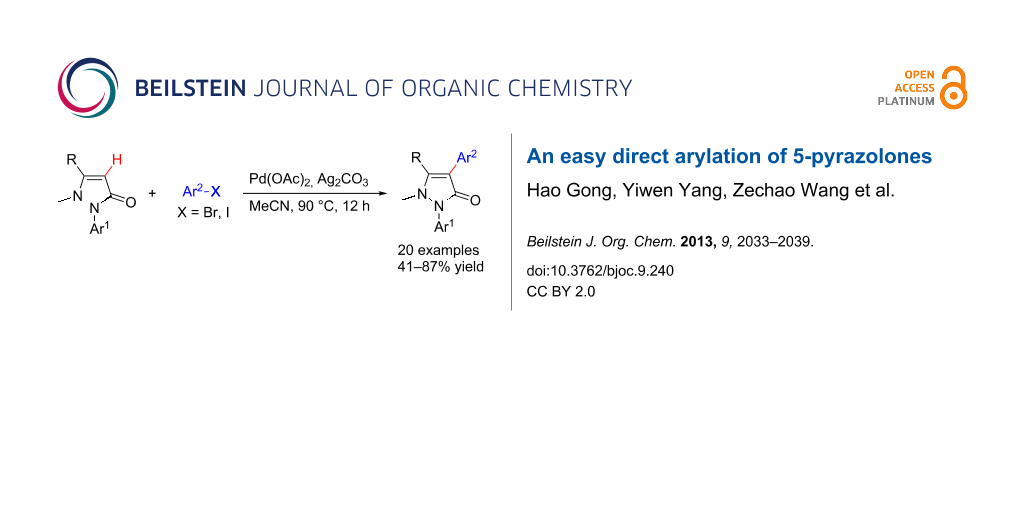
Abstract
A mild, efficient and catalytic ligand-free method for the direct arylation of 5-pyrazolones by Pd-catalyzed C–H bond activation is reported. The process smoothly proceeds and yields are moderate to excellent.
Graphical Abstract
Introduction
5-Pyrazolones are attracting considerable research interest because of their unique chemical properties and their structures that facilitate their application as biological and pharmaceutical intermediates and products [1-3]. Over the years, many of the biological activities of pyrazolones such as their antipyretic, analgesic [4,5], anti-inflammatory [6,7], antitumor [8,9], antiviral, antibacterial [10], and herbicidal [11] properties have been discovered and investigated. Pyrazolones are also potent inhibitors of telomerase, cyclooxygenase isoenzymes, platelet tromboxane synthesis, and prostanoid synthesis in humans [12,13]. Recently, pharmacologists have developed a novel class-II c-met inhibitor, whose structural unit is a pyrazolone ring [14]. The great medicinal significance and broad applications of pyrazolones prompted us to synthesize a new series of heterocyclic compounds containing the pyrazolone moiety.
The reaction of pyrazolones with arylboronic acids is an attractive approach for the synthesis of arylpyrazolone [15,16]. However, it often needs pre-formation of halo-pyrazolones. Transition metal-catalyzed direct arylation of (hetero)arenes has emerged over the past few years as a rapidly growing field of syntheses [17-26]. The direct arylation of pyrazolones by using aryl halides offers a cleaner and more efficient method of meeting such goals and rare examples of such transformations have been described [15].
In this paper, we report a convenient and catalytic ligand-free synthesis of a series of 4-aryl-5-pyrazolones 3 from 5-pyrazolones 1 and aryl halides 2 (Scheme 1). The direct arylation of 5-pyrazolones by Pd-catalyzed C–H bond activation was utilized.
Scheme 1: Direct arylation of 5-pyrazolones.
Scheme 1: Direct arylation of 5-pyrazolones.
Results and Discussion
We commenced this study by performing the direct arylation of phenazone (1a) in the presence of 2 equiv of iodobenzene (2a), 10 mol % of Pd(OAc)2 as a catalyst in acetonitrile in a sealed tube. The results are shown in Table 1. Gratifyingly, a 45% yield of the desired product 3a was achieved after stirring for 12 h at 90 °C. Encouraged by this preliminary result, we continued to optimize reaction conditions to further improve the chemical yield.
Table 1: Optimization of the synthesis of 3aa.
|
|
|||||
| entry | additive (2 equiv) | catalyst (0.1 equiv) | solvent | T (°C) | yield of 3ab |
|---|---|---|---|---|---|
| 1 | none | Pd(OAc)2 | CH3CN | 90 | 45 |
| 2 | K2CO3 | Pd(OAc)2 | CH3CN | 90 | 43 |
| 3 | Cs2CO3 | Pd(OAc)2 | CH3CN | 90 | 35 |
| 4 | Na2CO3 | Pd(OAc)2 | CH3CN | 90 | 27 |
| 5 | DBU | Pd(OAc)2 | CH3CN | 90 | 0 |
| 6 | K3PO4 | Pd(OAc)2 | CH3CN | 90 | 49 |
| 7 | Ph3P (0.25 equiv) | Pd(OAc)2 | CH3CN | 90 | 42 |
| 8 | none | Pd(OAc)2 (0.05 equiv) | CH3CN | 90 | 40 |
| 9 | none | Pd(OAc)2 (0.02 equiv) | CH3CN | 90 | 32 |
| 10 | none | Pd(OAc)2 | THF | 90 | traces |
| 11 | none | Pd(OAc)2 | DCE | 90 | 31 |
| 12 | none | Pd(OAc)2 | dioxane | 90 | 0 |
| 13 | none | Pd(OAc)2 | benzene | 90 | 22 |
| 14 | none | Pd(OAc)2 | CH3CN | 25 | 0 |
| 15 | none | Pd(OAc)2 | CH3CN | 60 | 31 |
| 16 | none | Pd(OAc)2 | CH3CN | 120 | 35 |
| 17 | O2(1atm) | Pd(OAc)2 | CH3CN | 90 | 55 |
| 18 | K2S2O8 | Pd(OAc)2 | CH3CN | 90 | 5 |
| 19 | benzoquinone | Pd(OAc)2 | CH3CN | 90 | 0 |
| 20 | Cu(OAc)2 | Pd(OAc)2 | CH3CN | 90 | 25 |
| 21 | Ag2CO3 | Pd(OAc)2 | CH3CN | 90 | 80 |
| 22 | none | FeCl3 (0.3 equiv) | CH3CN | 90 | 0 |
| 23 | none | Cu(OAc)2 (0.2 equiv) | CH3CN | 90 | 0 |
| 24 | none | none | CH3CN | 90 | 0 |
aReaction conditions: 1.0 equiv of 1a and 2.0 equiv of 2a were stirred for 12 h. bIsolated yield.
When 1a reacted with 2a in the presence of K2CO3 as a base in acetonitrile (90 °C, 12 h), the desired product 3a was generated in 43% yield (Table 1, entry 2). Changing K2CO3 to Cs2CO3, Na2CO3 and DBU (1,8-diazabicyclo(5.4.0)undec-7-ene), decreased the yield to 35%, 27% and 0%, respectively (Table 1, entry 3–5). Changing K2CO3 to K3PO4, the yield was increased to 49% (Table 1, entry 6). When Ph3P as a catalytic ligand was added to the reaction, the yield decreased to 42% (Table 1, entry 7). Reducing the dosage of Pd(OAc)2 to 0.05 equiv and 0.02 equiv, respectively, decreased the yield to 40% and 32% (Table 1, entries 8–9). Several solvents were examined under the conditions of entry 1. When the solvent was changed to THF, DCE, dioxane, and benzene, the yields decreased to trace, 31%, 0% and 22%, respectively (Table 1, entries 10–13). Other reaction parameters such as temperature and oxidants were also screened. When the reaction temperatures were 25 °C, 60 °C, and 120 °C, the yields decreased to 0%, 31% and 35%, respectively (Table 1, entries 14–16). When the reaction was under oxygen (1 atm) in a sealed tube and oxygen was used as an oxidant, product 3a was obtained in 55% yield (Table 1, entry 17). Changing the oxidant to K2S2O8, benzoquinone and Cu(OAc)2 decreased the yield to 5%, 0% and 25%, respectively (Table 1, entries 18–20). When Ag2CO3 was added to the reaction, the yield increased to 80% (Table 1, entry 21). Different catalysts were also examined. When Cu(OAc)2 or FeCl3 was used as a catalyst, or no catalyst was used in the reaction, product 3a was not obtained (Table 1, entries 22–24). Ultimately, the optimal reaction conditions were determined to be 0.1 equiv Pd(OAc)2 catalyst, 2.0 equiv Ag2CO3, acetonitrile, 90 °C, air atmosphere, 1:2 molar ratio of 1a to 2a, and 12 h reaction time.
Under the optimized conditions (Table 1, entry 10), the scope of aryl halides was examined and the results are summarized in Table 2. The reactions of aryl halides 2 with phenyl moieties carrying either an electron-donating group such as methyl (2d and 2i), ethyloxy (2e) or an electron-withdrawing substituent such as methoxycarbonyl (2c and 2g), trifluoromethyl (2f) or formyl (2h) proceeded smoothly with moderate to good yields (Table 2, entries 3–10). When the phenyl moiety of the aryl halides 2 carried an electron-donating group, higher yields were obtained (Table 2, entries 4, 5, 9). On the other hand, an electron-withdrawing group on the phenyl moiety of the aryl halides (2c, 2f, 2g and 2h) provided 4-aryl-5-pyrazolones 3 in relatively low yields (Table 2, entries 3, 6–8). Entries 1 and 2 show that the yield of products was lower when using aryl bromide than when using aryl iodide, and 2-bromopyridine also provided 3i in moderate yield (Table 2, entry 10).
Table 2: Synthesis of 4-aryl- 5-pyrazolones 3.
|
|
|||
| entry | Ar–X | product | yield of 3 (%)a |
|---|---|---|---|
| 1 |
2a |
3a |
80 |
| 2 |
2b |
3a |
67 |
| 3 |
2c |
3b |
71 |
| 4 |
2d |
3c |
81 |
| 5 |
2e |
3d |
84 |
| 6 |
2f |
3e |
71 |
| 7 |
2g |
3f |
78 |
| 8 |
2h |
3g |
70 |
| 9 |
2i |
3h |
82 |
| 10 |
2j |
3i |
64 |
aIsolated yield.
Next, we investigated the scope of 5-pyrazolone 1 substrates. Table 3 shows that in most cases, the desired pyrazolones 3 were generated smoothly in moderate to good yields. When the phenyl moiety of pyrazolones 1 carried an electron-donating substituent such as methoxy (1b) and methyl (1c), the reactions provided pyrazolones 3 in high yields (Table 3, entries 1, 2). On the other hand, when pyrazolones 1 carried an electron-withdrawing substituent such as nitro (1f) and halogens (1g, 1i and 1k) in the aromatic portion, relatively low yields were obtained (Table 3, entries 5, 6, 8, 10). Compared with 5-pyrazolones containing a butyl or a phenyl substituent on the 3-position of the heterocycle (1d and 1e), the methyl (1a) on the same position resulted in a higher yield (Table 3, entries 3 and 4). The cause might be the steric hindrance of phenyl or butyl. The same trend could be seen from 1g to 1l (cf. 3o, 3q and 3s with 3p, 3r and 3t) (Table 3, entries 6–11).
Conclusion
In summary, we developed a mild, simple and efficient method for the direct arylation of 5-pyrazolones by Pd-catalyzed C–H bond activation. This approach resulted in the construction of 4-aryl-5-pyrazolones, which are important heterocyclic compounds used in medicinal and biological research. The investigations on the reaction mechanism are still in progress.
Supporting Information
| Supporting Information File 1: Experimental details and characterization data for all compounds. | ||
| Format: PDF | Size: 1.5 MB | Download |
Acknowledgements
The present work was supported by the Natural Science Foundation of China (No. 21272174), the Key Projects of Shanghai in Biomedicine (No. 08431902700), and the Scientific Research Foundation of the State Education Ministry for Returned Overseas Chinese Scholars. We would also like to thank the Center for Instrumental Analysis, Tongji University, China.
References
-
Marinozzi, M.; Carotti, A.; Sansone, E.; Macchiarulo, A.; Rosatelli, E.; Sardella, R.; Natalini, B.; Rizzo, G.; Adorini, L.; Passeri, D.; De Franco, F.; Pruzanski, M.; Pellicciari, R. Bioorg. Med. Chem. 2012, 20, 3429–3445. doi:10.1016/j.bmc.2012.04.021
Return to citation in text: [1] -
Dow, R. L.; Carpino, P. A.; Gautreau, D.; Hadcock, J. R.; Iredale, P. A.; Kelly-Sullivan, D.; Lizano, J. S.; O’ Connor, R. E.; Schneider, S. R.; Scott, D. O.; Ward, K. M. ACS Med. Chem. Lett. 2012, 3, 397–401. doi:10.1021/ml3000325
Return to citation in text: [1] -
Panda, N.; Karmakar, S.; Jena, A. K. Chem. Heterocycl. Compd. 2011, 46, 1500–1508. doi:10.1007/s10593-011-0699-y
Return to citation in text: [1] -
Uramaru, N.; Shigematsu, H.; Toda, A.; Eyanagi, R.; Kitamura, S.; Ohta, S. J. Med. Chem. 2010, 53, 8727–8733. doi:10.1021/jm101208x
Return to citation in text: [1] -
Gold, M.; McKeen, C.; Beaver, W. T. Am. J. Med. Sci. 1965, 250, 577–604. doi:10.1097/00000441-196511000-00011
Return to citation in text: [1] -
Himly, M.; Jahn-Schmid, B.; Pittertschatscher, K.; Bohle, B.; Grubmayr, K.; Ferreira, F.; Ebner, H.; Ebner, C. J. Allergy Clin. Immunol. 2003, 111, 882–888. doi:10.1067/mai.2003.163
Return to citation in text: [1] -
Marković, V.; Erić, S.; Stanojković, T.; Gligorijević, N.; Arandelović, S.; Todorović, N.; Trifunović, S.; Manojlović, N.; Jelić, R.; Joksović, M. D. Bioorg. Med. Chem. Lett. 2011, 21, 4416–4421. doi:10.1016/j.bmcl.2011.06.025
Return to citation in text: [1] -
Braña, M. F.; Gradillas, A.; Ovalles, A. G.; López, B.; Acero, N.; Llinares, F.; Muñoz Mingarro, D. Bioorg. Med. Chem. 2006, 14, 9–16. doi:10.1016/j.bmc.2005.09.059
Return to citation in text: [1] -
Tripathy, R.; Ghose, A.; Singh, J.; Bacon, E. R.; Angeles, T. S.; Yang, S. X.; Albom, M. S.; Aimone, L. D.; Herman, J. L.; Mallamo, J. P. Bioorg. Med. Chem. Lett. 2007, 17, 1793–1798. doi:10.1016/j.bmcl.2006.12.054
Return to citation in text: [1] -
Sayed, G. H.; Shiba, S. A.; Radwan, A.; Mohamed, S. M.; Khalil, M. Chin. J. Chem. 1992, 10, 475–480. doi:10.1002/cjoc.19920100515
Return to citation in text: [1] -
Vassilev, G. N.; Yonova, P. A.; Bohland, H.; Vassilev, N. G.; Yordanov, B. Dokl. Bulg. Akad. Nauk. 1997, 50, 59–62.
Return to citation in text: [1] -
Costa, D.; Marques, A. P.; Reis, R. L.; Lima, J. L. F. C.; Fernandes, E. Free Radical Biol. Med. 2006, 40, 632–640. doi:10.1016/j.freeradbiomed.2005.09.017
Return to citation in text: [1] -
Kalyanaraman, B.; Sohnle, P. G. J. Clin. Invest. 1985, 75, 1618–1622. doi:10.1172/JCI111868
Return to citation in text: [1] -
Liu, L.; Norman, M. H.; Lee, M.; Xi, N.; Siegmund, A.; Boezio, A. A.; Booker, S.; Choquette, D.; D’Angelo, N. D.; Germain, J.; Yang, K.; Yang, Y.; Zhang, Y.; Bellon, S. F.; Whittington, D. A.; Harmange, J.-P.; Dominguez, C.; Kim, T.-S.; Dussault, I. J. Med. Chem. 2012, 55, 1868–1897. doi:10.1021/jm201331s
Return to citation in text: [1] -
Guckian, K.; Carter, M. B.; Lin, E. Y.-S.; Choi, M.; Sun, L.; Boriack-Sjodin, P. A.; Chuaqui, C.; Lane, B.; Cheung, K.; Ling, L.; Lee, W.-C. Bioorg. Med. Chem. Lett. 2010, 20, 326–329. doi:10.1016/j.bmcl.2009.10.108
Return to citation in text: [1] [2] -
Boriack-Sjodin, P. A.; Carter, M. B.; Choi, M. J.; Chuaqui, C.; Deng, Z.; Guckian, K.; Lee, W.; Lin, E. Y.; Sun, L. Substituted Pyrazolones. WO Patent 2007059359 A2, May 24, 2007.
Return to citation in text: [1] -
Cheng, C.; Shih, Y.-C.; Chen, H.-T.; Chien, T.-C. Tetrahedron 2013, 69, 1387–1396. doi:10.1016/j.tet.2012.11.001
Return to citation in text: [1] -
Sharma, A.; Vacchani, D.; Van der Eycken, E. Chem.–Eur. J. 2013, 19, 1158–1168. doi:10.1002/chem.201201868
Return to citation in text: [1] -
Kozhushkov, S. I.; Potukuchi, H. K.; Ackermann, L. Catal. Sci. Technol. 2013, 3, 562–571. doi:10.1039/c2cy20505j
Return to citation in text: [1] -
Mousseau, J. J.; Charrette, A. B. Acc. Chem. Res. 2013, 46, 412–424. doi:10.1021/ar300185z
Return to citation in text: [1] -
Neufeldt, S. R.; Sanford, M. S. Acc. Chem. Res. 2012, 45, 936–946. doi:10.1021/ar300014f
Return to citation in text: [1] -
Engle, K. M.; Mei, T.; Wasa, M.-S.; Yu, J.-Q. Acc. Chem. Res. 2012, 45, 788–802. doi:10.1021/ar200185g
Return to citation in text: [1] -
Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215–1292. doi:10.1021/cr100280d
Return to citation in text: [1] -
Sun, C.-L.; Li, B.-J.; Shi, Z.-J. Chem. Rev. 2011, 111, 1293–1314. doi:10.1021/cr100198w
Return to citation in text: [1] -
Baudoin, O. Chem. Soc. Rev. 2011, 40, 4902–4911. doi:10.1039/c1cs15058h
Return to citation in text: [1] -
Cho, S. H.; Kim, J. Y.; Kwak, J.; Chang, S. Chem. Soc. Rev. 2011, 40, 5068–5083. doi:10.1039/c1cs15082k
Return to citation in text: [1]
| 1. | Marinozzi, M.; Carotti, A.; Sansone, E.; Macchiarulo, A.; Rosatelli, E.; Sardella, R.; Natalini, B.; Rizzo, G.; Adorini, L.; Passeri, D.; De Franco, F.; Pruzanski, M.; Pellicciari, R. Bioorg. Med. Chem. 2012, 20, 3429–3445. doi:10.1016/j.bmc.2012.04.021 |
| 2. | Dow, R. L.; Carpino, P. A.; Gautreau, D.; Hadcock, J. R.; Iredale, P. A.; Kelly-Sullivan, D.; Lizano, J. S.; O’ Connor, R. E.; Schneider, S. R.; Scott, D. O.; Ward, K. M. ACS Med. Chem. Lett. 2012, 3, 397–401. doi:10.1021/ml3000325 |
| 3. | Panda, N.; Karmakar, S.; Jena, A. K. Chem. Heterocycl. Compd. 2011, 46, 1500–1508. doi:10.1007/s10593-011-0699-y |
| 10. | Sayed, G. H.; Shiba, S. A.; Radwan, A.; Mohamed, S. M.; Khalil, M. Chin. J. Chem. 1992, 10, 475–480. doi:10.1002/cjoc.19920100515 |
| 8. | Braña, M. F.; Gradillas, A.; Ovalles, A. G.; López, B.; Acero, N.; Llinares, F.; Muñoz Mingarro, D. Bioorg. Med. Chem. 2006, 14, 9–16. doi:10.1016/j.bmc.2005.09.059 |
| 9. | Tripathy, R.; Ghose, A.; Singh, J.; Bacon, E. R.; Angeles, T. S.; Yang, S. X.; Albom, M. S.; Aimone, L. D.; Herman, J. L.; Mallamo, J. P. Bioorg. Med. Chem. Lett. 2007, 17, 1793–1798. doi:10.1016/j.bmcl.2006.12.054 |
| 6. | Himly, M.; Jahn-Schmid, B.; Pittertschatscher, K.; Bohle, B.; Grubmayr, K.; Ferreira, F.; Ebner, H.; Ebner, C. J. Allergy Clin. Immunol. 2003, 111, 882–888. doi:10.1067/mai.2003.163 |
| 7. | Marković, V.; Erić, S.; Stanojković, T.; Gligorijević, N.; Arandelović, S.; Todorović, N.; Trifunović, S.; Manojlović, N.; Jelić, R.; Joksović, M. D. Bioorg. Med. Chem. Lett. 2011, 21, 4416–4421. doi:10.1016/j.bmcl.2011.06.025 |
| 4. | Uramaru, N.; Shigematsu, H.; Toda, A.; Eyanagi, R.; Kitamura, S.; Ohta, S. J. Med. Chem. 2010, 53, 8727–8733. doi:10.1021/jm101208x |
| 5. | Gold, M.; McKeen, C.; Beaver, W. T. Am. J. Med. Sci. 1965, 250, 577–604. doi:10.1097/00000441-196511000-00011 |
| 15. | Guckian, K.; Carter, M. B.; Lin, E. Y.-S.; Choi, M.; Sun, L.; Boriack-Sjodin, P. A.; Chuaqui, C.; Lane, B.; Cheung, K.; Ling, L.; Lee, W.-C. Bioorg. Med. Chem. Lett. 2010, 20, 326–329. doi:10.1016/j.bmcl.2009.10.108 |
| 16. | Boriack-Sjodin, P. A.; Carter, M. B.; Choi, M. J.; Chuaqui, C.; Deng, Z.; Guckian, K.; Lee, W.; Lin, E. Y.; Sun, L. Substituted Pyrazolones. WO Patent 2007059359 A2, May 24, 2007. |
| 15. | Guckian, K.; Carter, M. B.; Lin, E. Y.-S.; Choi, M.; Sun, L.; Boriack-Sjodin, P. A.; Chuaqui, C.; Lane, B.; Cheung, K.; Ling, L.; Lee, W.-C. Bioorg. Med. Chem. Lett. 2010, 20, 326–329. doi:10.1016/j.bmcl.2009.10.108 |
| 14. | Liu, L.; Norman, M. H.; Lee, M.; Xi, N.; Siegmund, A.; Boezio, A. A.; Booker, S.; Choquette, D.; D’Angelo, N. D.; Germain, J.; Yang, K.; Yang, Y.; Zhang, Y.; Bellon, S. F.; Whittington, D. A.; Harmange, J.-P.; Dominguez, C.; Kim, T.-S.; Dussault, I. J. Med. Chem. 2012, 55, 1868–1897. doi:10.1021/jm201331s |
| 12. | Costa, D.; Marques, A. P.; Reis, R. L.; Lima, J. L. F. C.; Fernandes, E. Free Radical Biol. Med. 2006, 40, 632–640. doi:10.1016/j.freeradbiomed.2005.09.017 |
| 13. | Kalyanaraman, B.; Sohnle, P. G. J. Clin. Invest. 1985, 75, 1618–1622. doi:10.1172/JCI111868 |
| 11. | Vassilev, G. N.; Yonova, P. A.; Bohland, H.; Vassilev, N. G.; Yordanov, B. Dokl. Bulg. Akad. Nauk. 1997, 50, 59–62. |
| 17. | Cheng, C.; Shih, Y.-C.; Chen, H.-T.; Chien, T.-C. Tetrahedron 2013, 69, 1387–1396. doi:10.1016/j.tet.2012.11.001 |
| 18. | Sharma, A.; Vacchani, D.; Van der Eycken, E. Chem.–Eur. J. 2013, 19, 1158–1168. doi:10.1002/chem.201201868 |
| 19. | Kozhushkov, S. I.; Potukuchi, H. K.; Ackermann, L. Catal. Sci. Technol. 2013, 3, 562–571. doi:10.1039/c2cy20505j |
| 20. | Mousseau, J. J.; Charrette, A. B. Acc. Chem. Res. 2013, 46, 412–424. doi:10.1021/ar300185z |
| 21. | Neufeldt, S. R.; Sanford, M. S. Acc. Chem. Res. 2012, 45, 936–946. doi:10.1021/ar300014f |
| 22. | Engle, K. M.; Mei, T.; Wasa, M.-S.; Yu, J.-Q. Acc. Chem. Res. 2012, 45, 788–802. doi:10.1021/ar200185g |
| 23. | Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215–1292. doi:10.1021/cr100280d |
| 24. | Sun, C.-L.; Li, B.-J.; Shi, Z.-J. Chem. Rev. 2011, 111, 1293–1314. doi:10.1021/cr100198w |
| 25. | Baudoin, O. Chem. Soc. Rev. 2011, 40, 4902–4911. doi:10.1039/c1cs15058h |
| 26. | Cho, S. H.; Kim, J. Y.; Kwak, J.; Chang, S. Chem. Soc. Rev. 2011, 40, 5068–5083. doi:10.1039/c1cs15082k |
© 2013 Gong et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)