Abstract
A straightforward synthesis of 6-substituted 1-phenyl-3-trifluoromethyl-1H-pyrazolo[4,3-c]pyridines and the corresponding 5-oxides is presented. Hence, microwave-assisted treatment of 5-chloro-1-phenyl-3-trifluoromethylpyrazole-4-carbaldehyde with various terminal alkynes in the presence of tert-butylamine under Sonogashira-type cross-coupling conditions affords the former title compounds in a one-pot multicomponent procedure. Oximes derived from (intermediate) 5-alkynyl-1-phenyl-3-trifluoromethyl-1H-pyrazole-4-carbaldehydes were transformed into the corresponding 1H-pyrazolo[4,3-c]pyridine 5-oxides by silver triflate-catalyzed cyclization. Detailed NMR spectroscopic investigations (1H, 13C, 15N and 19F) were undertaken with all obtained products.
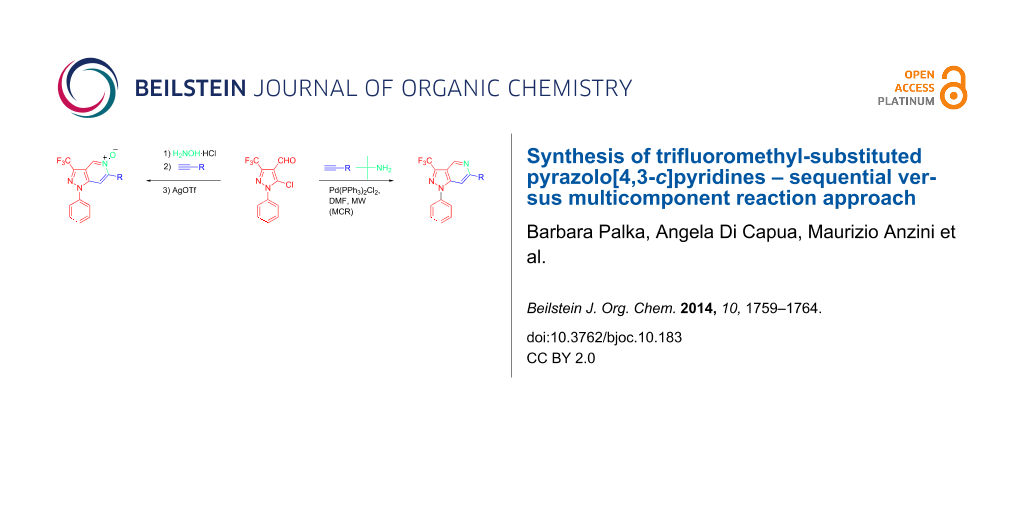
Graphical Abstract
Introduction
Fluorine-containing compounds play an important role in medicinal and pharmaceutical chemistry as well as in agrochemistry [1-4]. A popular approach for the modulation of activity consists in the introduction of one or more fluorine atoms into the structure of a bioactive compound. This variation frequently leads to a higher metabolic stability and can modulate some physicochemical properties such as basicity or lipophilicity [1,2]. Moreover, incorporation of fluorine often results in an increase of the binding affinity of drug molecules to the target protein [1,2]. As a consequence, a considerable amount – approximately 20% – of all the pharmaceuticals being currently on the market contain at least one fluorine substituent, including important drug molecules in different pharmaceutical classes [5]. Keeping in mind the above facts, the synthesis of fluorinated heterocyclic compounds, which can act as building blocks for the construction of biologically active fluorine-containing molecules, is of eminent interest. In the field of pyrazoles, pyridines and condensed systems thereof trifluoromethyl-substituted congeners can be found as partial structures in several pharmacologically active compounds. In the pyridine series the HIV protease inhibitor Tipranavir (Aptivus®) [6] may serve as an example, within the pyrazole-derived compounds the COX-2 inhibitor Celecoxib (Celebrex®) is an important representative (Figure 1) [7].
Figure 1: Important drug molecules containing a trifluoromethylpyridine, respectively a trifluoromethylpyrazole moiety.
Figure 1: Important drug molecules containing a trifluoromethylpyridine, respectively a trifluoromethylpyrazo...
In continuation of our program regarding the synthesis of fluoro- and trifluoromethyl-substituted pyrazoles and annulated pyrazoles [8,9] we here present the synthesis of trifluoromethyl-substituted pyrazolo[4,3-c]pyridines. The latter heterocyclic system represents the core of several biologically active compounds, acting, for instance, as SSAO inhibitors [10], or inhibitors of different kinases (LRRK2 [11,12], TYK2 [13], JAK [14,15]).
Results and Discussion
Chemistry
The construction of the pyrazolo[4,3-c]pyridine system can be mainly achieved through two different approaches. One strategy involves the annelation of a pyrazole ring onto an existing, suitable pyridine derivative [16]. Alternatively, the bicyclic system can be accessed by pyridine-ring formation with an accordant pyrazole precursor. Employing the latter approach we recently presented a novel method for the synthesis of the pyrazolo[4,3-c]pyridine system by Sonogashira-type cross-coupling reaction of easily obtainable 5-chloro-1-phenyl-1H-pyrazole-4-carbaldehydes with various alkynes and subsequent ring-closure reaction of the thus obtained 5-alkynyl-1H-pyrazole-4-carbaldehydes in the presence of tert-butylamine [17]. Furthermore, we showed that the oximes derived from the before mentioned 5-alkynylpyrazole-4-carbaldehydes can be transformed into the corresponding 1-phenylpyrazolo[4,3-c]pyridine 5-oxides [17].
For the synthesis of the title compounds a similar approach was envisaged. As starting material the commercially available 1-phenyl-3-trifluoromethyl-1H-pyrazol-5-ol (1) was employed which, after Vilsmaier formylation [18] and concomitant transformation of the hydroxy function into a chloro substituent by treatment with excessive POCl3, gave the chloroaldehyde 2 [19] (Scheme 1). Although Sonogashira-type cross-coupling reactions are preferably accomplished with iodo(hetero)arenes – considering the general reactivity I > Br/OTf >> Cl [20] – from related examples it was known that the chloro atom in 5-chloropyrazole-4-aldehydes is sufficiently activated to act as the leaving group in such kind of C–C linkages [17]. Indeed, reaction of chloroaldehyde 2 with different alkynes 3a–c under typical Sonogashira reaction conditions afforded the corresponding cross-coupling products 4a–c in good yields (Scheme 1). In some runs compounds of type 8 were determined as byproducts in differing yields, but mostly below 10%, obviously resulting from addition of water to the triple bond of 4 under the reaction conditions (or during work-up) and subsequent tautomerization of the thus formed enoles into the corresponding ketones. The hydration of C–C triple bonds under the influence of various catalytic systems, including also Pd-based catalysts, is a well-known reaction [21,22]. It should be emphasized that NMR investigations with compounds 8a,c unambiguously revealed the methylene group adjacent to the pyrazole nucleus and the carbonyl moiety attached to the substituent R originating from the employed alkyne.
Scheme 1: Synthesis of the title compounds.
Scheme 1: Synthesis of the title compounds.
In the next reaction step, alkynylaldehydes 4a,b were cyclized into the target pyrazolo[4,3-c]pyridines 5a,b in 71%, resp. 52% yield by reaction with tert-butylamine under microwave assistance [17]. In view of the fact, that the two-step conversion 2→4a,b→5a,b was characterized by only moderate overall yields (59%, resp. 43%) it was considered to merge these two steps into a one-pot multicomponent reaction. The latter type of reaction attracts increasing attention in organic chemistry due to its preeminent synthetic efficiency, also in the construction of heterocyclic and condensed heterocyclic systems [23-27]. After testing different reaction conditions we found that microwave heating of the chloroaldehyde 2 with tert-butylamine in the presence of 6 mol % of Pd(PPh3)2Cl2 afforded the desired pyrazolopyridines 5a–c in high (5a: 89%, 5c: 92%) respectively acceptable yields (5b: 51%) in a single one-pot and copper-free reaction step (Scheme 1). It should be mentioned that compounds 5 are also accessible by heating of ketones 8 with ammonium acetate in acetic acid according to a procedure described in [28]. Following this way, 5a and 5c were obtained in 70% yield from the corresponding ketones 8a and 8c. Although ketones 8 were only obtained as byproducts, the latter transformation allowed increasing the overall yield of compounds 5 through this ‘bypass’.
In order to gain access to the corresponding N-oxides of type 7, aldehydes 4a–c were transformed into the corresponding oximes 6a–c by reaction with hydroxylamine hydrochloride in ethanol in the presence of sodium acetate (Scheme 1). Subsequent treatment of the oximes with AgOTf in dichloromethane [29] finally afforded the corresponding pyrazolo[4,3-c]pyridine 5-oxides 7a–c by a regioselective 6-endo-dig cyclisation [30] in high yields. Moreover, we tested an alternative approach to access compounds 7 through multicomponent reactions (MCR). Attempts to react chloroaldehyde 2 with hydroxylamine hydrochloride and an alkyne 3 in the presence of a suitable catalytic system were not successful. However, after conversion of 2 into the corresponding aldoxime 9 the latter could be transformed into the N-oxides 7a and 7b by reaction with alkynes 3a and 3b, respectively, employing Pd(OAc)2 as the catalyst and under microwave irradiation (Scheme 1). Although a number of different reaction conditions were tested, we were not able to increase the yields in excess of 30%. Thus, with respect to the overall yields the successive approach 2→4→6→7 (overall yields: 6a: 62%, 6b: 43%) here is still advantageous compared to the multicomponent reaction following the path 2→9→7. Azine N-oxides of type 7 are estimated to be of particular interest due to the possibility of further functionalization adjacent to the nitrogen atom (position 4), for instance by palladium-catalyzed direct arylation reactions [31].
NMR spectroscopic investigations
In Supporting Information File 1 the NMR spectroscopic data of all compounds treated within this study are indicated. Full and unambiguous assignment of all 1H, 13C, 15N and 19F NMR resonances was achieved by combining standard NMR techniques [32], such as fully 1H-coupled 13C NMR spectra, APT, HMQC, gs-HSQC, gs-HMBC, COSY, TOCSY, NOESY and NOE-difference spectroscopy.
In compounds 4–7 the trifluoromethyl group exhibits very consistent chemical shifts, ranging from δ(F) −60.8 to −61.9 ppm. The fluorine resonance is split into a doublet by a small coupling (0.5–0.9 Hz) due to a through-space (or possibly 5J) interaction with spatially close protons (4: CHO; 6: CH=N; 5 and 7: H-4). Reversely, the signals of the latter protons are split into a quartet (not always well resolved). The corresponding carbon resonance of CF3 is located between 120.2 and 121.2 ppm with the relevant 1J(C,F) coupling constants being approximately 270 Hz (269.6–270.6 Hz). As well, the signal of C-3 is always split into a quartet (J ~ 40 Hz) due to the 2J(C,F3) coupling.
As the 15N NMR chemical shifts were determined by 15N,1H HMBC experiments the resonance of (pyrazole) N-2 was not captured owing to the fact that this nitrogen atom lacks of sufficient couplings to protons, thus disabling the necessary coherence transfer (19F,15N HMBC spectra were not possible with the equipment at hand). For N-1, with pyrazole derivatives 4 and 6 remarkably larger 15N chemical shifts were detected (−158.8 to −160.2 ppm) compared to the corresponding signals for pyrazolopyridines 5 and 7 (−182.2 to −185.9 ppm). When switching from an azine to an azine oxide partial structure (5→7) the N-5 resonance exhibits an explicit upfield shift (15.6–18.3 ppm), being typical for the changeover from pyridine to pyridine N-oxide [33].
NMR experiments also allowed the determination of the stereochemistry of oximes 6: considering the size of 1J(N=C-H) which is strongly dependent on lone-pair effects [34] as well as the comparison of chemical shifts with those of related, unambiguously assigned oximes [17] reveals E-configuration at the C=N double bond.
With byproduct 8a the position of the carbonyl group unequivocally follows from the correlations between phenyl protons and the carbonyl C-atom and, reversely, from those between the methylene protons with pyrazole C-4 and pyrazole C-5 (determined by 13C,1H HMBC).
In Figure 2 essential NMR data for the complete series of type c (4c, 5c, 6c, 7c) are displayed, which easily enables to compare the notable chemical shifts and allows following the trends described above.
Figure 2: 1H (in italics, red), 13C (black), 15N (in blue) and 19F NMR (green) chemical shifts of compounds 4c, 5c, 6c and 7c (in CDCl3).
Figure 2: 1H (in italics, red), 13C (black), 15N (in blue) and 19F NMR (green) chemical shifts of compounds 4c...
Full experimental details as well as spectral and microanalytical data of the obtained compounds are presented in Supporting Information File 1.
Conclusion
To sum up, the presented approach represents a simple method for the synthesis of 6-substituted 1-phenyl-3-trifluoromethyl-1H-pyrazolo[4,3-c]pyridines 5 and the analogous 5-oxides 7 starting from commercially available 1-phenyl-3-trifluoromethyl-1H-pyrazol-5-ol (1). In the case of the former (5) the described multicomponent reaction approach is superior compared to the sequential one, whereas the step-by-step synthesis of N-oxides 7 is still characterized by higher overall yields. In addition, in-depth NMR studies with all synthesized compounds were performed, affording full and unambiguous assignment of 1H, 13C, 15N and 19F resonances and the designation of ascertained heteronuclear spin-coupling constants.
Supporting Information
| Supporting Information File 1: Experimental details and characterization data. | ||
| Format: PDF | Size: 355.0 KB | Download |
References
-
Böhm, H.-J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Müller, K.; Obst-Sander, U.; Stahl, M. ChemBioChem 2004, 5, 637–643. doi:10.1002/cbic.200301023
Return to citation in text: [1] [2] [3] -
Hagmann, W. K. J. Med. Chem. 2008, 51, 4359–4369. doi:10.1021/jm800219f
Return to citation in text: [1] [2] [3] -
Liu, P.; Sharon, A.; Chu, C. K. J. Fluorine Chem. 2008, 129, 743–766. doi:10.1016/j.jfluchem.2008.06.007
Return to citation in text: [1] -
Berkowitz, D. B.; Karukurichi, K. R.; de la Salud-Bea, R.; Nelson, D. L.; McCune, C. D. J. Fluorine Chem. 2008, 129, 731–742. doi:10.1016/j.jfluchem.2008.05.016
Return to citation in text: [1] -
O’Hagan, D. J. Fluorine Chem. 2010, 131, 1071–1081. doi:10.1016/j.jfluchem.2010.03.003
Return to citation in text: [1] -
Poppe, S. M.; Slade, D. E.; Chong, K. T.; Hinshaw, R. R.; Pagano, P. J.; Markowitz, M.; Ho, D. D.; Mo, H.; Gorman, R. R., III; Dueweke, T. J.; Thaisrivongs, S.; Tarpley, W. G. Antimicrob. Agents Chemother. 1997, 41, 1058–1063.
Return to citation in text: [1] -
Penning, T. D.; Talley, J. J.; Bertenshaw, S. R.; Carter, J. S.; Collins, P. W.; Docter, S.; Graneto, M. J.; Lee, L. F.; Malecha, J. W.; Miyashiro, J. M.; Rogers, R. S.; Rogier, D. J.; Yu, S. S.; Anderson, G. D.; Burton, E. G.; Cogburn, J. N.; Gregory, S. A.; Koboldt, C. M.; Perkins, W. E.; Seibert, K.; Veenhuizen, A. W.; Zhang, Y. Y.; Isakson, P. C. J. Med. Chem. 1997, 40, 1347–1365. doi:10.1021/jm960803q
Return to citation in text: [1] -
Bieringer, S.; Holzer, W. Heterocycles 2006, 68, 1825–1836. doi:10.3987/COM-05-10502
Return to citation in text: [1] -
Holzer, W.; Ebner, A.; Schalle, K.; Batezila, G.; Eller, G. A. J. Fluorine Chem. 2010, 131, 1013–1024. doi:10.1016/j.jfluchem.2010.07.007
Return to citation in text: [1] -
Savory, E.; Simpson, I. New compounds II. WO Pat. Appl. WO/2010/031791 A1, March 25, 2010.
Return to citation in text: [1] -
Chan, B.; Estrada, A.; Sweeney, Z.; McIver, E. G.; McIver, S. Pyrazolopyridines as inhibitors of the kinase LRRK2. WO Pat. Appl. WO2011/141756 A1, Nov 17, 2011.
Return to citation in text: [1] -
Chan, B.; Chen, H.; Estrada, A.; Shore, D.; Sweeney, Z.; McIver, E. Pyrazolopyridines as inhibitors of the kinase LRRK2. WO Pat. Appl. WO2012/038743 A1, March 29, 2012.
Return to citation in text: [1] -
Blench, T.; Goodacre, S.; Lai, Y.; Liang, Y.; MacLeod, C.; Magnuson, S.; Tsui, V.; Williams, K.; Zhang, B. Pyrazolopyridines and pyrazolopyridines and their use as TYK2 inhibitors. WO Pat. Appl. WO2012/066061 A1, May 24, 2012.
Return to citation in text: [1] -
Oxenford, S.; Hobson, A.; Oliver, K.; Ratcliffe, A.; Ramsden, N. Pyrazolo[4,3-c]pyridine derivatives as JAK inhibitors. WO Pat. Appl. WO2013/017479 A1, Feb 7, 2013.
Return to citation in text: [1] -
Oxenford, S.; Hobson, A.; Oliver, K.; Ratcliffe, A.; Ramsden, N. Preparation of pyrazolo[4,3-c]pyridine derivatives as JAK inhibitors. WO Pat. Appl. WO2013/017480 A1, Feb 7, 2013.
Return to citation in text: [1] -
Lominac, W. J.; D’Angelo, M. L.; Smith, M. D.; Ollison, D. A.; Hanna, J. M., Jr. Tetrahedron Lett. 2012, 53, 906–909. doi:10.1016/j.tetlet.2011.12.055
Return to citation in text: [1] -
Vilkauskaité, G.; Šačkus, A.; Holzer, W. Eur. J. Org. Chem. 2011, 5123–5133. doi:10.1002/ejoc.201100626
Return to citation in text: [1] [2] [3] [4] [5] -
Meth-Cohn, O.; Stanforth, S. P. The Vilsmeier–Haack Reaction. In Comprehensive Organic Synthesis; Trost, B. M.; Fleming, I., Eds.; Pergamon: Oxford, 1991; pp 777–794. doi:10.1016/B978-0-08-052349-1.00049-4
Return to citation in text: [1] -
Gilbert, A. M.; Bursavich, M. G.; Lombardi, S.; Georgiadis, K. E.; Reifenberg, E.; Flannery, C. R.; Morris, E. A. Bioorg. Med. Chem. Lett. 2007, 17, 1189–1192. doi:10.1016/j.bmcl.2006.12.020
Return to citation in text: [1] -
Chinchilla, R.; Nájera, C. Chem. Rev. 2007, 107, 874–922. doi:10.1021/cr050992x
Return to citation in text: [1] -
Hintermann, L.; Labonne, A. Synthesis 2007, 1121–1150. doi:10.1055/s-2007-966002
Return to citation in text: [1] -
Li, X.; Hu, G.; Luo, P.; Tang, G.; Gao, Y.; Xu, P.; Zhao, Y. Adv. Synth. Catal. 2012, 354, 2427–2432. doi:10.1002/adsc.201200420
Return to citation in text: [1] -
Zhu, J.; Bienaymé, H., Eds. Multicomponent Reactions, 1st ed.; Wiley-VCH: Weinheim, 2005. doi:10.1002/3527605118
Return to citation in text: [1] -
Dömling, A.; Ugi, I. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. doi:10.1002/1521-3773(20000915)39:18<3168::AID-ANIE3168>3.0.CO;2-U
Return to citation in text: [1] -
D’Souza, D. M.; Müller, T. J. J. Chem. Soc. Rev. 2007, 36, 1095–1108. doi:10.1039/b608235c
Return to citation in text: [1] -
Müller, T. J. J. Top. Heterocycl. Chem. 2010, 25, 25–94. doi:10.1007/7081_2010_43
Return to citation in text: [1] -
Müller, T. J. J. Synthesis 2012, 159–174. doi:10.1055/s-0031-1289636
Return to citation in text: [1] -
Deady, L. W.; Smith, C. L. Aust. J. Chem. 2003, 56, 1219–1224. doi:10.1071/CH03136
Return to citation in text: [1] -
Yeom, H.-S.; Kim, S.; Shin, S. Synlett 2008, 924–928. doi:10.1055/s-2008-1042936
Return to citation in text: [1] -
Baldwin, J. E.; Thomas, R. C.; Kruse, L. I.; Silberman, L. J. Org. Chem. 1977, 42, 3846–3852. doi:10.1021/jo00444a011
Return to citation in text: [1] -
Campeau, L.-C.; Stuart, D. R.; Leclerc, J.-P.; Bertrand-Laperle, M.; Villemure, E.; Sun, H.-Y.; Lasserre, S.; Guimond, N.; Lecavallier, M.; Fagnou, K. J. Am. Chem. Soc. 2009, 131, 3291–3306. doi:10.1021/ja808332k
Return to citation in text: [1] -
Braun, S.; Kalinowski, H.-O.; Berger, S. 150 and More Basic NMR Experiments: A Practical Course – Second Expanded Edition; Wiley–VCH: Weinheim, Germany, 1998.
Return to citation in text: [1] -
Berger, S.; Braun, S.; Kalinowski, H.-O. 15N-NMR-Spektroskopie; NMR-Spektroskopie von Nichtmetallen, Vol. 2; Thieme: Stuttgart, New York, 1992; pp 42–43.
Return to citation in text: [1] -
Gil, V. M. S.; von Philipsborn, W. Magn. Reson. Chem. 1989, 27, 409–430. doi:10.1002/mrc.1260270502
Return to citation in text: [1]
| 34. | Gil, V. M. S.; von Philipsborn, W. Magn. Reson. Chem. 1989, 27, 409–430. doi:10.1002/mrc.1260270502 |
| 17. | Vilkauskaité, G.; Šačkus, A.; Holzer, W. Eur. J. Org. Chem. 2011, 5123–5133. doi:10.1002/ejoc.201100626 |
| 1. | Böhm, H.-J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Müller, K.; Obst-Sander, U.; Stahl, M. ChemBioChem 2004, 5, 637–643. doi:10.1002/cbic.200301023 |
| 2. | Hagmann, W. K. J. Med. Chem. 2008, 51, 4359–4369. doi:10.1021/jm800219f |
| 3. | Liu, P.; Sharon, A.; Chu, C. K. J. Fluorine Chem. 2008, 129, 743–766. doi:10.1016/j.jfluchem.2008.06.007 |
| 4. | Berkowitz, D. B.; Karukurichi, K. R.; de la Salud-Bea, R.; Nelson, D. L.; McCune, C. D. J. Fluorine Chem. 2008, 129, 731–742. doi:10.1016/j.jfluchem.2008.05.016 |
| 6. | Poppe, S. M.; Slade, D. E.; Chong, K. T.; Hinshaw, R. R.; Pagano, P. J.; Markowitz, M.; Ho, D. D.; Mo, H.; Gorman, R. R., III; Dueweke, T. J.; Thaisrivongs, S.; Tarpley, W. G. Antimicrob. Agents Chemother. 1997, 41, 1058–1063. |
| 18. | Meth-Cohn, O.; Stanforth, S. P. The Vilsmeier–Haack Reaction. In Comprehensive Organic Synthesis; Trost, B. M.; Fleming, I., Eds.; Pergamon: Oxford, 1991; pp 777–794. doi:10.1016/B978-0-08-052349-1.00049-4 |
| 5. | O’Hagan, D. J. Fluorine Chem. 2010, 131, 1071–1081. doi:10.1016/j.jfluchem.2010.03.003 |
| 19. | Gilbert, A. M.; Bursavich, M. G.; Lombardi, S.; Georgiadis, K. E.; Reifenberg, E.; Flannery, C. R.; Morris, E. A. Bioorg. Med. Chem. Lett. 2007, 17, 1189–1192. doi:10.1016/j.bmcl.2006.12.020 |
| 1. | Böhm, H.-J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Müller, K.; Obst-Sander, U.; Stahl, M. ChemBioChem 2004, 5, 637–643. doi:10.1002/cbic.200301023 |
| 2. | Hagmann, W. K. J. Med. Chem. 2008, 51, 4359–4369. doi:10.1021/jm800219f |
| 17. | Vilkauskaité, G.; Šačkus, A.; Holzer, W. Eur. J. Org. Chem. 2011, 5123–5133. doi:10.1002/ejoc.201100626 |
| 1. | Böhm, H.-J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Müller, K.; Obst-Sander, U.; Stahl, M. ChemBioChem 2004, 5, 637–643. doi:10.1002/cbic.200301023 |
| 2. | Hagmann, W. K. J. Med. Chem. 2008, 51, 4359–4369. doi:10.1021/jm800219f |
| 17. | Vilkauskaité, G.; Šačkus, A.; Holzer, W. Eur. J. Org. Chem. 2011, 5123–5133. doi:10.1002/ejoc.201100626 |
| 11. | Chan, B.; Estrada, A.; Sweeney, Z.; McIver, E. G.; McIver, S. Pyrazolopyridines as inhibitors of the kinase LRRK2. WO Pat. Appl. WO2011/141756 A1, Nov 17, 2011. |
| 12. | Chan, B.; Chen, H.; Estrada, A.; Shore, D.; Sweeney, Z.; McIver, E. Pyrazolopyridines as inhibitors of the kinase LRRK2. WO Pat. Appl. WO2012/038743 A1, March 29, 2012. |
| 14. | Oxenford, S.; Hobson, A.; Oliver, K.; Ratcliffe, A.; Ramsden, N. Pyrazolo[4,3-c]pyridine derivatives as JAK inhibitors. WO Pat. Appl. WO2013/017479 A1, Feb 7, 2013. |
| 15. | Oxenford, S.; Hobson, A.; Oliver, K.; Ratcliffe, A.; Ramsden, N. Preparation of pyrazolo[4,3-c]pyridine derivatives as JAK inhibitors. WO Pat. Appl. WO2013/017480 A1, Feb 7, 2013. |
| 10. | Savory, E.; Simpson, I. New compounds II. WO Pat. Appl. WO/2010/031791 A1, March 25, 2010. |
| 16. | Lominac, W. J.; D’Angelo, M. L.; Smith, M. D.; Ollison, D. A.; Hanna, J. M., Jr. Tetrahedron Lett. 2012, 53, 906–909. doi:10.1016/j.tetlet.2011.12.055 |
| 8. | Bieringer, S.; Holzer, W. Heterocycles 2006, 68, 1825–1836. doi:10.3987/COM-05-10502 |
| 9. | Holzer, W.; Ebner, A.; Schalle, K.; Batezila, G.; Eller, G. A. J. Fluorine Chem. 2010, 131, 1013–1024. doi:10.1016/j.jfluchem.2010.07.007 |
| 7. | Penning, T. D.; Talley, J. J.; Bertenshaw, S. R.; Carter, J. S.; Collins, P. W.; Docter, S.; Graneto, M. J.; Lee, L. F.; Malecha, J. W.; Miyashiro, J. M.; Rogers, R. S.; Rogier, D. J.; Yu, S. S.; Anderson, G. D.; Burton, E. G.; Cogburn, J. N.; Gregory, S. A.; Koboldt, C. M.; Perkins, W. E.; Seibert, K.; Veenhuizen, A. W.; Zhang, Y. Y.; Isakson, P. C. J. Med. Chem. 1997, 40, 1347–1365. doi:10.1021/jm960803q |
| 13. | Blench, T.; Goodacre, S.; Lai, Y.; Liang, Y.; MacLeod, C.; Magnuson, S.; Tsui, V.; Williams, K.; Zhang, B. Pyrazolopyridines and pyrazolopyridines and their use as TYK2 inhibitors. WO Pat. Appl. WO2012/066061 A1, May 24, 2012. |
| 21. | Hintermann, L.; Labonne, A. Synthesis 2007, 1121–1150. doi:10.1055/s-2007-966002 |
| 22. | Li, X.; Hu, G.; Luo, P.; Tang, G.; Gao, Y.; Xu, P.; Zhao, Y. Adv. Synth. Catal. 2012, 354, 2427–2432. doi:10.1002/adsc.201200420 |
| 20. | Chinchilla, R.; Nájera, C. Chem. Rev. 2007, 107, 874–922. doi:10.1021/cr050992x |
| 17. | Vilkauskaité, G.; Šačkus, A.; Holzer, W. Eur. J. Org. Chem. 2011, 5123–5133. doi:10.1002/ejoc.201100626 |
| 32. | Braun, S.; Kalinowski, H.-O.; Berger, S. 150 and More Basic NMR Experiments: A Practical Course – Second Expanded Edition; Wiley–VCH: Weinheim, Germany, 1998. |
| 33. | Berger, S.; Braun, S.; Kalinowski, H.-O. 15N-NMR-Spektroskopie; NMR-Spektroskopie von Nichtmetallen, Vol. 2; Thieme: Stuttgart, New York, 1992; pp 42–43. |
| 30. | Baldwin, J. E.; Thomas, R. C.; Kruse, L. I.; Silberman, L. J. Org. Chem. 1977, 42, 3846–3852. doi:10.1021/jo00444a011 |
| 31. | Campeau, L.-C.; Stuart, D. R.; Leclerc, J.-P.; Bertrand-Laperle, M.; Villemure, E.; Sun, H.-Y.; Lasserre, S.; Guimond, N.; Lecavallier, M.; Fagnou, K. J. Am. Chem. Soc. 2009, 131, 3291–3306. doi:10.1021/ja808332k |
| 28. | Deady, L. W.; Smith, C. L. Aust. J. Chem. 2003, 56, 1219–1224. doi:10.1071/CH03136 |
| 29. | Yeom, H.-S.; Kim, S.; Shin, S. Synlett 2008, 924–928. doi:10.1055/s-2008-1042936 |
| 17. | Vilkauskaité, G.; Šačkus, A.; Holzer, W. Eur. J. Org. Chem. 2011, 5123–5133. doi:10.1002/ejoc.201100626 |
| 23. | Zhu, J.; Bienaymé, H., Eds. Multicomponent Reactions, 1st ed.; Wiley-VCH: Weinheim, 2005. doi:10.1002/3527605118 |
| 24. | Dömling, A.; Ugi, I. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. doi:10.1002/1521-3773(20000915)39:18<3168::AID-ANIE3168>3.0.CO;2-U |
| 25. | D’Souza, D. M.; Müller, T. J. J. Chem. Soc. Rev. 2007, 36, 1095–1108. doi:10.1039/b608235c |
| 26. | Müller, T. J. J. Top. Heterocycl. Chem. 2010, 25, 25–94. doi:10.1007/7081_2010_43 |
| 27. | Müller, T. J. J. Synthesis 2012, 159–174. doi:10.1055/s-0031-1289636 |
© 2014 Palka et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)