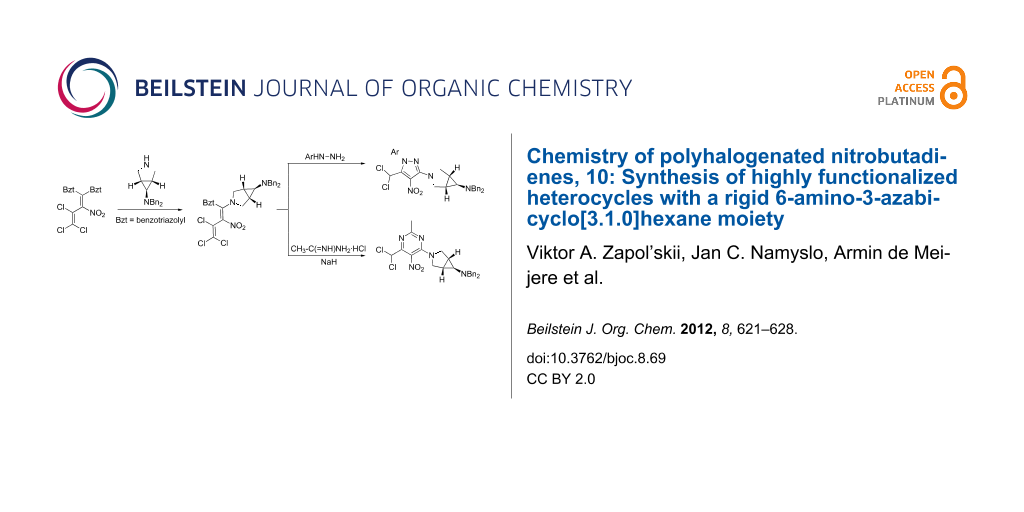
Abstract
The nitropolychlorobutadienes 3, 4 are valuable building blocks for various amination and successive heterocyclization products. Nucleophilic substitution reactions of the partially protected, bioactive amines 1, 2 with either vinyl, imidoyl or carbonyl chlorides result in the formation of the enamines 11, 12, 13, 16, 25, the amidine 6, and the amides 20, 21, respectively. In the following, cyclization to the highly functionalized pyrazoles 27, 28, pyrimidine 26 and pyridopyrimidine 24 succeeded. Deprotection of 21, 12 and 28 proved to be only partially feasible.
Graphical Abstract
Introduction
Nitropolychlorobutadienes are potent precursors for a variety of highly functionalized acyclic and (hetero)cyclic compounds. The readily accessible 2-nitroperchloro-1,3-butadiene (3) [1] is one of the most prominent members of this rather new class of dienes. During the past nine years we have published the syntheses of a wide range of diverse substance classes, applying this useful starting material [2-7].
The present work focuses on pharmacologically promising derivatives of the protected 6-amino-3-azabicyclo[3.1.0]hexanes 1 and 2, which are obtained upon reaction with polychloronitrobutadienes 3 and 4 (Figure 1).
Figure 1: Promising starting materials for biologically active compounds.
Figure 1: Promising starting materials for biologically active compounds.
The rigid bisamine 6-amino-3-azabicyclo[3.1.0]hexane is an essential building block of several pharmaceuticals, such as the potent gyrase inhibitor Trovafloxacin (Figure 2) [8,9]. As a 4th generation topoisomerase inhibitor, this fluoroquinolone anticipates replication of the bacterial DNA [10]. Other azabicyclo[3.1.0]hexane derivatives, for example with oxo-, oxazolidino-, quinolino-, oxobenzothiazolo[3,2-a]quinolino, or pyrrolidino substituents, exhibit remarkable antibacterial as well as antiprotozoal activity [11-15]. Furthermore, the 2-azabicyclo[3.1.0]hexane derivative of 3-hydroxyadamantylglycine, named Saxagliptin, is a pharmaceutical of the dipeptidyl peptidase IV (DPP-4) inhibitor class against type 2 diabetes mellitus and entered the market in 2009 (Figure 2) [16].
Figure 2: Pharmaceuticals bearing an azabicyclo[3.1.0]hexane unit.
Figure 2: Pharmaceuticals bearing an azabicyclo[3.1.0]hexane unit.
Results and Discussion
Driven by the promising stepwise reactivity of the highly substituted butadienes 3, 4 and with the above mentioned hints to biological activities in mind, we set out to develop structural conjunctions of the nitropolychlorobutadienes 3 and 4 with the 3-azabicyclo[3.1.0]hexane building blocks 1 and 2. Upon treatment with N,N-dibenzyl-3-azabicyclo[3.1.0]hexan-6-amine (1) in methanol the (tetrachloroallylidene)hydrazine 5 [4,7] derived from 3 reacted in a formal nucleophilic substitution at the imidoyl chloride unit to give the derivative 6 in 80% isolated yield (Scheme 1). The interest in such compounds derives from the fact that a number of similar hydrazones, e.g., phenyl(phenylchloromethylidene)hydrazine, exhibit fungicidal, antibacterial, and fungistatic activity [17,18].
Scheme 1: Synthesis of the azabicyclic hydrazone 6.
Scheme 1: Synthesis of the azabicyclic hydrazone 6.
In view of the insecticidal properties of some recently published [19] analogues of imidacloprid (N-[1-[(6-chloro-3-pyridyl)methyl]-4,5-dihydroimidazol-2-yl]nitramide), the substitutions of the imidazolidines 9 and 10 with 1 were also tested. Compounds 9 and 10 were prepared from the nitrodiene 3 and the ethylenediamines 7 and 8. The formal nucleophilic substitution of the α-chloro substituent within the trichlorovinyl group of 9 and 10 by the amine 1 proceeded smoothly to give both of the novel imidacloprid analogues 11 and 12, each in 90% yield (Scheme 2).
Scheme 2: Novel imidacloprid analogues 11, 12.
Scheme 2: Novel imidacloprid analogues 11, 12.
It is worth noting that all these nitroenamines 9–12 are formed as E-isomers, which are stabilized by an intramolecular hydrogen bridge bond in a six-membered pseudocycle (Figure 3).
Figure 3: Stabilizing hydrogen bond in nitrobutadiene-derived imidacloprid analogues 9–12.
Figure 3: Stabilizing hydrogen bond in nitrobutadiene-derived imidacloprid analogues 9–12.
Upon treatment of 1,3-dinitro-1,4,4-trichlorobutadiene (4), which was obtained in a four-step sequence from 1,2-dichloroethylene (mixture of diastereomers) [20,21], with a fourfold excess of the azabicyclo[3.1.0]hexane 1 in methanol at −40 °C, a twofold vinylic substitution led to the 4,4-bis(aminoazabicyclo[3.1.0]hexyl)-1-chloro-1,3-dinitrobutadiene 13 in 80% yield (Scheme 3).
Scheme 3: Synthesis of the 4,4-bis(aminoazabicyclo[3.1.0]hexyl)-1-chloro-1,3-dinitrobutadiene 13.
Scheme 3: Synthesis of the 4,4-bis(aminoazabicyclo[3.1.0]hexyl)-1-chloro-1,3-dinitrobutadiene 13.
In an analogous treatment of the pentachloronitrobutadiene 3 with a fourfold excess of 1,2,4-triazole in diethyl ether the 4,4-bistriazolyltrichloronitrobutadiene 14 (92% yield) was obtained [22] and turned out to be an appropriate substrate for a transamination, as the triazole is an excellent leaving group. Thus, by treatment of 14 with p-phenetidine the 4-triazolyl-4-(4-ethoxyphenylamino)butadiene 15 was obtained in 83% yield. Subsequent reaction of 15 with tert-butyl 6-amino-3-azabicyclo[3.1.0]hexane-3-carboxylate (2) provided the tris(amino)butadiene 16 in 70% yield (Scheme 4).
Scheme 4: Synthesis of the highly substituted trisaminodichloronitrobutadiene 16.
Scheme 4: Synthesis of the highly substituted trisaminodichloronitrobutadiene 16.
It is noteworthy that the diaminonitrovinyl moiety in compound 15 remains unaffected in the last substitution step. Apparently, the 2-nitroenamine substructure in 15 is less electrophilic at C-3 than its conceivable tautomeric structure 16A would suggest. After the third formal nucleophilic substitution at C-2, the resulting product 16 has a second enamine substructure as in 16A, rather than a dichloromethylimine subunit as in 16B. This is obvious from the 1H NMR spectrum of 16, which does not show a signal corresponding to a dichloromethyl proton, and in addition the 13C NMR spectrum shows four signals associated with olefinic carbon atoms (Figure 4).
Figure 4: Conceivable tautomeric structures of 16.
Figure 4: Conceivable tautomeric structures of 16.
In addition to the direct attachment of heteroatoms or even heterocycles to nitropolychlorobutadienes by formal vinylic nucleophilic substitution reactions as described above, it was of interest to incorporate a persubstituted diene unit as in 3 and 4 into a heterocycle. For example, the isothiazole 17 was obtained from the nitrodiene 3 upon treatment with elemental sulfur at 200 °C [23]. Subsequent reaction with fuming nitric acid provided the dichloroisothiazolocarboxylic acid 18 [24], which could be easily converted with thionyl chloride to the corresponding acid chloride 19 (93% yield). The latter smoothly reacted with the azabicyclohexane derivatives 1 and 2 to provide the corresponding amides 20 and 21, respectively (Scheme 5). These amides 20 and 21 are hot candidates for biological testing, as some known amides of 4-chloroisothiazol-3-carboxylic acid have been shown to exhibit high antibacterial activity [25-27].
Scheme 5: Syntheses of the perfunctionalized isothiazole derivatives 20, 21.
Scheme 5: Syntheses of the perfunctionalized isothiazole derivatives 20, 21.
The high number of heteroatoms in 20 and 21, accompanied by only a few hydrogen atoms, requires 1H/13C-2D as well as nitrogen NMR spectra for structural assignments. For example, aside from aromatic protons in 20, the methylene groups within the pyrrolidine ring each appear as a set of one single doublet (geminal coupling only) and a doublet of doublets (with additional coupling to the bridgehead proton due to an appropriate dihedral angle). Narrow shifts of the corresponding carbon atoms were assigned by means of an HSQC spectrum. The benzylic methylene protons give two slightly separated doublets (each with the expected 2J coupling of about 13 Hz). Appearing at 3.59 and 3.54 ppm, respectively, they are attached to isochronous carbon atoms at 59.0 ppm. Furthermore, the proton of the NCH fragment (13C NMR: δ = 47.3 ppm) of the cyclopropane ring appears as a triplet at 1.54 ppm (J = 2.3 Hz). Interestingly, the chemical shift of both of the bridgehead protons is 1.35 ppm (dd, J = 4.2, 2.3 Hz), whereas the corresponding carbon atoms have slightly different chemical shifts of 25.5 and 24.5 ppm, respectively. Most of the quarternary carbon shifts are unambiguous, whereas an HMBC spectrum was necessary for the assignment of two downfield signals: 160.5 ppm (C=O) and 160.4 ppm (SC). 14N NMR (one-dimensional, direct detection) and an inverse-detected 1H/15N-HMBC gave the nitrogen shifts (internal MeNO2 at 0.0 ppm): −65.8 ppm (C=N), −244.2 ppm (NCO), and −319.5 ppm (NBn).
In addition to the twofold triazole substitution, the nitrodiene 3 was treated with four equiv of benzotriazole in THF. Thus, the bisbenzotriazole derivative 22 was obtained in 76% yield [28]. Having the target of further compounds with insecticidal activities in mind, 22 was treated with 2-aminopyridine, but the simple substitution product, a benzotriazolyl-1-(pyrid-2-ylamino)diene, which must have been formed initially, apparently must have tautomerized to a pyridin-2(1H)-imine derivative, which then underwent cyclization by a formal nucleophilic substitution leading to the 4H-pyrido[1,2-a]pyrimidine 23. The remaining benzotriazole group in 23, which is activated by the adjacent nitro substituent allows for a further nucleophilic substitution. Therefore, upon treatment with the azabicyclohexane 1 under mild conditions (methanol, rt) the enamine 24 was formed in 86% yield (Scheme 6). Similar pyrido[1,2-a]pyrimidines show antiviral [29], antithrombotic [30] and antibacterial [31-34] activities.
Scheme 6: Preparation of the pyrazoles 27, 28, the pyrimidine 26 and the pyridopyrimidine 24.
Scheme 6: Preparation of the pyrazoles 27, 28, the pyrimidine 26 and the pyridopyrimidine 24.
Alternatively, conversion of bis(benzotriazolyl)butadiene 22 with one equiv of amine 1 led to transamination at C-1 of the butadiene to furnish the azabicyclohexyl-nitrobutadiene 25 in 95% yield. The latter, on one hand was converted by treatment with acetamidine hydrochloride in THF to the pyrimidine 26 (yield 58%), which apparently proceeds by transamination and subsequent intramolecular SNVin reaction. The structure of an analogous derivative of this heterocycle was previously confirmed by X-ray crystallography [5]. On the other hand, nitrodiene 25 was treated with arylhydrazines to give the persubstituted aminonitropyrazoles 27–28 in 75–90% yield (Scheme 6).
A conceivable mechanism for the cascade reaction that leads to the pyrazoles 27 and 28 is presented in Scheme 7. Initially, the trichlorobutene I is formed upon addition of the arylhydrazine to the nitro-substituted butadiene 25. Subsequent elimination of benzotriazole results in the diaminobutadiene II, which tautomerizes to the stable amidine III. The pyrazoline IV is then formed by an intramolecular SNVin reaction. Finally, HCl elimination affords the pyrazoles 27, 28. The stimulus to investigate such compounds originated from the known pharmacological activities of 4-nitropyrazoles [35-42].
Scheme 7: Proposed reaction mechanism for the formation of 27, 28.
Scheme 7: Proposed reaction mechanism for the formation of 27, 28.
At the end of our synthetic work, three of the intricate 6-amino-3-azabicyclo[3.1.0]hexane derivatives were subjected to common deprotection conditions on a micromole scale (Scheme 8). Interestingly, in the case of the removal of the N-Boc group from 21 by means of surplus trifluoroacetic acid under mild conditions, the corresponding free amine 29 was obtained in 83% yield, without any further optimization. However, accurately tailored conditions seemed to be necessary for the N-debenzylation of the protected amino compounds 12 and 28. The application of the usual reductive conditions (i.e., hydrogen under atmospheric pressure, palladium on charcoal suspended in, e.g., ethanol) to the protected amine 12 led to a multiple reaction involving the deprotection, a bisdechlorination and final hydrolysis of an intermediate imine. The resulting ketone 30 was isolated in 44% yield. On the other hand, the highly substituted dibenzylamino compound 28 showed another interesting reaction pathway: One of its benzyl groups was unmodified, even though the competing reduction of the dichloromethyl substituent took place to give the mono-N-benzylated rigid amine 31 in 45% yield. With Pd/C at ambient hydrogen pressure and ethanol as a solvent, no reduction of the nitro group or the aromatic chlorine atoms in 12 and 28 was observed. To avoid the described side reactions, further experiments should comprise the optimization of the hydrogen volume and pressure as well as some fine tuning of the catalyst/solvent system.
Scheme 8: Attempted deprotection of the azabicyclic compounds 21,12, and 28.
Scheme 8: Attempted deprotection of the azabicyclic compounds 21,12, and 28.
To the best of our knowledge, the transformation observed for compound 12, i.e., the reduction of a 1-amino-2,2-dichlorovinyl group to an acetyl substituent, is hitherto unprecedented. However, the individual parts of these multistep reactions, namely the conversion of the 1-amino-2,2-dichlorovinyl group to a dichloromethyl ketone and, in addition, the reductive bisdechlorination of a dichloromethyl group were recently published by our group [5,43].
Conclusion
Regioselective reactions of the nitrotrichlorobuta-1,3-dienes 3 and 4, some after initial transformations to other derivatives with exo-6-N,N-dibenzylamino-3-azabicyclo-[3.1.0]hexane (1) and exo-6-amino-3-(tert-butoxycarbonylaza)bicyclo[3.1.0]hexane (2), led to a series of potentially biologically active compounds, which are due to be tested in various assays.
Supporting Information
| Supporting Information File 1: Experimental section. | ||
| Format: PDF | Size: 337.8 KB | Download |
Acknowledgements
This work was supported financially by Bayer AG (Leverkusen, Germany). The authors are grateful to Dr. C. Stratmann (Georg-August-Universität Göttingen, Germany) for samples of the bicyclic diamine derivatives 1 and 2 as well as to Dr. H. Frauendorf for HR–ESI MS. We thank Dr. G. Dräger (Leibniz-Universität Hannover, Germany) for ESI and EI high-resolution mass spectra.
References
-
Potkin, V. I.; Zapol’skii, V. A.; Kaberdin, R. V. Izv. Akad. Nauk Bel., Ser. Khim. Nauk 1996, 40, 68–71.
Return to citation in text: [1] -
Kaberdin, R. V.; Potkin, V. I.; Zapol’skii, V. A. Russ. Chem. Rev. 1997, 66, 827–842. doi:10.1070/RC1997v066n10ABEH000310
Return to citation in text: [1] -
Zapol’skii, V. A.; Namyslo, J. C.; Adam, A. E. W.; Kaufmann, D. E. Heterocycles 2004, 63, 1281–1298. doi:10.3987/COM-04-10020
Return to citation in text: [1] -
Zapol’skii, V. A.; Nutz, E.; Namyslo, J. C.; Adam, A. E. W.; Kaufmann, D. E. Synthesis 2006, 2927–2933. doi:10.1055/s-2006-950187
Return to citation in text: [1] [2] -
Zapol’skii, V. A.; Namyslo, J. C.; Altug, C.; Gjikaj, M.; Kaufmann, D. E. Synthesis 2008, 304–310. doi:10.1055/s-2007-990948
Return to citation in text: [1] [2] [3] -
Zapol’skii, V. A.; Fischer, R.; Namyslo, J. C.; Kaufmann, D. E. Bioorg. Med. Chem. 2009, 17, 4206–4215. doi:10.1016/j.bmc.2009.01.001
Return to citation in text: [1] -
Zapol’skii, V. A.; Namyslo, J. C.; Gjikaj, M.; Kaufmann, D. E. Z. Naturforsch., B 2010, 65b, 843–860.
Return to citation in text: [1] [2] -
Brighty, K. E. Azabicyclo Quinolone Carboxylic Acids. WO Patent 91/02526, March 7, 1991.
Return to citation in text: [1] -
Fromtling, R. A.; Castañer, J. Drugs Future 1996, 21, 496–505.
Return to citation in text: [1] -
Gootz, T. D.; Zaniewski, R.; Haskell, S.; Schmieder, B.; Tankovic, J.; Girard, D.; Courvalin, P.; Polzer, R. J. Antimicrob. Agents Chemother. 1996, 40, 2691–2697.
Return to citation in text: [1] -
Komine, T.; Kojima, A.; Asahina, Y.; Saito, T.; Takano, H.; Shibue, T.; Fukuda, Y. J. Med. Chem. 2008, 51, 6558–6562. doi:10.1021/jm800800c
Return to citation in text: [1] -
Sattigeri, J. A.; Andappan, M. M. S.; Kishore, K.; Thangathirupathy, S.; Sundaram, S.; Singh, S.; Sharma, S.; Davis, J. A.; Chugh, A.; Bansal, V. S. Bioorg. Med. Chem. Lett. 2008, 18, 4087–4091. doi:10.1016/j.bmcl.2008.05.101
Return to citation in text: [1] -
Dinakaran, M.; Senthilkumar, P.; Yogeeswari, P.; China, A.; Nagaraja, V.; Sriram, D. Bioorg. Med. Chem. 2008, 16, 3408–3418. doi:10.1016/j.bmc.2007.11.016
Return to citation in text: [1] -
Kumar, N.; Kaur, K.; Aeron, S.; Dharmarajan, S.; Silamkoti, A. D. V.; Mehta, A.; Gupta, S.; Chugh, A.; Gupta, J. B.; Salman, M.; Palle, V. P.; Cliffe, I. A. Bioorg. Med. Chem. Lett. 2007, 17, 5256–5260. doi:10.1016/j.bmcl.2007.06.081
Return to citation in text: [1] -
Anquetin, G.; Greiner, J.; Mahmoudi, N.; Santillana-Hayat, M.; Gozalbes, R.; Farhati, K.; Derouin, F.; Aubry, A.; Cambau, E.; Vierling, P. Eur. J. Med. Chem. 2006, 41, 1478–1493. doi:10.1016/j.ejmech.2006.07.003
Return to citation in text: [1] -
Savage, S. A.; Jones, G. S.; Kolotuchin, S.; Ramrattan, S. A.; Vu, T.; Waltermire, R. E. Org. Process Res. Dev. 2009, 13, 1169–1176. doi:10.1021/op900226j
Return to citation in text: [1] -
Molodykh, Z. V.; Buzykin, B. I.; Kudrina, M. A.; Sysoeva, L. P.; Gazetdinova, N. G.; Neklesova, I. D.; Kitaev, Y. P. Pharm. Chem. J. 1980, 14, 162–169. doi:10.1007/BF00777380
Return to citation in text: [1] -
Rector, D. L.; Folz, S. D.; Conklin, R. D.; Nowakowski, L. H.; Kaugars, G. J. Med. Chem. 1981, 24, 532–538. doi:10.1021/jm00137a011
Return to citation in text: [1] -
Fischer, R.; Jeschke, P.; Erdelen, C.; Lösel, P.; Reckmann, U.; Kaufmann, D. E.; Zapol’skii, V. A. Halogenated Nitrobutadienes for Controlling Animal Pests. WO 03/040129 A1, May 15, 2003.
Return to citation in text: [1] -
Zapol’skii, V. A.; Potkin, V. I.; Kaberdin, R. V. Vestsi Nats. Akad. Navuk Belarusi, Ser. Khim. Navuk 1993, 3, 76–80.
Return to citation in text: [1] -
Zapol’skii, V. A.; Namyslo, J. C.; Blaschkowski, B.; Kaufmann, D. E. Synlett 2006, 3464–3468. doi:10.1055/s-2006-956490
Return to citation in text: [1] -
Zapol’skii, V. A.; Potkin, V. I.; Nechai, N. I.; Kaberdin, R. V.; Pevzner, M. S. Russ. J. Org. Chem. 1997, 33, 1632–1637.
Return to citation in text: [1] -
Kaberdin, R. V.; Potkin, V. I.; Ol'dekop, Y. A. Dokl. Chem. 1988, 300, 173–175.
Return to citation in text: [1] -
Kaberdin, R. V.; Potkin, V. I.; Ol'dekop, Y. A. Russ. J. Org. Chem. 1990, 26, 1347–1351.
Return to citation in text: [1] -
Li, J.; Wakefield, B. D.; Ruble, J. C.; Stiff, C. M.; Romero, D. L.; Marotti, K. R.; Sweeney, M. T.; Zurenko, G. E.; Rohrer, D. C.; Thorarensen, A. Bioorg. Med. Chem. Lett. 2007, 17, 2347–2350. doi:10.1016/j.bmcl.2006.12.055
Return to citation in text: [1] -
Kaizerman, J. A.; Gross, M. I.; Ge, Y.; White, S.; Hu, W.; Duan, J.-X.; Baird, E. E.; Johnson, K. W.; Tanaka, R. D.; Moser, H. E.; Bürli, R. W. J. Med. Chem. 2003, 46, 3914–3929. doi:10.1021/jm030097a
Return to citation in text: [1] -
Bürli, R. W.; Ge, Y.; White, S.; Baird, E. E.; Touami, S. M.; Taylor, M.; Kaizerman, J. A.; Moser, H. E. Bioorg. Med. Chem. Lett. 2002, 12, 2591–2594. doi:10.1016/S0960-894X(02)00515-2
Return to citation in text: [1] -
Zapol’skii, V. A.; Potkin, V. I.; Nechai, N. I.; Kaberdin, R. V. Russ. J. Org. Chem. 1993, 29, 731–734.
Return to citation in text: [1] -
Ukrainets, I. V.; Bereznyakova, N. L.; Turaibei, I. A. Chem. Heterocycl. Comp. 2008, 44, 50–63. doi:10.1007/s10593-008-0012-x
Return to citation in text: [1] -
Sturgeon, S. A.; Jones, C.; Angus, J. A.; Wright, C. E. Eur. J. Pharm. Sci. 2008, 587, 209–215. doi:10.1016/j.ejphar.2008.03.017
Return to citation in text: [1] -
Yoshida, K.-i.; Nakayama, K.; Ohtsuka, M.; Kuru, N.; Yokomizo, Y.; Sakamoto, A.; Takemura, M.; Hoshino, K.; Kanda, H.; Nitanai, H.; Namba, K.; Yoshida, K.; Inamura, Y.; Zhang, J. Z.; Lee, V. J.; Watkins, W. J. Bioorg. Med. Chem. 2007, 15, 7087–7097. doi:10.1016/j.bmc.2007.07.039
Return to citation in text: [1] -
La Motta, C.; Sartini, S.; Mugnaini, L.; Simorini, F.; Taliani, S.; Salerno, S.; Marini, A. M.; Da Settimo, F.; Lavecchia, A.; Novellino, E.; Cantore, M.; Failli, P.; Ciuffi, M. J. Med. Chem. 2007, 50, 4917–4927. doi:10.1021/jm070398a
Return to citation in text: [1] -
Barbeau, O. R.; Cano-Soumillac, C.; Griffin, R. J.; Hardcastle, I. R.; Smith, G. C. M.; Richardson, C.; Clegg, W.; Harrington, R. W.; Golding, B. T. Org. Biomol. Chem. 2007, 5, 2670–2677. doi:10.1039/b705095j
Return to citation in text: [1] -
Yoshida, K.-i.; Nakayama, K.; Kuru, N.; Kobayashi, S.; Ohtsuka, M.; Takemura, M.; Hoshino, K.; Kanda, H.; Zhang, J. Z.; Lee, V. J.; Watkins, W. J. Bioorg. Med. Chem. 2006, 14, 1993–2004. doi:10.1016/j.bmc.2005.10.043
Return to citation in text: [1] -
Aiello, E.; Aiello, S.; Mingoia, F.; Bacchi, A.; Pelizzi, G.; Musiu, C.; Setzu, M. G.; Pani, A.; La Colla, P.; Marongiu, M. E. Bioorg. Med. Chem. 2000, 8, 2719–2728. doi:10.1016/S0968-0896(00)00211-X
Return to citation in text: [1] -
Luo, C.; Xie, P.; Marmorstein, R. J. Med. Chem. 2008, 51, 6121–6127. doi:10.1021/jm800539g
Return to citation in text: [1] -
Firestine, S. M.; Wu, W.; Youn, H.; Davisson, V. J. Bioorg. Med. Chem. 2009, 17, 794–803. doi:10.1016/j.bmc.2008.11.057
Return to citation in text: [1] -
Schepetkin, I. A.; Khlebnikov, A. I.; Quinn, M. T. J. Med. Chem. 2007, 50, 4928–4938. doi:10.1021/jm070600+
Return to citation in text: [1] -
Baraldi, P. G.; Vicentini, C. B.; Simoni, D.; Menziani, E. Farmaco, Ed. Sci. 1983, 38, 508–513.
Return to citation in text: [1] -
Walczak, K.; Gondela, A.; Suwiński, J. Eur. J. Med. Chem. 2004, 39, 849–853. doi:10.1016/j.ejmech.2004.06.014
Return to citation in text: [1] -
Lu, I.-L.; Mahindroo, N.; Liang, P.-H.; Peng, Y.-H.; Kuo, C.-J.; Tsai, K.-C.; Hsieh, H.-P.; Chao, Y.-S.; Wu, S.-Y. J. Med. Chem. 2006, 49, 5154–5161. doi:10.1021/jm060207o
Return to citation in text: [1] -
Penning, T. D.; Talley, J. J.; Bertenshaw, S. R.; Carter, J. S.; Collins, P. W.; Docter, S.; Graneto, M. J.; Lee, L. F.; Malecha, J. W.; Miyashiro, J. M.; Rogers, R. S.; Rogier, D. J.; Yu, S. S.; Anderson, G. D.; Burton, E. G.; Cogburn, J. N.; Gregory, S. A.; Koboldt, C. M.; Perkins, W. E.; Seibert, K.; Veenhuizen, A. W.; Zhang, Y. Y.; Isakson, P. C. J. Med. Chem. 1997, 40, 1347–1365. doi:10.1021/jm960803q
Return to citation in text: [1] -
Zapol’skii, V. A.; Namyslo, J. C.; Gjikaj, M.; Kaufmann, D. E. Synlett 2007, 1507–1512. doi:10.1055/s-2007-982554
Return to citation in text: [1]
| 5. | Zapol’skii, V. A.; Namyslo, J. C.; Altug, C.; Gjikaj, M.; Kaufmann, D. E. Synthesis 2008, 304–310. doi:10.1055/s-2007-990948 |
| 30. | Sturgeon, S. A.; Jones, C.; Angus, J. A.; Wright, C. E. Eur. J. Pharm. Sci. 2008, 587, 209–215. doi:10.1016/j.ejphar.2008.03.017 |
| 31. | Yoshida, K.-i.; Nakayama, K.; Ohtsuka, M.; Kuru, N.; Yokomizo, Y.; Sakamoto, A.; Takemura, M.; Hoshino, K.; Kanda, H.; Nitanai, H.; Namba, K.; Yoshida, K.; Inamura, Y.; Zhang, J. Z.; Lee, V. J.; Watkins, W. J. Bioorg. Med. Chem. 2007, 15, 7087–7097. doi:10.1016/j.bmc.2007.07.039 |
| 32. | La Motta, C.; Sartini, S.; Mugnaini, L.; Simorini, F.; Taliani, S.; Salerno, S.; Marini, A. M.; Da Settimo, F.; Lavecchia, A.; Novellino, E.; Cantore, M.; Failli, P.; Ciuffi, M. J. Med. Chem. 2007, 50, 4917–4927. doi:10.1021/jm070398a |
| 33. | Barbeau, O. R.; Cano-Soumillac, C.; Griffin, R. J.; Hardcastle, I. R.; Smith, G. C. M.; Richardson, C.; Clegg, W.; Harrington, R. W.; Golding, B. T. Org. Biomol. Chem. 2007, 5, 2670–2677. doi:10.1039/b705095j |
| 34. | Yoshida, K.-i.; Nakayama, K.; Kuru, N.; Kobayashi, S.; Ohtsuka, M.; Takemura, M.; Hoshino, K.; Kanda, H.; Zhang, J. Z.; Lee, V. J.; Watkins, W. J. Bioorg. Med. Chem. 2006, 14, 1993–2004. doi:10.1016/j.bmc.2005.10.043 |
| 1. | Potkin, V. I.; Zapol’skii, V. A.; Kaberdin, R. V. Izv. Akad. Nauk Bel., Ser. Khim. Nauk 1996, 40, 68–71. |
| 11. | Komine, T.; Kojima, A.; Asahina, Y.; Saito, T.; Takano, H.; Shibue, T.; Fukuda, Y. J. Med. Chem. 2008, 51, 6558–6562. doi:10.1021/jm800800c |
| 12. | Sattigeri, J. A.; Andappan, M. M. S.; Kishore, K.; Thangathirupathy, S.; Sundaram, S.; Singh, S.; Sharma, S.; Davis, J. A.; Chugh, A.; Bansal, V. S. Bioorg. Med. Chem. Lett. 2008, 18, 4087–4091. doi:10.1016/j.bmcl.2008.05.101 |
| 13. | Dinakaran, M.; Senthilkumar, P.; Yogeeswari, P.; China, A.; Nagaraja, V.; Sriram, D. Bioorg. Med. Chem. 2008, 16, 3408–3418. doi:10.1016/j.bmc.2007.11.016 |
| 14. | Kumar, N.; Kaur, K.; Aeron, S.; Dharmarajan, S.; Silamkoti, A. D. V.; Mehta, A.; Gupta, S.; Chugh, A.; Gupta, J. B.; Salman, M.; Palle, V. P.; Cliffe, I. A. Bioorg. Med. Chem. Lett. 2007, 17, 5256–5260. doi:10.1016/j.bmcl.2007.06.081 |
| 15. | Anquetin, G.; Greiner, J.; Mahmoudi, N.; Santillana-Hayat, M.; Gozalbes, R.; Farhati, K.; Derouin, F.; Aubry, A.; Cambau, E.; Vierling, P. Eur. J. Med. Chem. 2006, 41, 1478–1493. doi:10.1016/j.ejmech.2006.07.003 |
| 28. | Zapol’skii, V. A.; Potkin, V. I.; Nechai, N. I.; Kaberdin, R. V. Russ. J. Org. Chem. 1993, 29, 731–734. |
| 10. | Gootz, T. D.; Zaniewski, R.; Haskell, S.; Schmieder, B.; Tankovic, J.; Girard, D.; Courvalin, P.; Polzer, R. J. Antimicrob. Agents Chemother. 1996, 40, 2691–2697. |
| 29. | Ukrainets, I. V.; Bereznyakova, N. L.; Turaibei, I. A. Chem. Heterocycl. Comp. 2008, 44, 50–63. doi:10.1007/s10593-008-0012-x |
| 8. | Brighty, K. E. Azabicyclo Quinolone Carboxylic Acids. WO Patent 91/02526, March 7, 1991. |
| 9. | Fromtling, R. A.; Castañer, J. Drugs Future 1996, 21, 496–505. |
| 24. | Kaberdin, R. V.; Potkin, V. I.; Ol'dekop, Y. A. Russ. J. Org. Chem. 1990, 26, 1347–1351. |
| 2. | Kaberdin, R. V.; Potkin, V. I.; Zapol’skii, V. A. Russ. Chem. Rev. 1997, 66, 827–842. doi:10.1070/RC1997v066n10ABEH000310 |
| 3. | Zapol’skii, V. A.; Namyslo, J. C.; Adam, A. E. W.; Kaufmann, D. E. Heterocycles 2004, 63, 1281–1298. doi:10.3987/COM-04-10020 |
| 4. | Zapol’skii, V. A.; Nutz, E.; Namyslo, J. C.; Adam, A. E. W.; Kaufmann, D. E. Synthesis 2006, 2927–2933. doi:10.1055/s-2006-950187 |
| 5. | Zapol’skii, V. A.; Namyslo, J. C.; Altug, C.; Gjikaj, M.; Kaufmann, D. E. Synthesis 2008, 304–310. doi:10.1055/s-2007-990948 |
| 6. | Zapol’skii, V. A.; Fischer, R.; Namyslo, J. C.; Kaufmann, D. E. Bioorg. Med. Chem. 2009, 17, 4206–4215. doi:10.1016/j.bmc.2009.01.001 |
| 7. | Zapol’skii, V. A.; Namyslo, J. C.; Gjikaj, M.; Kaufmann, D. E. Z. Naturforsch., B 2010, 65b, 843–860. |
| 25. | Li, J.; Wakefield, B. D.; Ruble, J. C.; Stiff, C. M.; Romero, D. L.; Marotti, K. R.; Sweeney, M. T.; Zurenko, G. E.; Rohrer, D. C.; Thorarensen, A. Bioorg. Med. Chem. Lett. 2007, 17, 2347–2350. doi:10.1016/j.bmcl.2006.12.055 |
| 26. | Kaizerman, J. A.; Gross, M. I.; Ge, Y.; White, S.; Hu, W.; Duan, J.-X.; Baird, E. E.; Johnson, K. W.; Tanaka, R. D.; Moser, H. E.; Bürli, R. W. J. Med. Chem. 2003, 46, 3914–3929. doi:10.1021/jm030097a |
| 27. | Bürli, R. W.; Ge, Y.; White, S.; Baird, E. E.; Touami, S. M.; Taylor, M.; Kaizerman, J. A.; Moser, H. E. Bioorg. Med. Chem. Lett. 2002, 12, 2591–2594. doi:10.1016/S0960-894X(02)00515-2 |
| 19. | Fischer, R.; Jeschke, P.; Erdelen, C.; Lösel, P.; Reckmann, U.; Kaufmann, D. E.; Zapol’skii, V. A. Halogenated Nitrobutadienes for Controlling Animal Pests. WO 03/040129 A1, May 15, 2003. |
| 22. | Zapol’skii, V. A.; Potkin, V. I.; Nechai, N. I.; Kaberdin, R. V.; Pevzner, M. S. Russ. J. Org. Chem. 1997, 33, 1632–1637. |
| 17. | Molodykh, Z. V.; Buzykin, B. I.; Kudrina, M. A.; Sysoeva, L. P.; Gazetdinova, N. G.; Neklesova, I. D.; Kitaev, Y. P. Pharm. Chem. J. 1980, 14, 162–169. doi:10.1007/BF00777380 |
| 18. | Rector, D. L.; Folz, S. D.; Conklin, R. D.; Nowakowski, L. H.; Kaugars, G. J. Med. Chem. 1981, 24, 532–538. doi:10.1021/jm00137a011 |
| 23. | Kaberdin, R. V.; Potkin, V. I.; Ol'dekop, Y. A. Dokl. Chem. 1988, 300, 173–175. |
| 4. | Zapol’skii, V. A.; Nutz, E.; Namyslo, J. C.; Adam, A. E. W.; Kaufmann, D. E. Synthesis 2006, 2927–2933. doi:10.1055/s-2006-950187 |
| 7. | Zapol’skii, V. A.; Namyslo, J. C.; Gjikaj, M.; Kaufmann, D. E. Z. Naturforsch., B 2010, 65b, 843–860. |
| 35. | Aiello, E.; Aiello, S.; Mingoia, F.; Bacchi, A.; Pelizzi, G.; Musiu, C.; Setzu, M. G.; Pani, A.; La Colla, P.; Marongiu, M. E. Bioorg. Med. Chem. 2000, 8, 2719–2728. doi:10.1016/S0968-0896(00)00211-X |
| 36. | Luo, C.; Xie, P.; Marmorstein, R. J. Med. Chem. 2008, 51, 6121–6127. doi:10.1021/jm800539g |
| 37. | Firestine, S. M.; Wu, W.; Youn, H.; Davisson, V. J. Bioorg. Med. Chem. 2009, 17, 794–803. doi:10.1016/j.bmc.2008.11.057 |
| 38. | Schepetkin, I. A.; Khlebnikov, A. I.; Quinn, M. T. J. Med. Chem. 2007, 50, 4928–4938. doi:10.1021/jm070600+ |
| 39. | Baraldi, P. G.; Vicentini, C. B.; Simoni, D.; Menziani, E. Farmaco, Ed. Sci. 1983, 38, 508–513. |
| 40. | Walczak, K.; Gondela, A.; Suwiński, J. Eur. J. Med. Chem. 2004, 39, 849–853. doi:10.1016/j.ejmech.2004.06.014 |
| 41. | Lu, I.-L.; Mahindroo, N.; Liang, P.-H.; Peng, Y.-H.; Kuo, C.-J.; Tsai, K.-C.; Hsieh, H.-P.; Chao, Y.-S.; Wu, S.-Y. J. Med. Chem. 2006, 49, 5154–5161. doi:10.1021/jm060207o |
| 42. | Penning, T. D.; Talley, J. J.; Bertenshaw, S. R.; Carter, J. S.; Collins, P. W.; Docter, S.; Graneto, M. J.; Lee, L. F.; Malecha, J. W.; Miyashiro, J. M.; Rogers, R. S.; Rogier, D. J.; Yu, S. S.; Anderson, G. D.; Burton, E. G.; Cogburn, J. N.; Gregory, S. A.; Koboldt, C. M.; Perkins, W. E.; Seibert, K.; Veenhuizen, A. W.; Zhang, Y. Y.; Isakson, P. C. J. Med. Chem. 1997, 40, 1347–1365. doi:10.1021/jm960803q |
| 16. | Savage, S. A.; Jones, G. S.; Kolotuchin, S.; Ramrattan, S. A.; Vu, T.; Waltermire, R. E. Org. Process Res. Dev. 2009, 13, 1169–1176. doi:10.1021/op900226j |
| 20. | Zapol’skii, V. A.; Potkin, V. I.; Kaberdin, R. V. Vestsi Nats. Akad. Navuk Belarusi, Ser. Khim. Navuk 1993, 3, 76–80. |
| 21. | Zapol’skii, V. A.; Namyslo, J. C.; Blaschkowski, B.; Kaufmann, D. E. Synlett 2006, 3464–3468. doi:10.1055/s-2006-956490 |
| 5. | Zapol’skii, V. A.; Namyslo, J. C.; Altug, C.; Gjikaj, M.; Kaufmann, D. E. Synthesis 2008, 304–310. doi:10.1055/s-2007-990948 |
| 43. | Zapol’skii, V. A.; Namyslo, J. C.; Gjikaj, M.; Kaufmann, D. E. Synlett 2007, 1507–1512. doi:10.1055/s-2007-982554 |
© 2012 Zapol’skii et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)