Abstract
The reaction of CpPd(η3-C3H5) with the new diphosphinoborane ligand derivative (o-PCy2-C6H4)2BPh CyDPBPh affords the T-shape complex (CyDPBPh)Pd(0) 9, which was characterized by X-ray analysis.

Graphical Abstract
Introduction
The amplification of traditional bidentate chelating L2-type ligands with a tethered borane functionality (e.g., Bourissou’s diphospinoborane (o-PR2-C6H4)2BR’ ligand RDPBR’) has received considerable attention [1-3], with first catalytic applications emerging [4]. The acyclic boron group in these ligands can adopt a variety of coordination modes (Figure 1) [5].
Figure 1: Selected M→B coordination modes 1–5 [6-10] and Hofmann’s Rucaphos complex 6 [11].
Figure 1: Selected M→B coordination modes 1–5 [6-10] and Hofmann’s Rucaphos complex 6 [11].
The borane can act as a σ-acceptor ligand in case of η1-B coordination (e.g., 1 [6] and 2 [7]), or as a boron containing π-ligand adopting η2-B,C (3) [8] or η3-B,C,C coordination (4 and 5) [5,9,10]. Changes of the hapticity appear to have significant influence onto the reactivity of the coordinated transition metal towards substrates [8]. For zerovalent palladium complexes only few examples featuring a η1-type Pd→B interaction have been reported [6,7]. However, these complexes require phosphines or pyridines as a stabilizing co-ligand, which can act as an inhibitor in catalytic transformations [7]. Similarly, monometallic 14 VE palladium complexes featuring a chelating diphosphine, such as in Hofmanns Rucaphos complexes 6, are very scarce [11]. While the dative Pd→B bond is strong in zerovalent Pd(0) DPB complexes such as 2, only weak Pd→B interactions have been observed for the respective Pd(II) complexes [7,12]. Discrimination by the borane functionality between the oxidations states Pd(0)/Pd(II) is of potential interest for organometallic transformations involved in homogeneous catalysis, such as the reductive elimination. Here we report the synthesis of the diphosphinoborane (o-PCy2-C6H4)2BPh ligand CyDPBPh. CyDPBPh reacts with CpPd(η3-C3H5) yielding monometallic zerovalent palladium complex 9 featuring a distinct η1-B coordination mode, without the need of a stabilizing co-ligand.
Findings
For the synthesis of CyDPBPh we adapted the known reaction sequence for the production of Bourissou’s (o-PPh2-C6H4)2BPh ligand PhDPBPh (Scheme 1) [13,14].
Scheme 1: Synthesis of diphosphinoborane CyDPBPh and complex 9.
Scheme 1: Synthesis of diphosphinoborane CyDPBPh and complex 9.
Starting material (2-bromophenyl)dicyclohexylphosphine (7) was produced by palladium catalyzed coupling of dicyclohexylphosphine with 1-iodo-2-bromobenzene [15]. Phosphine 7 was lithiated in diethyl ether with n-BuLi [16,17], affording the diethyl ether adduct 8. Reaction of 8 with 0.5 equiv of PhBCl2 in toluene at −78 °C produced the desired ligand CyDBPPh in 86% isolated yield. Typical resonances for a DPB ligand were observed in the 31P NMR spectrum at δ 1.70 and in the 11B NMR spectrum at δ 41 (w1/2 = 1300 ± 120 Hz), which are indicative for a dynamic P→B bond in solution [18].
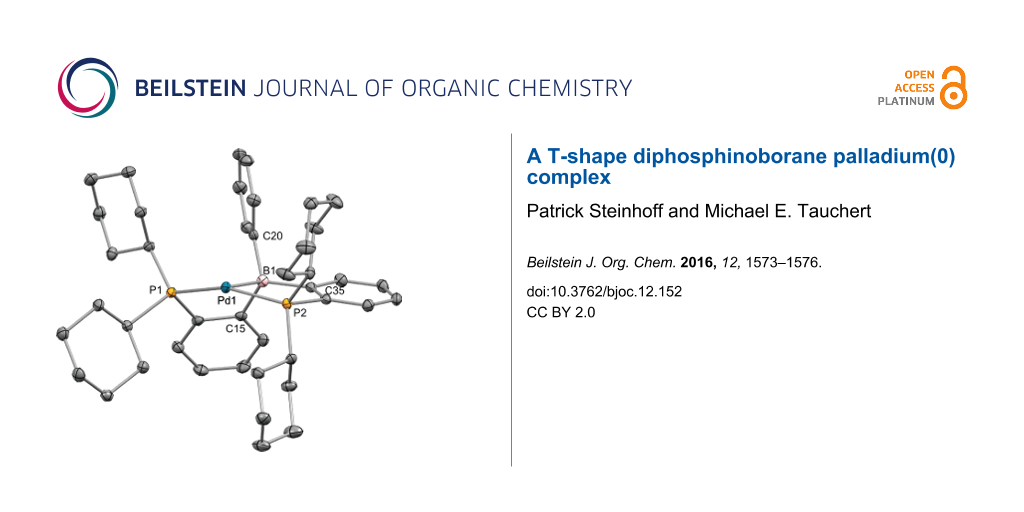
CyDPBPh was reacted with 1 equiv of CpPd(η3-C3H5) in benzene. Complete conversion towards complex 9 with equimolar formation of 5-allylcyclopenta-1,3-diene was reached within 18 h at 50 °C. Complex 9 showed a singlet resonance at δ 41.0 in the 31P NMR spectrum and a broad resonance at δ 22 (w1/2 = 800 ± 50 Hz) in the 11B NMR spectrum. High field shift and narrowing of the 11B NMR with respect to the free CyDPBPh ligand indicated the presence of a strong dative Pd(0)→B bond [7]. Despite the absence of a stabilizing co-ligand, we found complex 9 to be very stable in solution. The coordinating properties of CyDPBPh deviate from those observed for its aryl derivatives (PhDPBPh ((o-PPh2-C6H4)2BPh) and PhDPBMes ((o-PPh2-C6H4)2B(Mes))). For these ligands the reaction with one equivalent of CpPd(η3-C3H5) leads to 50% consumption of CpPd(η3-C3H5) with simultaneous formation of 5-allylcyclopenta-1,3-diene, but complete conversion of the ligand pointing towards the formation of a bisligand complex (DPB)2Pd [7]. Unlike complex 2 we were unable to form a pyridine adduct complex by treatment of 9 with 10 equiv of pyridine. Single crystals of complex 9 suitable for X-ray diffraction analysis were grown from hexane (Figure 2).
![[1860-5397-12-152-2]](/bjoc/content/figures/1860-5397-12-152-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Thermal ellipsoid plots of complex 9 at the 50% probability level. H atoms and one molecule of hexane have been omitted for clarity. Selected interatomic distances (Å) and angles (°): Pd1–B1 2.243(2), Pd1–P1 2.2761(6), Pd1–P2 2.3084(6), B1–Pd1–P1 85.82(6), B1–Pd1–P2 82.49(6), P1–Pd1–P2 157.72(2), C15–B1–C20 110.94(18), C15–B1–C35 116.58(18), C20–B1–C35 112.56(18).
Figure 2: Thermal ellipsoid plots of complex 9 at the 50% probability level. H atoms and one molecule of hexa...
The solid-state structure of 9 displayed a slightly distorted T-shape geometry around the palladium center. A short Pd1–B1 distance of 2.243(2) Å (cf. complex 2: 2.194(3) Å) and a significant pyramidalization at the boron center (ΣBα = 341°) is observed, indicating a strong Pd(0)→B bond. The distance between C20 and Pd1 was found to be 3.0805(22) Å. The η1-B coordination mode was well reproduced by DFT calculations (Supporting Information File 1). DFT calculations predict T-shape complexes with an almost linear P–Pd–P angle for model complexes (PMe3)2Pd → EX3 (E = B; X = H, F, Cl, Br, I) [17]. In complex 9 the trans-coordinated palladium center featured an obtuse P1–Pd1–P2 angle of 157.72(2)°.
Conclusion
In conclusion we synthesized the zerovalent palladium complex [{(o-PCy2-C6H4)2BPh}Pd(0)] 9. Complex 9 supplements the few known examples (e.g., 6 [11]) of 14 VE palladium complexes bearing a chelating diphosphine ligand by introduction of a borane acceptor functionality.
Supporting Information
| Supporting Information File 1: Experimental procedures and characterization data; crystallographic information for 9; 1H, 11B, 13C and 31P NMR spectra. | ||
| Format: PDF | Size: 1.2 MB | Download |
| Supporting Information File 2: CIF file of 9, CCDC 1471929. | ||
| Format: CIF | Size: 22.5 KB | Download |
Acknowledgements
We are grateful for financial support of this research by the Funds of the Chemical Industry (fellowships to PS and MET). We thank Q. Guo for collection of X-ray diffraction data and J. Wiesenthal for experimental assistance. We thank Prof. Dr. J. Okuda for his continuous and generous support.
References
-
Fontaine, F.-G.; Boudreau, J.; Thibault, M.-H. Eur. J. Inorg. Chem. 2008, 5439–5454. doi:10.1002/ejic.200800784
Return to citation in text: [1] -
Amgoune, A.; Bourissou, D. Chem. Commun. 2011, 47, 859–871. doi:10.1039/C0CC04109B
Return to citation in text: [1] -
Kameo, H.; Nakazawa, H. Chem. – Asian J. 2013, 8, 1720–1734. doi:10.1002/asia.201300184
Return to citation in text: [1] -
Bouhadir, G.; Bourissou, D. Chem. Soc. Rev. 2016, 45, 1065–1079. doi:10.1039/C5CS00697J
Return to citation in text: [1] -
Emslie, D. J. H.; Cowie, B. E.; Kolpin, K. B. Dalton Trans. 2012, 41, 1101–1117. doi:10.1039/C1DT11271F
Return to citation in text: [1] [2] -
Zech, A.; Haddow, M. F.; Othman, H.; Owen, G. R. Organometallics 2012, 31, 6753–6760. doi:10.1021/om300482m
Return to citation in text: [1] [2] [3] -
Schindler, T.; Lux, M.; Peters, M.; Scharf, L. T.; Osseili, H.; Maron, L.; Tauchert, M. E. Organometallics 2015, 34, 1978–1984. doi:10.1021/acs.organomet.5b00217
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] -
Harman, W. H.; Peters, J. C. J. Am. Chem. Soc. 2012, 134, 5080–5082. doi:10.1021/ja211419t
Return to citation in text: [1] [2] [3] -
Emslie, D. J. H.; Harrington, L. E.; Jenkins, H. A.; Robertson, C. M.; Britten, J. F. Organometallics 2008, 27, 5317–5325. doi:10.1021/om800670e
Return to citation in text: [1] [2] -
Cowie, B. E.; Emslie, D. J. H. Organometallics 2015, 34, 4093–4101. doi:10.1021/acs.organomet.5b00539
Return to citation in text: [1] [2] -
Schnetz, T.; Röder, M.; Rominger, F.; Hofmann, P. Dalton Trans. 2008, 2238–2240. doi:10.1039/b802684j
Return to citation in text: [1] [2] [3] -
Bontemps, S.; Sircoglou, M.; Bouhadir, G.; Puschmann, H.; Howard, J. A. K.; Dyer, P. W.; Miqueu, K.; Bourissou, D. Chem. – Eur. J. 2008, 14, 731–740. doi:10.1002/chem.200701027
Return to citation in text: [1] -
Sircoglou, M.; Bontemps, S.; Mercy, M.; Saffon, N.; Takahashi, M.; Bouhadir, G.; Maron, L.; Bourissou, D. Angew. Chem., Int. Ed. 2007, 46, 8583–8586. doi:10.1002/anie.200703518
Return to citation in text: [1] -
Conifer, C. M.; Law, D. J.; Sunley, G. J.; White, A. J. P.; Britovsek, G. J. P. Organometallics 2011, 30, 4060–4066. doi:10.1021/om200341t
Return to citation in text: [1] -
Murata, M.; Buchwald, S. L. Tetrahedron 2004, 60, 7397–7403. doi:10.1016/j.tet.2004.05.044
Return to citation in text: [1] -
Harder, S.; Brandsma, L.; Kanters, J. A.; Duisenberg, A.; van Lenthe, J. H. J. Organomet. Chem. 1991, 420, 143–154. doi:10.1016/0022-328X(91)80257-K
Return to citation in text: [1] -
Goedecke, C.; Hillebrecht, P.; Uhlemann, T.; Haunschild, R.; Frenking, G. Can. J. Chem. 2009, 87, 1470–1479. doi:10.1139/V09-099
Return to citation in text: [1] [2] -
Bontemps, S.; Bouhadir, G.; Dyer, P. W.; Miqueu, K.; Bourissou, D. Inorg. Chem. 2007, 46, 5149–5151. doi:10.1021/ic7006556
Return to citation in text: [1]
| 7. | Schindler, T.; Lux, M.; Peters, M.; Scharf, L. T.; Osseili, H.; Maron, L.; Tauchert, M. E. Organometallics 2015, 34, 1978–1984. doi:10.1021/acs.organomet.5b00217 |
| 16. | Harder, S.; Brandsma, L.; Kanters, J. A.; Duisenberg, A.; van Lenthe, J. H. J. Organomet. Chem. 1991, 420, 143–154. doi:10.1016/0022-328X(91)80257-K |
| 17. | Goedecke, C.; Hillebrecht, P.; Uhlemann, T.; Haunschild, R.; Frenking, G. Can. J. Chem. 2009, 87, 1470–1479. doi:10.1139/V09-099 |
| 18. | Bontemps, S.; Bouhadir, G.; Dyer, P. W.; Miqueu, K.; Bourissou, D. Inorg. Chem. 2007, 46, 5149–5151. doi:10.1021/ic7006556 |
| 1. | Fontaine, F.-G.; Boudreau, J.; Thibault, M.-H. Eur. J. Inorg. Chem. 2008, 5439–5454. doi:10.1002/ejic.200800784 |
| 2. | Amgoune, A.; Bourissou, D. Chem. Commun. 2011, 47, 859–871. doi:10.1039/C0CC04109B |
| 3. | Kameo, H.; Nakazawa, H. Chem. – Asian J. 2013, 8, 1720–1734. doi:10.1002/asia.201300184 |
| 11. | Schnetz, T.; Röder, M.; Rominger, F.; Hofmann, P. Dalton Trans. 2008, 2238–2240. doi:10.1039/b802684j |
| 13. | Sircoglou, M.; Bontemps, S.; Mercy, M.; Saffon, N.; Takahashi, M.; Bouhadir, G.; Maron, L.; Bourissou, D. Angew. Chem., Int. Ed. 2007, 46, 8583–8586. doi:10.1002/anie.200703518 |
| 14. | Conifer, C. M.; Law, D. J.; Sunley, G. J.; White, A. J. P.; Britovsek, G. J. P. Organometallics 2011, 30, 4060–4066. doi:10.1021/om200341t |
| 6. | Zech, A.; Haddow, M. F.; Othman, H.; Owen, G. R. Organometallics 2012, 31, 6753–6760. doi:10.1021/om300482m |
| 7. | Schindler, T.; Lux, M.; Peters, M.; Scharf, L. T.; Osseili, H.; Maron, L.; Tauchert, M. E. Organometallics 2015, 34, 1978–1984. doi:10.1021/acs.organomet.5b00217 |
| 8. | Harman, W. H.; Peters, J. C. J. Am. Chem. Soc. 2012, 134, 5080–5082. doi:10.1021/ja211419t |
| 9. | Emslie, D. J. H.; Harrington, L. E.; Jenkins, H. A.; Robertson, C. M.; Britten, J. F. Organometallics 2008, 27, 5317–5325. doi:10.1021/om800670e |
| 10. | Cowie, B. E.; Emslie, D. J. H. Organometallics 2015, 34, 4093–4101. doi:10.1021/acs.organomet.5b00539 |
| 15. | Murata, M.; Buchwald, S. L. Tetrahedron 2004, 60, 7397–7403. doi:10.1016/j.tet.2004.05.044 |
| 5. | Emslie, D. J. H.; Cowie, B. E.; Kolpin, K. B. Dalton Trans. 2012, 41, 1101–1117. doi:10.1039/C1DT11271F |
| 11. | Schnetz, T.; Röder, M.; Rominger, F.; Hofmann, P. Dalton Trans. 2008, 2238–2240. doi:10.1039/b802684j |
| 4. | Bouhadir, G.; Bourissou, D. Chem. Soc. Rev. 2016, 45, 1065–1079. doi:10.1039/C5CS00697J |
| 7. | Schindler, T.; Lux, M.; Peters, M.; Scharf, L. T.; Osseili, H.; Maron, L.; Tauchert, M. E. Organometallics 2015, 34, 1978–1984. doi:10.1021/acs.organomet.5b00217 |
| 12. | Bontemps, S.; Sircoglou, M.; Bouhadir, G.; Puschmann, H.; Howard, J. A. K.; Dyer, P. W.; Miqueu, K.; Bourissou, D. Chem. – Eur. J. 2008, 14, 731–740. doi:10.1002/chem.200701027 |
| 5. | Emslie, D. J. H.; Cowie, B. E.; Kolpin, K. B. Dalton Trans. 2012, 41, 1101–1117. doi:10.1039/C1DT11271F |
| 9. | Emslie, D. J. H.; Harrington, L. E.; Jenkins, H. A.; Robertson, C. M.; Britten, J. F. Organometallics 2008, 27, 5317–5325. doi:10.1021/om800670e |
| 10. | Cowie, B. E.; Emslie, D. J. H. Organometallics 2015, 34, 4093–4101. doi:10.1021/acs.organomet.5b00539 |
| 6. | Zech, A.; Haddow, M. F.; Othman, H.; Owen, G. R. Organometallics 2012, 31, 6753–6760. doi:10.1021/om300482m |
| 7. | Schindler, T.; Lux, M.; Peters, M.; Scharf, L. T.; Osseili, H.; Maron, L.; Tauchert, M. E. Organometallics 2015, 34, 1978–1984. doi:10.1021/acs.organomet.5b00217 |
| 11. | Schnetz, T.; Röder, M.; Rominger, F.; Hofmann, P. Dalton Trans. 2008, 2238–2240. doi:10.1039/b802684j |
| 8. | Harman, W. H.; Peters, J. C. J. Am. Chem. Soc. 2012, 134, 5080–5082. doi:10.1021/ja211419t |
| 7. | Schindler, T.; Lux, M.; Peters, M.; Scharf, L. T.; Osseili, H.; Maron, L.; Tauchert, M. E. Organometallics 2015, 34, 1978–1984. doi:10.1021/acs.organomet.5b00217 |
| 7. | Schindler, T.; Lux, M.; Peters, M.; Scharf, L. T.; Osseili, H.; Maron, L.; Tauchert, M. E. Organometallics 2015, 34, 1978–1984. doi:10.1021/acs.organomet.5b00217 |
| 7. | Schindler, T.; Lux, M.; Peters, M.; Scharf, L. T.; Osseili, H.; Maron, L.; Tauchert, M. E. Organometallics 2015, 34, 1978–1984. doi:10.1021/acs.organomet.5b00217 |
| 6. | Zech, A.; Haddow, M. F.; Othman, H.; Owen, G. R. Organometallics 2012, 31, 6753–6760. doi:10.1021/om300482m |
| 8. | Harman, W. H.; Peters, J. C. J. Am. Chem. Soc. 2012, 134, 5080–5082. doi:10.1021/ja211419t |
| 17. | Goedecke, C.; Hillebrecht, P.; Uhlemann, T.; Haunschild, R.; Frenking, G. Can. J. Chem. 2009, 87, 1470–1479. doi:10.1139/V09-099 |
© 2016 Steinhoff and Tauchert; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)