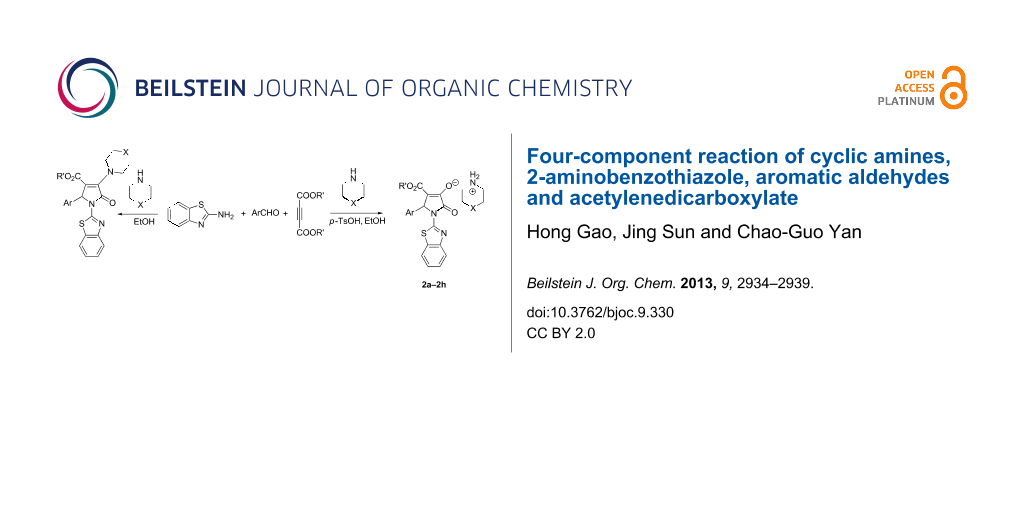
Abstract
The four-component reaction of 2-aminobenzothiazole, aromatic aldehydes, acetylenedicarboxylate and piperidine or pyrrolidine in ethanol afforded the functionalized 2-pyrrolidinones containing both benzothiazolyl and piperidinyl (or pyrrolidinyl) units in good yields. On the other hand, the similar four-component reactions resulted in the functionalized morpholinium or piperidinium 2-pyrrolidinon-3-olates in the presence of p-toluenesulfonic acid.
Graphical Abstract
Introduction
Over fifty years ago, Huisgen firstly described the addition reactions of nitrogen-containing heterocycles to electron-deficient alkynes to form 1,4-dipolar intermediates, which can reacted sequentially with other reagents to give cycloaddition products [1,2]. From then on much developments on the chemistry of Huisgen 1,4-dipoles have been achieved [3,4]. In the past few years, Huisgen 1,4-dipoles have been recognized as key components for designing practical multicomponent reactions and domino reactions, mainly due to their easy generation and versatile reactivity [5-10]. On the other hand, the similar reactive Huisgen 1,4-dipoles derived from the addition of primary or secondary amines to electron-deficient alkynes also provided many elegant procedures for the synthesis of various nitrogen-containing heterocycles [11-16]. In this hot research field, we also successfully developed a series of domino reactions containing primary amine, electron-deficient alkynes and the other components, and found several efficient synthetic protocols for versatile heterocycles and spiro compounds by using the in situ generated Huisgen 1,4-dipoles [17-24]. During these research works, we noticed that even through the cyclic secondary amines such as pyrrolidine, piperidine and morpholine also reacted with electron-deficient alkynes to give the Huisgen 1,4-dipoles very fast and in nearly quantitative yields [25,26]. But until now it seems that this kind of easily generated Huisgen 1,4-dipoles have not been utilized for the design of domino reactions. In continuation of our efforts to explore the practical applications of Huisgen 1,4-dipoles for the synthesis of a versatile heterocyclic system, herein we wish to report the interesting results of the four-component reaction of secondary cyclic amines, acetylenedicarboxylate, 2-aminobenzothiazole and aromatic aldehydes and the efficient synthesis of the complex 2-pyrrolidinones containing both benzothiazolyl and piperidinyl (or pyrrolidinyl) units.
Results and Discussion
Initially, we set out to investigate the reaction conditions by using piperidine to react with dimethyl acetylenedicarboxylate to give the expected β-enamino ester. It is interesting to find that the reaction of piperidine with acetylenedicarboxylate in ethanol at room temperature proceeded very quickly and could be finished to give the expected β-enamino ester in less than twenty minutes [27], while the reaction of normal primary arylamine with acetylenedicarboxylate or propiolate in ethanol at room temperature usually needed more than one day [28]. Thus we chose to employ a one-pot multicomponent reaction procedure to investigate our reaction. A mixture of dimethyl acetylenedicarboxylate, benzaldehyde, 2-aminobenzothiazole and excess piperidine in ethanol was stirred at room temperature for about twenty minutes and then was heated at 50–60 °C for about 48 hours. In this reaction the excess piperidine acted as base catalyst. After work-up, the expected polyfunctionalized 2-pyrrolidinone 1a was obtained in good yield (Table 1, entry 1). Under similar reaction conditions, various aromatic aldehydes were utilized in the reaction to give the polyfunctionalized 2-pyrrolidinone 1b–1f (Table 1, entries 2–6) in 53–72% yields, respectively. The four-component reaction containing diethyl acetylenedicarboxylate also successfully afforded the expected 2-pyrrolidinone 1g in 63% (Table 1, entry 7).
Table 1: Synthesis of pyrrolidinones 1a–1n via four-component reactionsa.
|
|
|||||
| Entry | Compd | X | R’ | Ar | Yieldb (%) |
|---|---|---|---|---|---|
| 1 | 1a | CH2 | CH3 | C6H5 | 58 |
| 2 | 1b | CH2 | CH3 | m-CH3C6H4 | 72 |
| 3 | 1c | CH2 | CH3 | p-CH(CH3)2C6H4 | 55 |
| 4 | 1d | CH2 | CH3 | p-ClC6H4 | 53 |
| 5 | 1e | CH2 | CH3 | m-ClC6H4 | 67 |
| 6 | 1f | CH2 | CH3 | m-NO2C6H4 | 70 |
| 7 | 1g | CH2 | CH2CH3 | p-CH3C6H4 | 63 |
| 8 | 1h | O | CH3 | C6H5 | 66c |
| 9 | 1i | O | CH3 | p-CH3OC6H4 | 55c |
| 10 | 1j | O | CH3 | p-ClC6H4 | 58c |
| 11 | 1k | O | CH3 | m-ClC6H4 | 63c |
| 12 | 1l | O | CH3 | m-NO2C6H4 | 68c |
| 13 | 1m | O | CH3 | p-NO2C6H4 | 52c |
| 14 | 1n | O | CH3 | p-CH3OC6H4 | 10c |
aReaction conditions: 2-aminobenzothiazole (2.0 mmol), acetylenedicarboxylate (2.0 mmol), aromatic aldehyde (2.0 mmol), piperidine (3.0 mmol) in EtOH (10.0 mL), rt, 20 min, 50–60 °C, 48 h; bIsolated yield; cPyrrolidine or morpholine (2.0 mmol) and DABCO (0.5 mmol) were used.
In view of the success of the above reaction, we explored the scope of this promising reaction by varying the structure of the secondary cyclic amines. When excess pyrrolidine was used in the reaction by using the above reaction procedure, it was surprising to find that only very low yields of 3-(pyrrolidin-1-yl)-2-pyrrolidinones were produced. After carefully optimizing the reaction conditions, we were pleased to find that the expected 3-(pyrrolidin-1-yl)-2-pyrrolidinones 1h–1m could be prepared in the satisfactory yields by adding the stronger base DABCO into the reaction as base catalyst (Table 1, entries 8–13). Another common cyclic amine morpholine still gave a very low yield of the desired 3-morpholinyl-2-pyrrolidinone 1n (Table 1, entry 14). It is known that pyrrolidine (pKb = 2.73) and piperidine (pKb = 2.88) have near similar basicity, while morpholine has a relative weak basicity (pKb = 5.64). At present, the exact reason for the different reactivity of piperidine, pyrrolidine and morpholine in this reaction is not very clear. The structures of the prepared 2-pyrrolidinones 1a–1n were fully characterized by 1H and 13C NMR, HRMS, IR spectra, and were further confirmed by single crystal structure determination of compound 1f (Figure 1). In 1H NMR spectra of compounds 1a–1n, the proton at the 5-position of the newly-formed 2-pyrrolidonyl ring usually displays a singlet at about 6.15 ppm. The piperidin-1yl or pyrrolidin-1-yl groups usually show two or three characteristic mixed peaks.
![[1860-5397-9-330-1]](/bjoc/content/figures/1860-5397-9-330-1.png?scale=2.6&max-width=1024&background=FFFFFF)
Figure 1: Molecular structure of compound 1f.
Figure 1: Molecular structure of compound 1f.
Two years ago, we reported that a p-toluenesulfonic acid-catalyzed three-component reaction of arylamine, aromatic aldehyde and acetylenedicarboxylate afforded 3-hydroxy-2-pyrrolidinone as main product [28]. In order to improve the reactivity of morpholine in this four-component reaction, p-toluenesulfonic acid was added in the four-component reaction of morpholine, p-methoxybenzaldehyde, 2-aminobenzothiazole and dimethyl acetylenedicarboxylate. After work-up, we found that the reaction afforded unexpected morpholinium 2-pyrrolidinon-3-olate 2a in 75% yield (Table 2, entry 1). Under similar conditions, the reactions with other aromatic aldehydes also gave the morpholinium 2-pyrrolidinon-3-olates 2b–2e (Table 2, entries 2–5) in 65–87% yields, respectively. The formation of morpholinium 2-pyrrolidinon-3-olates 2a–2e clearly indicated that the reaction initially gave the expected 3-hydroxy-2-pyrrolidinone, which in turn converted to enolate by deprotonation of basic morpholine. This result also showed that this four-component reaction has an interesting molecular diversity in basic or acidic solution. We also utilized piperidine in this acid-catalyzed four component reaction and obtained the corresponding piperidinium 2-pyrrolidinon-3-olates 2f–2h in good yields (Table 2, entries 6–8). The prepared piperidinium and morpholinium 2-pyrrolidinon-3-olates 2a–2h are very stable compounds. Their structures were fully characterized by 1H and 13C NMR, HRMS, IR spectra, and were also confirmed by single crystal structure determination of compounds 2a (Figure 2) and 2h (Figure 3).
Table 2: Synthesis of pyrrolidinones 2a–2h via four-component reactionsa.
|
|
||||
| Entry | Compd | X | Ar | Yieldb (%) |
|---|---|---|---|---|
| 1 | 2a | O | p-CH3OC6H4 | 75c |
| 2 | 2b | O | p-CH(CH3)2C6H4 | 73 |
| 3 | 2c | O | p-CH3C6H4 | 65 |
| 4 | 2d | O | C6H5 | 87 |
| 5 | 2e | O | m-NO2C6H4 | 79 |
| 6 | 2f | CH2 | p-CH3C6H4 | 75 |
| 7 | 2g | CH2 | m-CH3C6H4 | 72 |
| 8 | 2h | CH2 | p-(CH3)3CC6H4 | 80 |
aReaction condition: 2-aminobenzothiazole (2.0 mmol), acetylenedicarboxylate; (2.0 mmol), aromatic aldehyde (2.0 mmol), piperidine or piperidine (2.0 mmol), p-TsOH (0.5 mmol), in EtOH (10.0 mL), rt, 20 min., 50–60 °C 48 h; bIsolated yield.
![[1860-5397-9-330-2]](/bjoc/content/figures/1860-5397-9-330-2.png?scale=2.6&max-width=1024&background=FFFFFF)
Figure 2: Molecular structure of compound 2a.
Figure 2: Molecular structure of compound 2a.
![[1860-5397-9-330-3]](/bjoc/content/figures/1860-5397-9-330-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Molecular structure of compound 2h.
Figure 3: Molecular structure of compound 2h.
A plausible reaction mechanism for this four-component reaction both in basic media and in acid solution was proposed based on the previous reported similar reactions (Scheme 1) [29-34]. At first, piperidine adds to acetylenedicarboxylate to give 1,3-dipolar intermediate A. In the meantime, the condensation of the aromatic aldehyde with 2-aminobenzothiazole affords an aldimine B. Secondly, the nucleophilic addition of 1,3-dipole intermediate A to aldimine B gives an addition intermediate C. Thirdly, the intramolecular nucleophilic attack of the amino group to the carbonyl group produces the polyfunctionalized 2-pyrrolidinone 1. There is one reactive enamine unit in the obtained 2-pyrrolidinone 1. Under the catalysis of p-toluenesulfonic acid, the enamine moiety in 2-pyrrolidinone 1 was easily hydrolyzed to yield a 2, 3-pyrrolidinedione (D) and piperidine. Then 2,3-pyrrolidinedione D transforms to the more stable enol-form through the keto–enol tautomerism. Because the enol connects to both ester and amide groups, it has much stronger acidity and is deprotonated by piperidine in the solution to give the piperidinium 2-pyrrolidinon-3-olate 2 as the final product.
Scheme 1: The proposed reaction mechanism for the four-component reaction.
Scheme 1: The proposed reaction mechanism for the four-component reaction.
Conclusion
In summary, we have successfully developed a four-component reaction of an aromatic aldehyde, 2-aminobenzothiazole, secondary cyclic amines and acetylenedicarboxylate in basic or acidic soution. This four-component reaction provides a convenient procedure for the preparation of the mixed heterocyclic compounds containing units of benzothiazole, piperidine and 2-pyrrolidinone in satisfactory yields. The range of substrates and the reaction mechanism for this reaction were briefly discussed. This convenient synthetic reaction might be potentially used for complex heterocyclic systems in synthetic and medicinal chemistry.
Experimental
Reagents and apparatus: Melting points were taken on a hot-plate microscope apparatus. IR spectra were obtained on a Bruker Tensor 27 spectrometer (KBr disc). NMR spectra were recorded with a Bruker AV-600 spectrometer with DMSO-d6 as solvent and TMS as internal standard (600 and 150 MHz for 1H and 13C NMR spectra, respectively). HRMS were measured at UHR-TOF maXis instrument. X-ray data were collected on a Bruker Smart APEX-2 diffractometer. 2-Aminobenzothiazole, dimethyl or diethyl acetylenedicarboxylate, aromatic aldehyde and other reagents are commercial reagents and used as received. Solvents were purified by standard techniques. All reactions were monitored by TLC.
General procedure for the preparation of the functionalized 2-pyrrolidinones 1a–1n as a one-pot four-component reaction: A mixture of 2-aminobenzothiazole (2.0 mmol), acetylenedicarboxylate (2.0 mmol), aromatic aldehyde (2.0 mmol), piperidine (3.0 mmol) (in cases of pyrrolidine or morpholine was used in the reaction, pyrrolidine or morpholine (2.0 mmol), DABCO (0.5 mmol)) in ethanol (10.0 mL) was stirred at room temperature for about twenty minutes and then was heated at about 50–60 °C for about two days. After cooling to room temperature, the resulting precipitates were collected by filtration and washed with cold ethanol to give the crude product, which was recrystallized in ethanol to give the pure products 1a–1n for analysis.
General procedure for the preparation of functionalized 2-pyrrolidinon-3-olates 2a–2h as a one-pot reaction: A mixture of 2-aminobenzothiazole (2.0 mmol), acetylenedicarboxylate (2.0 mmol), aromatic aldehyde (2.0 mmol), morpholine or piperidine (3.0 mmol) and p-toluenesulfonic acid (0.5 mmol) in ethanol (10.0 mL) was stirred at room temperature for about twenty minutes and then was heated at about 50–60 °C for about two days. After cooling to room temperature, the resulting precipitates were collected by filtration and washed with cold ethanol to give the products 2a–2h.
X-ray crystallographic data: Single crystal data for compounds 1f (CCDC 950634), 2a (CCDC 950635) and 2h (CCDC 952039) have been deposited in the Cambridge Crystallographic Data Center. These data can be obtained free of charge via http://www.ccdc.ac.ck./data_request/cif .
Supporting Information
| Supporting Information File 1: Analytical data and 1H and 13C NMR spectra. | ||
| Format: PDF | Size: 1.0 MB | Download |
References
-
Huisgen, R.; Morikawa, M.; Herbig, K.; Brunn, E. Chem. Ber. 1967, 100, 1094–1106. doi:10.1002/cber.19671000406
Return to citation in text: [1] -
Huisgen, R. Z. Chem. 1968, 8, 290–298. doi:10.1002/zfch.19680080803
Return to citation in text: [1] -
Nair, V.; Rajesh, C.; Vinod, A. U.; Bindu, S.; Sreekanth, A. R.; Mathen, J. S.; Balagopal, L. Acc. Chem. Res. 2003, 36, 899–907. doi:10.1021/ar020258p
Return to citation in text: [1] -
Nair, V.; Menon, R. S.; Sreekanth, A.; Abhilash, N.; Biju, A. T. Acc. Chem. Res. 2006, 39, 520–530. doi:10.1021/ar0502026
Return to citation in text: [1] -
Kielland, N.; Lavilla, R. Top. Heterocycl. Chem. 2010, 25, 127–168. doi:10.1007/7081_2010_42
Return to citation in text: [1] -
Shaabani, A.; Maleki, A.; Rezayan, A. H.; Sarvary, A. Mol. Diversity 2011, 15, 41–68. doi:10.1007/s11030-010-9258-1
Return to citation in text: [1] -
Nair, V.; Devipriya, S.; Suresh, E. Tetrahedron 2008, 64, 3567–3577. doi:10.1016/j.tet.2008.01.106
Return to citation in text: [1] -
Nair, V.; Devipriya, S.; Suresh, E. Tetrahedron Lett. 2007, 48, 3667–3670. doi:10.1016/j.tetlet.2007.03.123
Return to citation in text: [1] -
Yavari, I.; Mirzaei, A.; Moradi, L.; Khalili, G. Tetrahedron Lett. 2010, 51, 396–398. doi:10.1016/j.tetlet.2009.11.040
Return to citation in text: [1] -
Yavari, I.; Seyfi, S.; Hossaini, Z. Tetrahedron Lett. 2010, 51, 2193–2194. doi:10.1016/j.tetlet.2010.02.107
Return to citation in text: [1] -
Yadav, J. S.; Reddy, B. V. S.; Yadav, N. N.; Gupta, M. K.; Sridhar, B. J. Org. Chem. 2008, 73, 6857–6859. doi:10.1021/jo8007034
Return to citation in text: [1] -
Ding, H.; Zhang, Y.; Bian, M.; Yao, W.; Ma, C. J. Org. Chem. 2008, 73, 578–584. doi:10.1021/jo702299m
Return to citation in text: [1] -
Srikrishna, A.; Sridharan, M.; Prasad, K. R. Tetrahedron 2010, 66, 3651–3654. doi:10.1016/j.tet.2010.03.084
Return to citation in text: [1] -
Bezenšek, J.; Koleša, T.; Grošelj, U.; Wagger, J.; Stare, K.; Meden, A.; Svete, J.; Stanovnik, B. Tetrahedron Lett. 2010, 51, 3392–3397. doi:10.1016/j.tetlet.2010.04.106
Return to citation in text: [1] -
Liu, W.; Jiang, H.; Huang, L. Org. Lett. 2010, 12, 312–315. doi:10.1021/ol9026478
Return to citation in text: [1] -
Yang, J.; Wang, C.; Xie, X.; Li, H.; Li, Y. Eur. J. Org. Chem. 2010, 4189–4193. doi:10.1002/ejoc.201000607
Return to citation in text: [1] -
Sun, J.; Xia, E.-Y.; Wu, Q.; Yan, C.-G. Org. Lett. 2010, 12, 3678–3681. doi:10.1021/ol101475b
Return to citation in text: [1] -
Sun, J.; Xia, E.-Y.; Wu, Q.; Yan, C.-G. ACS Comb. Sci. 2011, 13, 421–426. doi:10.1021/co200045t
Return to citation in text: [1] -
Sun, J.; Sun, Y.; Gao, H.; Yan, C.-G. Eur. J. Org. Chem. 2011, 6952–6956. doi:10.1002/ejoc.201101198
Return to citation in text: [1] -
Sun, J.; Sun, Y.; Xia, E.-Y.; Yan, C.-G. ACS Comb. Sci. 2011, 13, 436–441. doi:10.1021/co200071v
Return to citation in text: [1] -
Sun, J.; Sun, Y.; Gong, H.; Xie, Y.-J.; Yan, C.-G. Org. Lett. 2012, 14, 5172–5175. doi:10.1021/ol302530m
Return to citation in text: [1] -
Han, Y.; Sun, Y.; Sun, J.; Yan, C.-G. Tetrahedron 2012, 68, 8256–8260. doi:10.1016/j.tet.2012.07.056
Return to citation in text: [1] -
Han, Y.; Wu, Q.; Sun, J.; Yan, C.-G. Tetrahedron 2012, 68, 8539–8544. doi:10.1016/j.tet.2012.08.030
Return to citation in text: [1] -
Sun, J.; Sun, Y.; Gao, H.; Yan, C.-G. Eur. J. Org. Chem. 2012, 1976–1983. doi:10.1002/ejoc.201101737
Return to citation in text: [1] -
Schmidt, R. R.; Kast, J.; Speer, H. Synthesis 1983, 725–727. doi:10.1055/s-1983-30488
Return to citation in text: [1] -
Ziyaei-Halimehjani, A.; Saidi, M. R. Tetrahedron Lett. 2008, 49, 1244–1248. doi:10.1016/j.tetlet.2007.12.042
Return to citation in text: [1] -
Sun, J.; Gao, H.; Wu, Q.; Yan, C.-G. Beilstein J. Org. Chem. 2012, 8, 1839–1843. doi:10.3762/bjoc.8.211
Return to citation in text: [1] -
Sun, J.; Wu, Q.; Xia, E.-Y.; Yan, C.-G. Eur. J. Org. Chem. 2011, 2981–2986. doi:10.1002/ejoc.201100008
Return to citation in text: [1] [2] -
Zhu, Q.; Jiang, H.; Li, J.; Liu, S.; Xia, C.; Zhang, M. J. Comb. Chem. 2009, 11, 685–696. doi:10.1021/cc900046f
Return to citation in text: [1] -
Khan, A. T.; Ghosh, A.; Musawwer, M. M. Tetrahedron Lett. 2012, 53, 2622–2626. doi:10.1016/j.tetlet.2012.03.046
Return to citation in text: [1] -
Rana, S.; Brown, M.; Dutta, A.; Bhaumik, A.; Mukhopadhyay, C. Tetrahedron Lett. 2013, 54, 1371–1379. doi:10.1016/j.tetlet.2012.12.109
Return to citation in text: [1] -
Ramesh, K.; Murthy, S. N.; Karnakar, K.; Nageswar, Y. V. D. Tetrahedron Lett. 2011, 52, 3937–3941. doi:10.1016/j.tetlet.2011.05.100
Return to citation in text: [1] -
Gao, H.; Sun, J.; Yan, G.-C. Tetrahedron 2013, 69, 589–594. doi:10.1016/j.tet.2012.11.018
Return to citation in text: [1] -
Sarkar, R.; Mukhopadhyay, C. Tetrahedron Lett. 2013, 54, 3706–3711. doi:10.1016/j.tetlet.2013.05.017
Return to citation in text: [1]
| 1. | Huisgen, R.; Morikawa, M.; Herbig, K.; Brunn, E. Chem. Ber. 1967, 100, 1094–1106. doi:10.1002/cber.19671000406 |
| 2. | Huisgen, R. Z. Chem. 1968, 8, 290–298. doi:10.1002/zfch.19680080803 |
| 17. | Sun, J.; Xia, E.-Y.; Wu, Q.; Yan, C.-G. Org. Lett. 2010, 12, 3678–3681. doi:10.1021/ol101475b |
| 18. | Sun, J.; Xia, E.-Y.; Wu, Q.; Yan, C.-G. ACS Comb. Sci. 2011, 13, 421–426. doi:10.1021/co200045t |
| 19. | Sun, J.; Sun, Y.; Gao, H.; Yan, C.-G. Eur. J. Org. Chem. 2011, 6952–6956. doi:10.1002/ejoc.201101198 |
| 20. | Sun, J.; Sun, Y.; Xia, E.-Y.; Yan, C.-G. ACS Comb. Sci. 2011, 13, 436–441. doi:10.1021/co200071v |
| 21. | Sun, J.; Sun, Y.; Gong, H.; Xie, Y.-J.; Yan, C.-G. Org. Lett. 2012, 14, 5172–5175. doi:10.1021/ol302530m |
| 22. | Han, Y.; Sun, Y.; Sun, J.; Yan, C.-G. Tetrahedron 2012, 68, 8256–8260. doi:10.1016/j.tet.2012.07.056 |
| 23. | Han, Y.; Wu, Q.; Sun, J.; Yan, C.-G. Tetrahedron 2012, 68, 8539–8544. doi:10.1016/j.tet.2012.08.030 |
| 24. | Sun, J.; Sun, Y.; Gao, H.; Yan, C.-G. Eur. J. Org. Chem. 2012, 1976–1983. doi:10.1002/ejoc.201101737 |
| 11. | Yadav, J. S.; Reddy, B. V. S.; Yadav, N. N.; Gupta, M. K.; Sridhar, B. J. Org. Chem. 2008, 73, 6857–6859. doi:10.1021/jo8007034 |
| 12. | Ding, H.; Zhang, Y.; Bian, M.; Yao, W.; Ma, C. J. Org. Chem. 2008, 73, 578–584. doi:10.1021/jo702299m |
| 13. | Srikrishna, A.; Sridharan, M.; Prasad, K. R. Tetrahedron 2010, 66, 3651–3654. doi:10.1016/j.tet.2010.03.084 |
| 14. | Bezenšek, J.; Koleša, T.; Grošelj, U.; Wagger, J.; Stare, K.; Meden, A.; Svete, J.; Stanovnik, B. Tetrahedron Lett. 2010, 51, 3392–3397. doi:10.1016/j.tetlet.2010.04.106 |
| 15. | Liu, W.; Jiang, H.; Huang, L. Org. Lett. 2010, 12, 312–315. doi:10.1021/ol9026478 |
| 16. | Yang, J.; Wang, C.; Xie, X.; Li, H.; Li, Y. Eur. J. Org. Chem. 2010, 4189–4193. doi:10.1002/ejoc.201000607 |
| 5. | Kielland, N.; Lavilla, R. Top. Heterocycl. Chem. 2010, 25, 127–168. doi:10.1007/7081_2010_42 |
| 6. | Shaabani, A.; Maleki, A.; Rezayan, A. H.; Sarvary, A. Mol. Diversity 2011, 15, 41–68. doi:10.1007/s11030-010-9258-1 |
| 7. | Nair, V.; Devipriya, S.; Suresh, E. Tetrahedron 2008, 64, 3567–3577. doi:10.1016/j.tet.2008.01.106 |
| 8. | Nair, V.; Devipriya, S.; Suresh, E. Tetrahedron Lett. 2007, 48, 3667–3670. doi:10.1016/j.tetlet.2007.03.123 |
| 9. | Yavari, I.; Mirzaei, A.; Moradi, L.; Khalili, G. Tetrahedron Lett. 2010, 51, 396–398. doi:10.1016/j.tetlet.2009.11.040 |
| 10. | Yavari, I.; Seyfi, S.; Hossaini, Z. Tetrahedron Lett. 2010, 51, 2193–2194. doi:10.1016/j.tetlet.2010.02.107 |
| 3. | Nair, V.; Rajesh, C.; Vinod, A. U.; Bindu, S.; Sreekanth, A. R.; Mathen, J. S.; Balagopal, L. Acc. Chem. Res. 2003, 36, 899–907. doi:10.1021/ar020258p |
| 4. | Nair, V.; Menon, R. S.; Sreekanth, A.; Abhilash, N.; Biju, A. T. Acc. Chem. Res. 2006, 39, 520–530. doi:10.1021/ar0502026 |
| 28. | Sun, J.; Wu, Q.; Xia, E.-Y.; Yan, C.-G. Eur. J. Org. Chem. 2011, 2981–2986. doi:10.1002/ejoc.201100008 |
| 28. | Sun, J.; Wu, Q.; Xia, E.-Y.; Yan, C.-G. Eur. J. Org. Chem. 2011, 2981–2986. doi:10.1002/ejoc.201100008 |
| 27. | Sun, J.; Gao, H.; Wu, Q.; Yan, C.-G. Beilstein J. Org. Chem. 2012, 8, 1839–1843. doi:10.3762/bjoc.8.211 |
| 25. | Schmidt, R. R.; Kast, J.; Speer, H. Synthesis 1983, 725–727. doi:10.1055/s-1983-30488 |
| 26. | Ziyaei-Halimehjani, A.; Saidi, M. R. Tetrahedron Lett. 2008, 49, 1244–1248. doi:10.1016/j.tetlet.2007.12.042 |
| 29. | Zhu, Q.; Jiang, H.; Li, J.; Liu, S.; Xia, C.; Zhang, M. J. Comb. Chem. 2009, 11, 685–696. doi:10.1021/cc900046f |
| 30. | Khan, A. T.; Ghosh, A.; Musawwer, M. M. Tetrahedron Lett. 2012, 53, 2622–2626. doi:10.1016/j.tetlet.2012.03.046 |
| 31. | Rana, S.; Brown, M.; Dutta, A.; Bhaumik, A.; Mukhopadhyay, C. Tetrahedron Lett. 2013, 54, 1371–1379. doi:10.1016/j.tetlet.2012.12.109 |
| 32. | Ramesh, K.; Murthy, S. N.; Karnakar, K.; Nageswar, Y. V. D. Tetrahedron Lett. 2011, 52, 3937–3941. doi:10.1016/j.tetlet.2011.05.100 |
| 33. | Gao, H.; Sun, J.; Yan, G.-C. Tetrahedron 2013, 69, 589–594. doi:10.1016/j.tet.2012.11.018 |
| 34. | Sarkar, R.; Mukhopadhyay, C. Tetrahedron Lett. 2013, 54, 3706–3711. doi:10.1016/j.tetlet.2013.05.017 |
© 2013 Gao et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)