. doi:10.1021/ja021380m |
| 52. | According to Mayr's nucleophilicity scale, which compares nucleophilictity relative to benzhydrylium ions, the nucleophilicity of the tested dienes decreases in the order: cyclopentadiene>1,3-pentadiene>2,3-dimethyl-1,3-butadiene>1,3-cyclohexadiene. |
| 53. | Mayr, H.; Ofial, A. R. J. Phys. Org. Chem. 2008, 21, 584–595. doi:10.1002/poc.1325 |
| 54. | Mayr, H.; Ofial, A. R. Pure Appl. Chem. 2005, 77, 1807–1821. doi:10.1351/pac200577111807 |
| 55. | Mayr, H.; Kempf, B.; Ofial, A. R. Acc. Chem. Res. 2003, 36, 66–77. doi:10.1021/ar020094c |
© 2012 Rueping and Raja; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)