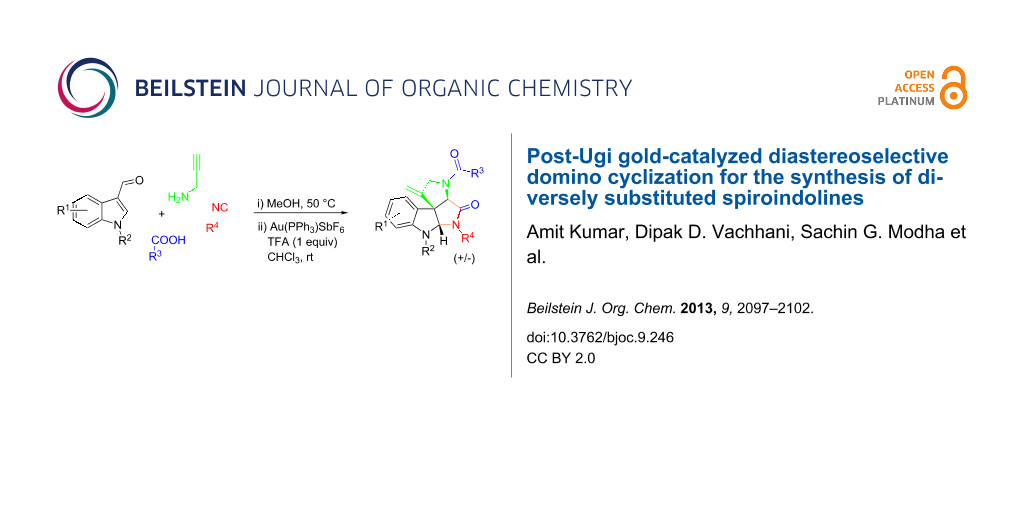
Abstract
An Ugi four-component reaction of propargylamine with 3-formylindole and various acids and isonitriles produces adducts which are subjected to a cationic gold-catalyzed diastereoselective domino cyclization to furnish diversely substituted spiroindolines. All the reactions run via an exo-dig attack in the hydroarylation step followed by an intramolecular diastereoselective trapping of the imminium ion. The whole sequence is atom economic and the application of a multicomponent reaction assures diversity.
Graphical Abstract
Introduction
The importance of nitrogen containing heterocyclic molecules in chemical biology is undisputed. The synthesis of such biologically interesting heterocycles is generally target-oriented, inspired by nature or randomly directed. In all these cases the design of a synthetic sequence to produce a library of diversely substituted molecules is the first and most important step. The basic concept of diversity-oriented synthesis (DOS) involves short reaction sequences, a strong focus on bond construction, and functional group compatibility [1-3]. Reactions that involve multiple bond formation, such as multicomponent reactions [4-9] and tandem reactions [10-16], are very useful in this context.
As an efficient activator of alkynes, gold has recently attracted a lot of attention [17-36]. Many tandem approaches have been reported which utilize this coinage metal for the construction of variously substituted complex molecules [37-43]. We have recently reported a post-Ugi gold-catalyzed intramolecular domino cyclization sequence which produces spiroindolines (Scheme 1) [44]. The first step in this sequence is an Ugi four-component reaction (Ugi-4CR) [4,5] with 2-alkynoic acid as an alkyne source. The second step is a cationic gold-catalyzed intramolecular hydroarylation tandem cyclization to produce spiroindolines with complete diastereoselectivity. This synthetic sequence is atom economic and mild conditions are applied to generate a very complex molecular structure from readily available starting materials. Based on this work and our continuous interest in transition metal catalysis [45-54], multicomponent reactions [55-57] and the chemistry of the indole core [58-60], we herein report a post-Ugi gold-catalyzed intramolecular domino cyclization sequence for the synthesis of spiroindolines with propargylamines as an alkyne source (Scheme 1).
Scheme 1: Gold-catalyzed approaches towards spiroindolines.
Scheme 1: Gold-catalyzed approaches towards spiroindolines.
Results and Discussion
The use of benzoic acid as an acid component in the Ugi-4CR did not produce the Ugi-adduct in good yield even after a prolonged reaction time. Therefore, we switched to phenylacetic acid. The Ugi-4CR of indole-3-carboxaldehyde (1a), propargylamine (2a), phenylacetic acid (3a) and tert-butylisonitrile (4a) in methanol at 50 °C gave the Ugi-adduct 5a with an excellent yield of 94%. With compound 5a in hand we were keen to apply the previously developed conditions for intramolecular hydroarylation [44]. Reaction of 5a with 5 mol % of Au(PPh3)SbF6 in chloroform at room temperature produced the desired spiroindoline 6a in a moderate yield of 55% along with some unidentified byproducts (Table 1, entry 1). The use of a protic acid with a gold catalyst is known in the literature [61-64]. To our delight, when the above reaction was carried out with 1 equivalent of trifluoroacetic acid (TFA) the yield was improved to 81% (Table 1, entry 2). Apart from being a good proton source TFA might be working as a coligand.
Table 1: Optimization for the intramolecular hydroarylation.a
|
|
||||
| Entry | Catalyst (mol %) | Acid (1 equiv) | Time h | % Yieldb |
|---|---|---|---|---|
| 1 | Au(PPh3)SbF6 (5) | — | 2 | 55c |
| 2 | Au(PPh3)SbF6 (5) | TFA | 2 | 81 |
| 3 | PtCl2 (5) | — | 10 | —d |
| 4 | PtCl2 (5) | TFA | 10 | —d |
| 5 | — | TFA | 10 | —d |
| 6 | Au(PPh3)SbF6 (5) | PTSA | 2 | 70 |
aAll the reactions were run on 0.1 mmol scale of 5a with chloroform (2 mL) as a solvent at rt. bIsolated yields. cUnidentified byproducts were formed. dNo conversion.
Experiments with PtCl2 as a catalyst did not show any conversion and the starting material was recovered quantitatively (Table 1, entries 3 and 4). In absence of the gold catalyst no product could be observed (Table 1, entry 5). The application of p-toluenesulfonic acid (PTSA) instead of TFA did not improve the outcome (Table 1, entry 6).
Having the optimized conditions in hand (Table 1, entry 2), various Ugi-adducts 5b–q were synthesized and subjected to this hydroarylation domino cyclization sequence (Table 2). Different substituents are well-tolerated and the sprioindolines were obtained in good to excellent yields. A methyl substituent on the indole nitrogen did not hamper the domino cyclization (Table 2, entries 4, 6, 11, 12, 14, 15). Substituents like tert-butyl, cyclohexyl and n-butyl on the isonitrile are well-tolerated for the domino cyclization on the second position of the indole (Table 2, entries 1–16). Regarding the substituents coming from the acid part, tert-butyl gave a decreased yield probably due to steric hindrance (Table 2, entry 5). It is noteworthy that the gold-catalyzed intramolecular hydroarylation exclusively gives the exo-dig product in all cases and with complete diastereoselectivity.
Table 2: Scope and limitations of intramolecular domino cyclization.a
| Entry | Ugi adduct 5 | Spiroindolines 6 (+/−) | Entry | Ugi adduct 5 | Spiroindolines 6 (+/−) |
|---|---|---|---|---|---|
| 1 |
5b, 87% |
6b, 70% |
9 |
5j, 89% |
6j, 75% |
| 2 |
5c, 86% |
6c, 60% |
10 |
5k, 63% |
6k, 80% |
| 3 |
5d, 69% |
6d, 76% |
11 |
5l, 77% |
6l, 74% |
| 4 |
5e, 72% |
6e, 66% |
12 |
5m, 74% |
6m, 68% |
| 5 |
5f, 68% |
6f, 40% |
13 |
5n, 65% |
6n, 72% |
| 6 |
5g, 77% |
6g, 71% |
14 |
5o, 68% |
6o, 60% |
| 7 |
5h, 79% |
6h, 83% |
15 |
5p, 58% |
6p, 84% |
| 8 |
5i, 67% |
6i, 69% |
16 |
5q, 59% |
6q, 69% |
aAll the reaction were run on a 0.2 mmol scale of 5 in a screw capped vial employing the optimal conditions of Table 1. Cy = cyclohexyl, Bn = benzyl, PMB = p-methoxybenzyl, Bu = n-butyl.
A plausible mechanism [30,44] is shown in Scheme 2 with only the R-isomer of the Ugi-adduct 5a to simplify the discussion. The cationic gold coordinates with the terminal alkyne which becomes activated for a nucleophilic attack. This can occur from both sides of the indole core. When the attack occurs from the back side of the indole core, spiro intermediate B will be formed. However, in this spiro intermediate the intramolecular trapping of the imminium ion by the amidic NH is sterically impossible and thus the intermediate reopens to intermediate A. If the attack takes place from the front side of the indole core, intermediate C is formed and trapping is possible. After deprotonation and protodeauration the desired spiroindoline 6a is formed with the stereochemistry of two new stereocenters S.
Scheme 2: Plausible mechanism for the domino sequence.
Scheme 2: Plausible mechanism for the domino sequence.
Conclusion
In conclusion we have developed a diversity-oriented post-Ugi gold-catalyzed intramolecular hydroarylation domino cyclization sequence for the diastereoselective synthesis of spiroindolines. The mild reaction conditions and short synthetic sequence are the merits of this method. The flexibility given by the multicomponent reaction assures the generation of diversity.
Experimental
General procedure for the synthesis of spiroindolines 6a–q
To a screw capped vial Au(PPh3)Cl (5 mol %) and AgSbF6 (5 mol %) were loaded along with chloroform (2 mL). Ugi product 5 (0.2 mmol) was added followed by TFA (1 equiv), and the reaction mixture was stirred at rt. After completion, the reaction mixture was partitioned between EtOAc (100 mL) and 2 N K2CO3 solution (2 × 50 mL). The organic layer was washed with brine (50 mL), dried over magnesium sulfate, and evaporated under reduced pressure. The obtained residue was purified by silica gel column chromatography (10% diethyl ether in dichloromethane) to afford compound 6a–q.
Supporting Information
| Supporting Information File 1: Experimental section. | ||
| Format: PDF | Size: 1.6 MB | Download |
Acknowledgements
The authors wish to thank the F.W.O [Fund for Scientific Research – Flanders (Belgium)] and the Research Fund of the University of Leuven (KU Leuven) for financial support. A.K. is thankful to EMA2experts (Erasmus Mundus Action 2, Lot 11 Asia: Experts) for providing a doctoral exchange scholarship, and D.D.V. is thankful to EMECW, lot 13 (Erasmus Mundus External Cooperation Window, Lot 13) for providing a doctoral scholarship. The authors thank Ir. B. Demarsin for HRMS measurements.
References
-
Ruijter, E.; Scheffelaar, R.; Orru, R. V. A. Angew. Chem., Int. Ed. 2011, 50, 6234–6246. doi:10.1002/anie.201006515
Return to citation in text: [1] -
Ganem, B. Acc. Chem. Res. 2009, 42, 463–472. doi:10.1021/ar800214s
Return to citation in text: [1] -
El Kaïm, L.; Grimaud, L. Mol. Diversity 2010, 14, 855–867. doi:10.1007/s11030-009-9175-3
Return to citation in text: [1] -
Dömling, A.; Ugi, I. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. doi:10.1002/1521-3773(20000915)39:18<3168::AID-ANIE3168>3.0.CO;2-U
Return to citation in text: [1] [2] -
Dömling, A. Chem. Rev. 2006, 106, 17–89. doi:10.1021/cr0505728
Return to citation in text: [1] [2] -
Ramón, D. J.; Yus, M. Angew. Chem., Int. Ed. 2005, 44, 1602–1634. doi:10.1002/anie.200460548
Return to citation in text: [1] -
Dömling, A.; Wang, W.; Wang, K. Chem. Rev. 2012, 112, 3083–3135. doi:10.1021/cr100233r
Return to citation in text: [1] -
Shriri, M. Chem. Rev. 2012, 112, 3508–3549. doi:10.1021/cr2003954
Return to citation in text: [1] -
Chen, Z.; Zheng, D.; Wu, J. Org. Lett. 2011, 13, 848–851. doi:10.1021/ol102775s
Return to citation in text: [1] -
Tietze, L. F.; Rackelmann, N. Pure Appl. Chem. 2004, 76, 1967–1983. doi:10.1351/pac200476111967
Return to citation in text: [1] -
Yang, J.; Xie, X.; Wang, Z.; Mei, R.; Zheng, H.; Wang, X.; Zhang, L.; Qi, J.; She, X. J. Org. Chem. 2013, 78, 1230–1235. doi:10.1021/jo302404v
Return to citation in text: [1] -
Cheng, H.-G.; Lu, L.-Q.; Wang, T.; Yang, Q.-Q.; Liu, X.-P.; Li, Y.; Deng, Q.-H.; Chen, J.-R.; Xiao, W.-J. Angew. Chem., Int. Ed. 2013, 52, 3250–3254. doi:10.1002/anie.201209998
Return to citation in text: [1] -
El Kaïm, L.; Grimaud, L.; Le Goff, X.-F.; Menes-Arzate, M.; Miranda, L. D. Chem. Commun. 2011, 47, 8145–8147. doi:10.1039/c1cc12236c
Return to citation in text: [1] -
Bai, B.; Li, D.-S.; Huang, S.-Z.; Ren, J.; Zhu, H.-J. Nat. Prod. Bioprospect. 2012, 2, 53–58. doi:10.1007/s13659-012-0003-6
Return to citation in text: [1] -
Lajiness, J. P.; Jiang, W.; Boger, D. L. Org. Lett. 2012, 14, 2078–2081. doi:10.1021/ol300599p
Return to citation in text: [1] -
Fan, F.; Xie, W.; Ma, D. Org. Lett. 2012, 14, 1405–1407. doi:10.1021/ol3003496
Return to citation in text: [1] -
Fürstner, A. Chem. Soc. Rev. 2009, 38, 3208–3221. doi:10.1039/b816696j
Return to citation in text: [1] -
Dyker, G. Angew. Chem., Int. Ed. 2000, 39, 4237–4239. doi:10.1002/1521-3773(20001201)39:23<4237::AID-ANIE4237>3.0.CO;2-A
Return to citation in text: [1] -
Hashmi, A. S. K.; Hutchings, G. Angew. Chem., Int. Ed. 2006, 45, 7896–7936. doi:10.1002/anie.200602454
Return to citation in text: [1] -
Fürstner, A.; Davies, P. W. Angew. Chem., Int. Ed. 2007, 46, 3410–3449. doi:10.1002/anie.200604335
Return to citation in text: [1] -
Gorin, D. J.; Sherry, B. D.; Toste, F. D. Chem. Rev. 2008, 108, 3351–3378. doi:10.1021/cr068430g
Return to citation in text: [1] -
Jiménez-Núñez, E.; Echavarren, A. M. Chem. Rev. 2008, 108, 3326–3350. doi:10.1021/cr0684319
Return to citation in text: [1] -
Li, Z. G.; Brouwer, C.; He, C. Chem. Rev. 2008, 108, 3239–3265. doi:10.1021/cr068434l
Return to citation in text: [1] -
Arcadi, A. Chem. Rev. 2008, 108, 3266–3325. doi:10.1021/cr068435d
Return to citation in text: [1] -
Hashmi, A. S. K.; Rudolph, M. Chem. Soc. Rev. 2008, 37, 1766–1775. doi:10.1039/b615629k
Return to citation in text: [1] -
Rudolph, M.; Hashmi, A. S. K. Chem. Soc. Rev. 2012, 41, 2448–2462. doi:10.1039/c1cs15279c
Return to citation in text: [1] -
Echavarren, A. M. Nat. Chem. 2009, 1, 431–433. doi:10.1038/nchem.344
Return to citation in text: [1] -
Jiménez-Núñez, E.; Echavarren, A. M. Chem. Commun. 2007, 333–346. doi:10.1039/b612008c
Return to citation in text: [1] -
Rudolph, M.; Hashmi, A. S. K. Chem. Commun. 2011, 47, 6536–6544. doi:10.1039/c1cc10780a
Return to citation in text: [1] -
Hashmi, A. S. K. Angew. Chem., Int. Ed. 2010, 49, 5232–5241. doi:10.1002/anie.200907078
Return to citation in text: [1] [2] -
Ferrer, C.; Echavarren, A. M. Angew. Chem., Int. Ed. 2006, 45, 1105–1109. doi:10.1002/anie.200503484
Return to citation in text: [1] -
Ferrer, C.; Amijs, C. H. M.; Echavarren, A. M. Chem.–Eur. J. 2007, 13, 1358–1373. doi:10.1002/chem.200601324
Return to citation in text: [1] -
Ferrer, C.; Escribano-Cuesta, A.; Echavarren, A. M. Tetrahedron 2009, 65, 9015–9020. doi:10.1016/j.tet.2009.08.067
Return to citation in text: [1] -
Hashmi, A. S. K.; Yang, W.; Rominger, F. Angew. Chem., Int. Ed. 2011, 50, 5762–5765. doi:10.1002/anie.201100989
Return to citation in text: [1] -
Hashmi, A. S. K.; Yang, W.; Rominger, F. Chem.–Eur. J. 2012, 18, 6576–6580. doi:10.1002/chem.201200314
Return to citation in text: [1] -
Chaładaj, W.; Corbet, M.; Fürstner, A. Angew. Chem., Int. Ed. 2012, 51, 6929–6933. doi:10.1002/anie.201203180
Return to citation in text: [1] -
Loh, C. C. J.; Badorrek, J.; Raabe, G.; Enders, D. Chem.–Eur. J. 2011, 17, 13409–13414. doi:10.1002/chem.201102793
Return to citation in text: [1] -
Lu, Y.; Du, X.; Jia, X.; Liu, Y. Adv. Synth. Catal. 2009, 351, 1517–1522. doi:10.1002/adsc.200900068
Return to citation in text: [1] -
Xie, X.; Du, X.; Chen, Y.; Liu, Y. J. Org. Chem. 2011, 76, 9175–9181. doi:10.1021/jo2017668
Return to citation in text: [1] -
Zhang, L. J. Am. Chem. Soc. 2005, 127, 16804–16805. doi:10.1021/ja056419c
Return to citation in text: [1] -
Cera, G.; Crispino, P.; Monari, M.; Bandini, M. Chem. Commun. 2011, 47, 7803–7805. doi:10.1039/c1cc12328a
Return to citation in text: [1] -
Cera, G.; Chiarucci, M.; Mazzanti, A.; Mancinelli, M.; Bandini, M. Org. Lett. 2012, 14, 1350–1353. doi:10.1021/ol300297t
Return to citation in text: [1] -
Liu, Y.; Xu, W.; Wang, W. Org. Lett. 2010, 12, 1448–1451. doi:10.1021/ol100153h
Return to citation in text: [1] -
Modha, S. G.; Kumar, A.; Vachhani, D. D.; Jacobs, J.; Sharma, S. K.; Parmar, V. S.; Van Meervelt, L.; Van der Eycken, E. V. Angew. Chem., Int. Ed. 2012, 51, 9572–9575. doi:10.1002/anie.201205052
Return to citation in text: [1] [2] [3] -
Modha, S. G.; Kumar, A.; Vachhani, D. D.; Sharma, S. K.; Parmar, V. S.; Van der Eycken, E. V. Chem. Commun. 2012, 48, 10916–10918. doi:10.1039/c2cc35900f
Return to citation in text: [1] -
Vachhani, D. D.; Galli, M.; Jacobs, J.; Van Meervelt, L.; Van der Eycken, E. V. Chem. Commun. 2013, 49, 7171–7173. doi:10.1039/c3cc43418d
Return to citation in text: [1] -
Modha, S. G.; Mehta, V. P.; Van der Eycken, E. V. Chem. Soc. Rev. 2013, 42, 5042–5055. doi:10.1039/c3cs60041f
Return to citation in text: [1] -
Kumar, A.; Vachhani, D. D.; Modha, S. G.; Sharma, S. K.; Parmar, V. S.; Van der Eycken, E. V. Eur. J. Org. Chem. 2013, 2288–2292. doi:10.1002/ejoc.201300132
Return to citation in text: [1] -
Vachhani, D. D.; Sharma, A.; Van der Eycken, E. J. Org. Chem. 2012, 77, 8768–8774. doi:10.1021/jo301401q
Return to citation in text: [1] -
Vachhani, D. D.; Mehta, V. P.; Modha, S. G.; Van Hecke, K.; Van Meervelt, L.; Van der Eycken, E. V. Adv. Synth. Catal. 2012, 354, 1593–1599. doi:10.1002/adsc.201100881
Return to citation in text: [1] -
Vachhani, D. D.; Kumar, A.; Modha, S. G.; Sharma, S. K.; Parmar, V. S.; Van der Eycken, E. V. Eur. J. Org. Chem. 2013, 1223–1227. doi:10.1002/ejoc.201201587
Return to citation in text: [1] -
Modha, S. G.; Trivedi, J. C.; Mehta, V. P.; Ermolat’ev, D. S.; Van der Eycken, E. V. J. Org. Chem. 2011, 76, 846–856. doi:10.1021/jo102089h
Return to citation in text: [1] -
Modha, S. G.; Mehta, V. P.; Ermolat’ev, D. S.; Balzarini, J.; Van Hecke, K.; Van Meervelt, L.; Van der Eycken, E. V. Mol. Diversity 2010, 14, 767–776. doi:10.1007/s11030-009-9221-1
Return to citation in text: [1] -
Kumar, A.; Li, Z.; Sharma, S. K.; Parmar, V. S.; Van der Eycken, E. V. Org. Lett. 2013, 15, 1874–1877. doi:10.1021/ol400526a
Return to citation in text: [1] -
Mehta, V. P.; Modha, S. G.; Ruijter, E.; Van Hecke, K.; Van Meervelt, L.; Pannecouque, C.; Balzarini, J.; Orru, R. V. A.; Van der Eycken, E. V. J. Org. Chem. 2011, 76, 2828–2839. doi:10.1021/jo200251q
Return to citation in text: [1] -
Peshkov, V. A.; Pereshivko, O. P.; Van der Eycken, E. V. Chem. Soc. Rev. 2012, 41, 3790–3807. doi:10.1039/c2cs15356d
Return to citation in text: [1] -
Vachhani, D. D.; Sharma, A.; Van der Eycken, E. V. Angew. Chem., Int. Ed. 2013, 52, 2547–2550. doi:10.1002/anie.201209312
Return to citation in text: [1] -
Modha, S. G.; Vachhani, D. D.; Jacobs, J.; Van Meervelt, L.; Van der Eycken, E. V. Chem. Commun. 2012, 48, 6550–6552. doi:10.1039/c2cc32586a
Return to citation in text: [1] -
Donets, P. A.; Van Hecke, K.; Van Meervelt, L.; Van der Eycken, E. V. Org. Lett. 2009, 11, 3618–3621. doi:10.1021/ol901356h
Return to citation in text: [1] -
Donets, P. A.; Van der Eycken, E. V. Synthesis 2011, 2147–2153. doi:10.1055/s-0030-1260057
Return to citation in text: [1] -
Zhou, C.-Y.; Chan, P. W. H.; Che, C.-M. Org. Lett. 2006, 8, 325–328. doi:10.1021/ol052696c
Return to citation in text: [1] -
Brand, J. P.; Waser, J. Angew. Chem., Int. Ed. 2010, 49, 7304–7307. doi:10.1002/anie.201003179
Return to citation in text: [1] -
Vachhani, D. D.; Modha, S. G.; Sharma, A.; Van der Eycken, E. V. Tetrahedron 2013, 69, 359–365. doi:10.1016/j.tet.2012.10.019
Return to citation in text: [1] -
Tubaro, C.; Baron, M.; Biffis, A.; Basato, M. Beilstein J. Org. Chem. 2013, 9, 246–253. doi:10.3762/bjoc.9.29
Return to citation in text: [1]
| 1. | Ruijter, E.; Scheffelaar, R.; Orru, R. V. A. Angew. Chem., Int. Ed. 2011, 50, 6234–6246. doi:10.1002/anie.201006515 |
| 2. | Ganem, B. Acc. Chem. Res. 2009, 42, 463–472. doi:10.1021/ar800214s |
| 3. | El Kaïm, L.; Grimaud, L. Mol. Diversity 2010, 14, 855–867. doi:10.1007/s11030-009-9175-3 |
| 37. | Loh, C. C. J.; Badorrek, J.; Raabe, G.; Enders, D. Chem.–Eur. J. 2011, 17, 13409–13414. doi:10.1002/chem.201102793 |
| 38. | Lu, Y.; Du, X.; Jia, X.; Liu, Y. Adv. Synth. Catal. 2009, 351, 1517–1522. doi:10.1002/adsc.200900068 |
| 39. | Xie, X.; Du, X.; Chen, Y.; Liu, Y. J. Org. Chem. 2011, 76, 9175–9181. doi:10.1021/jo2017668 |
| 40. | Zhang, L. J. Am. Chem. Soc. 2005, 127, 16804–16805. doi:10.1021/ja056419c |
| 41. | Cera, G.; Crispino, P.; Monari, M.; Bandini, M. Chem. Commun. 2011, 47, 7803–7805. doi:10.1039/c1cc12328a |
| 42. | Cera, G.; Chiarucci, M.; Mazzanti, A.; Mancinelli, M.; Bandini, M. Org. Lett. 2012, 14, 1350–1353. doi:10.1021/ol300297t |
| 43. | Liu, Y.; Xu, W.; Wang, W. Org. Lett. 2010, 12, 1448–1451. doi:10.1021/ol100153h |
| 17. | Fürstner, A. Chem. Soc. Rev. 2009, 38, 3208–3221. doi:10.1039/b816696j |
| 18. | Dyker, G. Angew. Chem., Int. Ed. 2000, 39, 4237–4239. doi:10.1002/1521-3773(20001201)39:23<4237::AID-ANIE4237>3.0.CO;2-A |
| 19. | Hashmi, A. S. K.; Hutchings, G. Angew. Chem., Int. Ed. 2006, 45, 7896–7936. doi:10.1002/anie.200602454 |
| 20. | Fürstner, A.; Davies, P. W. Angew. Chem., Int. Ed. 2007, 46, 3410–3449. doi:10.1002/anie.200604335 |
| 21. | Gorin, D. J.; Sherry, B. D.; Toste, F. D. Chem. Rev. 2008, 108, 3351–3378. doi:10.1021/cr068430g |
| 22. | Jiménez-Núñez, E.; Echavarren, A. M. Chem. Rev. 2008, 108, 3326–3350. doi:10.1021/cr0684319 |
| 23. | Li, Z. G.; Brouwer, C.; He, C. Chem. Rev. 2008, 108, 3239–3265. doi:10.1021/cr068434l |
| 24. | Arcadi, A. Chem. Rev. 2008, 108, 3266–3325. doi:10.1021/cr068435d |
| 25. | Hashmi, A. S. K.; Rudolph, M. Chem. Soc. Rev. 2008, 37, 1766–1775. doi:10.1039/b615629k |
| 26. | Rudolph, M.; Hashmi, A. S. K. Chem. Soc. Rev. 2012, 41, 2448–2462. doi:10.1039/c1cs15279c |
| 27. | Echavarren, A. M. Nat. Chem. 2009, 1, 431–433. doi:10.1038/nchem.344 |
| 28. | Jiménez-Núñez, E.; Echavarren, A. M. Chem. Commun. 2007, 333–346. doi:10.1039/b612008c |
| 29. | Rudolph, M.; Hashmi, A. S. K. Chem. Commun. 2011, 47, 6536–6544. doi:10.1039/c1cc10780a |
| 30. | Hashmi, A. S. K. Angew. Chem., Int. Ed. 2010, 49, 5232–5241. doi:10.1002/anie.200907078 |
| 31. | Ferrer, C.; Echavarren, A. M. Angew. Chem., Int. Ed. 2006, 45, 1105–1109. doi:10.1002/anie.200503484 |
| 32. | Ferrer, C.; Amijs, C. H. M.; Echavarren, A. M. Chem.–Eur. J. 2007, 13, 1358–1373. doi:10.1002/chem.200601324 |
| 33. | Ferrer, C.; Escribano-Cuesta, A.; Echavarren, A. M. Tetrahedron 2009, 65, 9015–9020. doi:10.1016/j.tet.2009.08.067 |
| 34. | Hashmi, A. S. K.; Yang, W.; Rominger, F. Angew. Chem., Int. Ed. 2011, 50, 5762–5765. doi:10.1002/anie.201100989 |
| 35. | Hashmi, A. S. K.; Yang, W.; Rominger, F. Chem.–Eur. J. 2012, 18, 6576–6580. doi:10.1002/chem.201200314 |
| 36. | Chaładaj, W.; Corbet, M.; Fürstner, A. Angew. Chem., Int. Ed. 2012, 51, 6929–6933. doi:10.1002/anie.201203180 |
| 10. | Tietze, L. F.; Rackelmann, N. Pure Appl. Chem. 2004, 76, 1967–1983. doi:10.1351/pac200476111967 |
| 11. | Yang, J.; Xie, X.; Wang, Z.; Mei, R.; Zheng, H.; Wang, X.; Zhang, L.; Qi, J.; She, X. J. Org. Chem. 2013, 78, 1230–1235. doi:10.1021/jo302404v |
| 12. | Cheng, H.-G.; Lu, L.-Q.; Wang, T.; Yang, Q.-Q.; Liu, X.-P.; Li, Y.; Deng, Q.-H.; Chen, J.-R.; Xiao, W.-J. Angew. Chem., Int. Ed. 2013, 52, 3250–3254. doi:10.1002/anie.201209998 |
| 13. | El Kaïm, L.; Grimaud, L.; Le Goff, X.-F.; Menes-Arzate, M.; Miranda, L. D. Chem. Commun. 2011, 47, 8145–8147. doi:10.1039/c1cc12236c |
| 14. | Bai, B.; Li, D.-S.; Huang, S.-Z.; Ren, J.; Zhu, H.-J. Nat. Prod. Bioprospect. 2012, 2, 53–58. doi:10.1007/s13659-012-0003-6 |
| 15. | Lajiness, J. P.; Jiang, W.; Boger, D. L. Org. Lett. 2012, 14, 2078–2081. doi:10.1021/ol300599p |
| 16. | Fan, F.; Xie, W.; Ma, D. Org. Lett. 2012, 14, 1405–1407. doi:10.1021/ol3003496 |
| 30. | Hashmi, A. S. K. Angew. Chem., Int. Ed. 2010, 49, 5232–5241. doi:10.1002/anie.200907078 |
| 44. | Modha, S. G.; Kumar, A.; Vachhani, D. D.; Jacobs, J.; Sharma, S. K.; Parmar, V. S.; Van Meervelt, L.; Van der Eycken, E. V. Angew. Chem., Int. Ed. 2012, 51, 9572–9575. doi:10.1002/anie.201205052 |
| 4. | Dömling, A.; Ugi, I. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. doi:10.1002/1521-3773(20000915)39:18<3168::AID-ANIE3168>3.0.CO;2-U |
| 5. | Dömling, A. Chem. Rev. 2006, 106, 17–89. doi:10.1021/cr0505728 |
| 6. | Ramón, D. J.; Yus, M. Angew. Chem., Int. Ed. 2005, 44, 1602–1634. doi:10.1002/anie.200460548 |
| 7. | Dömling, A.; Wang, W.; Wang, K. Chem. Rev. 2012, 112, 3083–3135. doi:10.1021/cr100233r |
| 8. | Shriri, M. Chem. Rev. 2012, 112, 3508–3549. doi:10.1021/cr2003954 |
| 9. | Chen, Z.; Zheng, D.; Wu, J. Org. Lett. 2011, 13, 848–851. doi:10.1021/ol102775s |
| 55. | Mehta, V. P.; Modha, S. G.; Ruijter, E.; Van Hecke, K.; Van Meervelt, L.; Pannecouque, C.; Balzarini, J.; Orru, R. V. A.; Van der Eycken, E. V. J. Org. Chem. 2011, 76, 2828–2839. doi:10.1021/jo200251q |
| 56. | Peshkov, V. A.; Pereshivko, O. P.; Van der Eycken, E. V. Chem. Soc. Rev. 2012, 41, 3790–3807. doi:10.1039/c2cs15356d |
| 57. | Vachhani, D. D.; Sharma, A.; Van der Eycken, E. V. Angew. Chem., Int. Ed. 2013, 52, 2547–2550. doi:10.1002/anie.201209312 |
| 44. | Modha, S. G.; Kumar, A.; Vachhani, D. D.; Jacobs, J.; Sharma, S. K.; Parmar, V. S.; Van Meervelt, L.; Van der Eycken, E. V. Angew. Chem., Int. Ed. 2012, 51, 9572–9575. doi:10.1002/anie.201205052 |
| 45. | Modha, S. G.; Kumar, A.; Vachhani, D. D.; Sharma, S. K.; Parmar, V. S.; Van der Eycken, E. V. Chem. Commun. 2012, 48, 10916–10918. doi:10.1039/c2cc35900f |
| 46. | Vachhani, D. D.; Galli, M.; Jacobs, J.; Van Meervelt, L.; Van der Eycken, E. V. Chem. Commun. 2013, 49, 7171–7173. doi:10.1039/c3cc43418d |
| 47. | Modha, S. G.; Mehta, V. P.; Van der Eycken, E. V. Chem. Soc. Rev. 2013, 42, 5042–5055. doi:10.1039/c3cs60041f |
| 48. | Kumar, A.; Vachhani, D. D.; Modha, S. G.; Sharma, S. K.; Parmar, V. S.; Van der Eycken, E. V. Eur. J. Org. Chem. 2013, 2288–2292. doi:10.1002/ejoc.201300132 |
| 49. | Vachhani, D. D.; Sharma, A.; Van der Eycken, E. J. Org. Chem. 2012, 77, 8768–8774. doi:10.1021/jo301401q |
| 50. | Vachhani, D. D.; Mehta, V. P.; Modha, S. G.; Van Hecke, K.; Van Meervelt, L.; Van der Eycken, E. V. Adv. Synth. Catal. 2012, 354, 1593–1599. doi:10.1002/adsc.201100881 |
| 51. | Vachhani, D. D.; Kumar, A.; Modha, S. G.; Sharma, S. K.; Parmar, V. S.; Van der Eycken, E. V. Eur. J. Org. Chem. 2013, 1223–1227. doi:10.1002/ejoc.201201587 |
| 52. | Modha, S. G.; Trivedi, J. C.; Mehta, V. P.; Ermolat’ev, D. S.; Van der Eycken, E. V. J. Org. Chem. 2011, 76, 846–856. doi:10.1021/jo102089h |
| 53. | Modha, S. G.; Mehta, V. P.; Ermolat’ev, D. S.; Balzarini, J.; Van Hecke, K.; Van Meervelt, L.; Van der Eycken, E. V. Mol. Diversity 2010, 14, 767–776. doi:10.1007/s11030-009-9221-1 |
| 54. | Kumar, A.; Li, Z.; Sharma, S. K.; Parmar, V. S.; Van der Eycken, E. V. Org. Lett. 2013, 15, 1874–1877. doi:10.1021/ol400526a |
| 61. | Zhou, C.-Y.; Chan, P. W. H.; Che, C.-M. Org. Lett. 2006, 8, 325–328. doi:10.1021/ol052696c |
| 62. | Brand, J. P.; Waser, J. Angew. Chem., Int. Ed. 2010, 49, 7304–7307. doi:10.1002/anie.201003179 |
| 63. | Vachhani, D. D.; Modha, S. G.; Sharma, A.; Van der Eycken, E. V. Tetrahedron 2013, 69, 359–365. doi:10.1016/j.tet.2012.10.019 |
| 64. | Tubaro, C.; Baron, M.; Biffis, A.; Basato, M. Beilstein J. Org. Chem. 2013, 9, 246–253. doi:10.3762/bjoc.9.29 |
| 4. | Dömling, A.; Ugi, I. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. doi:10.1002/1521-3773(20000915)39:18<3168::AID-ANIE3168>3.0.CO;2-U |
| 5. | Dömling, A. Chem. Rev. 2006, 106, 17–89. doi:10.1021/cr0505728 |
| 44. | Modha, S. G.; Kumar, A.; Vachhani, D. D.; Jacobs, J.; Sharma, S. K.; Parmar, V. S.; Van Meervelt, L.; Van der Eycken, E. V. Angew. Chem., Int. Ed. 2012, 51, 9572–9575. doi:10.1002/anie.201205052 |
| 58. | Modha, S. G.; Vachhani, D. D.; Jacobs, J.; Van Meervelt, L.; Van der Eycken, E. V. Chem. Commun. 2012, 48, 6550–6552. doi:10.1039/c2cc32586a |
| 59. | Donets, P. A.; Van Hecke, K.; Van Meervelt, L.; Van der Eycken, E. V. Org. Lett. 2009, 11, 3618–3621. doi:10.1021/ol901356h |
| 60. | Donets, P. A.; Van der Eycken, E. V. Synthesis 2011, 2147–2153. doi:10.1055/s-0030-1260057 |
© 2013 Kumar et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)