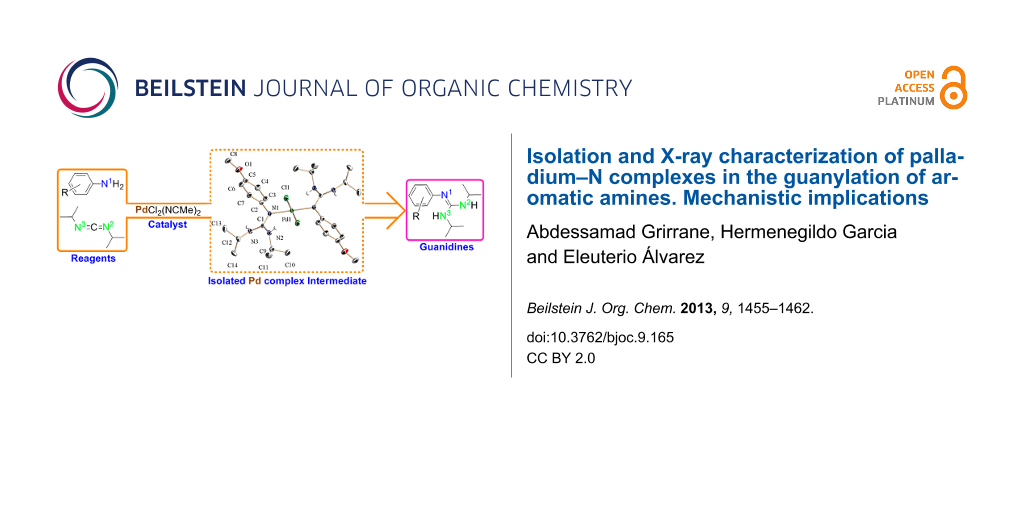
Abstract
In the context of palladium-catalyzed guanylation of anilines herein, we have been able to characterize and isolate bis(anilino) and bis(guanidino)Pd(II) complexes using reaction conditions under which stoichiometric amounts of palladium salts are used. Characterization of these palladium complexes strongly supports a mechanistic proposal for the catalytic guanylation of anilines using PdCl2(NCCH3)2 as catalyst that involves the intermediacy of these Pd(II) complexes.

Graphical Abstract
Introduction
N-Arylguanidines are important compounds with interesting biological activities [1,2] such as fungicides [3] and also in supramolecular chemistry as complementary partners of carboxylate and nitro groups [4-7]. Some of these guanidines are commercially used as antifouling agents in marine paints and in the formulation of protective surface coatings [8-10]. N-Arylguanidines can be obtained by aniline insertion into the corresponding carbodiimide [11-18]. This nucleophilic addition can be efficiently catalyzed by palladium salts [19], such as PdCl2 or Pd(OAc)2 in homogenous phase. Also recently we have reported that palladium nanoparticles supported on magnesia can be a solid catalyst for this process [20]. Working with PdCl2(NCCH3)2 in dichloromethane we were able to isolate two types of palladium complexes with iodoaniline and guanidine, respectively, (see Scheme 1) that give some clue about the reaction mechanism of the catalytic process.
Scheme 1: Isolation of trans-dichlorobis(4-iodoanilino-ĸN)palladium(II) and trans-dichlorobis[1,3-diisopropyl-2-(4-iodophenyl)guanidino-ĸN(aryl)]palladium(II) complexes formed by reaction of PdCl2(MeCN)2 with 4-iodoaniline (upper row) or with 4-iodoaniline and N,N’-diisopropylcarbodiimide (lower reaction).
Scheme 1: Isolation of trans-dichlorobis(4-iodoanilino-ĸN)palladium(II) and trans-dichlorobis[1,3-diisopropyl...
Results and Discussion
In order to provide further support to the mechanistic proposal for the C–N insertion promoted by palladium(II) suggested by us [20], in the present report we describe the study of palladium-catalyzed guanylation of three additional anilines (1a–c) with N,N’-diisopropylcarbodiimide (2). For these reactions we have been able to characterize three palladium complexes of the type of bis(anilino)Pd(II) (3a–c) as well as three palladium complexes of the type of bis(guanidino)Pd(II) (4a–c) (Scheme 2) whose structures have been characterized by single-crystal X-ray structural analysis, as well as to obtain evidence for the intermediacy of these complexes in the catalytic process. Overall the present data reinforce the previous proposal for the mechanism of aniline guanylation.
Scheme 2: Isolation of trans-dichlorobis[1,3-diisopropyl-2-(aryl)guanidino-ĸN(aryl)]palladium(II) complexes (4a–c) by reaction of PdCl2(MeCN)2 with anilines 1a–c and N,N’-diisopropylcarbodiimide (2). Dashed lines indicate the formation of trans-dichlorobis(anilino-ĸN)palladium(II) complexes (3a–c) as primary intermediates during the reaction, and their subsequent reaction with 2 to led 4a–c.
Scheme 2: Isolation of trans-dichlorobis[1,3-diisopropyl-2-(aryl)guanidino-ĸN(aryl)]palladium(II) complexes (...
When a stoichiometric (2:2:1) mixture of anilines 1a–c and carbodiimide 2 with PdCl2(MeCN)2 is stirred at 60 °C in CH2Cl2, evolution at initial reaction times of a solid precipitate is observed (Scheme 2). Filtration of these precipitates and subsequent washing with CH2Cl2 renders three solids whose combustion analysis is in accordance with the percentages expected for dichlorobis(anilino-ĸN)palladium(II) (3a–c) (see Supporting Information File 1, experimental section). IR spectra of complexes 3a–c show the characteristic absorption peaks due to the coordinated anilines 1a–c (see Supporting Information File 1, Figures S1–S3); these are compatible with the proposed structure for these intermediates. Complex 3a derived from 1a has been recently characterized by single-crystal X-ray diffraction [21] showing similar coordination to trans-dichlorobis(4-iodoaniline-ĸN)palladium(II) complex recently published by us [20]. Compounds 3a–c were also characterized by solid state 13C NMR spectroscopy that gave spectra showing carbon peaks compatible with the proposed structure (see Supporting Information File 1, Figures S4–S6).
After prolonging the reaction time, the initially evolved precipitate undergoes dissolution indicating that it has been transformed under the reaction conditions. At this stage, filtering of the transparent orange-red (4a,b) and red (4c) solutions followed by subsequent addition of ethyl ether or toluene and slow solvent evaporation at ambient temperature allows the formation of crystals with suitable quality for a crystallographic diffraction study. The structures of these intermediates solved by X-ray analysis showed that these compounds corresponding to trans-dichlorobis[arylguanidino-ĸN(aryl)]palladium(II). Figure 1, Figure 2 and Figure 3 present ORTEP views of complexes 4a–c as well as selected views along some crystallographic axes (see Tables S1–S3 and also Figures S7, S11 and S15 in Supporting Information File 1, and for full details of the crystallographic data see Supporting Information File 2).
![[1860-5397-9-165-1]](/bjoc/content/figures/1860-5397-9-165-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: (Top) ORTEP view of the centrosymmetric molecule 4a. (Bottom) Crystal packing detail of 4a viewed along the a-axis showing the presence of inter- and intramolecular hydrogen bonds between Cl and H (NH groups) atoms.
Figure 1: (Top) ORTEP view of the centrosymmetric molecule 4a. (Bottom) Crystal packing detail of 4a viewed a...
![[1860-5397-9-165-2]](/bjoc/content/figures/1860-5397-9-165-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: (Left) ORTEP representation of 4b. (Right) Crystal packing detail of 4b viewed along the a-axis showing the presence of intermolecular hydrogen bonds between O (from the dioxole moieties) and N (NH groups) atoms.
Figure 2: (Left) ORTEP representation of 4b. (Right) Crystal packing detail of 4b viewed along the a-axis sho...
![[1860-5397-9-165-3]](/bjoc/content/figures/1860-5397-9-165-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: (Left) ORTEP representation of 4c. (Right) Crystal-packing detail of 4c viewed along the a-axis showing the presence of intermolecular hydrogen bonds between Cl and N (NH groups) atoms.
Figure 3: (Left) ORTEP representation of 4c. (Right) Crystal-packing detail of 4c viewed along the a-axis sho...
Besides X-ray crystal structure analysis, palladium complexes 4a–c were also characterized by NMR spectroscopy, ESIMS and combustion analysis (see experimental section in Supporting Information File 1). 1H, 13C and 19F NMR spectroscopy of 4a–c in CD2Cl2 solution provides evidence showing that under the reaction conditions the starting reagents (PdCl2(MeCN)2, 1a–c and 2) including intermediates bis(anilino-ĸN)palladium(II) (3a–c) are completely converted into the corresponding bis(guanidino-ĸN)palladium(II) (4a–c) (see Supporting Information File 1, Figures S8 and S9 for 4a, Figures S12 and S13 for 4b and Figures S16–S18 for 4c). ESIMS of a solution obtained after dissolving complexes 4a–c in CH2Cl2/CH3CN (1:1) shows single positive MS peaks at 639.3, 667.2 and 867.1 attributable, respectively, to the complexes [C28H46Cl2N6O2Pd (4a) − Cl−]+, [C28H42Cl2N6O4Pd (4b) − Cl−]+ and [C26H38Cl2F2I2N6Pd (4c) − Cl−]+. Also negative MS shows single peaks at 711.2, 739.1 and 938.9 attributable, respectively, to the complexes [C28H46Cl2N6O2Pd (4a) + Cl−]−, [C28H42Cl2N6O4Pd (4b) + Cl−]− and [C26H38Cl2F2I2N6Pd (4c) + Cl−]− (see Supporting Information File 1, Figures S10, S14 and S19).
Similar reactions were carried out mixing anilines 1a–c and N,N’-diisopropylcarbodiimide (2), but in this case in the presence of only a catalytic amount of PdCl2(NCCH3)2 (4 mol %) (Scheme 3). Under these conditions no evidence for the formation of palladium complexes (3a–c) and (4a–c) could be obtained due to the low amount of palladium and no solid precipitates were observed. In contrast, in the presence of catalytic amounts of palladium, formation of the corresponding N-arylguanidines was observed in almost quantitative yield. These guanidines 5a–c formed by nucleophilic attack of anilines 1a–c to N,N’-diisopropylcarbodiimide (2) catalyzed by palladium were fully characterized by analytical and spectroscopic data (see Supporting Information File 1, experimental section).
Scheme 3: Guanylation reactions of anilines 1a–c by N,N’-diisopropylcarbodiimide (2) catalyzed by Pd(II) salt.
Scheme 3: Guanylation reactions of anilines 1a–c by N,N’-diisopropylcarbodiimide (2) catalyzed by Pd(II) salt....
Structure of guanidine 5a was confirmed by single-crystal X-ray analysis, Figure 4 shows the corresponding ORTEP for compound 5a as well as some views of the crystal packing (see also Supporting Information File 1, Table S4 and Figure S20 and for full details of crystallographic data see Supporting Information File 2). Beside X-ray crystal analysis of guanidine 5a, guanidines 5a–c were also characterized by 1H, 13C and 19F NMR spectroscopy and combustion analysis (see Figures S21 and S22 for 5a, Figures S24 and S25 for 5b, our recent published work for 5c [20] and experimental section in Supporting Information File 1). ESIMS and GC–MS of solutions obtained respectively after dissolving guanidines 5a and 5b in CH2Cl2/MeOH (1:1) and CH2Cl2 shows a single positive MS peak at 250.2 and 263.2 Da attributable, respectively, to the complexes [C14H23N3O (5a) + H+]+ and C14H21N3O2 (5b) (see Figure S23 and Figure S26 in Supporting Information File 1).
![[1860-5397-9-165-4]](/bjoc/content/figures/1860-5397-9-165-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: (Left) ORTEP representation of 5a. (Right) Crystal packing details of 5a viewed along the a-axis showing the presence of intermolecular hydrogen bonds between N atoms.
Figure 4: (Left) ORTEP representation of 5a. (Right) Crystal packing details of 5a viewed along the a-axis sh...
Overall the information obtained from the experiments performed in the presence of a large palladium excess, in which two kinds of palladium complexes have been detected and isolated, with the formation of guanidines under conditions in which a catalytic amount of palladium is present, allows us to make reasonable mechanistic proposals. Thus, upon contacting anilines 1a–c and PdCl2(MeCN)2, a rapid formation of dichlorobis(anilino-ĸN)palladium(II) (3a–c) complexes should take place. These palladium complexes will interact with the N,N’-diisopropylcarbodiimide (2) giving rise to the dichlorobis(guanidino-ĸN)palladium(II) (4a–c) complexes. When Pd is used in catalytic amounts, cleavage of this bis(guanidino-ĸN) complex by aniline will form another dichlorobis(anilino-ĸN)palladium(II) (3a–c) completing one cycle and liberating guanidines 5a–c as free products of this catalytic reaction with high yields and selectivities (see Scheme 4). In this mechanism the rate determining step will be the attack of dichlorobis(anilino-ĸN)palladium(II) (3a–c) to the N,N’-diisopropylcarbodiimide (2) (Scheme 4).
Scheme 4: Possible mechanisms for the C–N coupling catalyzed by PdCl2(NCMe)2 in homogeneous phase.
Scheme 4: Possible mechanisms for the C–N coupling catalyzed by PdCl2(NCMe)2 in homogeneous phase.
In this process, coordination of nitrogen to palladium should strongly reduce the nucleophilicity of the corresponding nitrogen atom and, therefore, the attack at the carbodiimide would be significantly slowed down compared to free uncoordinated aniline. However, this negative effect of palladium coordination to aniline should be overcompensated by coordination of carbodiimide to palladium in close proximity to aniline in fixed geometry as indicated in the presumed intermediates 6a–c. There are precedents in the literature [22] showing that palladium(II) can interact weakly with accumulated carbodiimide bonds, but this type of complex is typically very labile and difficult to isolate under the reaction conditions due the presence of an aniline excess, and for this reason we have been unable to isolate labile intermediates 6a–c. This preassociation between complexes 3a–c and carbodiimide 2 to form intermediates 6a–c would make easier the key step of C=N insertion leading to guanidines (Scheme 4).
Alternatively, it can be also envisioned that the acidity of hydrogen atoms bonded to nitrogen in intermediates 3a–c increases sufficiently to protonate the nitrogen atom of the carbodiimide that subsequently would be activated to accept the nucleophilic attack of the resulting anilide anion or aniline (see Scheme 4). This mechanism would be similar to that accepted for peptide-bond formation mediated by carbodiimides [23-26]. It can also be possible that these two steps, i.e., protonation and nucleophilic attack occur in a quasi-concerted manner around the intermediates 6a–c.
Conclusion
In conclusion, we show the possibility to isolate and characterize palladium complexes by performing some reactions using large amounts of palladium salts. The structures of these complexes shed light onto the reaction mechanism of the palladium-catalyzed reaction. In this case, we have applied this methodology to isolate and characterize bis(anilino)- and bis(guanidino)palladium complexes that are proposed to be reaction intermediates, together with the still not isolated aniline–carbodiimide palladium complex 6, in the mechanism of the guanylation of anilines. Our study opens the way to apply a similar methodology to study the reaction mechanism of other catalytic reactions.
Supporting Information
| Supporting Information File 1: Experimental details of preparation, isolation and full characterization of new palladium compounds 3a–c, 4a–c as well as guanidine compounds 5a,b, including IR, NMR, ESIMS and GC–MS spectra for new compounds. | ||
| Format: PDF | Size: 3.1 MB | Download |
| Supporting Information File 2: X-Ray structure analysis data for 4a (CCDC-931786, 4b (CCDC-931787), 4c (CCDC-931788) and 5a (CCDC-931789) are given. These data can also be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. | ||
| Format: CIF | Size: 79.3 KB | Download |
References
-
Membrino, A.; Paramasivam, M.; Cogoi, S.; Alzeer, J.; Luedtke, N. W.; Xodo, L. E. Chem. Commun. 2010, 46, 625–627. doi:10.1039/b918964e
Return to citation in text: [1] -
Blondeau, P.; Segura, M.; Pérez-Fernández, R.; de Mendoza, J. Chem. Soc. Rev. 2007, 36, 198–210. doi:10.1039/b603089k
Return to citation in text: [1] -
Thevissen, K.; Pellens, K.; De Brucker, K.; François, I. E. J. A.; Chow, K. K.; Meert, E. M. K.; Meert, W.; Van Minnebruggen, G.; Borgers, M.; Vroome, V.; Levin, J.; De Vos, D.; Maes, L.; Cos, P.; Cammue, B. P. A. Bioorg. Med. Chem. Lett. 2011, 21, 3686–3692. doi:10.1016/j.bmcl.2011.04.075
Return to citation in text: [1] -
Guanidines: Historical, biological, biochemical and clinical aspects of the naturally occurring guanidino compounds. In Proceedings of the international symposium on guanidino compounds, Tokyo, Japan, Sept 5–7, 1983; Mori, A.; Cohen, B. D.; Lowenthal, A., Eds.; Plenum Press: New York, NY, USA, 1985.
Return to citation in text: [1] -
Berlinck, R. G. S.; Burtoloso, A. C. B.; Kossuga, M. H. Nat. Prod. Rep. 2008, 25, 919–954. doi:10.1039/b507874c
Return to citation in text: [1] -
Berlinck, R. G. S.; Kossuga, M. H. Nat. Prod. Rep. 2005, 22, 516–550. doi:10.1039/b209227c
Return to citation in text: [1] -
Bae, I.; Han, H.; Chang, S. J. Am. Chem. Soc. 2005, 127, 2038–2039. doi:10.1021/ja0432968
Return to citation in text: [1] -
Tamura, A.; Tamura, N.; Shibuya, H.; Ouchi, S.; Kuwahara, M. Antifouling agent for underwater noxious attached organism. Japanese Patent 2000128717, May 9, 2000.
Return to citation in text: [1] -
Denneman, M. A.; Karzijn, W. Process for removing fouling. WO 2010076259, July 8, 2010.
Return to citation in text: [1] -
Omae, I. Chem. Rev. 2003, 103, 3431–3448. doi:10.1021/cr030669z
Return to citation in text: [1] -
Liu, C.; Zhou, S.; Wang, S.; Zhang, L.; Yang, G. Dalton Trans. 2010, 39, 8994–8999. doi:10.1039/c0dt00246a
Return to citation in text: [1] -
Ong, T. G.; Yap, G. P. A.; Richeson, D. S. J. Am. Chem. Soc. 2003, 125, 8100–8101. doi:10.1021/ja035716j
Return to citation in text: [1] -
Montilla, F.; Pastor, A.; Galindo, A. J. Organomet. Chem. 2004, 689, 993–996. doi:10.1016/j.jorganchem.2004.01.005
Return to citation in text: [1] -
Zhang, W.-X.; Li, D.; Wang, Z.; Xi, Z. Organometallics 2009, 28, 882–887. doi:10.1021/om801035t
Return to citation in text: [1] -
Rowley, C. N.; Ong, T.-G.; Priem, J.; Richeson, D. S.; Woo, T. K. Inorg. Chem. 2008, 47, 12024–12031. doi:10.1021/ic801739a
Return to citation in text: [1] -
Ong, T.-G.; O'Brien, J. S.; Korobkov, I.; Richeson, D. S. Organometallics 2006, 25, 4728–4730. doi:10.1021/om060539r
Return to citation in text: [1] -
Zhu, X.; Xu, F.; Shen, Q. Chin. J. Chem. 2009, 27, 19–22. doi:10.1002/cjoc.200990017
Return to citation in text: [1] -
Wu, Y.; Wang, S.; Zhang, L.; Yang, G.; Zhu, X.; Liu, C.; Yin, C.; Rong, J. Inorg. Chim. Acta 2009, 362, 2814–2819. doi:10.1016/j.ica.2008.12.030
Return to citation in text: [1] -
Li, D.; Guang, J.; Zhang, W.-X.; Wang, Y.; Xi, Z. Org. Biomol. Chem. 2010, 8, 1816–1820. doi:10.1039/b923249b
Return to citation in text: [1] -
Grirrane, A.; Garcia, H.; Corma, A.; Alvarez, E. Chem.–Eur. J. 2012, 18, 14934–14938. doi:10.1002/chem.201202823
Return to citation in text: [1] [2] [3] [4] -
Bon, V.; Orysyk, S.; Pekhnyo, V. Acta Crystallogr., Sect. E 2009, 65, M673. doi:10.1107/S1600536809018509
Return to citation in text: [1] -
Anderson, R. A.; Einstein, F. W. B. Acta Crystallogr., Sect. B 1978, 34, 271–272. doi:10.1107/S0567740878002770
Return to citation in text: [1] -
Wendlberger, G. Houben–Weyl: Methoden der Organischen Chemie; Georg Thieme Verlag: Stuttgart, Germany, 1974; Vol. 15/2, p 101.
Return to citation in text: [1] -
Rich, D. H.; Singh, J. In The Peptides: Analysis, Synthesis, Biology; Gross, E.; Meienhofer, J., Eds.; Academic Press: New York, NY, USA, 1979; pp 241–261.
Return to citation in text: [1] -
Jones, J. The Chemical Synthesis of Peptides; Oxford University Press: Oxford, U.K., 1991.
Return to citation in text: [1] -
Bodanszky, M. Peptide Chemistry. A Practical Textbook, 2nd ed.; Springer Verlag: Berlin, Germany, 1993. doi:10.1007/978-3-642-78206-0
Return to citation in text: [1]
| 1. | Membrino, A.; Paramasivam, M.; Cogoi, S.; Alzeer, J.; Luedtke, N. W.; Xodo, L. E. Chem. Commun. 2010, 46, 625–627. doi:10.1039/b918964e |
| 2. | Blondeau, P.; Segura, M.; Pérez-Fernández, R.; de Mendoza, J. Chem. Soc. Rev. 2007, 36, 198–210. doi:10.1039/b603089k |
| 11. | Liu, C.; Zhou, S.; Wang, S.; Zhang, L.; Yang, G. Dalton Trans. 2010, 39, 8994–8999. doi:10.1039/c0dt00246a |
| 12. | Ong, T. G.; Yap, G. P. A.; Richeson, D. S. J. Am. Chem. Soc. 2003, 125, 8100–8101. doi:10.1021/ja035716j |
| 13. | Montilla, F.; Pastor, A.; Galindo, A. J. Organomet. Chem. 2004, 689, 993–996. doi:10.1016/j.jorganchem.2004.01.005 |
| 14. | Zhang, W.-X.; Li, D.; Wang, Z.; Xi, Z. Organometallics 2009, 28, 882–887. doi:10.1021/om801035t |
| 15. | Rowley, C. N.; Ong, T.-G.; Priem, J.; Richeson, D. S.; Woo, T. K. Inorg. Chem. 2008, 47, 12024–12031. doi:10.1021/ic801739a |
| 16. | Ong, T.-G.; O'Brien, J. S.; Korobkov, I.; Richeson, D. S. Organometallics 2006, 25, 4728–4730. doi:10.1021/om060539r |
| 17. | Zhu, X.; Xu, F.; Shen, Q. Chin. J. Chem. 2009, 27, 19–22. doi:10.1002/cjoc.200990017 |
| 18. | Wu, Y.; Wang, S.; Zhang, L.; Yang, G.; Zhu, X.; Liu, C.; Yin, C.; Rong, J. Inorg. Chim. Acta 2009, 362, 2814–2819. doi:10.1016/j.ica.2008.12.030 |
| 8. | Tamura, A.; Tamura, N.; Shibuya, H.; Ouchi, S.; Kuwahara, M. Antifouling agent for underwater noxious attached organism. Japanese Patent 2000128717, May 9, 2000. |
| 9. | Denneman, M. A.; Karzijn, W. Process for removing fouling. WO 2010076259, July 8, 2010. |
| 10. | Omae, I. Chem. Rev. 2003, 103, 3431–3448. doi:10.1021/cr030669z |
| 4. | Guanidines: Historical, biological, biochemical and clinical aspects of the naturally occurring guanidino compounds. In Proceedings of the international symposium on guanidino compounds, Tokyo, Japan, Sept 5–7, 1983; Mori, A.; Cohen, B. D.; Lowenthal, A., Eds.; Plenum Press: New York, NY, USA, 1985. |
| 5. | Berlinck, R. G. S.; Burtoloso, A. C. B.; Kossuga, M. H. Nat. Prod. Rep. 2008, 25, 919–954. doi:10.1039/b507874c |
| 6. | Berlinck, R. G. S.; Kossuga, M. H. Nat. Prod. Rep. 2005, 22, 516–550. doi:10.1039/b209227c |
| 7. | Bae, I.; Han, H.; Chang, S. J. Am. Chem. Soc. 2005, 127, 2038–2039. doi:10.1021/ja0432968 |
| 23. | Wendlberger, G. Houben–Weyl: Methoden der Organischen Chemie; Georg Thieme Verlag: Stuttgart, Germany, 1974; Vol. 15/2, p 101. |
| 24. | Rich, D. H.; Singh, J. In The Peptides: Analysis, Synthesis, Biology; Gross, E.; Meienhofer, J., Eds.; Academic Press: New York, NY, USA, 1979; pp 241–261. |
| 25. | Jones, J. The Chemical Synthesis of Peptides; Oxford University Press: Oxford, U.K., 1991. |
| 26. | Bodanszky, M. Peptide Chemistry. A Practical Textbook, 2nd ed.; Springer Verlag: Berlin, Germany, 1993. doi:10.1007/978-3-642-78206-0 |
| 3. | Thevissen, K.; Pellens, K.; De Brucker, K.; François, I. E. J. A.; Chow, K. K.; Meert, E. M. K.; Meert, W.; Van Minnebruggen, G.; Borgers, M.; Vroome, V.; Levin, J.; De Vos, D.; Maes, L.; Cos, P.; Cammue, B. P. A. Bioorg. Med. Chem. Lett. 2011, 21, 3686–3692. doi:10.1016/j.bmcl.2011.04.075 |
| 21. | Bon, V.; Orysyk, S.; Pekhnyo, V. Acta Crystallogr., Sect. E 2009, 65, M673. doi:10.1107/S1600536809018509 |
| 20. | Grirrane, A.; Garcia, H.; Corma, A.; Alvarez, E. Chem.–Eur. J. 2012, 18, 14934–14938. doi:10.1002/chem.201202823 |
| 20. | Grirrane, A.; Garcia, H.; Corma, A.; Alvarez, E. Chem.–Eur. J. 2012, 18, 14934–14938. doi:10.1002/chem.201202823 |
| 22. | Anderson, R. A.; Einstein, F. W. B. Acta Crystallogr., Sect. B 1978, 34, 271–272. doi:10.1107/S0567740878002770 |
| 20. | Grirrane, A.; Garcia, H.; Corma, A.; Alvarez, E. Chem.–Eur. J. 2012, 18, 14934–14938. doi:10.1002/chem.201202823 |
| 19. | Li, D.; Guang, J.; Zhang, W.-X.; Wang, Y.; Xi, Z. Org. Biomol. Chem. 2010, 8, 1816–1820. doi:10.1039/b923249b |
| 20. | Grirrane, A.; Garcia, H.; Corma, A.; Alvarez, E. Chem.–Eur. J. 2012, 18, 14934–14938. doi:10.1002/chem.201202823 |
© 2013 Grirrane et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)