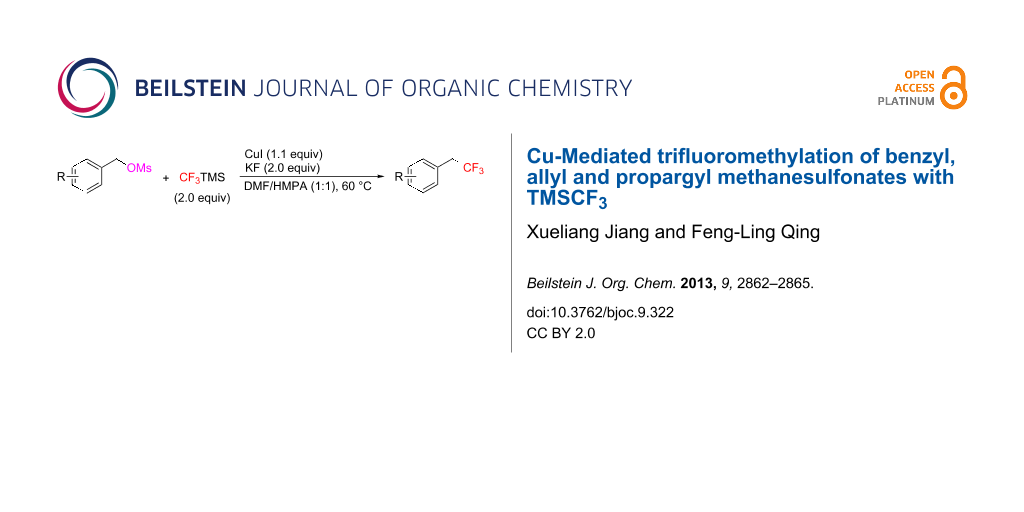
Abstract
A Cu-mediated trifluoromethylation of benzyl, allyl and propargyl methanesulfonates with TMSCF3 was developed for the first time. This method offers a convenient and economical approach to various trifluoroethyl-containing compounds.
Graphical Abstract
Introduction
Fluorinated organic molecules are extremely important in agrochemicals, pharmaceuticals and materials [1-6]. In recent years, (trifluoroethyl)arenes have drawn increasing attention in medicinal chemistry and related fields [7-9]. Different methods have been developed for the synthesis of (trifluoroethyl)arenes, such as Cl–F exchange of the trichloroethyl derivatives [10], reduction of the (trifluoroethyl)aryl derivatives [11] and addition of 2,2-difluorostyrene derivatives [12]. Compared to these methods, the direct transition metal-mediated trifluoroethylation of arylboronic acids [13,14] (Scheme 1a) and trifluoromethylation of benzyl halides [15-21] (Scheme 1b) are more convenient. Especially trifluoromethylations of benzyl bromides with a [CuCF3] species, which are generated from different precursors, are generally employed to afford various (trifluoroethyl)arenes. Although these methods are proven efficient, it is still highly desirable to develop new protocols from economic consideration. In continuation of our research on transition metal-mediated trifluoromethylation [22-27], we report here the first example of Cu-mediated trifluoromethylation of benzyl methanesulfonates (Scheme 1c).
Scheme 1: Transition metal-mediated methods for the preparation of (trifluoroethyl)arenes.
Scheme 1: Transition metal-mediated methods for the preparation of (trifluoroethyl)arenes.
Results and Discussion
We initiated our investigation by reacting benzyl methanesulfonate 1a with TMSCF3 (2.0 equiv) in the presence of KF (2.0 equiv) and CuI (0.2 equiv) in DMF (2.0 mL) at 60 °C under Ar atmosphere. However, only 17% yield of the desired product 2a was observed in this case (Table 1, entry 1). The yield was improved to 31% when the reaction was carried out in the presence of 1,10-phenanthroline (phen) (Table 1, entry 2). Increasing the substrate concentration (from 0.1 M to 0.4 M) could further improve the product yield to 49% (Table 1, entries 3 and 4). Other copper salts such as CuBr, CuCl, CuTc and CuOAc, were next screened, but none of them was better than CuI (Table 1, entries 5–8). Interestingly, when the benzyl methanesulfonate reacted with [CuCF3] generated in situ from TMSCF3 and a stoichiometric amount of CuI (1.1 equiv) without phen, the desired product 2a was formed in 68% yield (Table 1, entry 9). Decreasing or increasing the amount of CuI resulted in a lower yield (Table 1, entries 10 and 11). The solvent was next screened and, to our delight, the highest yield of the product was achieved when using DMF/HMPA (1:1) as the mixed solvent (Table 1, entry 14).
Table 1: Optimization of the reaction conditions.a
|
|
||||
| entry | CuX (equiv) | ligand | solvent | yield of 2a (%)b |
|---|---|---|---|---|
| 1c | CuI (0.2) | – | DMF | 17 |
| 2c | CuI (0.2) | phen | DMF | 31 |
| 3d | CuI (0.2) | phen | DMF | 32 |
| 4 | CuI (0.2) | phen | DMF | 49 |
| 5 | CuBr (0.2) | phen | DMF | 40 |
| 6 | CuCl (0.2) | phen | DMF | trace |
| 7e | CuTc (0.2) | phen | DMF | trace |
| 8 | CuOAc | phen | DMF | trace |
| 9 | CuI (1.1) | – | DMF | 68 |
| 10 | CuI (1.5) | – | DMF | 66 |
| 11 | CuI (1.0) | – | DMF | 62 |
| 12 | CuI (1.1) | – | DMSO | 38 |
| 13 | CuI (1.1) | – | HMPA | 9 |
| 14 | CuI (1.1) | – | DMF/HMPA (1:1) | 76 |
aReaction conditions: 1a (0.2 mmol), ligand (0.2 mmol), TMSCF3 (0.4 mmol), KF (0.4 mmol), DMF (0.5 mL), 60 °C, under Ar atmosphere. bYield was determined by 19F NMR using benzotrifluoride as an internal standard. c2.0 mL of DMF. d1.0 mL of DMF. eCuTc is copper(I) thiophene-2-carboxylate.
With the optimal conditions in hand, we next examined the substrate scope of the Cu-mediated trifluoromethylation of benzyl methanesulfonates with TMSCF3 (Scheme 2). This method tolerates various functional groups. A wide range of benzyl methanesulfonates bearing electron-withdrawing groups, such as nitro (1f), cyano (1g), trifluoromethyl (1h) and ester (1i), as well as electron-donating groups such as phenyl (1b), smoothly underwent the transformation, affording the desired products in moderate to good yield. Importantly, both chloro (1d) and bromo (1e) substituents are also compatible with this method. It is particularly noteworthy that the reaction can be scaled up efficiently. 2a and 2c were successfully prepared on 10 mmol scale, indicating the good reliability of the process.
Scheme 2: Cu-mediated trifluoromethylation of benzyl methanesulfonates. Reaction conditions: 1 (2.0 mmol), CuI (2.2 mmol), TMSCF3 (4.0 mmol), KF (4.0 mmol), DMF/HMPA (1:1, 5.0 mL), 60 °C, under Ar atmosphere; Isolated yield. aIsolated yield after distillation on 10.0 mmol scale.
Scheme 2: Cu-mediated trifluoromethylation of benzyl methanesulfonates. Reaction conditions: 1 (2.0 mmol), Cu...
The present reaction could also be expanded to the trifluoromethylation of allylic methanesulfonates (Scheme 3). Treatment of the substrate 1k under the standard reaction conditions afforded the linear trifluoromethylated product 2k in 78% yield with a trace amount of Z isomer. Interesting, the reactions with the allylic methanesulfonates 1l and 1m gave the same regioselectivity and stereoselectivity with good yields. These observations indicate that a π-allyl/Cuш complex might be involved in the Csp3–CF3 bond formation, but the detailed mechanism remains to be elucidated.
Scheme 3: Cu-Mediated trifluoromethylation of allyl methanesulfonates.
Scheme 3: Cu-Mediated trifluoromethylation of allyl methanesulfonates.
We were next interested in the trifluoromethylation of propargyl methanesulfonate derivates. Both aliphatic and aryl-substituted linear propargyl methanesulfonates under standard reaction conditions afforded the corresponding trifluoromethylated propargylic products in moderate yields (Scheme 4a). However, the reaction of the branched substrates under identical conditions gave the trifluoromethylated allenylic products in good to excellent yields, without any trifluoromethylated propargylic products (Scheme 4b). Thus, this reaction provides an efficient protocol for the synthesis of allenylic-CF3 derivatives, which are useful building blocks for pharmaceuticals [28,29].
Scheme 4: Cu-Mediated trifluoromethylation of propargyl methanesulfonates.
Scheme 4: Cu-Mediated trifluoromethylation of propargyl methanesulfonates.
Conclusion
In summary, we have developed an efficient copper-mediated trifluoromethylation of benzyl methanesulfonates at the benzylic position under mild conditions. The reaction can be easily scaled up and allows for the efficient synthesis of a series of (trifluoroethyl)arenes with excellent functional group compatibility. Furthermore, the method could also be extended to the trifluoromethylation of allyl and progargyl methanesulfonates, affording the corresponding allylic-, progargylic- and allenylic-CF3 derivatives.
Experimental
General procedure for the Cu-mediated trifluoromethylation of benzyl methanesulfonates: CuI (2.2 mmol) and KF (4.0 mmol) were added into a Schlenk tube equipped with a magnetic stirring bar under Ar atmosphere. DMF (5.0 mL) and Me3SiCF3 (2.0 equiv) were added. After stirring for 20 minutes, the mixture was heated to 60 °C and then benzyl methanesulfonate (2.0 mmol) was added under N2 atmosphere. The reaction mixture was kept at 60 °C for 4 hours and then cooled to room temperature. The resulting mixture was diluted with diethyl ether, washed with water and brine, dried over sodium sulfate, and concentrated. The crude products were purified by column chromatography on silica gel to give the products.
Supporting Information
| Supporting Information File 1: Experimental details, characterization data of all products and copies of NMR spectra. | ||
| Format: PDF | Size: 1.8 MB | Download |
References
-
Kirsch, P. Modern Fluoroorganic Chemistry; Wiley-VCH: Weinheim, 2004. doi:10.1002/352760393X
Return to citation in text: [1] -
Uneyama, K. Organofluorine Chemistry; Blackwell: Oxford, U.K., 2006. doi:10.1002/9780470988589
Return to citation in text: [1] -
Ojima, I. Fluorine in Medicinal Chemistry and Chemical Biology; Wiley-Blackwell: Chichester, U.K., 2009.
Return to citation in text: [1] -
Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881–1886. doi:10.1126/science.1131943
Return to citation in text: [1] -
Kirk, K. L. Org. Process Res. Dev. 2008, 12, 305–321. doi:10.1021/op700134j
Return to citation in text: [1] -
O'Hagan, D. Chem. Soc. Rev. 2008, 37, 308–319. doi:10.1039/b711844a
Return to citation in text: [1] -
Shi, G. Q.; Dropinski, J. F.; Zhang, Y.; Santini, C.; Sahoo, S. P.; Berger, J. P.; MacNaul, K. L.; Zhou, G.; Agrawal, A.; Alvaro, R.; Cai, T.-Q.; Hernandez, M.; Wright, S. D.; Moller, D. E.; Heck, J. V.; Meinke, P. T. J. Med. Chem. 2005, 48, 5589–5599. doi:10.1021/jm050373g
Return to citation in text: [1] -
Parrish, C. A.; Adams, N. D.; Auger, K. R.; Burgess, J. L.; Carson, J. D.; Chaudhari, A. M.; Copeland, R. A.; Diamond, M. A.; Donatelli, C. A.; Duffy, K. J.; Faucette, L. F.; Finer, J. T.; Huffman, W. F.; Hugger, E. D.; Jackson, J. R.; Knight, S. D.; Luo, L.; Moore, M. L.; Newlander, K. A.; Ridgers, L. H.; Sakowicz, R.; Shaw, A. N.; Sung, C.-M. M.; Sutton, D.; Wood, K. W.; Zhang, S.-Y.; Zimmerman, M. N.; Dhanak, D. J. Med. Chem. 2007, 50, 4939–4952. doi:10.1021/jm070435y
Return to citation in text: [1] -
Macsari, I.; Besidski, Y.; Csjernyik, G.; Nilsson, L. I.; Sandberg, L.; Yngve, U.; Åhlin, K.; Bueters, T.; Eriksson, A. B.; Lund, P.-E.; Venyike, E.; Oerther, S.; Hygge Blakeman, K.; Luo, L.; Arvidsson, P. I. J. Med. Chem. 2012, 55, 6866–6880. doi:10.1021/jm300623u
Return to citation in text: [1] -
Ando, A.; Miki, T.; Kumadaki, I. J. Org. Chem. 1988, 53, 3637–3639. doi:10.1021/jo00250a049
Return to citation in text: [1] -
Uneyama, K.; Momota, M.; Hayashida, K.; Itoh, T. J. Org. Chem. 1990, 55, 5364–5368. doi:10.1021/jo00306a013
Return to citation in text: [1] -
Nguyen, B. V.; Burton, D. J. J. Org. Chem. 1997, 62, 7758–7764. doi:10.1021/jo971019w
Return to citation in text: [1] -
Zhao, Y.; Hu, J. Angew. Chem., Int. Ed. 2012, 51, 1033–1036. doi:10.1002/anie.201106742
Return to citation in text: [1] -
Liang, A.; Li, X.; Liu, D.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Chem. Commun. 2012, 48, 8273–8275. doi:10.1039/c2cc31651j
Return to citation in text: [1] -
Kobayashi, Y.; Yamamoto, K.; Kumadaki, I. Tetrahedron Lett. 1979, 20, 4071–4072. doi:10.1016/S0040-4039(01)86506-0
Return to citation in text: [1] -
Urata, H.; Fuchikami, T. Tetrahedron Lett. 1991, 32, 91–94. doi:10.1016/S0040-4039(00)71226-3
Return to citation in text: [1] -
Chen, Q.-Y.; Duan, J.-X. J. Chem. Soc., Chem. Commun. 1993, 1389–1391. doi:10.1039/C39930001389
Return to citation in text: [1] -
Kim, J.; Shreeve, J. M. Org. Biomol. Chem. 2004, 2, 2728–2734. doi:10.1039/b412480b
Return to citation in text: [1] -
Dubinina, G. G.; Furutachi, H.; Vicic, D. A. J. Am. Chem. Soc. 2008, 130, 8600–8601. doi:10.1021/ja802946s
Return to citation in text: [1] -
Dubinina, G. G.; Ogikubo, J.; Vicic, D. A. Organometallics 2008, 27, 6233–6235. doi:10.1021/om800794m
Return to citation in text: [1] -
Kawai, H.; Furukawa, T.; Nomura, Y.; Tokunaga, E.; Shibata, N. Org. Lett. 2011, 13, 3596–3599. doi:10.1021/ol201205t
Return to citation in text: [1] -
Chu, L.; Qing, F.-L. J. Am. Chem. Soc. 2010, 132, 7262–7263. doi:10.1021/ja102175w
Return to citation in text: [1] -
Chu, L.; Qing, F.-L. Org. Lett. 2010, 12, 5060–5063. doi:10.1021/ol1023135
Return to citation in text: [1] -
Chu, L.; Qing, F.-L. J. Am. Chem. Soc. 2012, 134, 1298–1304. doi:10.1021/ja209992w
Return to citation in text: [1] -
Jiang, X.; Chu, L.; Qing, F.-L. J. Org. Chem. 2012, 77, 1251–1257. doi:10.1021/jo202566h
Return to citation in text: [1] -
Chu, L.; Qing, F.-L. Org. Lett. 2012, 14, 2106–2109. doi:10.1021/ol300639a
Return to citation in text: [1] -
Wu, X.; Chu, L.; Qing, F.-L. Angew. Chem., Int. Ed. 2013, 52, 2198–2202. doi:10.1002/anie.201208971
Return to citation in text: [1] -
Zhao, T. S. N.; Szabó, K. J. Org. Lett. 2012, 14, 3966–3969. doi:10.1021/ol3017287
Return to citation in text: [1] -
Miyake, Y.; Ota, S.; Shibata, M.; Nakajima, K.; Nishibayashi, Y. Chem. Commun. 2013, 49, 7809–7811. doi:10.1039/c3cc44434a
Return to citation in text: [1]
| 1. | Kirsch, P. Modern Fluoroorganic Chemistry; Wiley-VCH: Weinheim, 2004. doi:10.1002/352760393X |
| 2. | Uneyama, K. Organofluorine Chemistry; Blackwell: Oxford, U.K., 2006. doi:10.1002/9780470988589 |
| 3. | Ojima, I. Fluorine in Medicinal Chemistry and Chemical Biology; Wiley-Blackwell: Chichester, U.K., 2009. |
| 4. | Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881–1886. doi:10.1126/science.1131943 |
| 5. | Kirk, K. L. Org. Process Res. Dev. 2008, 12, 305–321. doi:10.1021/op700134j |
| 6. | O'Hagan, D. Chem. Soc. Rev. 2008, 37, 308–319. doi:10.1039/b711844a |
| 12. | Nguyen, B. V.; Burton, D. J. J. Org. Chem. 1997, 62, 7758–7764. doi:10.1021/jo971019w |
| 11. | Uneyama, K.; Momota, M.; Hayashida, K.; Itoh, T. J. Org. Chem. 1990, 55, 5364–5368. doi:10.1021/jo00306a013 |
| 10. | Ando, A.; Miki, T.; Kumadaki, I. J. Org. Chem. 1988, 53, 3637–3639. doi:10.1021/jo00250a049 |
| 7. | Shi, G. Q.; Dropinski, J. F.; Zhang, Y.; Santini, C.; Sahoo, S. P.; Berger, J. P.; MacNaul, K. L.; Zhou, G.; Agrawal, A.; Alvaro, R.; Cai, T.-Q.; Hernandez, M.; Wright, S. D.; Moller, D. E.; Heck, J. V.; Meinke, P. T. J. Med. Chem. 2005, 48, 5589–5599. doi:10.1021/jm050373g |
| 8. | Parrish, C. A.; Adams, N. D.; Auger, K. R.; Burgess, J. L.; Carson, J. D.; Chaudhari, A. M.; Copeland, R. A.; Diamond, M. A.; Donatelli, C. A.; Duffy, K. J.; Faucette, L. F.; Finer, J. T.; Huffman, W. F.; Hugger, E. D.; Jackson, J. R.; Knight, S. D.; Luo, L.; Moore, M. L.; Newlander, K. A.; Ridgers, L. H.; Sakowicz, R.; Shaw, A. N.; Sung, C.-M. M.; Sutton, D.; Wood, K. W.; Zhang, S.-Y.; Zimmerman, M. N.; Dhanak, D. J. Med. Chem. 2007, 50, 4939–4952. doi:10.1021/jm070435y |
| 9. | Macsari, I.; Besidski, Y.; Csjernyik, G.; Nilsson, L. I.; Sandberg, L.; Yngve, U.; Åhlin, K.; Bueters, T.; Eriksson, A. B.; Lund, P.-E.; Venyike, E.; Oerther, S.; Hygge Blakeman, K.; Luo, L.; Arvidsson, P. I. J. Med. Chem. 2012, 55, 6866–6880. doi:10.1021/jm300623u |
| 28. | Zhao, T. S. N.; Szabó, K. J. Org. Lett. 2012, 14, 3966–3969. doi:10.1021/ol3017287 |
| 29. | Miyake, Y.; Ota, S.; Shibata, M.; Nakajima, K.; Nishibayashi, Y. Chem. Commun. 2013, 49, 7809–7811. doi:10.1039/c3cc44434a |
| 22. | Chu, L.; Qing, F.-L. J. Am. Chem. Soc. 2010, 132, 7262–7263. doi:10.1021/ja102175w |
| 23. | Chu, L.; Qing, F.-L. Org. Lett. 2010, 12, 5060–5063. doi:10.1021/ol1023135 |
| 24. | Chu, L.; Qing, F.-L. J. Am. Chem. Soc. 2012, 134, 1298–1304. doi:10.1021/ja209992w |
| 25. | Jiang, X.; Chu, L.; Qing, F.-L. J. Org. Chem. 2012, 77, 1251–1257. doi:10.1021/jo202566h |
| 26. | Chu, L.; Qing, F.-L. Org. Lett. 2012, 14, 2106–2109. doi:10.1021/ol300639a |
| 27. | Wu, X.; Chu, L.; Qing, F.-L. Angew. Chem., Int. Ed. 2013, 52, 2198–2202. doi:10.1002/anie.201208971 |
| 15. | Kobayashi, Y.; Yamamoto, K.; Kumadaki, I. Tetrahedron Lett. 1979, 20, 4071–4072. doi:10.1016/S0040-4039(01)86506-0 |
| 16. | Urata, H.; Fuchikami, T. Tetrahedron Lett. 1991, 32, 91–94. doi:10.1016/S0040-4039(00)71226-3 |
| 17. | Chen, Q.-Y.; Duan, J.-X. J. Chem. Soc., Chem. Commun. 1993, 1389–1391. doi:10.1039/C39930001389 |
| 18. | Kim, J.; Shreeve, J. M. Org. Biomol. Chem. 2004, 2, 2728–2734. doi:10.1039/b412480b |
| 19. | Dubinina, G. G.; Furutachi, H.; Vicic, D. A. J. Am. Chem. Soc. 2008, 130, 8600–8601. doi:10.1021/ja802946s |
| 20. | Dubinina, G. G.; Ogikubo, J.; Vicic, D. A. Organometallics 2008, 27, 6233–6235. doi:10.1021/om800794m |
| 21. | Kawai, H.; Furukawa, T.; Nomura, Y.; Tokunaga, E.; Shibata, N. Org. Lett. 2011, 13, 3596–3599. doi:10.1021/ol201205t |
| 13. | Zhao, Y.; Hu, J. Angew. Chem., Int. Ed. 2012, 51, 1033–1036. doi:10.1002/anie.201106742 |
| 14. | Liang, A.; Li, X.; Liu, D.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Chem. Commun. 2012, 48, 8273–8275. doi:10.1039/c2cc31651j |
© 2013 Jiang and Qing; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)