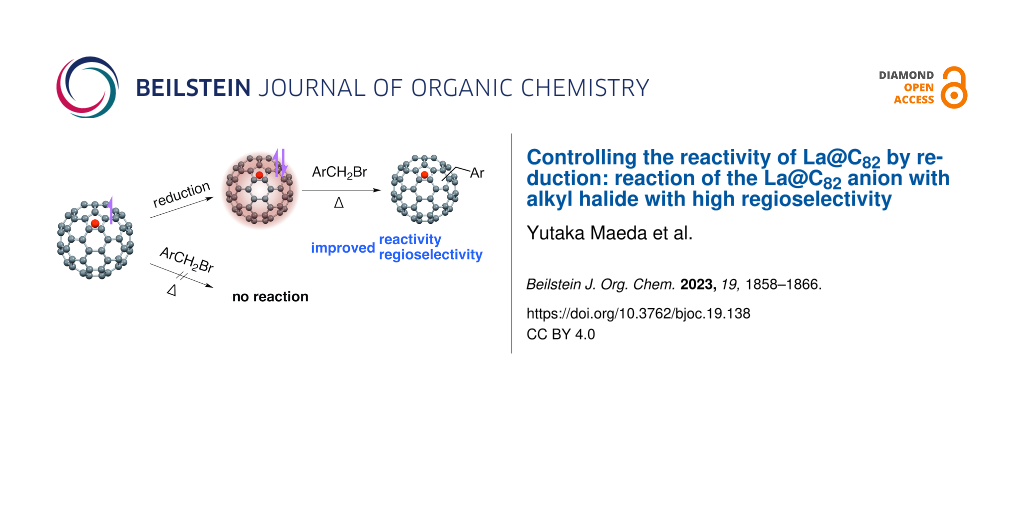
Abstract
Endohedral metallofullerenes have excellent redox properties, which can be used to vary their reactivity to certain classes of molecules, such as alkyl halides. In this study, the thermal reaction of the La@C2v-C82 anion with benzyl bromide derivatives 1 at 110 °C afforded single-bonded adducts 2–5 with high regioselectivity. The products were characterized by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and visible–near infrared spectroscopy. The reaction of La@C2v-C82 with alkyl halides using the same conditions showed no consumption of La@C2v-C82, indicating that the reactivity of La@C2v-C82 toward alkyl halides was effectively increased by one-electron reduction. Single-crystal X-ray diffraction analysis of the single-bonded adduct 3a revealed the addition site of the p-methoxybenzyl group on La@C2v-C82. Theoretical calculations indicated that the addition site carbons in neutral La@C2v-C82 have high spin density, whereas those in the La@C2v-C82 anion do not have high charge densities. Thus, the reaction is believed to occur via electron transfer, followed by the radical coupling of La@C2v-C82 and benzyl radicals, rather than by bimolecular nucleophilic substitution reaction of La@C2v-C82 anion with 1.

Graphical Abstract
Introduction
Fullerenes, the third carbon allotrope, have unique spherical molecular structures and exhibit high reactivity as electron-deficient polyolefins. The excellent redox properties of fullerenes are useful for their chemical derivatization and practical applications [1-5]. Fullerene anions can be easily produced chemically or electrochemically. C602− is a strong electron donor and potential nucleophile that reacts with electrophiles [6-11]. The mechanism for the reaction of C602− with alkyl halides has been studied in detail by Fukuzumi et al., who found that the reaction occurs via electron transfer, followed by bimolecular nucleophilic substitution (SN2) reaction [8].
Endohedral metallofullerenes, wherein one or more metal atoms are encapsulated inside a fullerene cage, have garnered research interest [12-15]. The encapsulation of metal atoms can result in electron transfer from the metal atoms to the fullerene cage. Because of this intramolecular electron transfer, the characteristic properties of metallofullerenes, such as their redox potentials, are significantly different from those of empty fullerenes. For example, La@C82 has paramagnetic properties, and its formal electronic structure is described as La3+C823−. We previously investigated the reaction of M@C2v-C82 ions (M = Y, La, Ce) with disilirane, which possesses high reactivity toward electron acceptors [16,17]. Interestingly, the reactivity of M@C2v-C82 toward disilirane was increased by the one-electron oxidation of M@C2v-C82. Moreover, the reaction was suppressed by the one-electron reduction of M@C2v-C82. These results suggest that oxidation and reduction reactions are useful for tuning the reactivity of metallofullerenes. Recently, remarkable reactivity of [M3N@Ih-C80]2− (M = Lu, Sc) toward benzal bromide was reported, demonstrating one possible reaction of the anion species of closed-shell endohedral metallofullerenes [18]. Although [Lu3N@Ih-C80]2− reacts with benzal bromide to afford a methanofullerene, [Sc3N@Ih-C80]2− did not react under the same conditions (Eox [Lu3N@Ih-C80]2− = 1.80 V vs Fc+/Fc; Eox [Sc3N@Ih-C80]2− = −1.62 V vs Fc+/Fc; C602− = −1.50 V vs Fc+/Fc)). The different reactivity of [M3N@Ih-C80]2− was explained by theoretical calculations. The charge density of the highest occupied molecular orbital (HOMO) was more highly localized on the fullerene cage for [Lu3N@Ih-C80]2−, whereas it was more localized on the inside of the cluster for [Sc3N@Ih-C80]2−.
A previous study reported that thermal treatment of La@C2v-C82 in the presence of 3-triphenylmethyl-5-oxazolidinone in toluene afforded four different benzylated La@C2v-C82 isomers [19]. Benzyl radicals may have been generated due to the involvement of azomethine ylide; however, the detailed mechanism has not been elucidated. In this article, we describe the thermal reaction of the La@C2v-C82 anion, activated by one-electron reduction, with benzyl bromide derivatives.
Results and Discussion
The La@C2v-C82 anion [20] was prepared by chemical reduction [21] using a degassed tetrabutylammonium hexafluorophosphate (TBAF) pyridine solution. After stirring for 3 h, a dark green solution was obtained. CS2 was added to precipitate TBAF, and the solution was filtered to collect the La@C2v-C82 anion solution. The solvent was then removed under reduced pressure and replaced with 1,2-dichlorobenzene (ODCB). The characteristic absorption peak at 1000 nm assigned to La@C2v-C82 decreased, and the new absorption peak at 934 nm assigned to the La@C2v-C82 anion increased. Reactions of the La@C2v-C82 anion with 4-methylbenzyl bromide (1a) were conducted at 110 °C for 2 h (Scheme 1). Figure 1 depicts the changes in the visible–near infrared (vis–NIR) absorption spectra during the reaction, showing gradual changes with isosbestic points. Since the electrolyte interferes with the high-performance liquid chromatography (HPLC) separation and anionic species may not be eluted under typical fullerene HPLC separation conditions, trifluoroacetic acid was added to the reaction mixture. Notably, La@C2v-C82 is produced after the addition of trifluoroacetic acid to the La@C2v-C82 anion [20]. After removing the solvent under vacuum, the electrolyte was removed by adding CS2 and then filtering. Subsequent HPLC separation of the reaction mixture with 1a afforded products 2a, 3a, 4a, and 5a in yields of 40, 37, 9, and 1%, respectively, based on the consumption of La@C2v-C82 (Figure 2a and Supporting Information File 1, Figure S1). The yield was estimated from the absorption intensity ratio at 330 nm. On the other hand, no consumption of La@C2v-C82 was observed in the reaction of La@ C2v-C82 with 1a (Figure 2b). The reaction of the La@C2v-C82 anion toward 2a–5a requires heating, therefore the reactivity of La@C2vC82 anion is lower than that of the C602− and C60 anion radicals [10,11], which react even at room temperature. However, the one-electron reduction of La@C2v-C82 is effective for the activating its reactivity toward alkyl halides in the thermal reaction. Recently, Zhou et al. reported that the reaction of Gd@C2v-C82 with benzyl bromide requires a three-electron reduction of Gd@C2v-C82 for the addition reaction to occur at room temperature [22].
Scheme 1: Reaction of the La@C2v-C82 anion with benzyl bromide derivatives.
Scheme 1: Reaction of the La@C2v-C82 anion with benzyl bromide derivatives.
![[1860-5397-19-138-1]](/bjoc/content/figures/1860-5397-19-138-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Changes in absorption spectra during the reaction of La@C2v-C82 anion with 1a.
Figure 1: Changes in absorption spectra during the reaction of La@C2v-C82 anion with 1a.
![[1860-5397-19-138-2]](/bjoc/content/figures/1860-5397-19-138-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: HPLC profiles of the reaction mixture. Conditions: Buckyprep column (⌀ = 4.6 × 250 mm); eluent, toluene; flow rate, 1 mL/min; UV detector, 330 nm. (a) La@C2v-C82 anion with 1a. 110 °C, 2 h. (b) La@C2v-C82 with 1a. 110 °C, 2 h. (c) La@C2v-C82 anion with 1a. hv > 350 nm, 1 h. (d) La@C2v-C82 with 1a. hv > 350 nm, 1 h. Black line is the HPLC profiles of La@C2V-C82 at the same concentration as the reaction. Reaction mixtures with La@C2v-C82 anions were treated with dichloroacetic acid before injection.
Figure 2: HPLC profiles of the reaction mixture. Conditions: Buckyprep column (⌀ = 4.6 × 250 mm); eluent, tol...
Supporting Information File 1, Figure S1 depicts the three HPLC separation steps including recycling for the isolation. The matrix-assisted laser desorption/ionization time-of-flight (MALDI–TOF) mass spectra of 2a–5a displayed the molecular ion peaks at m/z 1229, as expected for the 1:1 adducts of La@C2v-C82 and the 4-methylbenzyl group [MH]+ (Figure 3). Fragment peaks were observed at m/z 1123, corresponding to the mass of the fragment ion [La@C2v-C82]+. Similarly, the reaction of 1b gave 2b–5b in yields of 39, 42, 5, and 5%, respectively, and that of 1c gave 2c–5c in yields of 34, 33, 16, and 10%, respectively, based on the consumption of La@C2v-C82 (see Supporting Information File 1, Figures S2–S6).
![[1860-5397-19-138-3]](/bjoc/content/figures/1860-5397-19-138-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: HPLC profiles (Buckyprep column (⌀ = 4.6 × 250 mm); eluent, toluene; flow rate, 1.0 mL/min; UV detector, 330 nm.) and MALDI–TOF mass spectra (matrix: dithranol, positive mode) of 2a–5a.
Figure 3: HPLC profiles (Buckyprep column (⌀ = 4.6 × 250 mm); eluent, toluene; flow rate, 1.0 mL/min; UV dete...
For the comparison, the photoreaction of the La@C2v-C82 anion with 1a was performed in ODCB using a high-pressure mercury arc lamp (cutoff < 350 nm, 1 h). The HPLC profile after the photoreaction indicates that several products other than 2a–5a were present (Figure 2c), similar to the photoreaction of La@C2v-C82 with 1a (Figure 2d). A previous study reported that the reaction of La@C2v-C82 with benzyl bromide under photolytic conditions affords eight monoadducts [19]. Therefore, one-electron reduction and the subsequent thermal reaction of La@C2v-C82 were effective for its functionalization in terms of both regioselectivity and reactivity compared to the thermal and photoreactions of La@C2v-C82 reported previously [19].
Figure 4 shows the absorption spectra of 2–5. Their absorption onsets move to shorter wavelengths relative to those of La@C2v-C82, which are characteristic features of single-bonded La@C2v-C82 derivatives [19,23], indicating that 2–5 have larger HOMO–lowest unoccupied molecular orbital energy gaps owing to their closed shell structures. As previous studies have shown that the absorption spectra of fullerene derivatives sensitively reflect the addition site, the absorption spectra can be regarded as powerful tools to determine the addition site in fullerene adducts [19,23-25]. Regardless of the substituents (a–c) of benzyl bromide, 2, 3, 4, and 5 exhibited similar characteristic absorption features, respectively, supporting that the addition site of each isomer (e.g., 2a–c) are the same.
![[1860-5397-19-138-4]](/bjoc/content/figures/1860-5397-19-138-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Absorption spectra of 2, 3, 4, and 5 in CS2 and absorption spectra of C14, C10, C18, and C9 in Ce@C2v-C82(CH2C6H3Me2), which is attributed to the 1b, 1d, 1c, and 1a isomers in [25]. The carbon atom numbers correspond to that shown in Figure 6d.
Figure 4: Absorption spectra of 2, 3, 4, and 5 in CS2 and absorption spectra of C14, C10, C18, and C9 in Ce@C2...
We determined the addition sites of the single-bonded La@C2v-C82 derivatives, La@C2v-C82(CHClC6H3Cl2) [19] and La@C2v-C82(CBr(CO2Et)2) [23], by single-crystal X-ray diffraction (SC-XRD) analysis. Based on the similarity in the absorption spectra of La@C2v-C82(CHClC6H3Cl2), the addition site of 3a–c was expected to be at the C10 (for the numbering of carbon atoms in La@C2v-C82; see Figure 6d). Takano et al. estimated the addition sites of the 3,5-dimethylphenylmethyl group on Ce@C2v-C82 (Ce@C2v-C82(CH2C6H3Me2)) through temperature-dependent paramagnetic shifts of its nuclear magnetic resonance signals [25]. The similarity in the HPLC separation behavior and absorption spectra between the La@C2v-C82 adducts (2a–c, 3a–c, and 4a–c) [19] and the Ce@C2v-C82(CH2C6H3Me2) isomers [25] reported by Takano et al. was observed. Based on this observation, the plausible addition sites of 2a–c, 3a–c, and 4a–c were estimated to be at the C14, C10, and C18 positions. The molecular structure of 3a was confirmed by the SC-XRD analysis, which showed that the addition site of addendum was indeed at the C10 position of La@C2v-C82 (Figure 5).
![[1860-5397-19-138-5]](/bjoc/content/figures/1860-5397-19-138-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: ORTEP drawing of 3a with thermal ellipsoids shown at 50% probability level. Only an independent unit of 3a is shown. This crystal has two independent units of 3a and three CS2 molecules as guest solvents. The difference between the two independent units is the direction of the tolyl group in the crystal (Supporting Information File 1, Figure S7).
Figure 5: ORTEP drawing of 3a with thermal ellipsoids shown at 50% probability level. Only an independent uni...
The La@C2v-C82 anion can act as an electron donor and a nucleophile. To confirm the reaction mechanism, charge density and the p-orbital axis vector (POAV) values [26] of the carbon atoms (θσπ-90˚) of the La@C2v-C82 anion were calculated using density functional theory (DFT) [27-33]. As shown in Table 1 and Figure 6, the C1, C2, and C3 atoms have large negative charge densities (C1: −0.1498, C2: −0.1828, C3: −0.1126), and C1 and C2 atoms have high POAV values (C1: 11.2, C2: 11.3) in the La@C2v-C82 anion. Meanwhile, the C10, C14, and C18 atoms have moderate or small negative charge densities (C10: −0.0317, C14: −0.0137, C18: −0.0128) and high POAV values (C10: 11.0, C14: 11.3, C18: 11.2) in the La@C2v-C82 anion. On the other hand, the C10, C14, and C18 atoms have larger spin densities (C10: 0.032, C14: 0.023, C18: 0.030) [34,35] than the C1 and C2 atoms (C1: 0.002, C2: 0.016) in La@C2v-C82 (Figure 6b). These results suggest that the reaction mechanism involving the electron transfer from the La@C2v-C82 anion to benzyl bromide derivatives followed by the radical coupling reaction is more plausible for the formation of the corresponding adducts rather than the SN2 reaction mechanism of the La@C2v-C82 anion with benzyl bromide derivatives.
![[1860-5397-19-138-6]](/bjoc/content/figures/1860-5397-19-138-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: (a) Charge density of La@C2v-C82 anion as a function of its POAV values and (b) an enlarged part view of blue region in (a). (c) Spin density of La@C2v-C82 as a function of its POAV values [34,35]. (d) Molecular structure of La@C2v-C82 and numbering carbon atoms.
Figure 6: (a) Charge density of La@C2v-C82 anion as a function of its POAV values and (b) an enlarged part vi...
Table 1: Charge densities and POAV values of carbon atoms for La@C2v-C82 anion, and spin densities and POAV values of carbon atoms for La@C2v-C82 [34,35]. The carbon atom numbers correspond to that shown in Figure 6d.
| Carbon atom number | La@C2v-C82 anion | La@C2v-C82 [34,35] | ||
| POAV | Charge density | POAV | Spin density | |
| 1 | 11.2 | −0.1498 | 11.2 | 0.002 |
| 2 | 11.3 | −0.1828 | 11.3 | 0.016 |
| 3 | 8.8 | −0.1126 | 8.8 | 0.021 |
| 4 | 9.8 | −0.0778 | 9.6 | 0.015 |
| 5 | 9.0 | −0.0629 | 9.2 | 0.000 |
| 6 | 10.4 | −0.0491 | 10.5 | 0.004 |
| 7 | 8.9 | −0.1041 | 9.1 | 0.001 |
| 8 | 8.9 | −0.0672 | 8.9 | 0.000 |
| 9 | 10.2 | −0.0353 | 9.9 | 0.026 |
| 10 | 11.0 | −0.0317 | 10.7 | 0.032 |
| 11 | 10.4 | −0.0386 | 10.6 | 0.009 |
| 12 | 7.5 | −0.0554 | 7.7 | 0.005 |
| 13 | 10.7 | −0.0196 | 10.9 | 0.003 |
| 14 | 11.3 | −0.0137 | 11.0 | 0.023 |
| 15 | 7.3 | −0.0369 | 7.4 | 0.014 |
| 16 | 10.4 | −0.0244 | 10.6 | 0.013 |
| 17 | 8.0 | −0.0443 | 8.2 | 0.000 |
| 18 | 11.2 | −0.0128 | 11.0 | 0.030 |
| 19 | 11.1 | −0.0201 | 10.9 | 0.024 |
| 20 | 8.3 | −0.0332 | 8.4 | 0.000 |
| 21 | 10.3 | −0.0119 | 10.5 | 0.001 |
| 22 | 10.6 | −0.0186 | 10.7 | 0.000 |
| 23 | 11.1 | −0.0084 | 10.7 | 0.025 |
| 24 | 8.2 | −0.0314 | 8.3 | 0.000 |
Conclusion
The reaction of La@C2v-C82 anion with benzyl bromide derivatives 1 at 110 °C afforded the corresponding single-bonded adducts 2–5 with high regioselectivity. One-electron reduction of La@C2v-C82 increased its reactivity during thermal reaction relative to that of neutral La@C2v-C82. Structural analysis of the two major products indicated that the characteristic absorption features were strongly affected by the addition sites. Based on theoretical studies and considering the identified addition sites, a plausible reaction mechanism for the reaction is the electron transfer from La@C2v-C82 anion to benzyl bromide, followed by radical coupling. This demonstrates that one-electron reduction of La@C2v-C82 is an easy and effective method for controlling its reactivity and selectivity via ionization for the production of La@C2v-C82 derivatives.
Experimental
General: All chemicals and solvents were obtained from Wako, TCI, and Aldrich and were used without further purification unless otherwise stated. ODCB was distilled over P2O5 under vacuum prior to use. HPLC was performed on an LC-9201 instrument (Japan Analytical Industry Co., Ltd.) by monitoring the UV absorption at 330 nm with toluene as the eluent. Mass spectrometry was performed using a Bruker AUTOFLEX III smart beam with dithranol as the matrix. Optical absorption spectra were recorded using a Pyrex cell with a 10 mm path length and a spectrophotometer (V-670; Jasco Corp.).
Preparation of La@C2v-C82
As described in [19], soot containing endohedral metallofullerenes were produced through the standard arc vaporization method using a composite anode rod containing graphite and metal oxide. The composite rod was subjected to an arc discharge under a He atmosphere at 50 Torr. Raw soot was collected and suspended in 1,2,4-trichlorobenzene (TCB). The mixture was refluxed for 16 h. The TCB solution was collected and injected into the HPLC instrument to separate the endohedral metallofullerenes using a PBB column (⌀ 20 mm × 250 mm; Cosmoses, Nacalai Tesque Inc.) with chlorobenzene as the mobile phase in the first step and a Buckyprep column (⌀ 20 mm × 250 mm × 2; Cosmoses, Nacalai Tesque Inc.) with toluene as the mobile phase in the second step.
Preparation of the La@C2v-C82 anion
As described in [21], La@C2v-C82 (0.34 × 10−6 mol) was dissolved in 10 mL of a pyridine solution containing TBAF (0.54 × 10−3 mol) and then stirred for 2 h under an Ar atmosphere. The resulting green solution was concentrated to 2.0 mL. CS2 was added to the solution to precipitate excess TBAF which was then removed by filtration. The La@C2v-C82 anion ([La@C2v-C82]PF6) was collected as the filtrate (with a 78% yield estimated from the molar absorbance coefficient). This step was repeated to ensure a sufficient amount of La@C2v-C82 anions for the next step.
Reaction of the La@C2v-C82 anion with 1a
1a was added to 12 mL of the La@C2v-C82 anion (0.89 × 10−6 mol) ODCB solution. The solution was degassed using freeze-pump-thaw cycles. The solution was then heated at 110 °C for 2 h. After the reaction, dichloroacetic acid was added to recover the unreacted La@C2v-C82 in its neutral form. Four isomers, 2a, 3a, 4a, and 5a, and La@C2v-C82 were isolated from the reaction mixture using multistep HPLC, as shown in Supporting Information File 1, Figures S2, S4, and S6. The yields were calculated from the HPLC peak areas monitored at 330 nm, assuming that La@C2v-C82 and the monoadducts have the same absorption coefficients.
X-ray crystallography
Black crystalline rods of 3a were obtained using the liquid–liquid bilayer diffusion method with 3a in a CS2 solution and an n-hexane solution in a glass tube (⌀ = 7 mm) at room temperature. The SC-XRD measurement was performed at 90 K on a Bruker AXS instrument equipped with an Apex II CCD detector with Mo Kα radiation (λ = 0.71073 Å). The multi-scan method was used for absorption corrections. Structures were solved using direct methods and refined using SHELXL-2014/7 [36-38]. Deposition Number 2299232 (for 3a) contains the supplementary crystallographic data for this study. This data was provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures Service.
Theoretical calculations
POAV (θσπ−90º) and charge densities values were calculated using the Gaussian 03 program with DFT at the B3LYP/3-21G for C and H [33], and the LanL2DZ basis set and effective core potential (ECP) for La [29,32].
La@C2v-C82(CH2C6H4CH3) (2a): vis–NIR (CS2): λmax = 572, 741, 1304 nm; MALDI–TOF MS (m/z): [MH]+ calcd for LaC90H9, 1228.98; found, 1229.04.
La@C2v-C82(CH2C6H4CH3) (3a): vis–NIR (CS2): λmax = 560, 851, 991, 1271 nm; MALDI–TOF MS (m/z): [MH]+ calcd for LaC90H9, 1228.98; found, 1228.99.
La@C2v-C82(CH2C6H4CH3) (4a): vis–NIR (CS2): λmax = 525, 986, 1410 nm; MALDI–TOF MS (m/z): [MH]+ calcd for LaC90H9, 1228.98; found, 1229.15.
La@C2v-C82(CH2C6H4CH3) (5a): vis–NIR (CS2): λmax = 809, 997, 1454 nm; MALDI–TOF MS (m/z): [MH]+ calcd for LaC90H9, 1228.98; found, 1229.04.
La@C2v-C82(CH2C6H4CCSi(CH3)3) (2b): vis–NIR (CS2): λmax = 568, 762, 1307 nm; MALDI–TOF MS (m/z): [MH]+ calcd for LaC94H16Si, 1311.01; found, 1311.25.
La@C2v-C82(CH2C6H4CCSi(CH3)3) (3b): vis–NIR (CS2): λmax = 559, 883, 995, 1242 nm; MALDI–TOF MS (m/z): [MH]+ calcd for LaC94H16Si, 1311.01; found, 1311.37.
La@C2v-C82(CH2C6H4CCSi(CH3)3) (4b): vis–NIR (CS2): λmax = 521, 986, 1410 nm; MALDI–TOF MS (m/z): [MH]+ calcd for LaC94H16Si, 1311.01; found, 1311.29.
La@C2v-C82(CH2C6H4CCSi(CH3)3) (5b): vis–NIR (CS2): λmax = 809, 998, 1447 nm; MALDI–TOF MS (m/z): [MH]+ calcd for LaC94H16Si, 1311.01; found, 1311.25.
La@C2v-C82(CH2C6H5) (2c): vis–NIR (CS2): λmax = 583, 786, 1305 nm; MALDI–TOF MS (m/z): [MH]+ calcd for LaC89H7, 1214.97; found, 1214.83.
La@C2v-C82(CH2C6H5) (3c): vis–NIR (CS2): λmax = 561, 891, 993, 1270 nm; MALDI–TOF MS (m/z): [MH]+ calcd for LaC89H7, 1214.97; found, 1214.96.
La@C2v-C82(CH2C6H5) (4c): vis–NIR (CS2): λmax = 521, 986, 1410 nm; MALDI–TOF MS (m/z): [MH]+ calcd for LaC89H7, 1214.97; found, 1214.82.
La@C2v-C82(CH2C6H5) (5c): vis–NIR (CS2): λmax = 818, 999, 1451 nm; MALDI–TOF MS (m/z): [MH]+ calcd for LaC89H7, 1214.97; found, 1214.98.
Supporting Information
Supporting Information features HPLC chromatographs and MS spectra of fullerene derivatives, changes in absorption spectra during the reaction of La@C2v-C82 with 1b and 1c, X-ray crystallographic data of 3a, and ORTEP drawings of the independent unit of 3a.
| Supporting Information File 1: Additional experimental data. | ||
| Format: PDF | Size: 1.2 MB | Download |
References
-
Hirsch, A.; Brettreich, M. Fullerenes: Chemistry and Reactions; Wiley-VCH: Weinheim, Germany, 2004. doi:10.1002/3527603492
Return to citation in text: [1] -
Kadish, K. M.; Ruoff, R. S., Eds. Fullerenes: Chemistry, Physics, and Technology; John Wiley & Sons: New York, NY, USA, 2000.
Return to citation in text: [1] -
Prato, M.; Maggini, M. Acc. Chem. Res. 1998, 31, 519–526. doi:10.1021/ar970210p
Return to citation in text: [1] -
Thilgen, C.; Diederich, F. Chem. Rev. 2006, 106, 5049–5135. doi:10.1021/cr0505371
Return to citation in text: [1] -
Vostrowsky, O.; Hirsch, A. Chem. Rev. 2006, 106, 5191–5207. doi:10.1021/cr050561e
Return to citation in text: [1] -
Caron, C.; Subramanian, R.; D'Souza, F.; Kim, J.; Kutner, W.; Jones, M. T.; Kadish, K. M. J. Am. Chem. Soc. 1993, 115, 8505–8506. doi:10.1021/ja00071a093
Return to citation in text: [1] -
Subramanian, R.; Boulas, P.; Vijayashree, M. N.; D’Souza, F.; Jones, M. T.; Kadish, K. M. J. Chem. Soc., Chem. Commun. 1994, 1847–1848. doi:10.1039/c39940001847
Return to citation in text: [1] -
Fukuzumi, S.; Suenobu, T.; Hirasaka, T.; Arakawa, R.; Kadish, K. M. J. Am. Chem. Soc. 1998, 120, 9220–9227. doi:10.1021/ja9815430
Return to citation in text: [1] [2] -
Allard, E.; Rivière, L.; Delaunay, J.; Dubois, D.; Cousseau, J. Tetrahedron Lett. 1999, 40, 7223–7226. doi:10.1016/s0040-4039(99)01467-7
Return to citation in text: [1] -
Wabra, I.; Holzwarth, J.; Hauke, F.; Hirsch, A. Chem. – Eur. J. 2019, 25, 5186–5201. doi:10.1002/chem.201805777
Return to citation in text: [1] [2] -
Maeda, Y.; Sanno, M.; Morishita, T.; Sakamoto, K.; Sugiyama, E.; Akita, S.; Yamada, M.; Suzuki, M. New J. Chem. 2019, 43, 6457–6460. doi:10.1039/c9nj01043b
Return to citation in text: [1] [2] -
Akasaka, T.; Nagase, S., Eds. Endofullerenes: A New Family of Carbon Clusters; Kluwer Academic Publisher: Dordrecht, Netherlands, 2002.
Return to citation in text: [1] -
Maeda, Y.; Tsuchiya, T.; Lu, X.; Takano, Y.; Akasaka, T.; Nagase, S. Nanoscale 2011, 3, 2421–2429. doi:10.1039/c0nr00968g
Return to citation in text: [1] -
Lu, X.; Feng, L.; Akasaka, T.; Nagase, S. Chem. Soc. Rev. 2012, 41, 7723–7760. doi:10.1039/c2cs35214a
Return to citation in text: [1] -
Popov, A. A.; Yang, S.; Dunsch, L. Chem. Rev. 2013, 113, 5989–6113. doi:10.1021/cr300297r
Return to citation in text: [1] -
Akasaka, T.; Kato, T.; Kobayashi, K.; Nagase, S.; Yamamoto, K.; Funasaka, H.; Takahashi, T. Nature 1995, 374, 600–601. doi:10.1038/374600a0
Return to citation in text: [1] -
Maeda, Y.; Miyashita, J.; Hasegawa, T.; Wakahara, T.; Tsuchiya, T.; Feng, L.; Lian, Y.; Akasaka, T.; Kobayashi, K.; Nagase, S.; Kako, M.; Yamamoto, K.; Kadish, K. M. J. Am. Chem. Soc. 2005, 127, 2143–2146. doi:10.1021/ja043986b
Return to citation in text: [1] -
Li, F.-F.; Rodríguez-Fortea, A.; Poblet, J. M.; Echegoyen, L. J. Am. Chem. Soc. 2011, 133, 2760–2765. doi:10.1021/ja110160j
Return to citation in text: [1] -
Takano, Y.; Yomogida, A.; Nikawa, H.; Yamada, M.; Wakahara, T.; Tsuchiya, T.; Ishitsuka, M. O.; Maeda, Y.; Akasaka, T.; Kato, T.; Slanina, Z.; Mizorogi, N.; Nagase, S. J. Am. Chem. Soc. 2008, 130, 16224–16230. doi:10.1021/ja802748q
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] [8] -
Akasaka, T.; Wakahara, T.; Nagase, S.; Kobayashi, K.; Waelchli, M.; Yamamoto, K.; Kondo, M.; Shirakura, S.; Okubo, S.; Maeda, Y.; Kato, T.; Kako, M.; Nakadaira, Y.; Nagahata, R.; Gao, X.; Van Caemelbecke, E.; Kadish, K. M. J. Am. Chem. Soc. 2000, 122, 9316–9317. doi:10.1021/ja001586s
Return to citation in text: [1] [2] -
Tsuchiya, T.; Wakahara, T.; Lian, Y.; Maeda, Y.; Akasaka, T.; Kato, T.; Mizorogi, N.; Nagase, S. J. Phys. Chem. B 2006, 110, 22517–22520. doi:10.1021/jp0650679
Return to citation in text: [1] [2] -
Zhou, X.; Yao, Y.-R.; Hu, Y.; Yang, L.; Yang, S.; Zhang, Y.; Zhang, Q.; Peng, P.; Jin, P.; Li, F.-F. Inorganics 2023, 11, 349. doi:10.3390/inorganics11090349
Return to citation in text: [1] -
Feng, L.; Nakahodo, T.; Wakahara, T.; Tsuchiya, T.; Maeda, Y.; Akasaka, T.; Kato, T.; Horn, E.; Yoza, K.; Mizorogi, N.; Nagase, S. J. Am. Chem. Soc. 2005, 127, 17136–17137. doi:10.1021/ja055484j
Return to citation in text: [1] [2] [3] -
Hirsch, A.; Grösser, T.; Skiebe, A.; Soi, A. Chem. Ber. 1993, 126, 1061–1067. doi:10.1002/cber.19931260428
Return to citation in text: [1] -
Takano, Y.; Tashita, R.; Suzuki, M.; Nagase, S.; Imahori, H.; Akasaka, T. J. Am. Chem. Soc. 2016, 138, 8000–8006. doi:10.1021/jacs.6b04037
Return to citation in text: [1] [2] [3] [4] -
Haddon, R. C. J. Am. Chem. Soc. 1990, 112, 3385–3389. doi:10.1021/ja00165a020
Return to citation in text: [1] -
Maeda, Y.; Matsunaga, Y.; Wakahara, T.; Takahashi, S.; Tsuchiya, T.; Ishitsuka, M. O.; Hasegawa, T.; Akasaka, T.; Liu, M. T. H.; Kokura, K.; Horn, E.; Yoza, K.; Kato, T.; Okubo, S.; Kobayashi, K.; Nagase, S.; Yamamoto, K. J. Am. Chem. Soc. 2004, 126, 6858–6859. doi:10.1021/ja0316115
Return to citation in text: [1] -
Gaussian 03, Revision C.02; Gaussian, Inc.: Wallingford, CT, 2004.
Return to citation in text: [1] -
Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785–789. doi:10.1103/physrevb.37.785
Return to citation in text: [1] [2] -
Becke, A. D. Phys. Rev. A 1988, 38, 3098–3100. doi:10.1103/physreva.38.3098
Return to citation in text: [1] -
Becke, A. D. J. Chem. Phys. 1993, 98, 5648–5652. doi:10.1063/1.464913
Return to citation in text: [1] -
Hay, P. J.; Wadt, W. R. J. Chem. Phys. 1985, 82, 299–310. doi:10.1063/1.448975
Return to citation in text: [1] [2] -
Binkley, J. S.; Pople, J. A.; Hehre, W. J. J. Am. Chem. Soc. 1980, 102, 939–947. doi:10.1021/ja00523a008
Return to citation in text: [1] [2] -
Sato, S.; Maeda, Y.; Guo, J.-D.; Yamada, M.; Mizorogi, N.; Nagase, S.; Akasaka, T. J. Am. Chem. Soc. 2013, 135, 5582–5587. doi:10.1021/ja309763f
Return to citation in text: [1] [2] [3] [4] -
Maeda, Y.; Sato, S.; Inada, K.; Nikawa, H.; Yamada, M.; Mizorogi, N.; Hasegawa, T.; Tsuchiya, T.; Akasaka, T.; Kato, T.; Slanina, Z.; Nagase, S. Chem. – Eur. J. 2010, 16, 2193–2197. doi:10.1002/chem.200902512
Return to citation in text: [1] [2] [3] [4] -
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, 64, 112–122. doi:10.1107/s0108767307043930
Return to citation in text: [1] -
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3–8. doi:10.1107/s2053273314026370
Return to citation in text: [1] -
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8. doi:10.1107/s2053229614024218
Return to citation in text: [1]
| 34. | Sato, S.; Maeda, Y.; Guo, J.-D.; Yamada, M.; Mizorogi, N.; Nagase, S.; Akasaka, T. J. Am. Chem. Soc. 2013, 135, 5582–5587. doi:10.1021/ja309763f |
| 35. | Maeda, Y.; Sato, S.; Inada, K.; Nikawa, H.; Yamada, M.; Mizorogi, N.; Hasegawa, T.; Tsuchiya, T.; Akasaka, T.; Kato, T.; Slanina, Z.; Nagase, S. Chem. – Eur. J. 2010, 16, 2193–2197. doi:10.1002/chem.200902512 |
| 19. | Takano, Y.; Yomogida, A.; Nikawa, H.; Yamada, M.; Wakahara, T.; Tsuchiya, T.; Ishitsuka, M. O.; Maeda, Y.; Akasaka, T.; Kato, T.; Slanina, Z.; Mizorogi, N.; Nagase, S. J. Am. Chem. Soc. 2008, 130, 16224–16230. doi:10.1021/ja802748q |
| 21. | Tsuchiya, T.; Wakahara, T.; Lian, Y.; Maeda, Y.; Akasaka, T.; Kato, T.; Mizorogi, N.; Nagase, S. J. Phys. Chem. B 2006, 110, 22517–22520. doi:10.1021/jp0650679 |
| 1. | Hirsch, A.; Brettreich, M. Fullerenes: Chemistry and Reactions; Wiley-VCH: Weinheim, Germany, 2004. doi:10.1002/3527603492 |
| 2. | Kadish, K. M.; Ruoff, R. S., Eds. Fullerenes: Chemistry, Physics, and Technology; John Wiley & Sons: New York, NY, USA, 2000. |
| 3. | Prato, M.; Maggini, M. Acc. Chem. Res. 1998, 31, 519–526. doi:10.1021/ar970210p |
| 4. | Thilgen, C.; Diederich, F. Chem. Rev. 2006, 106, 5049–5135. doi:10.1021/cr0505371 |
| 5. | Vostrowsky, O.; Hirsch, A. Chem. Rev. 2006, 106, 5191–5207. doi:10.1021/cr050561e |
| 16. | Akasaka, T.; Kato, T.; Kobayashi, K.; Nagase, S.; Yamamoto, K.; Funasaka, H.; Takahashi, T. Nature 1995, 374, 600–601. doi:10.1038/374600a0 |
| 17. | Maeda, Y.; Miyashita, J.; Hasegawa, T.; Wakahara, T.; Tsuchiya, T.; Feng, L.; Lian, Y.; Akasaka, T.; Kobayashi, K.; Nagase, S.; Kako, M.; Yamamoto, K.; Kadish, K. M. J. Am. Chem. Soc. 2005, 127, 2143–2146. doi:10.1021/ja043986b |
| 19. | Takano, Y.; Yomogida, A.; Nikawa, H.; Yamada, M.; Wakahara, T.; Tsuchiya, T.; Ishitsuka, M. O.; Maeda, Y.; Akasaka, T.; Kato, T.; Slanina, Z.; Mizorogi, N.; Nagase, S. J. Am. Chem. Soc. 2008, 130, 16224–16230. doi:10.1021/ja802748q |
| 23. | Feng, L.; Nakahodo, T.; Wakahara, T.; Tsuchiya, T.; Maeda, Y.; Akasaka, T.; Kato, T.; Horn, E.; Yoza, K.; Mizorogi, N.; Nagase, S. J. Am. Chem. Soc. 2005, 127, 17136–17137. doi:10.1021/ja055484j |
| 12. | Akasaka, T.; Nagase, S., Eds. Endofullerenes: A New Family of Carbon Clusters; Kluwer Academic Publisher: Dordrecht, Netherlands, 2002. |
| 13. | Maeda, Y.; Tsuchiya, T.; Lu, X.; Takano, Y.; Akasaka, T.; Nagase, S. Nanoscale 2011, 3, 2421–2429. doi:10.1039/c0nr00968g |
| 14. | Lu, X.; Feng, L.; Akasaka, T.; Nagase, S. Chem. Soc. Rev. 2012, 41, 7723–7760. doi:10.1039/c2cs35214a |
| 15. | Popov, A. A.; Yang, S.; Dunsch, L. Chem. Rev. 2013, 113, 5989–6113. doi:10.1021/cr300297r |
| 19. | Takano, Y.; Yomogida, A.; Nikawa, H.; Yamada, M.; Wakahara, T.; Tsuchiya, T.; Ishitsuka, M. O.; Maeda, Y.; Akasaka, T.; Kato, T.; Slanina, Z.; Mizorogi, N.; Nagase, S. J. Am. Chem. Soc. 2008, 130, 16224–16230. doi:10.1021/ja802748q |
| 23. | Feng, L.; Nakahodo, T.; Wakahara, T.; Tsuchiya, T.; Maeda, Y.; Akasaka, T.; Kato, T.; Horn, E.; Yoza, K.; Mizorogi, N.; Nagase, S. J. Am. Chem. Soc. 2005, 127, 17136–17137. doi:10.1021/ja055484j |
| 24. | Hirsch, A.; Grösser, T.; Skiebe, A.; Soi, A. Chem. Ber. 1993, 126, 1061–1067. doi:10.1002/cber.19931260428 |
| 25. | Takano, Y.; Tashita, R.; Suzuki, M.; Nagase, S.; Imahori, H.; Akasaka, T. J. Am. Chem. Soc. 2016, 138, 8000–8006. doi:10.1021/jacs.6b04037 |
| 8. | Fukuzumi, S.; Suenobu, T.; Hirasaka, T.; Arakawa, R.; Kadish, K. M. J. Am. Chem. Soc. 1998, 120, 9220–9227. doi:10.1021/ja9815430 |
| 19. | Takano, Y.; Yomogida, A.; Nikawa, H.; Yamada, M.; Wakahara, T.; Tsuchiya, T.; Ishitsuka, M. O.; Maeda, Y.; Akasaka, T.; Kato, T.; Slanina, Z.; Mizorogi, N.; Nagase, S. J. Am. Chem. Soc. 2008, 130, 16224–16230. doi:10.1021/ja802748q |
| 6. | Caron, C.; Subramanian, R.; D'Souza, F.; Kim, J.; Kutner, W.; Jones, M. T.; Kadish, K. M. J. Am. Chem. Soc. 1993, 115, 8505–8506. doi:10.1021/ja00071a093 |
| 7. | Subramanian, R.; Boulas, P.; Vijayashree, M. N.; D’Souza, F.; Jones, M. T.; Kadish, K. M. J. Chem. Soc., Chem. Commun. 1994, 1847–1848. doi:10.1039/c39940001847 |
| 8. | Fukuzumi, S.; Suenobu, T.; Hirasaka, T.; Arakawa, R.; Kadish, K. M. J. Am. Chem. Soc. 1998, 120, 9220–9227. doi:10.1021/ja9815430 |
| 9. | Allard, E.; Rivière, L.; Delaunay, J.; Dubois, D.; Cousseau, J. Tetrahedron Lett. 1999, 40, 7223–7226. doi:10.1016/s0040-4039(99)01467-7 |
| 10. | Wabra, I.; Holzwarth, J.; Hauke, F.; Hirsch, A. Chem. – Eur. J. 2019, 25, 5186–5201. doi:10.1002/chem.201805777 |
| 11. | Maeda, Y.; Sanno, M.; Morishita, T.; Sakamoto, K.; Sugiyama, E.; Akita, S.; Yamada, M.; Suzuki, M. New J. Chem. 2019, 43, 6457–6460. doi:10.1039/c9nj01043b |
| 19. | Takano, Y.; Yomogida, A.; Nikawa, H.; Yamada, M.; Wakahara, T.; Tsuchiya, T.; Ishitsuka, M. O.; Maeda, Y.; Akasaka, T.; Kato, T.; Slanina, Z.; Mizorogi, N.; Nagase, S. J. Am. Chem. Soc. 2008, 130, 16224–16230. doi:10.1021/ja802748q |
| 21. | Tsuchiya, T.; Wakahara, T.; Lian, Y.; Maeda, Y.; Akasaka, T.; Kato, T.; Mizorogi, N.; Nagase, S. J. Phys. Chem. B 2006, 110, 22517–22520. doi:10.1021/jp0650679 |
| 10. | Wabra, I.; Holzwarth, J.; Hauke, F.; Hirsch, A. Chem. – Eur. J. 2019, 25, 5186–5201. doi:10.1002/chem.201805777 |
| 11. | Maeda, Y.; Sanno, M.; Morishita, T.; Sakamoto, K.; Sugiyama, E.; Akita, S.; Yamada, M.; Suzuki, M. New J. Chem. 2019, 43, 6457–6460. doi:10.1039/c9nj01043b |
| 29. | Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785–789. doi:10.1103/physrevb.37.785 |
| 32. | Hay, P. J.; Wadt, W. R. J. Chem. Phys. 1985, 82, 299–310. doi:10.1063/1.448975 |
| 20. | Akasaka, T.; Wakahara, T.; Nagase, S.; Kobayashi, K.; Waelchli, M.; Yamamoto, K.; Kondo, M.; Shirakura, S.; Okubo, S.; Maeda, Y.; Kato, T.; Kako, M.; Nakadaira, Y.; Nagahata, R.; Gao, X.; Van Caemelbecke, E.; Kadish, K. M. J. Am. Chem. Soc. 2000, 122, 9316–9317. doi:10.1021/ja001586s |
| 22. | Zhou, X.; Yao, Y.-R.; Hu, Y.; Yang, L.; Yang, S.; Zhang, Y.; Zhang, Q.; Peng, P.; Jin, P.; Li, F.-F. Inorganics 2023, 11, 349. doi:10.3390/inorganics11090349 |
| 19. | Takano, Y.; Yomogida, A.; Nikawa, H.; Yamada, M.; Wakahara, T.; Tsuchiya, T.; Ishitsuka, M. O.; Maeda, Y.; Akasaka, T.; Kato, T.; Slanina, Z.; Mizorogi, N.; Nagase, S. J. Am. Chem. Soc. 2008, 130, 16224–16230. doi:10.1021/ja802748q |
| 36. | Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, 64, 112–122. doi:10.1107/s0108767307043930 |
| 37. | Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3–8. doi:10.1107/s2053273314026370 |
| 38. | Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8. doi:10.1107/s2053229614024218 |
| 18. | Li, F.-F.; Rodríguez-Fortea, A.; Poblet, J. M.; Echegoyen, L. J. Am. Chem. Soc. 2011, 133, 2760–2765. doi:10.1021/ja110160j |
| 20. | Akasaka, T.; Wakahara, T.; Nagase, S.; Kobayashi, K.; Waelchli, M.; Yamamoto, K.; Kondo, M.; Shirakura, S.; Okubo, S.; Maeda, Y.; Kato, T.; Kako, M.; Nakadaira, Y.; Nagahata, R.; Gao, X.; Van Caemelbecke, E.; Kadish, K. M. J. Am. Chem. Soc. 2000, 122, 9316–9317. doi:10.1021/ja001586s |
| 33. | Binkley, J. S.; Pople, J. A.; Hehre, W. J. J. Am. Chem. Soc. 1980, 102, 939–947. doi:10.1021/ja00523a008 |
| 23. | Feng, L.; Nakahodo, T.; Wakahara, T.; Tsuchiya, T.; Maeda, Y.; Akasaka, T.; Kato, T.; Horn, E.; Yoza, K.; Mizorogi, N.; Nagase, S. J. Am. Chem. Soc. 2005, 127, 17136–17137. doi:10.1021/ja055484j |
| 25. | Takano, Y.; Tashita, R.; Suzuki, M.; Nagase, S.; Imahori, H.; Akasaka, T. J. Am. Chem. Soc. 2016, 138, 8000–8006. doi:10.1021/jacs.6b04037 |
| 19. | Takano, Y.; Yomogida, A.; Nikawa, H.; Yamada, M.; Wakahara, T.; Tsuchiya, T.; Ishitsuka, M. O.; Maeda, Y.; Akasaka, T.; Kato, T.; Slanina, Z.; Mizorogi, N.; Nagase, S. J. Am. Chem. Soc. 2008, 130, 16224–16230. doi:10.1021/ja802748q |
| 34. | Sato, S.; Maeda, Y.; Guo, J.-D.; Yamada, M.; Mizorogi, N.; Nagase, S.; Akasaka, T. J. Am. Chem. Soc. 2013, 135, 5582–5587. doi:10.1021/ja309763f |
| 35. | Maeda, Y.; Sato, S.; Inada, K.; Nikawa, H.; Yamada, M.; Mizorogi, N.; Hasegawa, T.; Tsuchiya, T.; Akasaka, T.; Kato, T.; Slanina, Z.; Nagase, S. Chem. – Eur. J. 2010, 16, 2193–2197. doi:10.1002/chem.200902512 |
| 34. | Sato, S.; Maeda, Y.; Guo, J.-D.; Yamada, M.; Mizorogi, N.; Nagase, S.; Akasaka, T. J. Am. Chem. Soc. 2013, 135, 5582–5587. doi:10.1021/ja309763f |
| 35. | Maeda, Y.; Sato, S.; Inada, K.; Nikawa, H.; Yamada, M.; Mizorogi, N.; Hasegawa, T.; Tsuchiya, T.; Akasaka, T.; Kato, T.; Slanina, Z.; Nagase, S. Chem. – Eur. J. 2010, 16, 2193–2197. doi:10.1002/chem.200902512 |
| 27. | Maeda, Y.; Matsunaga, Y.; Wakahara, T.; Takahashi, S.; Tsuchiya, T.; Ishitsuka, M. O.; Hasegawa, T.; Akasaka, T.; Liu, M. T. H.; Kokura, K.; Horn, E.; Yoza, K.; Kato, T.; Okubo, S.; Kobayashi, K.; Nagase, S.; Yamamoto, K. J. Am. Chem. Soc. 2004, 126, 6858–6859. doi:10.1021/ja0316115 |
| 28. | Gaussian 03, Revision C.02; Gaussian, Inc.: Wallingford, CT, 2004. |
| 29. | Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785–789. doi:10.1103/physrevb.37.785 |
| 30. | Becke, A. D. Phys. Rev. A 1988, 38, 3098–3100. doi:10.1103/physreva.38.3098 |
| 31. | Becke, A. D. J. Chem. Phys. 1993, 98, 5648–5652. doi:10.1063/1.464913 |
| 32. | Hay, P. J.; Wadt, W. R. J. Chem. Phys. 1985, 82, 299–310. doi:10.1063/1.448975 |
| 33. | Binkley, J. S.; Pople, J. A.; Hehre, W. J. J. Am. Chem. Soc. 1980, 102, 939–947. doi:10.1021/ja00523a008 |
| 34. | Sato, S.; Maeda, Y.; Guo, J.-D.; Yamada, M.; Mizorogi, N.; Nagase, S.; Akasaka, T. J. Am. Chem. Soc. 2013, 135, 5582–5587. doi:10.1021/ja309763f |
| 35. | Maeda, Y.; Sato, S.; Inada, K.; Nikawa, H.; Yamada, M.; Mizorogi, N.; Hasegawa, T.; Tsuchiya, T.; Akasaka, T.; Kato, T.; Slanina, Z.; Nagase, S. Chem. – Eur. J. 2010, 16, 2193–2197. doi:10.1002/chem.200902512 |
| 25. | Takano, Y.; Tashita, R.; Suzuki, M.; Nagase, S.; Imahori, H.; Akasaka, T. J. Am. Chem. Soc. 2016, 138, 8000–8006. doi:10.1021/jacs.6b04037 |
| 26. | Haddon, R. C. J. Am. Chem. Soc. 1990, 112, 3385–3389. doi:10.1021/ja00165a020 |
| 25. | Takano, Y.; Tashita, R.; Suzuki, M.; Nagase, S.; Imahori, H.; Akasaka, T. J. Am. Chem. Soc. 2016, 138, 8000–8006. doi:10.1021/jacs.6b04037 |
| 19. | Takano, Y.; Yomogida, A.; Nikawa, H.; Yamada, M.; Wakahara, T.; Tsuchiya, T.; Ishitsuka, M. O.; Maeda, Y.; Akasaka, T.; Kato, T.; Slanina, Z.; Mizorogi, N.; Nagase, S. J. Am. Chem. Soc. 2008, 130, 16224–16230. doi:10.1021/ja802748q |
© 2023 Maeda et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.