Abstract
The last decade has witnessed the emergence of innovative synthetic tools for the synthesis of fluorinated molecules. Among these approaches, the transition-metal-catalyzed functionalization of various scaffolds with a panel of fluorinated groups (XRF, X = S, Se, O) offered straightforward access to high value-added compounds. This review will highlight the main advances made in the field with the transition-metal-catalyzed functionalization of C(sp2) and C(sp3) centers with SCF3, SeCF3, or OCH2CF3 groups among others, by C–H bond activation. The scope and limitations of these transformations are discussed in this review.
Graphical Abstract
Introduction
Nowadays, fluorinated molecules represent an indispensable class of molecules in chemistry and in chemical biology. Thanks to the unique properties of the fluorine atom or fluorinated groups to modulate biological and physicochemical properties of the parent non-fluorinated molecules [1,2], their incorporation in various scaffolds afforded high value-added compounds as demonstrated by their numerous applications in the industrial sector such as drugs, agrochemicals, and materials. To further illustrate the ubiquity of fluorinated compounds, almost 20% of pharmaceuticals and 30–40% of agrochemicals [3-5] contain at least one fluorine atom. Because of the exceptional features of fluorinated derivatives, tremendous developments and discoveries have been made in this blossoming research area, with a high interest for fundamental research as well as the industry [6-11]. Among them [2,5,12-18], the direct functionalization of a simple C–H bond by transition-metal catalysis [19-43] became an important tool offering new retrosynthetic disconnections. In this context, a strong interest from the scientific community was shown towards the challenging synthesis of fluorinated molecules by transition-metal-catalyzed C–H bond activation [44-50], allowing the functionalization of complex molecules and even for late-stage functionalization strategy [51-53] for the synthesis of natural products [42,54-59].
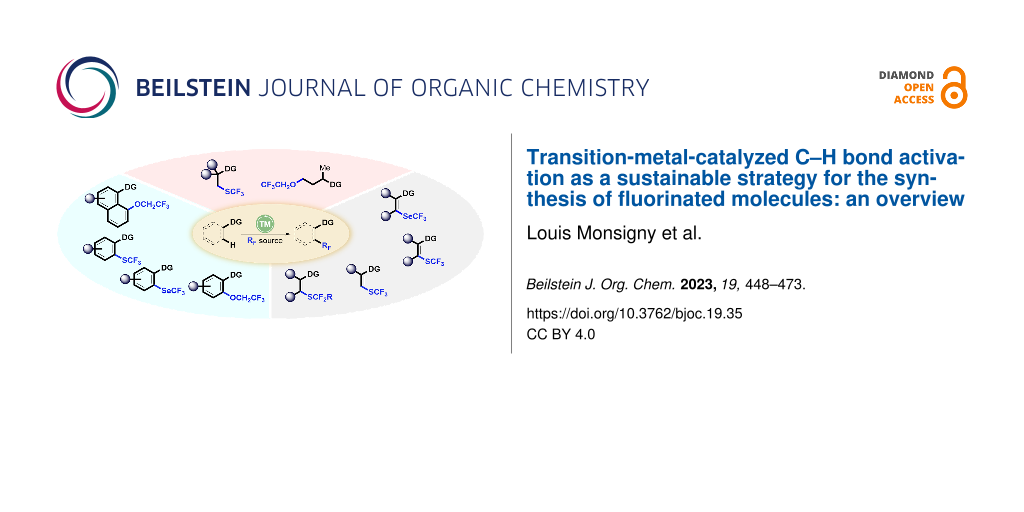
The goal of this review is to highlight and discuss the recent approaches for the synthesis of fluorinated derivatives by the direct incorporation of a fluorinated group of XRF type (e.g., SCF3, SeCF3, SCF2CO2Et, OCH2CF3) by transition-metal-catalyzed C–H bond activation (Scheme 1). The review will be organized in two main parts, dedicated to the construction of a C–SCF2R/SeCF3 and C–OCH2CF3 bond. This review does not aim to be exhaustive and key examples were carefully chosen to provide the reader a nice overview. Since reviews dealing with transition-metal-catalyzed functionalization of compounds by C–H bond activation with fluorinated reagents [60-77] and vinylic, allylic or propargylic fluorinated building blocks [49] have been already reported, these reactions will not be included.
Scheme 1: Transition-metal-catalyzed C–XRF bond formation by C–H bond activation: an overview.
Scheme 1: Transition-metal-catalyzed C–XRF bond formation by C–H bond activation: an overview.
Review
I. Transition-metal-catalyzed directed C–chalcogen bond formation (C–S, C–Se) by C–H bond activation
In the past decade, particular attention has been paid to the development of new methodologies for the incorporation of sulfur-containing fluorinated groups. Although the transition-metal-catalyzed direct C–H bond functionalization appeared to be a powerful tool for the installation of C–C, C–N, or C–O bonds, the direct formation of a C(sp2)–SRF or a C(sp3)–SRF bond remains a challenging task. In this context, key players in the field have been interested in the design of original methodologies for the trifluoromethylthiolation and more recently the difluoromethylthiolation of various compounds by transition-metal catalysis [78]. Moreover, a recent interest was devoted to the trifluoromethylselenolation reaction as depicted in this section.
I.1) Transition-metal-catalyzed C–H trifluoromethylthiolation of aromatic C(sp2) centers
Thanks to its unique features such as an interesting lipophilicity (Hansch parameter = 1.44) [79,80] and a strong withdrawing character, the development of new methodologies for the incorporation of the SCF3 residue on various molecules has known a tremendous expansion [81-113].
Copper catalysis: In 2012, Daugulis and co-workers reported the copper-promoted trifluoromethylthiolation of benzamide derivatives 1 at the ortho-position by C–H bond activation [114]. Indeed, using a bidentate directing group (amide derived from the 8-aminoquinoline), the mono- and difunctionalized compounds were obtained when Cu(OAc)2 (0.5 equiv) and the toxic and volatile disulfide F3CS–SCF3 were employed (Scheme 2, 10 examples, up to 76% yield). With this approach, derivatives bearing an aromatic part substituted at the para-position with electron-donating substituents (1a,b), halogens (1c) as well as electron-withdrawing groups (1d) were difunctionalized in good yields. The substitution pattern on the aromatic ring did not affect the reaction efficiency, the meta-substituted derivative 2e as well as the ortho-substituted derivative 2f were obtained in high yields (70% and 63% yields, respectively). It should be noted that the presence of ortho-substituents on the aryl residue allowed the monofunctionalization to occur selectively. Also, amide 1g bearing a disubstituted arene was successfully functionalized in 59% yield. Finally, the difunctionalized thiophene derivative 2h was obtained in 56% yield.
Scheme 2: Cu(OAc)2-promoted mono- and ditrifluoromethylthiolation of benzamide derivatives derived from 8-aminoquinoline reported by the group of Daugulis [114].
Scheme 2: Cu(OAc)2-promoted mono- and ditrifluoromethylthiolation of benzamide derivatives derived from 8-ami...
In 2016, Wang's group developed another methodology for the trifluoromethylthiolation of azacalix[1]arene[3]pyridines by C–H bond activation using a complex of Cu(ClO4)2·6H2O and the shelf-stable Me4NSCF3 [115,116] as a nucleophilic source of SCF3 (Scheme 3) [100]. Within these conditions, a set of six azacalix[1]arene[3]pyridines bearing electron-donating groups, halogens or electron-withdrawing groups were functionalized and the expected products were isolated in moderate to high yields (4a–f, 58–91%).
Scheme 3: Trifluoromethylthiolation of azacalix[1]arene[3]pyridines using copper salts and a nucleophilic SCF3 source reported by Wang and co-workers [100]. aA mixture of CHCl3/MeOH 1:1 was used as solvent.
Scheme 3: Trifluoromethylthiolation of azacalix[1]arene[3]pyridines using copper salts and a nucleophilic SCF3...
Palladium catalysis: Several works have been reported for the palladium-catalyzed trifluoromethylthiolation reaction of various aromatic compounds 5 by C–H bond activation and involved in most cases an electrophilic SCF3 source (R1R2NSCF3). For these transformations, the following working hypothesis was generally suggested (Scheme 4). After coordination of the palladium catalyst to a directing group, the metallacycle A is formed. This latter undergoes an oxidative addition in the presence of an electrophilic source or an oxidation/ligand exchange in the presence of a nucleophilic source (i.e., AgSCF3) and an oxidant (B in Scheme 4). Finally, after a reductive elimination step, the expected functionalized product 6 is obtained and the palladium catalyst is regenerated.
Scheme 4: Working hypothesis for the palladium-catalyzed C–H trifluoromethylthiolation reaction.
Scheme 4: Working hypothesis for the palladium-catalyzed C–H trifluoromethylthiolation reaction.
In 2014, Shen and Xu [117] developed a new methodology for the selective functionalization of 2-arylpyridine derivatives using an electrophilic SCF3 reagent, the Haas reagent I (Scheme 5) [118]. A broad range of 2-arylpyridine derivatives were trifluoromethylthiolated in good to high yields (18 examples, from 52 to 91% yields). The substitution pattern of the aromatic ring had no impact on the outcome of the reaction as illustrated with substrates substituted by a methyl group (7b, 7d, and 7f) at the para-, meta- and ortho-positions, which were readily functionalized in 71%, 84% and 78% yields, respectively. This reaction was also tolerant of a 2-naphthyl group, the palladium-catalyzed trifluromethylthiolation afforded the corresponding product 8h in 76% yield. Also, the 2,4-dimethoxylated substrate 7g and the benzothiophene derivative 7i were successfully trifluoromethylthiolated in 76% and 63% yields, respectively. This reaction proved to be compatible with the presence of an ester (8c) or a halogen (8e). Other directing groups, such as substituted pyridines (9a and 9b) and pyrimidine (9c) turned out to be also efficient in this transformation (Scheme 5, 4 examples, up to 84% yield).
Scheme 5: Trifluoromethylthiolation of 2-arylpyridine derivatives and analogs by means of palladium-catalyzed C–H activation reported by the group of Shen [117]. a6 equiv of I were used at 150 °C for 24 h. bThe reaction was conducted at 150 °C for 24 h.
Scheme 5: Trifluoromethylthiolation of 2-arylpyridine derivatives and analogs by means of palladium-catalyzed...
The same year, the group of Huang reported an elegant and straightforward palladium(II)-catalyzed ortho-selective trifluoromethylthiolation of arenes bearing various directing groups using the nucleophilic trifluoromethylthiolating source AgSCF3 in combination with Selectfluor® as oxidant (Scheme 6, 29 examples, up to 91% yield) [119]. 2-Arylpyridine derivatives bearing electron-donating groups, electron-withdrawing groups or halogen at the para- and meta-positions of the aromatic ring were readily functionalized (11a–g, 58–85% yields). Also 2-(2-methoxyphenyl)pyridine (11h) and 2-(2-naphthyl)pyridine (11i) were found to be suitable substrates leading to the corresponding products 12h and 12i in 91% and 83% yields, respectively. The use of other directing groups was also suitable for this transformation such as methyl and cyano-substituted pyridines 13a,b, pyrimidine (13c), pyrazole (13d), as well as the amide derived from 8-aminoquinoline 13e (Scheme 6, 13 examples, up to 75% yield).
Scheme 6: C(sp2)–SCF3 bond formation by Pd-catalyzed C–H bond activation using AgSCF3 and Selectfluor® as reported by the group of Huang [119]. a20 equiv of Cl2CHCOOH were used instead of AcOH.
Scheme 6: C(sp2)–SCF3 bond formation by Pd-catalyzed C–H bond activation using AgSCF3 and Selectfluor® as rep...
In this study, two mechanisms were reported. The first one suggested that a palladacycle C is formed after the irreversible chelation of the 2-phenylpyridine substrate with palladium, which is the rate-determining step (KIE = 2.7). Subsequently palladacycle C is oxidized by Selectfluor® to form a palladium(IV) complex D. After a ligand exchange with AgSCF3, the intermediate E is obtained, which, after reductive elimination, releases the desired product 12 and regenerates the catalyst. Alternatively, a ligand exchange with AgSCF3 occurs before the oxidation step, generating the palladium(II) complex F. After an oxidative addition in the presence of Selectfluor®, the palladium(IV) intermediate E is generated. Finally, after reductive elimination step, the desired product 12 is released and the catalyst regenerated. Note that, in this process, Selectfluor® is playing a key role. Indeed, using this electrophilic fluorinating source as oxidant generates a Pd(IV)(ppy)F(OAc)2 (ppy = 2-phenylpyridine) complex as intermediate. As the competitive C–F bond formation was disfavored (slow reductive elimination step), the desired trifluoromethylthiolated product 12 is selectively afforded after a F/SCF3 ligand exchange.
In 2015, Ye and Liu reported the palladium-catalyzed trifluoromethylthiolation of 2-arylpyridine derivatives using the Billard reagent II (Scheme 7) [120]. Unlike Shen's methodology (Scheme 5), the use of benzoyl chloride was necessary to activate the trifluoromethylthiolated reagent [120]. Unsubstituted and pyridines substituted derivatives 15 were very efficiently ortho-trifluoromethylthiolated (17 examples, up to 91% yield). This methodology was tolerant to electron-donating and electron-withdrawing groups as well as halogens (16b–f, 62–91% yields). The meta-substituted (16f) and disubstituted (16j) products were obtained in high yields (73% and 79%, respectively).
Scheme 7: Palladium-catalyzed ortho-trifluoromethylthiolation of 2-arylpyridine derivatives reported by the group of Ye and Liu [120].
Scheme 7: Palladium-catalyzed ortho-trifluoromethylthiolation of 2-arylpyridine derivatives reported by the g...
In 2018, Anbarasan and co-workers described a palladium-catalyzed trifluoromethylthiolation of arenes by C–H bond activation bearing several directing groups (Scheme 8) [121]. With this methodology, the functionalization of 2-phenylpyridine derivatives was possible (10 examples, up to 81% yield) using the Billard reagents III or IV [122]. The trifluoromethylthiolation of 2-arylpyridines substituted by electron-donating groups such as methyl, methoxy or methylthio groups (17b–d) or by halogen (17e) was achieved (Scheme 8, up to 77% yield). Note that in case of disubstituted 2-(4-ethoxy-3-fluorophenyl)pyridine (17h), the expected product 18h was isolated in 31% yield. Moreover, selective oxidation of the SCF3 residue into the corresponding sulfoxide and the sulfone was possible and the corresponding products were obtained in 98% and 95% yields, respectively.
Scheme 8: Palladium-catalyzed ortho-trifluoromethylthiolation of 2-arylpyridine and analogs reported by Anbarasan [121]. aThe reaction was conducted at 120 °C using 1.1 equiv of Billard reagent IV (R = Me).
Scheme 8: Palladium-catalyzed ortho-trifluoromethylthiolation of 2-arylpyridine and analogs reported by Anbar...
This reaction was successfully expanded to the trifluoromethylthiolation of derivatives bearing various directing groups such as substituted pyridines (4 examples, up to 84% yield, 19a, 84% yield), substituted pyrazole derivatives (6 examples, up to 55% yield, 19b, 55% yield), pyrimidine (19c, 31% yield) as well as quinoline and isoquinoline (19d and 19e, 65% and 66% yields, respectively). In addition, the trifluoromethylthiolated benzo[h]quinoline 20f was obtained in good yield (63%).
The same year, Besset and co-workers reported a palladium-catalyzed C(sp2)–SCF3 bond formation on amides derived from 8-aminoquinoline as a cleavable directing group in the presence of the Munavalli reagent V (Scheme 9, 12 examples, up to 71% yield) [106]. Depending on the substitution pattern on the aromatic ring, the amides were mono- or difunctionalized. Indeed, meta- and ortho-substituted derivatives (21a–d) were selectively trifluoromethylthiolated while para-substituted substrates led to the difunctionalized products 22e and 22f. Within these reaction conditions, the polysubstituted derivative 21g was also functionalized in high yield (71%).
Scheme 9: Mono- and ditrifluoromethylthiolation of benzamide derivatives derived from 8-aminoquinoline using PdCl2 as catalyst reported by Besset and co-workers [106]. a2.2 equiv of the fluorinated source V were used.
Scheme 9: Mono- and ditrifluoromethylthiolation of benzamide derivatives derived from 8-aminoquinoline using ...
Pleasingly, other metals have been also successfully applied for the trifluoromethylthiolation of aromatic derivatives by C(sp2)–H bond activation such as Rh(III) and Co(III)-based catalysts as depicted below.
Rhodium catalysis: In 2015, the group of Li disclosed the Cp*Rh(III)-catalyzed regioselective trifluoromethylthiolation of N-substituted indoles with (substituted) pyridines or pyrimidine as the directing groups (Scheme 10) [123]. The selective trifluoromethylthiolation of indoles at the C2 position was achieved in the presence of N-(trifluoromethylthio)saccharine (VI, Shen’s reagent) as both oxidant and electrophilic source (18 examples, up to 91% yield). Indoles bearing various electron-donating and electron-withdrawing groups as well as halogens at the C5-position and at the C6-position were functionalized in high yields (24a–f, 82–87% yields). The substitution of the indole at the C3-position did not impact the reaction and the product 24g was obtained in 91% yield. Substituted pyridines and pyrimidine (24h and 24i) were also used as directing groups (7 examples, up to 86% yield). This methodology was extended to the functionalization of other heteroaromatic derivatives (24j, 87% yield). It should be noted that the presence of zinc triflate, a Lewis acid, was used for the activation of the electrophilic source VI.
Scheme 10: Regioselective Cp*Rh(III)-catalyzed directed trifluoromethylthiolation reported by the group of Li [123]. Cp* = pentamethylcyclopentadienyl.
Scheme 10: Regioselective Cp*Rh(III)-catalyzed directed trifluoromethylthiolation reported by the group of Li [123]...
Cobalt catalysis: In 2017, Wang described the Cp*Co(III)-catalyzed trifluoromethylthiolation of 2-phenylpyridine derivatives using AgSCF3 (Scheme 11) [124]. This methodology allowed the functionalization of several aromatic compounds bearing a pyridine or a pyrimidine as a directing group (20 examples, up to 65% yield). The reaction proceeded smoothly with substrates bearing an electron-donating group (25b,c), halogen (25d) or withdrawing group (25e) and the desired SCF3-containing products were obtained in moderate to good yields. The functionalization of trisubstituted arene 25g and heteroarene 25h was also possible leading to the corresponding products 26g and 26h in moderate yields (23% and 42% yields, respectively). Regarding the reaction mechanism, the active Co(III) complex G was obtained from the dimeric catalyst [Cp*CoI2]2 in the presence AgSCF3 and/or NaOPiv·H2O. Then, the reversible formation of the metallacycle H occurs, which after a ligand exchange in the presence of AgSCF3 leads to the formation of adduct J. The product 26 is released via a reductive elimination step, generating at the same time the reduced cobalt Cp*Co(I), which is converted to the active catalyst after oxidation.
Scheme 11: Cp*Co(III)-catalyzed ortho-trifluoromethylthiolation of 2-phenylpyridine and 2-phenylpyrimidine derivatives reported by Wang and co-workers [124]. aCH2Cl2 was used as solvent.
Scheme 11: Cp*Co(III)-catalyzed ortho-trifluoromethylthiolation of 2-phenylpyridine and 2-phenylpyrimidine der...
The same year, Yoshino and Matsunaga described a similar methodology, using the cobalt(III) complex [Cp*Co(CH3CN)3](SbF6)2 and N-trifluoromethylthiodibenzenesulfonimide VII as electrophilic SCF3 source (Scheme 12) [125]. Under these reaction conditions, 2-arylpyridines (9 examples, up to 94% yield) or 6-arylpurines (10 examples, up to 72% yield) were ortho-trifluoromethylthiolated. The substitution pattern of the aryl part did not impact the outcome of the reaction. This methodology was also tolerant to a large range of functional groups (ester, halogen) as illustrated by the products 28c, 28j, 28d, 28k, and 28g.
Scheme 12: Cp*Co(III)-catalyzed ortho-trifluoromethylthiolation of 2-phenylpyridine and 6-phenylpurine derivatives described by Yoshino and Matsunaga [125]. aWithout AgSbF6. b2.0 equiv of VII, 10 mol % of AgOAc and 30 mol % of Gd(OTf)3 were used instead of AgSbF6.
Scheme 12: Cp*Co(III)-catalyzed ortho-trifluoromethylthiolation of 2-phenylpyridine and 6-phenylpurine derivat...
I.2) Transition-metal-catalyzed C–H trifluoromethylthiolation of vinylic C(sp2) centers
Several research groups have been interested in the development of strategies for the formation of vinylic C(sp2)–SCF3 bonds, offering an efficient tool towards the synthesis of challenging Z-isomers.
Palladium catalysis: In 2018, Bouillon and Besset described the first example of a selective palladium-catalyzed trifluoromethylthiolation of α-arylacrylamides derived from 8-aminoquinolines 29 by C–H bond activation (Scheme 13) [104]. Using the Munavalli reagent V as an electrophilic SCF3 source, this diastereoselective method selectively led to the formation of Z-isomers and turned out to be robust (not air or moisture sensitive). Under these mild reaction conditions, a panel of α-(hetero)arylacrylamides were trifluoromethylthiolated in good to high yields. Acrylamides substituted at the α-position by an aryl bearing an electron-donating group (OMe) or halogen (Cl) at the para-position were readily functionalized (30b and 30c, 70% and 75% yields, respectively). The substitution of the arene with a CF3 residue at the meta-position or with a methoxy group at the ortho-position did not have any impact to the outcome of the reaction (30e and 30f, 62% and 78% yields, respectively). It should be noted that acrylamides bearing a disubstituted arene (29g,h) and an heteroaryl (29i) at the α-position were also suitable substrates for this reaction. Finally, acrylamides bearing a methyl group at the α-position (29j) or the α,β-dimethylated acrylamide (29k) were suitable substrates albeit the corresponding products were obtained in 30% and 20% yields, respectively. Several mechanistic experiments revealed that the C(sp2)–H bond activation step was reversible and represented the rate-determining step (KIE = 2.4). First, the chelation of the palladium(II) catalyst with the bidentate directing group, followed by the C(sp2)–H bond activation involving a concerted metalation–deprotonation pathway affords the metallacycle K. After an oxidative addition in the N-SCF3 bond of the Munavalli reagent V, the palladium(IV) species L is obtained. Finally, the reductive elimination affords the product and regenerates the catalyst. The same year, Besset and co-workers extended this methodology to a larger class of acrylamides [106].
Scheme 13: Diastereoselective trifluoromethylthiolation of acrylamide derivatives derived from 8-aminoquinoline using PdCl2 reported by Bouillon and Besset [104,106]. a20 mol % of PdCl2 and 2.0 equiv of SCF3 source V were used for 36 h. bThe reaction was conducted with 30 mol % of PdCl2 and 2.0 equiv of reagent V were used.
Scheme 13: Diastereoselective trifluoromethylthiolation of acrylamide derivatives derived from 8-aminoquinolin...
I.3) Transition-metal-catalyzed trifluoromethylthiolation of aliphatic C(sp3)–H bonds
Despite the important progresses presented in the previous section, some limitations of the trifluoromethylthiolation persist. In particular, the functionalization of a C(sp3)–H bond with a trifluoromethylthiolated moiety by transition-metal-catalyzed C–H activation remains a challenging task both in terms of reactivity and selectivity.
In 2015, Besset reported the first C(sp3)–SCF3 bond formation of unactivated primary C(sp3) centers by transition-metal-catalyzed C–H activation with the Munavalli or the Billard reagents as the trifluoromethylthiolation source (Scheme 14) [126]. Using a bidentate directing group, this methodology allowed the functionalization of a large range of aliphatic amides with a primary β-C(sp3)–H bond (21 examples, up to 53% yield). The methodology was applied to the functionalization of a series of amides having an α-quaternary center (α,α-dialkyl (31a), α-alkyl,α-benzyl derivatives 31c–f) as well as to an amide with an α-tertiary center (31b) and pleasingly, the presence of α-C–H bonds did not have a significant impact on the outcome of the reaction. It should be noted that this methodology afforded the products with a high regioselectivity, and no incorporation of the SCF3 moiety on the benzylic or at the C5 position of the quinoline part of the directing group was observed. Note that in 2018, Besset and Lebel developed a more efficient process for the palladium-catalyzed trifluoromethylthiolation by C–H bond activation under continuous flow conditions [127].
Scheme 14: C(sp3)–SCF3 bond formation on aliphatic amide derivatives derived from 8-aminoquinoline by palladium-catalyzed C–H bond activation described by Besset and co-workers [126]. Product 32d was contaminated with 10% of an inseparable impurity.
Scheme 14: C(sp3)–SCF3 bond formation on aliphatic amide derivatives derived from 8-aminoquinoline by palladiu...
I.4) Difluoromethylthiolation of aromatic and vinylic C(sp2)–H bonds (C–SCF2H and C–SCF2CO2Et bonds)
More recently, researchers became interested in the synthesis of molecules substituted with original and functionalized fluorinated moieties such as SCF2FG [128-136] (FG = functional group). In sharp contrast with the trifluoromethylthiolation reaction, only a handful of reports dealt with the incorporation of such high value-added fluorinated moieties onto C(sp2) centers by transition-metal-catalyzed C–H bond activation.
In 2022, He and Pan reported the first example of a difluoromethylthiolation achieved by transition-metal-catalyzed C–H bond activation (Scheme 15) [137]. Using the reagent IX and a catalytic amount of PdCl2, they succeeded in the functionalization of acrylamides 33 derived from 8-aminoquinoline. Within these mild conditions, α-arylacrylamides substituted at para-, meta-, and ortho-positions were readily difluoromethylthiolated (34a–g, 81–95% yields). This reaction was also tolerant to a large class of functional groups (halogens, cyano, trifluoromethyl), affording products 34c,d and 34e in high yields (86%, 89% and 81%, respectively). The α-methyl-substituted acrylamide 33j also underwent difluoromethylthiolation to give product 34j in 81% isolated yield. The α,β- and β-substituted acrylamides were functionalized in high yields (34h,i and 34k–m, 71–86%). The plausible mechanism is similar as the one reported by Besset for the trifluoromethylthiolation of acrylamides derived from 8-aminoquinoline (Scheme 13).
Scheme 15: Regio- and diastereoselective difluoromethylthiolation of acrylamides under palladium catalysis reported by He and Pan [137].
Scheme 15: Regio- and diastereoselective difluoromethylthiolation of acrylamides under palladium catalysis rep...
The same year, Besset and co-workers reported the first palladium-catalyzed C(sp2)–SCF2CO2Et bond formation by C–H bond activation (Scheme 16) [138]. In the presence of the electrophilic SCF2CO2Et source X, the methodology was successfully applied for the functionalization of 2-arylpyridine derivatives 35a–i as well as 2-vinylpyridine derivatives 35j–m (35 examples, up to 87% yield). The substitution pattern of the aryl substituent of the 2-phenylpyridine derivatives did not influence the reaction as for instance products 36b, 36h, and 36i were obtained in good yields (70%, 63% and 61% yield, respectively). This reaction was tolerant to a broad range of functional groups such as halogens, ester, aldehyde, cyano, and nitro (36c–g, 36–74% yield). It is noteworthy that a disubstituted compound 35j and a thiophene derivative 35k were also efficiently difluoromethylthiolated (36j and 36k, 72% and 65%, respectively). α-Substituted vinylpyridines with electron-donating or electron-withdrawing groups on the aromatic ring were functionalized and 36l and 36m were easily isolated in 73% and 81% yields, respectively. Even an α,β-disubstituted vinylpyridine 35n and the benzoquinoline 35o were smoothly functionalized showing the efficiency of the approach. Of high interest, the modularity of the SCF2CO2Et was highlighted by its conversion into various other fluorinated residues (amide, carboxylic acid) and its selective oxidation into the corresponding sulfoxide and sulfone.
Scheme 16: Palladium-catalyzed (ethoxycarbonyl)difluoromethylthiolation reaction of 2-(hetero)aryl and 2-(α-aryl-vinyl)pyridine derivatives reported by Besset [138].
Scheme 16: Palladium-catalyzed (ethoxycarbonyl)difluoromethylthiolation reaction of 2-(hetero)aryl and 2-(α-ar...
I.5) Trifluoromethylselenolation of aromatic and vinylic C(sp2)–H bonds by palladium catalysis
Very recently, the palladium-catalyzed trifluoromethylselenolation of (hetero)aromatic and olefinic derivatives has been investigated by the group of Billard using similar catalytic systems as those depicted for the trifluoromethylthiolation reactions. Indeed, using amides 37 derived from 5-methoxy-8-aminoquinoline, the functionalization of (hetero)aromatic compounds was achieved using 20 mol % of Pd(CH3CN)4Cl2 in the presence of TolSO2SeCF3 as the fluorinating source (14 examples, Scheme 17) [139]. Of note, when the reaction was carried out on derivatives bearing substituents at the meta- (37c and 37d) as well as at the para- (37a and 37b) positions, the corresponding products 38a–d were obtained as a mixture of mono- and disubstituted trifluoromethylselenolated derivatives with global yields ranging from 48% to 70%. When one of the ortho-positions of the aromatic ring was substituted with a Me (37e), a OMe (37f), a Cl (37g) or a CF3 (37h) group, the corresponding compounds 38e, 38f, 38g, and 38h were isolated in moderate to high yields (43% to 80%). The methodology also allowed the trifluoromethylselenolation of the furan derivative 37i, which led to the desired product 38i in 30% yield. A careful monitoring of the reaction unveiled the rapid formation of the CF3SeSeCF3 dimer, which could be the active trifluoromethylselenolating reagent in this transformation.
Scheme 17: Pd(II)-catalyzed trifluoromethylselenolation of benzamides derived from 5-methoxy-8-aminoquinoline reported by the group of Billard [139]. aThe yields given are the sum of the yields of mono- and ditrifluoromethylselenolated products.
Scheme 17: Pd(II)-catalyzed trifluoromethylselenolation of benzamides derived from 5-methoxy-8-aminoquinoline ...
In 2022, Magnier, Billard and co-workers applied the previous methodology to the trifluoromethylselenolation of acrylamide derivatives [140]. Using the same directing group, a panel of α-arylacrylamide derivatives 39a–f was successfully functionalized with a high Z-selectivity (yields up to 98%, Scheme 18). Both, thermal reaction conditions (DMSO at 70 °C for 16 h) and microwave irradiation (100 °C using microwaves in only 1 h) turned out to be efficient in the process. α-Methyl- and α,β-dimethylacrylamides 39g and 39h were also functionalized. Furthermore, a series of β-substituted acrylamides 39i–m with various substituents readily underwent the trifluoromethylselenolation reaction with high selectivity in moderate yields. Finally, the SeCF3-containing β-methylacrylamide 40n was also obtained in 37% yield.
Scheme 18: Pd(II)-catalyzed trifluoromethylselenolation of acrylamide derivatives derived from 5-methoxy-8-aminoquinoline reported by the groups of Magnier and Billard [140]. aMicrowave, 100 °C, 1 h. b70 °C for 16 h. c20 mol % of Pd(CH3CN)2(Cl)2, 100 °C for 24 h.
Scheme 18: Pd(II)-catalyzed trifluoromethylselenolation of acrylamide derivatives derived from 5-methoxy-8-ami...
II. Transition-metal-catalyzed fluoroalkoxylation of (hetero)arenes by C–H bond activation
II.1) Fluoroalkoxylation of aromatic C(sp2)–H bonds by transition-metal catalysis
Over the last years, key advances have been made for the formation of a C(sp2)–OCHRCF3 bond by transition-metal-catalyzed C–H bond activation. Indeed, fluorinated ethers [71,141-153] are key compounds, with especially molecules substituted with the 2,2,2-trifluoroethoxy moiety (OCH2CF3), an important fluorinated group found in several bioactive compounds such as flecanide [154,155] and lansoprazole [156], as flagship molecules. Although the transition-metal-catalyzed hydroxylation and alkoxylation have been studied especially under palladium catalysis [157,158], the direct dehydrogenative 2,2,2-trifluoroethoxylation of (hetero)arenes, often using 2,2,2-trifluoroethanol as a readily available, inexpensive, and green fluorination source [159,160], is still underexplored.
In 2004, Sanford and co-workers reported the dehydrogenative 2,2,2-trifluoroethoxylation of benzo[h]quinoline under palladium catalysis in the presence of PhI(OAc)2 as oxidant (Scheme 19) [161]. Since this seminal work and in the course of their investigation towards the development of new methods for the alkoxylation of C(sp2) centers by transition-metal catalysis, few examples of transition-metal-catalyzed dehydrogenative 2,2,2-trifluoroethoxylation reactions have been reported. In 2021, the palladium-catalyzed ortho-2,2,2-trifluoroethoxylation of 3-arylcoumarins was depicted by the group of Kumar (6 examples, up to 69% yield) [162]. Further developments unveiled the use of copper catalysts for such functionalization. In 2013, the group of Daugulis described the copper-catalyzed ortho-2,2,2-trifluoroethoxylation of a 3-trifluoromethylated benzamide derived from 8-aminoquinoline, giving the corresponding product in 73% yield [149]. The group of Baidya showed that the dehydrogenative 2,2,2-trifluoroethoxylation of benzamide with another bidentate directing group was also possible in the presence of Cu(OAc)2 and hexamethyldisilane [163]. Using N,N- and N,O-bidentate directing groups, the construction of C(sp2)–OCH2CF3 bonds by C–H bond activation was also reported using Ni [164] and Co catalysis [165-167]. In 2022, Volla and co-workers reported the ortho-2,2,2-trifluoroethoxylation of benzamide using an N,O-bidentate directing group by merging Co- and visible light organophotocatalysis [168].
Scheme 19: Transition-metal-catalyzed dehydrogenative 2,2,2-trifluoroethoxylation of (hetero)aromatic derivatives by C–H bond activation [149]. aCF3CH2OH was used as solvent. bPd cat. = [Pd(μ-κ1-OAc)(κ2-N,C10-benzo[h]quinoline)]2. c5 equiv of CF3CH2OH. d15 equiv of CF3CH2OH. e50 equiv of CF3CH2OH. fCF3CH2OH or (CF3)2CHOH was used as solvent with o-chlorotoluene with a ratio of 1:1. AdCOOH = adamantanecarboxylic acid, DMAP = 4-(dimethylamino)pyridine, ORF = OCH2CF3.
Scheme 19: Transition-metal-catalyzed dehydrogenative 2,2,2-trifluoroethoxylation of (hetero)aromatic derivati...
Palladium catalysis: In 2017, Ji, Li and co-workers reported a thorough study on the Pd-catalyzed 2,2,2-trifluoroethoxylation of N-sulfonylbenzamides in the presence of PhI(OAc)2 as oxidant and an excess of TFA (Scheme 20) [150]. The functionalization of diversely substituted derivatives bearing either electron-donating groups (41b, 41d, and 41f) and electron-withdrawing groups (41c, 41e, and 41g) was achieved (19 examples, up to 93% yield). Of note, the transformation was also efficient with disubstituted substrates such as 41h–j. The authors suggested the following mechanism. After formation of the metallacycle O, the latter is oxidized leading to the Pd(IV) species P. After a ligand exchange, the intermediate Q is generated. Finally, a reductive elimination step affords the expected functionalized product 42 and regenerates the catalyst.
Scheme 20: Pd(II)-catalyzed ortho-2,2,2-trifluoroethoxylation of N-sulfonylbenzamides reported by the group of Ji and Li [150]. a50 °C. b15 equiv TFA. c5 equiv TFA. dN-acetylglycine (60 mol %). Ns: 4-nitrobenzenesulfonyl.
Scheme 20: Pd(II)-catalyzed ortho-2,2,2-trifluoroethoxylation of N-sulfonylbenzamides reported by the group of...
The 2,2,2-trifluoroethoxylation reaction is not restricted to amides. In 2020, Yorimitsu and co-workers developed a methodology allowing the formation of a C(sp2)–ORF bond through palladium-catalyzed C–H bond activation [169]. Using Pd(OPiv)2 as the catalyst in the presence of PhI(OAc)2 (PIDA), the naphthalene sulfoxide 43a was 2,2,2-trifluoroethoxylated in 82% yield (Scheme 21). The substituent of the sulfoxide part does not impact the efficiency of the reaction as illustrated by the synthesis of compounds 44b and 44c. The presence of an electron-donating substituent in the para-position of the directing group was found to be deleterious for the reaction since 44d was obtained in 31% yield while its brominated analog 44e was isolated in 70% yield. Mechanistic studies indicated that the C–H bond activation event was the rate-limiting step and the authors suggested a similar mechanism to the one depicted in Scheme 20: formation of a palladacycle thanks to a concerted metalation deprotonation (CMD) process followed by oxidation, ligand exchange with CF3CH2OH, and finally, reductive elimination affording the expected product and regenerating the catalyst. Gratifyingly, the approach was applied to the incorporation of other fluorinated moieties such as OCH2CF2H and OCH2(CF2)nH (n = 2 or 4) and gave compounds 46a–c.
Scheme 21: Pd(II)-catalyzed selective 2,2,2-trifluoroethoxylation and other fluoroalkoxylations of naphthalene sulfoxide derivatives reported by the group of Yorimitsu [169]. a80 °C for 1 h. bRFOH/AcOH 1:7.
Scheme 21: Pd(II)-catalyzed selective 2,2,2-trifluoroethoxylation and other fluoroalkoxylations of naphthalene...
Thanks to the transient directing group strategy [35,170-182], efficient methodologies for the functionalization of previously reluctant compounds such as benzaldehyde derivatives were developed, and key advances are depicted below.
In 2021, Wang and co-workers described the selective palladium-catalyzed ortho-2,2,2-trifluoroethoxylation of a series of benzaldehydes (Scheme 22, 35 examples) using the amino acid ʟ-valine in the presence of K2S2O8 and TFA at 80 °C [153]. This reaction proved to be highly tolerant to various substituents including a CF3 group at the ortho-, meta- and para-positions (48m, 48j, and 48f, respectively), halogens (48d, 48h, and 48l), an ester moiety (48e and 48i), and a methoxy group (48c). Note that even di- and trisubstituted benzaldehydes 47n–q were smoothly functionalized under these conditions. The authors also suggested a plausible mechanism. The amino acid acts as an organocatalyst and first reacts with the benzaldehyde 47 to generate the transient directing group (47’). Then, formation of the palladacycle (species R) followed by its oxidation to a Pd(IV) intermediate and a ligand exchange with 2,2,2-trifluoroethanol leads to the formation of the species S. The latter complex S undergoes a reductive elimination leading to the compound 48’ along with the regeneration of the palladium catalyst. Finally, after hydrolysis of 48’, the expected product 48 is afforded together with the organocatalyst.
Scheme 22: Pd(II)-catalyzed selective ortho-2,2,2-trifluoroethoxylation of benzaldehyde derivatives by means of the transient directing group strategy as reported by the group of Wang [153].
Scheme 22: Pd(II)-catalyzed selective ortho-2,2,2-trifluoroethoxylation of benzaldehyde derivatives by means o...
Then, the group of Sun and Wang used a similar approach for the 2,2,2-trifluoroethoxylation of benzaldehydes under palladium catalysis using the amino acid 51 as organic catalyst in the presence of the fluoropyridinium salt 52 (19 examples, up to 88% yield, Scheme 23) [183]. Pleasingly, the methodology was extended to the formation of C(sp2)–ORF bonds starting from benzaldehyde (ORF = 2,2-difluoroethoxy 50g, 2,2-difluoropropoxy 50h, and 1,1,1,3,3,3-hexafluoroisopropoxy 50i).
Scheme 23: Pd(II)-catalyzed selective ortho-2,2,2-trifluoroethoxylation (and other fluoroalkoxylations) of benzaldehyde derivatives via the assistance of a transient directing group reported by the group of Sun and Wang [183].
Scheme 23: Pd(II)-catalyzed selective ortho-2,2,2-trifluoroethoxylation (and other fluoroalkoxylations) of ben...
II.2) Fluoroalkoxylation of aliphatic C(sp3)–H bonds by transition-metal-catalysis
The functionalization of C(sp3) centers by transition-metal-catalyzed C–H bond activation remains highly challenging [24,184-191]. In particular, the 2,2,2-trifluoroethoxylation of aliphatic derivatives is still limited to a handful of examples as illustrated by the two examples depicted in Scheme 24 [71,192]. Using a bidentate directing group (namely NHPA and CONHPIP for 53 and 55, respectively), the groups of Chen [71] and Shi [192] independently reported the palladium-catalyzed selective 2,2,2-trifluoroethoxylation of aliphatic amines and amides at the γ and β positions, respectively, using trifluoroethanol as fluorination source in the presence of PIDA. Hence, an efficient access to the corresponding monoether derivatives 54 and 56 in 71% and 65%, respectively, were obtained.
Scheme 24: Pd(II)-catalyzed selective 2,2,2-trifluoroethoxylation of aliphatic amides using a bidentate directing group reported by the groups of Chen [71] and Shi [192].
Scheme 24: Pd(II)-catalyzed selective 2,2,2-trifluoroethoxylation of aliphatic amides using a bidentate direct...
Conclusion
In summary, this review provides an overview of the major developments made over the last years for the synthesis of fluorinated compounds by transition-metal-catalyzed C–H bond activation. This review focused on the construction of C(sp2)–XRF bonds and C(sp3)–XRF bonds with an emphasis on the trifluoromethylation reaction and transformations using emergent fluorinated residues (SCF3, SeCF3, SCF2H, SCF2CO2Et or OCH2CF3 groups). Well-designed catalytic systems and suitable fluorinating sources were the key of success for these major developments.
Despite these advances, synthetic challenges still need to be overcome. These synthetic tools are so far still restricted to some fluorinated moieties and extension to other high value-added fluorinated residues [131,136,193-208] is of high importance. Besides, in comparison with the functionalization of C(sp2) centers on aromatic and vinylic derivatives, transition-metal-catalyzed functionalization of C(sp3)–H bonds remains largely underexplored to date. Furthermore, the development of enantioselective transformations allowing the synthesis of enantioenriched fluorine-containing compounds by transition-metal-catalyzed C–H bond activation will have a significant impact as for instance an access to pharmaceutically relevant derivatives. Finally, the use of abundant non-noble transition metals [209-211] in such reactions combined or not with modern technologies (photocatalysis and electrocatalysis) is still underexplored and any advances will be of high importance especially from a sustainability point of view aiming at developing greener synthetic routes towards fluorinated molecules.
Funding
This work has been partially supported by University of Rouen Normandy, INSA Rouen Normandy, the Centre National de la Recherche Scientifique (CNRS), European Regional Development Fund (ERDF), Labex SynOrg (ANR-11-LABX-0029), Carnot Institute I2C, the graduate school for research XL-Chem (ANR-18-EURE-0020 XL CHEM), and Region Normandie. L.M. and T.B. thank the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 758710). F.D. thanks Labex SynOrg (ANR-11-LABX-0029) and the Region Normandy (RIN 50% program) for a doctoral fellowship.
References
-
Smart, B. E. J. Fluorine Chem. 2001, 109, 3–11. doi:10.1016/s0022-1139(01)00375-x
Return to citation in text: [1] -
O'Hagan, D. Chem. Soc. Rev. 2008, 37, 308–319. doi:10.1039/b711844a
Return to citation in text: [1] [2] -
Inoue, M.; Sumii, Y.; Shibata, N. ACS Omega 2020, 5, 10633–10640. doi:10.1021/acsomega.0c00830
Return to citation in text: [1] -
Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432–2506. doi:10.1021/cr4002879
Return to citation in text: [1] -
Landelle, G.; Panossian, A.; Pazenok, S.; Vors, J.-P.; Leroux, F. R. Beilstein J. Org. Chem. 2013, 9, 2476–2536. doi:10.3762/bjoc.9.287
Return to citation in text: [1] [2] -
Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320–330. doi:10.1039/b610213c
Return to citation in text: [1] -
Berger, R.; Resnati, G.; Metrangolo, P.; Weber, E.; Hulliger, J. Chem. Soc. Rev. 2011, 40, 3496–3508. doi:10.1039/c0cs00221f
Return to citation in text: [1] -
Fujiwara, T.; O’Hagan, D. J. Fluorine Chem. 2014, 167, 16–29. doi:10.1016/j.jfluchem.2014.06.014
Return to citation in text: [1] -
Ilardi, E. A.; Vitaku, E.; Njardarson, J. T. J. Med. Chem. 2014, 57, 2832–2842. doi:10.1021/jm401375q
Return to citation in text: [1] -
Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315–8359. doi:10.1021/acs.jmedchem.5b00258
Return to citation in text: [1] -
Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J. L.; Soloshonok, V. A.; Izawa, K.; Liu, H. Chem. Rev. 2016, 116, 422–518. doi:10.1021/acs.chemrev.5b00392
Return to citation in text: [1] -
Landelle, G.; Panossian, A.; Leroux, F. R. Curr. Top. Med. Chem. 2014, 14, 941–951. doi:10.2174/1568026614666140202210016
Return to citation in text: [1] -
Besset, T.; Poisson, T.; Pannecoucke, X. Chem. – Eur. J. 2014, 20, 16830–16845. doi:10.1002/chem.201404537
Return to citation in text: [1] -
Merino, E.; Nevado, C. Chem. Soc. Rev. 2014, 43, 6598–6608. doi:10.1039/c4cs00025k
Return to citation in text: [1] -
Champagne, P. A.; Desroches, J.; Hamel, J.-D.; Vandamme, M.; Paquin, J.-F. Chem. Rev. 2015, 115, 9073–9174. doi:10.1021/cr500706a
Return to citation in text: [1] -
Ni, C.; Hu, J. Chem. Soc. Rev. 2016, 45, 5441–5454. doi:10.1039/c6cs00351f
Return to citation in text: [1] -
Pan, Y. ACS Med. Chem. Lett. 2019, 10, 1016–1019. doi:10.1021/acsmedchemlett.9b00235
Return to citation in text: [1] -
Nobile, E.; Castanheiro, T.; Besset, T. Angew. Chem., Int. Ed. 2021, 60, 12170–12191. doi:10.1002/anie.202009995
Return to citation in text: [1] -
Chen, X.; Engle, K. M.; Wang, D.-H.; Yu, J.-Q. Angew. Chem., Int. Ed. 2009, 48, 5094–5115. doi:10.1002/anie.200806273
Return to citation in text: [1] -
Giri, R.; Shi, B.-F.; Engle, K. M.; Maugel, N.; Yu, J.-Q. Chem. Soc. Rev. 2009, 38, 3242–3272. doi:10.1039/b816707a
Return to citation in text: [1] -
Jazzar, R.; Hitce, J.; Renaudat, A.; Sofack-Kreutzer, J.; Baudoin, O. Chem. – Eur. J. 2010, 16, 2654–2672. doi:10.1002/chem.200902374
Return to citation in text: [1] -
Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 110, 1147–1169. doi:10.1021/cr900184e
Return to citation in text: [1] -
Wencel-Delord, J.; Dröge, T.; Liu, F.; Glorius, F. Chem. Soc. Rev. 2011, 40, 4740–4761. doi:10.1039/c1cs15083a
Return to citation in text: [1] -
Li, H.; Li, B.-J.; Shi, Z.-J. Catal. Sci. Technol. 2011, 1, 191–206. doi:10.1039/c0cy00076k
Return to citation in text: [1] [2] -
Ackermann, L. Chem. Rev. 2011, 111, 1315–1345. doi:10.1021/cr100412j
Return to citation in text: [1] -
Kozhushkov, S. I.; Potukuchi, H. K.; Ackermann, L. Catal. Sci. Technol. 2013, 3, 562–571. doi:10.1039/c2cy20505j
Return to citation in text: [1] -
Wang, K.; Hu, F.; Zhang, Y.; Wang, J. Sci. China: Chem. 2015, 58, 1252–1265. doi:10.1007/s11426-015-5362-5
Return to citation in text: [1] -
Crabtree, R. H.; Lei, A. Chem. Rev. 2017, 117, 8481–8482. doi:10.1021/acs.chemrev.7b00307
Return to citation in text: [1] -
Pototschnig, G.; Maulide, N.; Schnürch, M. Chem. – Eur. J. 2017, 23, 9206–9232. doi:10.1002/chem.201605657
Return to citation in text: [1] -
Dong, Z.; Ren, Z.; Thompson, S. J.; Xu, Y.; Dong, G. Chem. Rev. 2017, 117, 9333–9403. doi:10.1021/acs.chemrev.6b00574
Return to citation in text: [1] -
Sambiagio, C.; Schönbauer, D.; Blieck, R.; Dao-Huy, T.; Pototschnig, G.; Schaaf, P.; Wiesinger, T.; Zia, M. F.; Wencel-Delord, J.; Besset, T.; Maes, B. U. W.; Schnürch, M. Chem. Soc. Rev. 2018, 47, 6603–6743. doi:10.1039/c8cs00201k
Return to citation in text: [1] -
Rej, S.; Ano, Y.; Chatani, N. Chem. Rev. 2020, 120, 1788–1887. doi:10.1021/acs.chemrev.9b00495
Return to citation in text: [1] -
Chen, S.; Ranjan, P.; Voskressensky, L. G.; Van der Eycken, E. V.; Sharma, U. K. Molecules 2020, 25, 4970. doi:10.3390/molecules25214970
Return to citation in text: [1] -
Lam, N. Y. S.; Wu, K.; Yu, J.-Q. Angew. Chem., Int. Ed. 2021, 60, 15767–15790. doi:10.1002/anie.202011901
Return to citation in text: [1] -
Jacob, C.; Maes, B. U. W.; Evano, G. Chem. – Eur. J. 2021, 27, 13899–13952. doi:10.1002/chem.202101598
Return to citation in text: [1] [2] -
Dhawa, U.; Kaplaneris, N.; Ackermann, L. Org. Chem. Front. 2021, 8, 4886–4913. doi:10.1039/d1qo00727k
Return to citation in text: [1] -
Dalton, T.; Faber, T.; Glorius, F. ACS Cent. Sci. 2021, 7, 245–261. doi:10.1021/acscentsci.0c01413
Return to citation in text: [1] -
Dutta, U.; Maiti, S.; Bhattacharya, T.; Maiti, D. Science 2021, 372, 658–663. doi:10.1126/science.abd5992
Return to citation in text: [1] -
Zhang, J.; Lu, X.; Shen, C.; Xu, L.; Ding, L.; Zhong, G. Chem. Soc. Rev. 2021, 50, 3263–3314. doi:10.1039/d0cs00447b
Return to citation in text: [1] -
Rej, S.; Das, A.; Chatani, N. Coord. Chem. Rev. 2021, 431, 213683. doi:10.1016/j.ccr.2020.213683
Return to citation in text: [1] -
Ahmad, M. S.; Meguellati, K. ChemistrySelect 2022, 7, e202103716. doi:10.1002/slct.202103716
Return to citation in text: [1] -
Phukon, J.; Jyoti Borah, A.; Gogoi, S. Asian J. Org. Chem. 2022, 11, e202200581. doi:10.1002/ajoc.202200581
Return to citation in text: [1] [2] -
Mondal, A.; van Gemmeren, M. Angew. Chem., Int. Ed. 2022, 61, e202210825. doi:10.1002/anie.202210825
Return to citation in text: [1] -
Petrone, D. A.; Ye, J.; Lautens, M. Chem. Rev. 2016, 116, 8003–8104. doi:10.1021/acs.chemrev.6b00089
Return to citation in text: [1] -
Li, X.; Shi, X.; Li, X.; Shi, D. Beilstein J. Org. Chem. 2019, 15, 2213–2270. doi:10.3762/bjoc.15.218
Return to citation in text: [1] -
Mazeh, S.; Lapuh, M. I.; Besset, T. Chimia 2020, 74, 871. doi:10.2533/chimia.2020.871
Return to citation in text: [1] -
Egami, H. Chem. Pharm. Bull. 2020, 68, 491–511. doi:10.1248/cpb.c19-00856
Return to citation in text: [1] -
Ruyet, L.; Besset, T. Beilstein J. Org. Chem. 2020, 16, 1051–1065. doi:10.3762/bjoc.16.92
Return to citation in text: [1] -
Sindhe, H.; Chaudhary, B.; Chowdhury, N.; Kamble, A.; Kumar, V.; Lad, A.; Sharma, S. Org. Chem. Front. 2022, 9, 1742–1775. doi:10.1039/d1qo01544c
Return to citation in text: [1] [2] -
Liu, H.; Gu, Z.; Jiang, X. Adv. Synth. Catal. 2013, 355, 617–626. doi:10.1002/adsc.201200764
Return to citation in text: [1] -
Cernak, T.; Dykstra, K. D.; Tyagarajan, S.; Vachal, P.; Krska, S. W. Chem. Soc. Rev. 2016, 45, 546–576. doi:10.1039/c5cs00628g
Return to citation in text: [1] -
Moir, M.; Danon, J. J.; Reekie, T. A.; Kassiou, M. Expert Opin. Drug Discovery 2019, 14, 1137–1149. doi:10.1080/17460441.2019.1653850
Return to citation in text: [1] -
Börgel, J.; Ritter, T. Chem 2020, 6, 1877–1887. doi:10.1016/j.chempr.2020.07.007
Return to citation in text: [1] -
Chen, D. Y.-K.; Youn, S. W. Chem. – Eur. J. 2012, 18, 9452–9474. doi:10.1002/chem.201201329
Return to citation in text: [1] -
Yamaguchi, J.; Yamaguchi, A. D.; Itami, K. Angew. Chem., Int. Ed. 2012, 51, 8960–9009. doi:10.1002/anie.201201666
Return to citation in text: [1] -
Qiu, Y.; Gao, S. Nat. Prod. Rep. 2016, 33, 562–581. doi:10.1039/c5np00122f
Return to citation in text: [1] -
Basu, D.; Kumar, S.; V, S. S.; Bandichhor, R. J. Chem. Sci. 2018, 130, 71. doi:10.1007/s12039-018-1468-6
Return to citation in text: [1] -
Karimov, R. R.; Hartwig, J. F. Angew. Chem., Int. Ed. 2018, 57, 4234–4241. doi:10.1002/anie.201710330
Return to citation in text: [1] -
Baudoin, O. Angew. Chem., Int. Ed. 2020, 59, 17798–17809. doi:10.1002/anie.202001224
Return to citation in text: [1] -
Liang, T.; Neumann, C. N.; Ritter, T. Angew. Chem., Int. Ed. 2013, 52, 8214–8264. doi:10.1002/anie.201206566
Return to citation in text: [1] -
Lin, A.; Huehls, C. B.; Yang, J. Org. Chem. Front. 2014, 1, 434–438. doi:10.1039/c4qo00020j
Return to citation in text: [1] -
Li, Y.; Wu, Y.; Li, G.-S.; Wang, X.-S. Adv. Synth. Catal. 2014, 356, 1412–1418. doi:10.1002/adsc.201400101
Return to citation in text: [1] -
Campbell, M. G.; Hoover, A. J.; Ritter, T. Transition Metal-Mediated and Metal-Catalyzed Carbon–Fluorine Bond Formation. In Organometallic Fluorine Chemistry; Braun, T.; Hughes, R. P., Eds.; Springer International Publishing: Cham, 2015; pp 1–53. doi:10.1007/3418_2014_88
Return to citation in text: [1] -
Szpera, R.; Moseley, D. F. J.; Smith, L. B.; Sterling, A. J.; Gouverneur, V. Angew. Chem., Int. Ed. 2019, 58, 14824–14848. doi:10.1002/anie.201814457
Return to citation in text: [1] -
McMurtrey, K. B.; Racowski, J. M.; Sanford, M. S. Org. Lett. 2012, 14, 4094–4097. doi:10.1021/ol301739f
Return to citation in text: [1] -
Wu, T.; Cheng, J.; Chen, P.; Liu, G. Chem. Commun. 2013, 49, 8707–8709. doi:10.1039/c3cc44711a
Return to citation in text: [1] -
Lou, S.-J.; Xu, D.-Q.; Xia, A.-B.; Wang, Y.-F.; Liu, Y.-K.; Du, X.-H.; Xu, Z.-Y. Chem. Commun. 2013, 49, 6218–6220. doi:10.1039/c3cc42220h
Return to citation in text: [1] -
Brückl, T.; Baxter, R. D.; Ishihara, Y.; Baran, P. S. Acc. Chem. Res. 2012, 45, 826–839. doi:10.1021/ar200194b
Return to citation in text: [1] -
Wang, X.; Truesdale, L.; Yu, J.-Q. J. Am. Chem. Soc. 2010, 132, 3648–3649. doi:10.1021/ja909522s
Return to citation in text: [1] -
Ye, Y.; Ball, N. D.; Kampf, J. W.; Sanford, M. S. J. Am. Chem. Soc. 2010, 132, 14682–14687. doi:10.1021/ja107780w
Return to citation in text: [1] -
Zhang, S.-Y.; He, G.; Zhao, Y.; Wright, K.; Nack, W. A.; Chen, G. J. Am. Chem. Soc. 2012, 134, 7313–7316. doi:10.1021/ja3023972
Return to citation in text: [1] [2] [3] [4] [5] -
Zhang, L.-S.; Chen, K.; Chen, G.; Li, B.-J.; Luo, S.; Guo, Q.-Y.; Wei, J.-B.; Shi, Z.-J. Org. Lett. 2013, 15, 10–13. doi:10.1021/ol302814x
Return to citation in text: [1] -
Miura, M.; Feng, C.-G.; Ma, S.; Yu, J.-Q. Org. Lett. 2013, 15, 5258–5261. doi:10.1021/ol402471y
Return to citation in text: [1] -
Zou, L.; Li, P.; Wang, B.; Wang, L. Chem. Commun. 2019, 55, 3737–3740. doi:10.1039/c9cc01014a
Return to citation in text: [1] -
Cai, S.; Chen, C.; Sun, Z.; Xi, C. Chem. Commun. 2013, 49, 4552–4554. doi:10.1039/c3cc41331d
Return to citation in text: [1] -
Feng, C.; Loh, T.-P. Angew. Chem., Int. Ed. 2013, 52, 12414–12417. doi:10.1002/anie.201307245
Return to citation in text: [1] -
Besset, T.; Cahard, D.; Pannecoucke, X. J. Org. Chem. 2014, 79, 413–418. doi:10.1021/jo402385g
Return to citation in text: [1] -
Vuagnat, M.; Jubault, P.; Besset, T. Pd-Catalyzed C–Chalcogen Bond Formation (C–S, C–Se) by C–H Bond Activation. In Handbook of CH-Functionalization; Maiti, D., Ed.; Wiley-VCH: Germany, 2022; pp 1–25. doi:10.1002/9783527834242.chf0017
Return to citation in text: [1] -
Leo, A.; Hansch, C.; Elkins, D. Chem. Rev. 1971, 71, 525–616. doi:10.1021/cr60274a001
Return to citation in text: [1] -
Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165–195. doi:10.1021/cr00002a004
Return to citation in text: [1] -
Toulgoat, F.; Alazet, S.; Billard, T. Eur. J. Org. Chem. 2014, 2415–2428. doi:10.1002/ejoc.201301857
Return to citation in text: [1] -
Xu, X.-H.; Matsuzaki, K.; Shibata, N. Chem. Rev. 2015, 115, 731–764. doi:10.1021/cr500193b
Return to citation in text: [1] -
Barata-Vallejo, S.; Bonesi, S.; Postigo, A. Org. Biomol. Chem. 2016, 14, 7150–7182. doi:10.1039/c6ob00763e
Return to citation in text: [1] -
Li, M.; Guo, J.; Xue, X.-S.; Cheng, J.-P. Org. Lett. 2016, 18, 264–267. doi:10.1021/acs.orglett.5b03433
Return to citation in text: [1] -
Zheng, H.; Huang, Y.; Weng, Z. Tetrahedron Lett. 2016, 57, 1397–1409. doi:10.1016/j.tetlet.2016.02.073
Return to citation in text: [1] -
Boiko, V. N. Beilstein J. Org. Chem. 2010, 6, 880–921. doi:10.3762/bjoc.6.88
Return to citation in text: [1] -
Li, X.; Zhao, J.; Zhang, L.; Hu, M.; Wang, L.; Hu, J. Org. Lett. 2015, 17, 298–301. doi:10.1021/ol5034018
Return to citation in text: [1] -
Ye, K.-Y.; Zhang, X.; Dai, L.-X.; You, S.-L. J. Org. Chem. 2014, 79, 12106–12110. doi:10.1021/jo5019393
Return to citation in text: [1] -
Lefebvre, Q.; Fava, E.; Nikolaienko, P.; Rueping, M. Chem. Commun. 2014, 50, 6617–6619. doi:10.1039/c4cc02060j
Return to citation in text: [1] -
Liu, J.-B.; Xu, X.-H.; Chen, Z.-H.; Qing, F.-L. Angew. Chem., Int. Ed. 2015, 54, 897–900. doi:10.1002/anie.201409983
Return to citation in text: [1] -
Jiang, L.; Qian, J.; Yi, W.; Lu, G.; Cai, C.; Zhang, W. Angew. Chem., Int. Ed. 2015, 54, 14965–14969. doi:10.1002/anie.201508495
Return to citation in text: [1] -
Zheng, J.; Wang, L.; Lin, J.-H.; Xiao, J.-C.; Liang, S. H. Angew. Chem., Int. Ed. 2015, 54, 13236–13240. doi:10.1002/anie.201505446
Return to citation in text: [1] -
Yin, G.; Kalvet, I.; Schoenebeck, F. Angew. Chem., Int. Ed. 2015, 54, 6809–6813. doi:10.1002/anie.201501617
Return to citation in text: [1] -
Candish, L.; Pitzer, L.; Gómez-Suárez, A.; Glorius, F. Chem. – Eur. J. 2016, 22, 4753–4756. doi:10.1002/chem.201600421
Return to citation in text: [1] -
Matheis, C.; Wagner, V.; Goossen, L. J. Chem. – Eur. J. 2016, 22, 79–82. doi:10.1002/chem.201503524
Return to citation in text: [1] -
Jarrige, L.; Carboni, A.; Dagousset, G.; Levitre, G.; Magnier, E.; Masson, G. Org. Lett. 2016, 18, 2906–2909. doi:10.1021/acs.orglett.6b01257
Return to citation in text: [1] -
Liu, X.; An, R.; Zhang, X.; Luo, J.; Zhao, X. Angew. Chem., Int. Ed. 2016, 55, 5846–5850. doi:10.1002/anie.201601713
Return to citation in text: [1] -
Xiong, H.-Y.; Pannecoucke, X.; Besset, T. Org. Chem. Front. 2016, 3, 620–624. doi:10.1039/c6qo00064a
Return to citation in text: [1] -
Yang, Y.; Xu, L.; Yu, S.; Liu, X.; Zhang, Y.; Vicic, D. A. Chem. – Eur. J. 2016, 22, 858–863. doi:10.1002/chem.201504790
Return to citation in text: [1] -
Wang, F.; Zhao, L.; You, J.; Wang, M.-X. Org. Chem. Front. 2016, 3, 880–886. doi:10.1039/c6qo00161k
Return to citation in text: [1] [2] [3] -
Bu, M.-j.; Lu, G.-p.; Cai, C. Org. Chem. Front. 2017, 4, 266–270. doi:10.1039/c6qo00622a
Return to citation in text: [1] -
Lübcke, M.; Yuan, W.; Szabó, K. J. Org. Lett. 2017, 19, 4548–4551. doi:10.1021/acs.orglett.7b02139
Return to citation in text: [1] -
Carbonnel, E.; Besset, T.; Poisson, T.; Labar, D.; Pannecoucke, X.; Jubault, P. Chem. Commun. 2017, 53, 5706–5709. doi:10.1039/c7cc02652h
Return to citation in text: [1] -
Zhao, Q.; Poisson, T.; Pannecoucke, X.; Bouillon, J.-P.; Besset, T. Org. Lett. 2017, 19, 5106–5109. doi:10.1021/acs.orglett.7b02384
Return to citation in text: [1] [2] [3] -
Gelat, F.; Poisson, T.; Biju, A. T.; Pannecoucke, X.; Besset, T. Eur. J. Org. Chem. 2018, 3693–3696. doi:10.1002/ejoc.201800418
Return to citation in text: [1] -
Zhao, Q.; Chen, M.-Y.; Poisson, T.; Pannecoucke, X.; Bouillon, J.-P.; Besset, T. Eur. J. Org. Chem. 2018, 6167–6175. doi:10.1002/ejoc.201801071
Return to citation in text: [1] [2] [3] [4] [5] -
Ghiazza, C.; Khrouz, L.; Monnereau, C.; Billard, T.; Tlili, A. Chem. Commun. 2018, 54, 9909–9912. doi:10.1039/c8cc05256e
Return to citation in text: [1] -
Saravanan, P.; Anbarasan, P. Adv. Synth. Catal. 2018, 360, 2894–2899. doi:10.1002/adsc.201800366
Return to citation in text: [1] -
Xi, C.-C.; Chen, Z.-M.; Zhang, S.-Y.; Tu, Y.-Q. Org. Lett. 2018, 20, 4227–4230. doi:10.1021/acs.orglett.8b01627
Return to citation in text: [1] -
He, J.; Chen, C.; Fu, G. C.; Peters, J. C. ACS Catal. 2018, 8, 11741–11748. doi:10.1021/acscatal.8b04094
Return to citation in text: [1] -
Lindberg, E.; Angerani, S.; Anzola, M.; Winssinger, N. Nat. Commun. 2018, 9, 3539. doi:10.1038/s41467-018-05916-9
Return to citation in text: [1] -
Zhang, J.; Yang, J.-D.; Zheng, H.; Xue, X.-S.; Mayr, H.; Cheng, J.-P. Angew. Chem., Int. Ed. 2018, 57, 12690–12695. doi:10.1002/anie.201805859
Return to citation in text: [1] -
Luo, Z.; Yang, X.; Tsui, G. C. Org. Lett. 2020, 22, 6155–6159. doi:10.1021/acs.orglett.0c02235
Return to citation in text: [1] -
Tran, L. D.; Popov, I.; Daugulis, O. J. Am. Chem. Soc. 2012, 134, 18237–18240. doi:10.1021/ja3092278
Return to citation in text: [1] [2] -
Tyrra, W.; Naumann, D.; Hoge, B.; Yagupolskii, Y. L. J. Fluorine Chem. 2003, 119, 101–107. doi:10.1016/s0022-1139(02)00276-2
Return to citation in text: [1] -
Scattolin, T.; Pu, M.; Schoenebeck, F. Chem. – Eur. J. 2018, 24, 567–571. doi:10.1002/chem.201705240
Return to citation in text: [1] -
Xu, C.; Shen, Q. Org. Lett. 2014, 16, 2046–2049. doi:10.1021/ol5006533
Return to citation in text: [1] [2] -
Whitfield, S. R.; Sanford, M. S. J. Am. Chem. Soc. 2007, 129, 15142–15143. doi:10.1021/ja077866q
Return to citation in text: [1] -
Yin, W.; Wang, Z.; Huang, Y. Adv. Synth. Catal. 2014, 356, 2998–3006. doi:10.1002/adsc.201400362
Return to citation in text: [1] [2] -
Xu, J.; Chen, P.; Ye, J.; Liu, G. Acta Chim. Sin. (Chin. Ed.) 2015, 73, 1294–1297. doi:10.6023/a15030211
Return to citation in text: [1] [2] [3] -
Kesavan, A.; Chaitanya, M.; Anbarasan, P. Eur. J. Org. Chem. 2018, 3276–3279. doi:10.1002/ejoc.201800451
Return to citation in text: [1] [2] -
Alazet, S.; Zimmer, L.; Billard, T. Chem. – Eur. J. 2014, 20, 8589–8593. doi:10.1002/chem.201403409
Return to citation in text: [1] -
Wang, Q.; Xie, F.; Li, X. J. Org. Chem. 2015, 80, 8361–8366. doi:10.1021/acs.joc.5b00940
Return to citation in text: [1] [2] -
Liu, X.-G.; Li, Q.; Wang, H. Adv. Synth. Catal. 2017, 359, 1942–1946. doi:10.1002/adsc.201700066
Return to citation in text: [1] [2] -
Yoshida, M.; Kawai, K.; Tanaka, R.; Yoshino, T.; Matsunaga, S. Chem. Commun. 2017, 53, 5974–5977. doi:10.1039/c7cc03072j
Return to citation in text: [1] [2] -
Xiong, H.-Y.; Besset, T.; Cahard, D.; Pannecoucke, X. J. Org. Chem. 2015, 80, 4204–4212. doi:10.1021/acs.joc.5b00505
Return to citation in text: [1] [2] -
Bouchard, A.; Kairouz, V.; Manneveau, M.; Xiong, H.-Y.; Besset, T.; Pannecoucke, X.; Lebel, H. J. Flow Chem. 2019, 9, 9–12. doi:10.1007/s41981-018-0023-4
Return to citation in text: [1] -
Xiong, H.-Y.; Pannecoucke, X.; Besset, T. Chem. – Eur. J. 2016, 22, 16734–16749. doi:10.1002/chem.201603438
Return to citation in text: [1] -
Xiao, X.; Zheng, Z.-T.; Li, T.; Zheng, J.-L.; Tao, T.; Chen, L.-M.; Gu, J.-Y.; Yao, X.; Lin, J.-H.; Xiao, J.-C. Synthesis 2020, 52, 197–207. doi:10.1055/s-0039-1690714
Return to citation in text: [1] -
Pannecoucke, X.; Besset, T. Org. Biomol. Chem. 2019, 17, 1683–1693. doi:10.1039/c8ob02995d
Return to citation in text: [1] -
Besset, T.; Poisson, T. Extension to the SCF2H, SCH2F, and SCF2R Motifs (R = PO(OEt)2, CoO2R, RF). In Emerging Fluorinated Motifs; Ma, J.-A.; Cahard, D., Eds.; Wiley VCH: Germany, 2020; pp 449–475. doi:10.1002/9783527824342.ch16
Return to citation in text: [1] [2] -
Xiong, H.-Y.; Bayle, A.; Pannecoucke, X.; Besset, T. Angew. Chem., Int. Ed. 2016, 55, 13490–13494. doi:10.1002/anie.201607231
Return to citation in text: [1] -
Shen, F.; Zhang, P.; Lu, L.; Shen, Q. Org. Lett. 2017, 19, 1032–1035. doi:10.1021/acs.orglett.7b00010
Return to citation in text: [1] -
Ismalaj, E.; Glenadel, Q.; Billard, T. Eur. J. Org. Chem. 2017, 1911–1914. doi:10.1002/ejoc.201700103
Return to citation in text: [1] -
Wang, J.; Xiong, H.-Y.; Petit, E.; Bailly, L.; Pannecoucke, X.; Poisson, T.; Besset, T. Chem. Commun. 2019, 55, 8784–8787. doi:10.1039/c9cc01851d
Return to citation in text: [1] -
Petit-Cancelier, F.; François, B.; Pannecoucke, X.; Couve-Bonnaire, S.; Besset, T. Adv. Synth. Catal. 2020, 362, 760–764. doi:10.1002/adsc.201901454
Return to citation in text: [1] [2] -
Xiang, T.; Liu, Y.; Xu, Q.; He, K.; Pan, F. J. Org. Chem. 2022, 87, 3135–3144. doi:10.1021/acs.joc.1c02881
Return to citation in text: [1] [2] -
Doche, F.; Escudero, J.; Petit-Cancelier, F.; Xiong, H.-Y.; Couve-Bonnaire, S.; Audisio, D.; Poisson, T.; Besset, T. Chem. – Eur. J. 2022, 28, e202202099. doi:10.1002/chem.202202099
Return to citation in text: [1] [2] -
Grollier, K.; Chefdeville, E.; Jeanneau, E.; Billard, T. Chem. – Eur. J. 2021, 27, 12910–12916. doi:10.1002/chem.202102121
Return to citation in text: [1] [2] -
de Zordo-Banliat, A.; Grollier, K.; Vigier, J.; Jeanneau, E.; Dagousset, G.; Pegot, B.; Magnier, E.; Billard, T. Chem. – Eur. J. 2022, 28, e202202299. doi:10.1002/chem.202202299
Return to citation in text: [1] [2] -
Huang, Y.; Huang, R.; Weng, Z. Synlett 2015, 26, 2327–2331. doi:10.1055/s-0035-1560054
Return to citation in text: [1] -
Pethő, B.; Novák, Z. Asian J. Org. Chem. 2019, 8, 568–575. doi:10.1002/ajoc.201800414
Return to citation in text: [1] -
Jeschke, P.; Baston, E.; Leroux, R. F. Mini-Rev. Med. Chem. 2007, 7, 1027–1034. doi:10.2174/138955707782110150
Return to citation in text: [1] -
Idoux, J. P.; Madenwald, M. L.; Garcia, B. S.; Chu, D. L.; Gupton, J. T. J. Org. Chem. 1985, 50, 1876–1878. doi:10.1021/jo00211a018
Return to citation in text: [1] -
Shen, X.; Neumann, C. N.; Kleinlein, C.; Goldberg, N. W.; Ritter, T. Angew. Chem., Int. Ed. 2015, 54, 5662–5665. doi:10.1002/anie.201500902
Return to citation in text: [1] -
Pethő, B.; Zwillinger, M.; Csenki, J. T.; Káncz, A. E.; Krámos, B.; Müller, J.; Balogh, G. T.; Novák, Z. Chem. – Eur. J. 2017, 23, 15628–15632. doi:10.1002/chem.201704205
Return to citation in text: [1] -
Vuluga, D.; Legros, J.; Crousse, B.; Bonnet-Delpon, D. Eur. J. Org. Chem. 2009, 3513–3518. doi:10.1002/ejoc.200900303
Return to citation in text: [1] -
Zhang, K.; Xu, X.-H.; Qing, F.-L. J. Fluorine Chem. 2017, 196, 24–31. doi:10.1016/j.jfluchem.2016.07.008
Return to citation in text: [1] -
Roane, J.; Daugulis, O. Org. Lett. 2013, 15, 5842–5845. doi:10.1021/ol402904d
Return to citation in text: [1] [2] [3] -
Yang, L.; Li, S.; Cai, L.; Ding, Y.; Fu, L.; Cai, Z.; Ji, H.; Li, G. Org. Lett. 2017, 19, 2746–2749. doi:10.1021/acs.orglett.7b01103
Return to citation in text: [1] [2] [3] -
Maiti, S.; Alam, T.; Mal, P. Asian J. Org. Chem. 2018, 7, 715–719. doi:10.1002/ajoc.201800069
Return to citation in text: [1] -
Xu, X.; Xia, C.; Li, X.; Sun, J.; Hao, L. RSC Adv. 2020, 10, 2016–2026. doi:10.1039/c9ra10194b
Return to citation in text: [1] -
Jin, L.; Zhang, X.-L.; Guo, R.-L.; Wang, M.-Y.; Gao, Y.-R.; Wang, Y.-Q. Org. Lett. 2021, 23, 1921–1927. doi:10.1021/acs.orglett.1c00365
Return to citation in text: [1] [2] [3] -
Tamargo, J.; Le Heuzey, J.-Y.; Mabo, P. Eur. J. Clin. Pharmacol. 2015, 71, 549–567. doi:10.1007/s00228-015-1832-0
Return to citation in text: [1] -
Paolini, E.; Stronati, G.; Guerra, F.; Capucci, A. Pharmacol. Res. 2019, 148, 104443. doi:10.1016/j.phrs.2019.104443
Return to citation in text: [1] -
Matheson, A. J.; Jarvis, B. Drugs 2001, 61, 1801–1833. doi:10.2165/00003495-200161120-00011
Return to citation in text: [1] -
Liu, B.; Shi, B.-F. Tetrahedron Lett. 2015, 56, 15–22. doi:10.1016/j.tetlet.2014.11.039
Return to citation in text: [1] -
Enthaler, S.; Company, A. Chem. Soc. Rev. 2011, 40, 4912–4924. doi:10.1039/c1cs15085e
Return to citation in text: [1] -
Kauffman, G. B. Angew. Chem., Int. Ed. 2008, 47, 8155–8156. doi:10.1002/anie.200885643
Return to citation in text: [1] -
Shuklov, I. A.; Dubrovina, N. V.; Börner, A. Synthesis 2007, 2925–2943. doi:10.1055/s-2007-983902
Return to citation in text: [1] -
Dick, A. R.; Hull, K. L.; Sanford, M. S. J. Am. Chem. Soc. 2004, 126, 2300–2301. doi:10.1021/ja031543m
Return to citation in text: [1] -
Shinde, V. N.; Rangan, K.; Kumar, D.; Kumar, A. J. Org. Chem. 2021, 86, 9755–9770. doi:10.1021/acs.joc.1c01097
Return to citation in text: [1] -
Selvakumar, J.; Grandhi, G. S.; Sahoo, H.; Baidya, M. RSC Adv. 2016, 6, 79361–79365. doi:10.1039/c6ra18861c
Return to citation in text: [1] -
Rajesh, N.; Sundararaju, B. Asian J. Org. Chem. 2018, 7, 1368–1371. doi:10.1002/ajoc.201800286
Return to citation in text: [1] -
Zhang, L.-B.; Hao, X.-Q.; Zhang, S.-K.; Liu, Z.-J.; Zheng, X.-X.; Gong, J.-F.; Niu, J.-L.; Song, M.-P. Angew. Chem., Int. Ed. 2015, 54, 272–275. doi:10.1002/anie.201409751
Return to citation in text: [1] -
Zhang, T.; Zhu, H.; Yang, F.; Wu, Y.; Wu, Y. Tetrahedron 2019, 75, 1541–1547. doi:10.1016/j.tet.2019.02.002
Return to citation in text: [1] -
Guo, X.-K.; Zhang, L.-B.; Wei, D.; Niu, J.-L. Chem. Sci. 2015, 6, 7059–7071. doi:10.1039/c5sc01807b
Return to citation in text: [1] -
Kumar, S.; Nair, A. M.; Volla, C. M. R. Chem. – Asian J. 2022, 17, e202200801. doi:10.1002/asia.202200801
Return to citation in text: [1] -
Sato, T.; Nogi, K.; Yorimitsu, H. ChemCatChem 2020, 12, 3467–3471. doi:10.1002/cctc.202000485
Return to citation in text: [1] [2] -
Gong, W.; Zhang, G.; Liu, T.; Giri, R.; Yu, J.-Q. J. Am. Chem. Soc. 2014, 136, 16940–16946. doi:10.1021/ja510233h
Return to citation in text: [1] -
Sun, H.; Guimond, N.; Huang, Y. Org. Biomol. Chem. 2016, 14, 8389–8397. doi:10.1039/c6ob01258b
Return to citation in text: [1] -
Afewerki, S.; Córdova, A. Chem. Rev. 2016, 116, 13512–13570. doi:10.1021/acs.chemrev.6b00226
Return to citation in text: [1] -
Kim, D.-S.; Park, W.-J.; Jun, C.-H. Chem. Rev. 2017, 117, 8977–9015. doi:10.1021/acs.chemrev.6b00554
Return to citation in text: [1] -
Zhao, Q.; Poisson, T.; Pannecoucke, X.; Besset, T. Synthesis 2017, 49, 4808–4826. doi:10.1055/s-0036-1590878
Return to citation in text: [1] -
Gandeepan, P.; Ackermann, L. Chem 2018, 4, 199–222. doi:10.1016/j.chempr.2017.11.002
Return to citation in text: [1] -
Bhattacharya, T.; Pimparkar, S.; Maiti, D. RSC Adv. 2018, 8, 19456–19464. doi:10.1039/c8ra03230k
Return to citation in text: [1] -
St John-Campbell, S.; Bull, J. A. Org. Biomol. Chem. 2018, 16, 4582–4595. doi:10.1039/c8ob00926k
Return to citation in text: [1] -
Rasheed, O. K.; Sun, B. ChemistrySelect 2018, 3, 5689–5708. doi:10.1002/slct.201801097
Return to citation in text: [1] -
Wu, Y.; Shi, B. Chin. J. Org. Chem. 2020, 40, 3517. doi:10.6023/cjoc202003057
Return to citation in text: [1] -
Niu, B.; Yang, K.; Lawrence, B.; Ge, H. ChemSusChem 2019, 12, 2955–2969. doi:10.1002/cssc.201900151
Return to citation in text: [1] -
Lapuh, M. I.; Mazeh, S.; Besset, T. ACS Catal. 2020, 10, 12898–12919. doi:10.1021/acscatal.0c03317
Return to citation in text: [1] -
Higham, J. I.; Bull, J. A. Org. Biomol. Chem. 2020, 18, 7291–7315. doi:10.1039/d0ob01587c
Return to citation in text: [1] -
Tian, M.; Shao, L.; Su, X.; Zhou, X.; Zhang, H.; Wei, K.; Sun, R.; Wang, J. RSC Adv. 2022, 12, 18722–18727. doi:10.1039/d2ra00241h
Return to citation in text: [1] [2] -
Xu, Y.; Dong, G. Chem. Sci. 2018, 9, 1424–1432. doi:10.1039/c7sc04768a
Return to citation in text: [1] -
He, C.; Whitehurst, W. G.; Gaunt, M. J. Chem 2019, 5, 1031–1058. doi:10.1016/j.chempr.2018.12.017
Return to citation in text: [1] -
Zhang, M.; Wang, Q.; Peng, Y.; Chen, Z.; Wan, C.; Chen, J.; Zhao, Y.; Zhang, R.; Zhang, A. Q. Chem. Commun. 2019, 55, 13048–13065. doi:10.1039/c9cc06609h
Return to citation in text: [1] -
Uttry, A.; van Gemmeren, M. Synthesis 2020, 52, 479–488. doi:10.1055/s-0039-1690720
Return to citation in text: [1] -
Liu, B.; Romine, A. M.; Rubel, C. Z.; Engle, K. M.; Shi, B.-F. Chem. Rev. 2021, 121, 14957–15074. doi:10.1021/acs.chemrev.1c00519
Return to citation in text: [1] -
Panda, P.; Pal, K.; Chakroborty, S. Results Chem. 2021, 3, 100154. doi:10.1016/j.rechem.2021.100154
Return to citation in text: [1] -
Sen, S.; Das, J.; Maiti, D. Tetrahedron Chem 2022, 1, 100005. doi:10.1016/j.tchem.2022.100005
Return to citation in text: [1] -
Xie, J.; Zhu, C. Transition Metal-Catalyzed, Directing Group-Assisted C(Sp3)–H Bond Functionalization. In Sustainable C(sp3)–H Bond Functionalization; Xie, J.; Zhu, C., Eds.; SpringerBriefs in Molecular Science; Springer: Berlin, Heidelberg, 2016; pp 1–23. doi:10.1007/978-3-662-49496-7_1
Return to citation in text: [1] -
Chen, F.-J.; Zhao, S.; Hu, F.; Chen, K.; Zhang, Q.; Zhang, S.-Q.; Shi, B.-F. Chem. Sci. 2013, 4, 4187–4192. doi:10.1039/c3sc51993g
Return to citation in text: [1] [2] [3] -
Ma, J.-A.; Cahard, D., Eds. Emerging Fluorinated Motifs: Synthesis, Properties and Applications; Wiley-VCH: Germany, 2020; Vol. 2. doi:10.1002/9783527824342
Return to citation in text: [1] -
Li, Y.; Yang, Y.; Xin, J.; Tang, P. Nat. Commun. 2020, 11, 755. doi:10.1038/s41467-020-14598-1
Return to citation in text: [1] -
Duhail, T.; Bortolato, T.; Mateos, J.; Anselmi, E.; Jelier, B.; Togni, A.; Magnier, E.; Dagousset, G.; Dell’Amico, L. Org. Lett. 2021, 23, 7088–7093. doi:10.1021/acs.orglett.1c02494
Return to citation in text: [1] -
Wang, Q.; Zhang, X.; Sorochinsky, A. E.; Butler, G.; Han, J.; Soloshonok, V. A. Symmetry 2021, 13, 2380. doi:10.3390/sym13122380
Return to citation in text: [1] -
Barata-Vallejo, S.; Bonesi, S. M.; Postigo, A. Chem. – Eur. J. 2022, 28, e202201776. doi:10.1002/chem.202201776
Return to citation in text: [1] -
Lee, K. N.; Lee, J. W.; Ngai, M.-Y. Tetrahedron 2018, 74, 7127–7135. doi:10.1016/j.tet.2018.09.020
Return to citation in text: [1] -
Han, Q.; Zhao, C.; Zhang, C. Chin. J. Org. Chem. 2019, 39, 84. doi:10.6023/cjoc201808029
Return to citation in text: [1] -
Tóth, B. L.; Kovács, S.; Sályi, G.; Novák, Z. Angew. Chem., Int. Ed. 2016, 55, 1988–1992. doi:10.1002/anie.201510555
Return to citation in text: [1] -
Ruyet, L.; Lapuh, M. I.; Koshti, V. S.; Földesi, T.; Jubault, P.; Poisson, T.; Novák, Z.; Besset, T. Chem. Commun. 2021, 57, 6241–6244. doi:10.1039/d1cc02007b
Return to citation in text: [1] -
Dong, L.; Feng, T.; Xiong, D.; Xu, Z.; Cheng, J.; Xu, X.; Shao, X.; Li, Z. Org. Lett. 2022, 24, 1913–1917. doi:10.1021/acs.orglett.2c00245
Return to citation in text: [1] -
Solas, D.; Hale, R. L.; Patel, D. V. J. Org. Chem. 1996, 61, 1537–1539. doi:10.1021/jo9517508
Return to citation in text: [1] -
Lu, Y.; Liu, C.; Chen, Q.-Y. Curr. Org. Chem. 2015, 19, 1638–1650. doi:10.2174/1385272819666150615235605
Return to citation in text: [1] -
Rong, J.; Ni, C.; Hu, J. Asian J. Org. Chem. 2017, 6, 139–152. doi:10.1002/ajoc.201600509
Return to citation in text: [1] -
Yerien, D. E.; Barata-Vallejo, S.; Postigo, A. Chem. – Eur. J. 2017, 23, 14676–14701. doi:10.1002/chem.201702311
Return to citation in text: [1] -
Feng, Z.; Xiao, Y.-L.; Zhang, X. Acc. Chem. Res. 2018, 51, 2264–2278. doi:10.1021/acs.accounts.8b00230
Return to citation in text: [1] -
Lemos, A.; Lemaire, C.; Luxen, A. Adv. Synth. Catal. 2019, 361, 1500–1537. doi:10.1002/adsc.201801121
Return to citation in text: [1] -
Gandeepan, P.; Müller, T.; Zell, D.; Cera, G.; Warratz, S.; Ackermann, L. Chem. Rev. 2019, 119, 2192–2452. doi:10.1021/acs.chemrev.8b00507
Return to citation in text: [1] -
Planas, O.; Peciukenas, V.; Leutzsch, M.; Nöthling, N.; Pantazis, D. A.; Cornella, J. J. Am. Chem. Soc. 2022, 144, 14489–14504. doi:10.1021/jacs.2c01072
Return to citation in text: [1] -
Carvalho, R. L.; de Miranda, A. S.; Nunes, M. P.; Gomes, R. S.; Jardim, G. A. M.; Júnior, E. N. d. S. Beilstein J. Org. Chem. 2021, 17, 1849–1938. doi:10.3762/bjoc.17.126
Return to citation in text: [1]
| 122. | Alazet, S.; Zimmer, L.; Billard, T. Chem. – Eur. J. 2014, 20, 8589–8593. doi:10.1002/chem.201403409 |
| 121. | Kesavan, A.; Chaitanya, M.; Anbarasan, P. Eur. J. Org. Chem. 2018, 3276–3279. doi:10.1002/ejoc.201800451 |
| 183. | Tian, M.; Shao, L.; Su, X.; Zhou, X.; Zhang, H.; Wei, K.; Sun, R.; Wang, J. RSC Adv. 2022, 12, 18722–18727. doi:10.1039/d2ra00241h |
| 106. | Zhao, Q.; Chen, M.-Y.; Poisson, T.; Pannecoucke, X.; Bouillon, J.-P.; Besset, T. Eur. J. Org. Chem. 2018, 6167–6175. doi:10.1002/ejoc.201801071 |
| 183. | Tian, M.; Shao, L.; Su, X.; Zhou, X.; Zhang, H.; Wei, K.; Sun, R.; Wang, J. RSC Adv. 2022, 12, 18722–18727. doi:10.1039/d2ra00241h |
| 153. | Jin, L.; Zhang, X.-L.; Guo, R.-L.; Wang, M.-Y.; Gao, Y.-R.; Wang, Y.-Q. Org. Lett. 2021, 23, 1921–1927. doi:10.1021/acs.orglett.1c00365 |
| 153. | Jin, L.; Zhang, X.-L.; Guo, R.-L.; Wang, M.-Y.; Gao, Y.-R.; Wang, Y.-Q. Org. Lett. 2021, 23, 1921–1927. doi:10.1021/acs.orglett.1c00365 |
| 35. | Jacob, C.; Maes, B. U. W.; Evano, G. Chem. – Eur. J. 2021, 27, 13899–13952. doi:10.1002/chem.202101598 |
| 170. | Gong, W.; Zhang, G.; Liu, T.; Giri, R.; Yu, J.-Q. J. Am. Chem. Soc. 2014, 136, 16940–16946. doi:10.1021/ja510233h |
| 171. | Sun, H.; Guimond, N.; Huang, Y. Org. Biomol. Chem. 2016, 14, 8389–8397. doi:10.1039/c6ob01258b |
| 172. | Afewerki, S.; Córdova, A. Chem. Rev. 2016, 116, 13512–13570. doi:10.1021/acs.chemrev.6b00226 |
| 173. | Kim, D.-S.; Park, W.-J.; Jun, C.-H. Chem. Rev. 2017, 117, 8977–9015. doi:10.1021/acs.chemrev.6b00554 |
| 174. | Zhao, Q.; Poisson, T.; Pannecoucke, X.; Besset, T. Synthesis 2017, 49, 4808–4826. doi:10.1055/s-0036-1590878 |
| 175. | Gandeepan, P.; Ackermann, L. Chem 2018, 4, 199–222. doi:10.1016/j.chempr.2017.11.002 |
| 176. | Bhattacharya, T.; Pimparkar, S.; Maiti, D. RSC Adv. 2018, 8, 19456–19464. doi:10.1039/c8ra03230k |
| 177. | St John-Campbell, S.; Bull, J. A. Org. Biomol. Chem. 2018, 16, 4582–4595. doi:10.1039/c8ob00926k |
| 178. | Rasheed, O. K.; Sun, B. ChemistrySelect 2018, 3, 5689–5708. doi:10.1002/slct.201801097 |
| 179. | Wu, Y.; Shi, B. Chin. J. Org. Chem. 2020, 40, 3517. doi:10.6023/cjoc202003057 |
| 180. | Niu, B.; Yang, K.; Lawrence, B.; Ge, H. ChemSusChem 2019, 12, 2955–2969. doi:10.1002/cssc.201900151 |
| 181. | Lapuh, M. I.; Mazeh, S.; Besset, T. ACS Catal. 2020, 10, 12898–12919. doi:10.1021/acscatal.0c03317 |
| 182. | Higham, J. I.; Bull, J. A. Org. Biomol. Chem. 2020, 18, 7291–7315. doi:10.1039/d0ob01587c |
| 125. | Yoshida, M.; Kawai, K.; Tanaka, R.; Yoshino, T.; Matsunaga, S. Chem. Commun. 2017, 53, 5974–5977. doi:10.1039/c7cc03072j |
| 104. | Zhao, Q.; Poisson, T.; Pannecoucke, X.; Bouillon, J.-P.; Besset, T. Org. Lett. 2017, 19, 5106–5109. doi:10.1021/acs.orglett.7b02384 |
| 124. | Liu, X.-G.; Li, Q.; Wang, H. Adv. Synth. Catal. 2017, 359, 1942–1946. doi:10.1002/adsc.201700066 |
| 71. | Zhang, S.-Y.; He, G.; Zhao, Y.; Wright, K.; Nack, W. A.; Chen, G. J. Am. Chem. Soc. 2012, 134, 7313–7316. doi:10.1021/ja3023972 |
| 125. | Yoshida, M.; Kawai, K.; Tanaka, R.; Yoshino, T.; Matsunaga, S. Chem. Commun. 2017, 53, 5974–5977. doi:10.1039/c7cc03072j |
| 123. | Wang, Q.; Xie, F.; Li, X. J. Org. Chem. 2015, 80, 8361–8366. doi:10.1021/acs.joc.5b00940 |
| 71. | Zhang, S.-Y.; He, G.; Zhao, Y.; Wright, K.; Nack, W. A.; Chen, G. J. Am. Chem. Soc. 2012, 134, 7313–7316. doi:10.1021/ja3023972 |
| 124. | Liu, X.-G.; Li, Q.; Wang, H. Adv. Synth. Catal. 2017, 359, 1942–1946. doi:10.1002/adsc.201700066 |
| 192. | Chen, F.-J.; Zhao, S.; Hu, F.; Chen, K.; Zhang, Q.; Zhang, S.-Q.; Shi, B.-F. Chem. Sci. 2013, 4, 4187–4192. doi:10.1039/c3sc51993g |
| 106. | Zhao, Q.; Chen, M.-Y.; Poisson, T.; Pannecoucke, X.; Bouillon, J.-P.; Besset, T. Eur. J. Org. Chem. 2018, 6167–6175. doi:10.1002/ejoc.201801071 |
| 24. | Li, H.; Li, B.-J.; Shi, Z.-J. Catal. Sci. Technol. 2011, 1, 191–206. doi:10.1039/c0cy00076k |
| 184. | Xu, Y.; Dong, G. Chem. Sci. 2018, 9, 1424–1432. doi:10.1039/c7sc04768a |
| 185. | He, C.; Whitehurst, W. G.; Gaunt, M. J. Chem 2019, 5, 1031–1058. doi:10.1016/j.chempr.2018.12.017 |
| 186. | Zhang, M.; Wang, Q.; Peng, Y.; Chen, Z.; Wan, C.; Chen, J.; Zhao, Y.; Zhang, R.; Zhang, A. Q. Chem. Commun. 2019, 55, 13048–13065. doi:10.1039/c9cc06609h |
| 187. | Uttry, A.; van Gemmeren, M. Synthesis 2020, 52, 479–488. doi:10.1055/s-0039-1690720 |
| 188. | Liu, B.; Romine, A. M.; Rubel, C. Z.; Engle, K. M.; Shi, B.-F. Chem. Rev. 2021, 121, 14957–15074. doi:10.1021/acs.chemrev.1c00519 |
| 189. | Panda, P.; Pal, K.; Chakroborty, S. Results Chem. 2021, 3, 100154. doi:10.1016/j.rechem.2021.100154 |
| 190. | Sen, S.; Das, J.; Maiti, D. Tetrahedron Chem 2022, 1, 100005. doi:10.1016/j.tchem.2022.100005 |
| 191. | Xie, J.; Zhu, C. Transition Metal-Catalyzed, Directing Group-Assisted C(Sp3)–H Bond Functionalization. In Sustainable C(sp3)–H Bond Functionalization; Xie, J.; Zhu, C., Eds.; SpringerBriefs in Molecular Science; Springer: Berlin, Heidelberg, 2016; pp 1–23. doi:10.1007/978-3-662-49496-7_1 |
| 123. | Wang, Q.; Xie, F.; Li, X. J. Org. Chem. 2015, 80, 8361–8366. doi:10.1021/acs.joc.5b00940 |
| 71. | Zhang, S.-Y.; He, G.; Zhao, Y.; Wright, K.; Nack, W. A.; Chen, G. J. Am. Chem. Soc. 2012, 134, 7313–7316. doi:10.1021/ja3023972 |
| 192. | Chen, F.-J.; Zhao, S.; Hu, F.; Chen, K.; Zhang, Q.; Zhang, S.-Q.; Shi, B.-F. Chem. Sci. 2013, 4, 4187–4192. doi:10.1039/c3sc51993g |
| 106. | Zhao, Q.; Chen, M.-Y.; Poisson, T.; Pannecoucke, X.; Bouillon, J.-P.; Besset, T. Eur. J. Org. Chem. 2018, 6167–6175. doi:10.1002/ejoc.201801071 |
| 104. | Zhao, Q.; Poisson, T.; Pannecoucke, X.; Bouillon, J.-P.; Besset, T. Org. Lett. 2017, 19, 5106–5109. doi:10.1021/acs.orglett.7b02384 |
| 106. | Zhao, Q.; Chen, M.-Y.; Poisson, T.; Pannecoucke, X.; Bouillon, J.-P.; Besset, T. Eur. J. Org. Chem. 2018, 6167–6175. doi:10.1002/ejoc.201801071 |
| 126. | Xiong, H.-Y.; Besset, T.; Cahard, D.; Pannecoucke, X. J. Org. Chem. 2015, 80, 4204–4212. doi:10.1021/acs.joc.5b00505 |
| 209. | Gandeepan, P.; Müller, T.; Zell, D.; Cera, G.; Warratz, S.; Ackermann, L. Chem. Rev. 2019, 119, 2192–2452. doi:10.1021/acs.chemrev.8b00507 |
| 210. | Planas, O.; Peciukenas, V.; Leutzsch, M.; Nöthling, N.; Pantazis, D. A.; Cornella, J. J. Am. Chem. Soc. 2022, 144, 14489–14504. doi:10.1021/jacs.2c01072 |
| 211. | Carvalho, R. L.; de Miranda, A. S.; Nunes, M. P.; Gomes, R. S.; Jardim, G. A. M.; Júnior, E. N. d. S. Beilstein J. Org. Chem. 2021, 17, 1849–1938. doi:10.3762/bjoc.17.126 |
| 192. | Chen, F.-J.; Zhao, S.; Hu, F.; Chen, K.; Zhang, Q.; Zhang, S.-Q.; Shi, B.-F. Chem. Sci. 2013, 4, 4187–4192. doi:10.1039/c3sc51993g |
| 131. | Besset, T.; Poisson, T. Extension to the SCF2H, SCH2F, and SCF2R Motifs (R = PO(OEt)2, CoO2R, RF). In Emerging Fluorinated Motifs; Ma, J.-A.; Cahard, D., Eds.; Wiley VCH: Germany, 2020; pp 449–475. doi:10.1002/9783527824342.ch16 |
| 136. | Petit-Cancelier, F.; François, B.; Pannecoucke, X.; Couve-Bonnaire, S.; Besset, T. Adv. Synth. Catal. 2020, 362, 760–764. doi:10.1002/adsc.201901454 |
| 193. | Ma, J.-A.; Cahard, D., Eds. Emerging Fluorinated Motifs: Synthesis, Properties and Applications; Wiley-VCH: Germany, 2020; Vol. 2. doi:10.1002/9783527824342 |
| 194. | Li, Y.; Yang, Y.; Xin, J.; Tang, P. Nat. Commun. 2020, 11, 755. doi:10.1038/s41467-020-14598-1 |
| 195. | Duhail, T.; Bortolato, T.; Mateos, J.; Anselmi, E.; Jelier, B.; Togni, A.; Magnier, E.; Dagousset, G.; Dell’Amico, L. Org. Lett. 2021, 23, 7088–7093. doi:10.1021/acs.orglett.1c02494 |
| 196. | Wang, Q.; Zhang, X.; Sorochinsky, A. E.; Butler, G.; Han, J.; Soloshonok, V. A. Symmetry 2021, 13, 2380. doi:10.3390/sym13122380 |
| 197. | Barata-Vallejo, S.; Bonesi, S. M.; Postigo, A. Chem. – Eur. J. 2022, 28, e202201776. doi:10.1002/chem.202201776 |
| 198. | Lee, K. N.; Lee, J. W.; Ngai, M.-Y. Tetrahedron 2018, 74, 7127–7135. doi:10.1016/j.tet.2018.09.020 |
| 199. | Han, Q.; Zhao, C.; Zhang, C. Chin. J. Org. Chem. 2019, 39, 84. doi:10.6023/cjoc201808029 |
| 200. | Tóth, B. L.; Kovács, S.; Sályi, G.; Novák, Z. Angew. Chem., Int. Ed. 2016, 55, 1988–1992. doi:10.1002/anie.201510555 |
| 201. | Ruyet, L.; Lapuh, M. I.; Koshti, V. S.; Földesi, T.; Jubault, P.; Poisson, T.; Novák, Z.; Besset, T. Chem. Commun. 2021, 57, 6241–6244. doi:10.1039/d1cc02007b |
| 202. | Dong, L.; Feng, T.; Xiong, D.; Xu, Z.; Cheng, J.; Xu, X.; Shao, X.; Li, Z. Org. Lett. 2022, 24, 1913–1917. doi:10.1021/acs.orglett.2c00245 |
| 203. | Solas, D.; Hale, R. L.; Patel, D. V. J. Org. Chem. 1996, 61, 1537–1539. doi:10.1021/jo9517508 |
| 204. | Lu, Y.; Liu, C.; Chen, Q.-Y. Curr. Org. Chem. 2015, 19, 1638–1650. doi:10.2174/1385272819666150615235605 |
| 205. | Rong, J.; Ni, C.; Hu, J. Asian J. Org. Chem. 2017, 6, 139–152. doi:10.1002/ajoc.201600509 |
| 206. | Yerien, D. E.; Barata-Vallejo, S.; Postigo, A. Chem. – Eur. J. 2017, 23, 14676–14701. doi:10.1002/chem.201702311 |
| 207. | Feng, Z.; Xiao, Y.-L.; Zhang, X. Acc. Chem. Res. 2018, 51, 2264–2278. doi:10.1021/acs.accounts.8b00230 |
| 208. | Lemos, A.; Lemaire, C.; Luxen, A. Adv. Synth. Catal. 2019, 361, 1500–1537. doi:10.1002/adsc.201801121 |
| 138. | Doche, F.; Escudero, J.; Petit-Cancelier, F.; Xiong, H.-Y.; Couve-Bonnaire, S.; Audisio, D.; Poisson, T.; Besset, T. Chem. – Eur. J. 2022, 28, e202202099. doi:10.1002/chem.202202099 |
| 139. | Grollier, K.; Chefdeville, E.; Jeanneau, E.; Billard, T. Chem. – Eur. J. 2021, 27, 12910–12916. doi:10.1002/chem.202102121 |
| 137. | Xiang, T.; Liu, Y.; Xu, Q.; He, K.; Pan, F. J. Org. Chem. 2022, 87, 3135–3144. doi:10.1021/acs.joc.1c02881 |
| 138. | Doche, F.; Escudero, J.; Petit-Cancelier, F.; Xiong, H.-Y.; Couve-Bonnaire, S.; Audisio, D.; Poisson, T.; Besset, T. Chem. – Eur. J. 2022, 28, e202202099. doi:10.1002/chem.202202099 |
| 128. | Xiong, H.-Y.; Pannecoucke, X.; Besset, T. Chem. – Eur. J. 2016, 22, 16734–16749. doi:10.1002/chem.201603438 |
| 129. | Xiao, X.; Zheng, Z.-T.; Li, T.; Zheng, J.-L.; Tao, T.; Chen, L.-M.; Gu, J.-Y.; Yao, X.; Lin, J.-H.; Xiao, J.-C. Synthesis 2020, 52, 197–207. doi:10.1055/s-0039-1690714 |
| 130. | Pannecoucke, X.; Besset, T. Org. Biomol. Chem. 2019, 17, 1683–1693. doi:10.1039/c8ob02995d |
| 131. | Besset, T.; Poisson, T. Extension to the SCF2H, SCH2F, and SCF2R Motifs (R = PO(OEt)2, CoO2R, RF). In Emerging Fluorinated Motifs; Ma, J.-A.; Cahard, D., Eds.; Wiley VCH: Germany, 2020; pp 449–475. doi:10.1002/9783527824342.ch16 |
| 132. | Xiong, H.-Y.; Bayle, A.; Pannecoucke, X.; Besset, T. Angew. Chem., Int. Ed. 2016, 55, 13490–13494. doi:10.1002/anie.201607231 |
| 133. | Shen, F.; Zhang, P.; Lu, L.; Shen, Q. Org. Lett. 2017, 19, 1032–1035. doi:10.1021/acs.orglett.7b00010 |
| 134. | Ismalaj, E.; Glenadel, Q.; Billard, T. Eur. J. Org. Chem. 2017, 1911–1914. doi:10.1002/ejoc.201700103 |
| 135. | Wang, J.; Xiong, H.-Y.; Petit, E.; Bailly, L.; Pannecoucke, X.; Poisson, T.; Besset, T. Chem. Commun. 2019, 55, 8784–8787. doi:10.1039/c9cc01851d |
| 136. | Petit-Cancelier, F.; François, B.; Pannecoucke, X.; Couve-Bonnaire, S.; Besset, T. Adv. Synth. Catal. 2020, 362, 760–764. doi:10.1002/adsc.201901454 |
| 137. | Xiang, T.; Liu, Y.; Xu, Q.; He, K.; Pan, F. J. Org. Chem. 2022, 87, 3135–3144. doi:10.1021/acs.joc.1c02881 |
| 127. | Bouchard, A.; Kairouz, V.; Manneveau, M.; Xiong, H.-Y.; Besset, T.; Pannecoucke, X.; Lebel, H. J. Flow Chem. 2019, 9, 9–12. doi:10.1007/s41981-018-0023-4 |
| 126. | Xiong, H.-Y.; Besset, T.; Cahard, D.; Pannecoucke, X. J. Org. Chem. 2015, 80, 4204–4212. doi:10.1021/acs.joc.5b00505 |
| 140. | de Zordo-Banliat, A.; Grollier, K.; Vigier, J.; Jeanneau, E.; Dagousset, G.; Pegot, B.; Magnier, E.; Billard, T. Chem. – Eur. J. 2022, 28, e202202299. doi:10.1002/chem.202202299 |
| 140. | de Zordo-Banliat, A.; Grollier, K.; Vigier, J.; Jeanneau, E.; Dagousset, G.; Pegot, B.; Magnier, E.; Billard, T. Chem. – Eur. J. 2022, 28, e202202299. doi:10.1002/chem.202202299 |
| 139. | Grollier, K.; Chefdeville, E.; Jeanneau, E.; Billard, T. Chem. – Eur. J. 2021, 27, 12910–12916. doi:10.1002/chem.202102121 |
| 1. | Smart, B. E. J. Fluorine Chem. 2001, 109, 3–11. doi:10.1016/s0022-1139(01)00375-x |
| 2. | O'Hagan, D. Chem. Soc. Rev. 2008, 37, 308–319. doi:10.1039/b711844a |
| 19. | Chen, X.; Engle, K. M.; Wang, D.-H.; Yu, J.-Q. Angew. Chem., Int. Ed. 2009, 48, 5094–5115. doi:10.1002/anie.200806273 |
| 20. | Giri, R.; Shi, B.-F.; Engle, K. M.; Maugel, N.; Yu, J.-Q. Chem. Soc. Rev. 2009, 38, 3242–3272. doi:10.1039/b816707a |
| 21. | Jazzar, R.; Hitce, J.; Renaudat, A.; Sofack-Kreutzer, J.; Baudoin, O. Chem. – Eur. J. 2010, 16, 2654–2672. doi:10.1002/chem.200902374 |
| 22. | Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 110, 1147–1169. doi:10.1021/cr900184e |
| 23. | Wencel-Delord, J.; Dröge, T.; Liu, F.; Glorius, F. Chem. Soc. Rev. 2011, 40, 4740–4761. doi:10.1039/c1cs15083a |
| 24. | Li, H.; Li, B.-J.; Shi, Z.-J. Catal. Sci. Technol. 2011, 1, 191–206. doi:10.1039/c0cy00076k |
| 25. | Ackermann, L. Chem. Rev. 2011, 111, 1315–1345. doi:10.1021/cr100412j |
| 26. | Kozhushkov, S. I.; Potukuchi, H. K.; Ackermann, L. Catal. Sci. Technol. 2013, 3, 562–571. doi:10.1039/c2cy20505j |
| 27. | Wang, K.; Hu, F.; Zhang, Y.; Wang, J. Sci. China: Chem. 2015, 58, 1252–1265. doi:10.1007/s11426-015-5362-5 |
| 28. | Crabtree, R. H.; Lei, A. Chem. Rev. 2017, 117, 8481–8482. doi:10.1021/acs.chemrev.7b00307 |
| 29. | Pototschnig, G.; Maulide, N.; Schnürch, M. Chem. – Eur. J. 2017, 23, 9206–9232. doi:10.1002/chem.201605657 |
| 30. | Dong, Z.; Ren, Z.; Thompson, S. J.; Xu, Y.; Dong, G. Chem. Rev. 2017, 117, 9333–9403. doi:10.1021/acs.chemrev.6b00574 |
| 31. | Sambiagio, C.; Schönbauer, D.; Blieck, R.; Dao-Huy, T.; Pototschnig, G.; Schaaf, P.; Wiesinger, T.; Zia, M. F.; Wencel-Delord, J.; Besset, T.; Maes, B. U. W.; Schnürch, M. Chem. Soc. Rev. 2018, 47, 6603–6743. doi:10.1039/c8cs00201k |
| 32. | Rej, S.; Ano, Y.; Chatani, N. Chem. Rev. 2020, 120, 1788–1887. doi:10.1021/acs.chemrev.9b00495 |
| 33. | Chen, S.; Ranjan, P.; Voskressensky, L. G.; Van der Eycken, E. V.; Sharma, U. K. Molecules 2020, 25, 4970. doi:10.3390/molecules25214970 |
| 34. | Lam, N. Y. S.; Wu, K.; Yu, J.-Q. Angew. Chem., Int. Ed. 2021, 60, 15767–15790. doi:10.1002/anie.202011901 |
| 35. | Jacob, C.; Maes, B. U. W.; Evano, G. Chem. – Eur. J. 2021, 27, 13899–13952. doi:10.1002/chem.202101598 |
| 36. | Dhawa, U.; Kaplaneris, N.; Ackermann, L. Org. Chem. Front. 2021, 8, 4886–4913. doi:10.1039/d1qo00727k |
| 37. | Dalton, T.; Faber, T.; Glorius, F. ACS Cent. Sci. 2021, 7, 245–261. doi:10.1021/acscentsci.0c01413 |
| 38. | Dutta, U.; Maiti, S.; Bhattacharya, T.; Maiti, D. Science 2021, 372, 658–663. doi:10.1126/science.abd5992 |
| 39. | Zhang, J.; Lu, X.; Shen, C.; Xu, L.; Ding, L.; Zhong, G. Chem. Soc. Rev. 2021, 50, 3263–3314. doi:10.1039/d0cs00447b |
| 40. | Rej, S.; Das, A.; Chatani, N. Coord. Chem. Rev. 2021, 431, 213683. doi:10.1016/j.ccr.2020.213683 |
| 41. | Ahmad, M. S.; Meguellati, K. ChemistrySelect 2022, 7, e202103716. doi:10.1002/slct.202103716 |
| 42. | Phukon, J.; Jyoti Borah, A.; Gogoi, S. Asian J. Org. Chem. 2022, 11, e202200581. doi:10.1002/ajoc.202200581 |
| 43. | Mondal, A.; van Gemmeren, M. Angew. Chem., Int. Ed. 2022, 61, e202210825. doi:10.1002/anie.202210825 |
| 114. | Tran, L. D.; Popov, I.; Daugulis, O. J. Am. Chem. Soc. 2012, 134, 18237–18240. doi:10.1021/ja3092278 |
| 162. | Shinde, V. N.; Rangan, K.; Kumar, D.; Kumar, A. J. Org. Chem. 2021, 86, 9755–9770. doi:10.1021/acs.joc.1c01097 |
| 2. | O'Hagan, D. Chem. Soc. Rev. 2008, 37, 308–319. doi:10.1039/b711844a |
| 5. | Landelle, G.; Panossian, A.; Pazenok, S.; Vors, J.-P.; Leroux, F. R. Beilstein J. Org. Chem. 2013, 9, 2476–2536. doi:10.3762/bjoc.9.287 |
| 12. | Landelle, G.; Panossian, A.; Leroux, F. R. Curr. Top. Med. Chem. 2014, 14, 941–951. doi:10.2174/1568026614666140202210016 |
| 13. | Besset, T.; Poisson, T.; Pannecoucke, X. Chem. – Eur. J. 2014, 20, 16830–16845. doi:10.1002/chem.201404537 |
| 14. | Merino, E.; Nevado, C. Chem. Soc. Rev. 2014, 43, 6598–6608. doi:10.1039/c4cs00025k |
| 15. | Champagne, P. A.; Desroches, J.; Hamel, J.-D.; Vandamme, M.; Paquin, J.-F. Chem. Rev. 2015, 115, 9073–9174. doi:10.1021/cr500706a |
| 16. | Ni, C.; Hu, J. Chem. Soc. Rev. 2016, 45, 5441–5454. doi:10.1039/c6cs00351f |
| 17. | Pan, Y. ACS Med. Chem. Lett. 2019, 10, 1016–1019. doi:10.1021/acsmedchemlett.9b00235 |
| 18. | Nobile, E.; Castanheiro, T.; Besset, T. Angew. Chem., Int. Ed. 2021, 60, 12170–12191. doi:10.1002/anie.202009995 |
| 115. | Tyrra, W.; Naumann, D.; Hoge, B.; Yagupolskii, Y. L. J. Fluorine Chem. 2003, 119, 101–107. doi:10.1016/s0022-1139(02)00276-2 |
| 116. | Scattolin, T.; Pu, M.; Schoenebeck, F. Chem. – Eur. J. 2018, 24, 567–571. doi:10.1002/chem.201705240 |
| 6. | Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320–330. doi:10.1039/b610213c |
| 7. | Berger, R.; Resnati, G.; Metrangolo, P.; Weber, E.; Hulliger, J. Chem. Soc. Rev. 2011, 40, 3496–3508. doi:10.1039/c0cs00221f |
| 8. | Fujiwara, T.; O’Hagan, D. J. Fluorine Chem. 2014, 167, 16–29. doi:10.1016/j.jfluchem.2014.06.014 |
| 9. | Ilardi, E. A.; Vitaku, E.; Njardarson, J. T. J. Med. Chem. 2014, 57, 2832–2842. doi:10.1021/jm401375q |
| 10. | Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315–8359. doi:10.1021/acs.jmedchem.5b00258 |
| 11. | Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J. L.; Soloshonok, V. A.; Izawa, K.; Liu, H. Chem. Rev. 2016, 116, 422–518. doi:10.1021/acs.chemrev.5b00392 |
| 81. | Toulgoat, F.; Alazet, S.; Billard, T. Eur. J. Org. Chem. 2014, 2415–2428. doi:10.1002/ejoc.201301857 |
| 82. | Xu, X.-H.; Matsuzaki, K.; Shibata, N. Chem. Rev. 2015, 115, 731–764. doi:10.1021/cr500193b |
| 83. | Barata-Vallejo, S.; Bonesi, S.; Postigo, A. Org. Biomol. Chem. 2016, 14, 7150–7182. doi:10.1039/c6ob00763e |
| 84. | Li, M.; Guo, J.; Xue, X.-S.; Cheng, J.-P. Org. Lett. 2016, 18, 264–267. doi:10.1021/acs.orglett.5b03433 |
| 85. | Zheng, H.; Huang, Y.; Weng, Z. Tetrahedron Lett. 2016, 57, 1397–1409. doi:10.1016/j.tetlet.2016.02.073 |
| 86. | Boiko, V. N. Beilstein J. Org. Chem. 2010, 6, 880–921. doi:10.3762/bjoc.6.88 |
| 87. | Li, X.; Zhao, J.; Zhang, L.; Hu, M.; Wang, L.; Hu, J. Org. Lett. 2015, 17, 298–301. doi:10.1021/ol5034018 |
| 88. | Ye, K.-Y.; Zhang, X.; Dai, L.-X.; You, S.-L. J. Org. Chem. 2014, 79, 12106–12110. doi:10.1021/jo5019393 |
| 89. | Lefebvre, Q.; Fava, E.; Nikolaienko, P.; Rueping, M. Chem. Commun. 2014, 50, 6617–6619. doi:10.1039/c4cc02060j |
| 90. | Liu, J.-B.; Xu, X.-H.; Chen, Z.-H.; Qing, F.-L. Angew. Chem., Int. Ed. 2015, 54, 897–900. doi:10.1002/anie.201409983 |
| 91. | Jiang, L.; Qian, J.; Yi, W.; Lu, G.; Cai, C.; Zhang, W. Angew. Chem., Int. Ed. 2015, 54, 14965–14969. doi:10.1002/anie.201508495 |
| 92. | Zheng, J.; Wang, L.; Lin, J.-H.; Xiao, J.-C.; Liang, S. H. Angew. Chem., Int. Ed. 2015, 54, 13236–13240. doi:10.1002/anie.201505446 |
| 93. | Yin, G.; Kalvet, I.; Schoenebeck, F. Angew. Chem., Int. Ed. 2015, 54, 6809–6813. doi:10.1002/anie.201501617 |
| 94. | Candish, L.; Pitzer, L.; Gómez-Suárez, A.; Glorius, F. Chem. – Eur. J. 2016, 22, 4753–4756. doi:10.1002/chem.201600421 |
| 95. | Matheis, C.; Wagner, V.; Goossen, L. J. Chem. – Eur. J. 2016, 22, 79–82. doi:10.1002/chem.201503524 |
| 96. | Jarrige, L.; Carboni, A.; Dagousset, G.; Levitre, G.; Magnier, E.; Masson, G. Org. Lett. 2016, 18, 2906–2909. doi:10.1021/acs.orglett.6b01257 |
| 97. | Liu, X.; An, R.; Zhang, X.; Luo, J.; Zhao, X. Angew. Chem., Int. Ed. 2016, 55, 5846–5850. doi:10.1002/anie.201601713 |
| 98. | Xiong, H.-Y.; Pannecoucke, X.; Besset, T. Org. Chem. Front. 2016, 3, 620–624. doi:10.1039/c6qo00064a |
| 99. | Yang, Y.; Xu, L.; Yu, S.; Liu, X.; Zhang, Y.; Vicic, D. A. Chem. – Eur. J. 2016, 22, 858–863. doi:10.1002/chem.201504790 |
| 100. | Wang, F.; Zhao, L.; You, J.; Wang, M.-X. Org. Chem. Front. 2016, 3, 880–886. doi:10.1039/c6qo00161k |
| 101. | Bu, M.-j.; Lu, G.-p.; Cai, C. Org. Chem. Front. 2017, 4, 266–270. doi:10.1039/c6qo00622a |
| 102. | Lübcke, M.; Yuan, W.; Szabó, K. J. Org. Lett. 2017, 19, 4548–4551. doi:10.1021/acs.orglett.7b02139 |
| 103. | Carbonnel, E.; Besset, T.; Poisson, T.; Labar, D.; Pannecoucke, X.; Jubault, P. Chem. Commun. 2017, 53, 5706–5709. doi:10.1039/c7cc02652h |
| 104. | Zhao, Q.; Poisson, T.; Pannecoucke, X.; Bouillon, J.-P.; Besset, T. Org. Lett. 2017, 19, 5106–5109. doi:10.1021/acs.orglett.7b02384 |
| 105. | Gelat, F.; Poisson, T.; Biju, A. T.; Pannecoucke, X.; Besset, T. Eur. J. Org. Chem. 2018, 3693–3696. doi:10.1002/ejoc.201800418 |
| 106. | Zhao, Q.; Chen, M.-Y.; Poisson, T.; Pannecoucke, X.; Bouillon, J.-P.; Besset, T. Eur. J. Org. Chem. 2018, 6167–6175. doi:10.1002/ejoc.201801071 |
| 107. | Ghiazza, C.; Khrouz, L.; Monnereau, C.; Billard, T.; Tlili, A. Chem. Commun. 2018, 54, 9909–9912. doi:10.1039/c8cc05256e |
| 108. | Saravanan, P.; Anbarasan, P. Adv. Synth. Catal. 2018, 360, 2894–2899. doi:10.1002/adsc.201800366 |
| 109. | Xi, C.-C.; Chen, Z.-M.; Zhang, S.-Y.; Tu, Y.-Q. Org. Lett. 2018, 20, 4227–4230. doi:10.1021/acs.orglett.8b01627 |
| 110. | He, J.; Chen, C.; Fu, G. C.; Peters, J. C. ACS Catal. 2018, 8, 11741–11748. doi:10.1021/acscatal.8b04094 |
| 111. | Lindberg, E.; Angerani, S.; Anzola, M.; Winssinger, N. Nat. Commun. 2018, 9, 3539. doi:10.1038/s41467-018-05916-9 |
| 112. | Zhang, J.; Yang, J.-D.; Zheng, H.; Xue, X.-S.; Mayr, H.; Cheng, J.-P. Angew. Chem., Int. Ed. 2018, 57, 12690–12695. doi:10.1002/anie.201805859 |
| 113. | Luo, Z.; Yang, X.; Tsui, G. C. Org. Lett. 2020, 22, 6155–6159. doi:10.1021/acs.orglett.0c02235 |
| 159. | Kauffman, G. B. Angew. Chem., Int. Ed. 2008, 47, 8155–8156. doi:10.1002/anie.200885643 |
| 160. | Shuklov, I. A.; Dubrovina, N. V.; Börner, A. Synthesis 2007, 2925–2943. doi:10.1055/s-2007-983902 |
| 3. | Inoue, M.; Sumii, Y.; Shibata, N. ACS Omega 2020, 5, 10633–10640. doi:10.1021/acsomega.0c00830 |
| 4. | Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432–2506. doi:10.1021/cr4002879 |
| 5. | Landelle, G.; Panossian, A.; Pazenok, S.; Vors, J.-P.; Leroux, F. R. Beilstein J. Org. Chem. 2013, 9, 2476–2536. doi:10.3762/bjoc.9.287 |
| 114. | Tran, L. D.; Popov, I.; Daugulis, O. J. Am. Chem. Soc. 2012, 134, 18237–18240. doi:10.1021/ja3092278 |
| 161. | Dick, A. R.; Hull, K. L.; Sanford, M. S. J. Am. Chem. Soc. 2004, 126, 2300–2301. doi:10.1021/ja031543m |
| 60. | Liang, T.; Neumann, C. N.; Ritter, T. Angew. Chem., Int. Ed. 2013, 52, 8214–8264. doi:10.1002/anie.201206566 |
| 61. | Lin, A.; Huehls, C. B.; Yang, J. Org. Chem. Front. 2014, 1, 434–438. doi:10.1039/c4qo00020j |
| 62. | Li, Y.; Wu, Y.; Li, G.-S.; Wang, X.-S. Adv. Synth. Catal. 2014, 356, 1412–1418. doi:10.1002/adsc.201400101 |
| 63. | Campbell, M. G.; Hoover, A. J.; Ritter, T. Transition Metal-Mediated and Metal-Catalyzed Carbon–Fluorine Bond Formation. In Organometallic Fluorine Chemistry; Braun, T.; Hughes, R. P., Eds.; Springer International Publishing: Cham, 2015; pp 1–53. doi:10.1007/3418_2014_88 |
| 64. | Szpera, R.; Moseley, D. F. J.; Smith, L. B.; Sterling, A. J.; Gouverneur, V. Angew. Chem., Int. Ed. 2019, 58, 14824–14848. doi:10.1002/anie.201814457 |
| 65. | McMurtrey, K. B.; Racowski, J. M.; Sanford, M. S. Org. Lett. 2012, 14, 4094–4097. doi:10.1021/ol301739f |
| 66. | Wu, T.; Cheng, J.; Chen, P.; Liu, G. Chem. Commun. 2013, 49, 8707–8709. doi:10.1039/c3cc44711a |
| 67. | Lou, S.-J.; Xu, D.-Q.; Xia, A.-B.; Wang, Y.-F.; Liu, Y.-K.; Du, X.-H.; Xu, Z.-Y. Chem. Commun. 2013, 49, 6218–6220. doi:10.1039/c3cc42220h |
| 68. | Brückl, T.; Baxter, R. D.; Ishihara, Y.; Baran, P. S. Acc. Chem. Res. 2012, 45, 826–839. doi:10.1021/ar200194b |
| 69. | Wang, X.; Truesdale, L.; Yu, J.-Q. J. Am. Chem. Soc. 2010, 132, 3648–3649. doi:10.1021/ja909522s |
| 70. | Ye, Y.; Ball, N. D.; Kampf, J. W.; Sanford, M. S. J. Am. Chem. Soc. 2010, 132, 14682–14687. doi:10.1021/ja107780w |
| 71. | Zhang, S.-Y.; He, G.; Zhao, Y.; Wright, K.; Nack, W. A.; Chen, G. J. Am. Chem. Soc. 2012, 134, 7313–7316. doi:10.1021/ja3023972 |
| 72. | Zhang, L.-S.; Chen, K.; Chen, G.; Li, B.-J.; Luo, S.; Guo, Q.-Y.; Wei, J.-B.; Shi, Z.-J. Org. Lett. 2013, 15, 10–13. doi:10.1021/ol302814x |
| 73. | Miura, M.; Feng, C.-G.; Ma, S.; Yu, J.-Q. Org. Lett. 2013, 15, 5258–5261. doi:10.1021/ol402471y |
| 74. | Zou, L.; Li, P.; Wang, B.; Wang, L. Chem. Commun. 2019, 55, 3737–3740. doi:10.1039/c9cc01014a |
| 75. | Cai, S.; Chen, C.; Sun, Z.; Xi, C. Chem. Commun. 2013, 49, 4552–4554. doi:10.1039/c3cc41331d |
| 76. | Feng, C.; Loh, T.-P. Angew. Chem., Int. Ed. 2013, 52, 12414–12417. doi:10.1002/anie.201307245 |
| 77. | Besset, T.; Cahard, D.; Pannecoucke, X. J. Org. Chem. 2014, 79, 413–418. doi:10.1021/jo402385g |
| 78. | Vuagnat, M.; Jubault, P.; Besset, T. Pd-Catalyzed C–Chalcogen Bond Formation (C–S, C–Se) by C–H Bond Activation. In Handbook of CH-Functionalization; Maiti, D., Ed.; Wiley-VCH: Germany, 2022; pp 1–25. doi:10.1002/9783527834242.chf0017 |
| 156. | Matheson, A. J.; Jarvis, B. Drugs 2001, 61, 1801–1833. doi:10.2165/00003495-200161120-00011 |
| 42. | Phukon, J.; Jyoti Borah, A.; Gogoi, S. Asian J. Org. Chem. 2022, 11, e202200581. doi:10.1002/ajoc.202200581 |
| 54. | Chen, D. Y.-K.; Youn, S. W. Chem. – Eur. J. 2012, 18, 9452–9474. doi:10.1002/chem.201201329 |
| 55. | Yamaguchi, J.; Yamaguchi, A. D.; Itami, K. Angew. Chem., Int. Ed. 2012, 51, 8960–9009. doi:10.1002/anie.201201666 |
| 56. | Qiu, Y.; Gao, S. Nat. Prod. Rep. 2016, 33, 562–581. doi:10.1039/c5np00122f |
| 57. | Basu, D.; Kumar, S.; V, S. S.; Bandichhor, R. J. Chem. Sci. 2018, 130, 71. doi:10.1007/s12039-018-1468-6 |
| 58. | Karimov, R. R.; Hartwig, J. F. Angew. Chem., Int. Ed. 2018, 57, 4234–4241. doi:10.1002/anie.201710330 |
| 59. | Baudoin, O. Angew. Chem., Int. Ed. 2020, 59, 17798–17809. doi:10.1002/anie.202001224 |
| 79. | Leo, A.; Hansch, C.; Elkins, D. Chem. Rev. 1971, 71, 525–616. doi:10.1021/cr60274a001 |
| 80. | Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165–195. doi:10.1021/cr00002a004 |
| 157. | Liu, B.; Shi, B.-F. Tetrahedron Lett. 2015, 56, 15–22. doi:10.1016/j.tetlet.2014.11.039 |
| 158. | Enthaler, S.; Company, A. Chem. Soc. Rev. 2011, 40, 4912–4924. doi:10.1039/c1cs15085e |
| 51. | Cernak, T.; Dykstra, K. D.; Tyagarajan, S.; Vachal, P.; Krska, S. W. Chem. Soc. Rev. 2016, 45, 546–576. doi:10.1039/c5cs00628g |
| 52. | Moir, M.; Danon, J. J.; Reekie, T. A.; Kassiou, M. Expert Opin. Drug Discovery 2019, 14, 1137–1149. doi:10.1080/17460441.2019.1653850 |
| 53. | Börgel, J.; Ritter, T. Chem 2020, 6, 1877–1887. doi:10.1016/j.chempr.2020.07.007 |
| 71. | Zhang, S.-Y.; He, G.; Zhao, Y.; Wright, K.; Nack, W. A.; Chen, G. J. Am. Chem. Soc. 2012, 134, 7313–7316. doi:10.1021/ja3023972 |
| 141. | Huang, Y.; Huang, R.; Weng, Z. Synlett 2015, 26, 2327–2331. doi:10.1055/s-0035-1560054 |
| 142. | Pethő, B.; Novák, Z. Asian J. Org. Chem. 2019, 8, 568–575. doi:10.1002/ajoc.201800414 |
| 143. | Jeschke, P.; Baston, E.; Leroux, R. F. Mini-Rev. Med. Chem. 2007, 7, 1027–1034. doi:10.2174/138955707782110150 |
| 144. | Idoux, J. P.; Madenwald, M. L.; Garcia, B. S.; Chu, D. L.; Gupton, J. T. J. Org. Chem. 1985, 50, 1876–1878. doi:10.1021/jo00211a018 |
| 145. | Shen, X.; Neumann, C. N.; Kleinlein, C.; Goldberg, N. W.; Ritter, T. Angew. Chem., Int. Ed. 2015, 54, 5662–5665. doi:10.1002/anie.201500902 |
| 146. | Pethő, B.; Zwillinger, M.; Csenki, J. T.; Káncz, A. E.; Krámos, B.; Müller, J.; Balogh, G. T.; Novák, Z. Chem. – Eur. J. 2017, 23, 15628–15632. doi:10.1002/chem.201704205 |
| 147. | Vuluga, D.; Legros, J.; Crousse, B.; Bonnet-Delpon, D. Eur. J. Org. Chem. 2009, 3513–3518. doi:10.1002/ejoc.200900303 |
| 148. | Zhang, K.; Xu, X.-H.; Qing, F.-L. J. Fluorine Chem. 2017, 196, 24–31. doi:10.1016/j.jfluchem.2016.07.008 |
| 149. | Roane, J.; Daugulis, O. Org. Lett. 2013, 15, 5842–5845. doi:10.1021/ol402904d |
| 150. | Yang, L.; Li, S.; Cai, L.; Ding, Y.; Fu, L.; Cai, Z.; Ji, H.; Li, G. Org. Lett. 2017, 19, 2746–2749. doi:10.1021/acs.orglett.7b01103 |
| 151. | Maiti, S.; Alam, T.; Mal, P. Asian J. Org. Chem. 2018, 7, 715–719. doi:10.1002/ajoc.201800069 |
| 152. | Xu, X.; Xia, C.; Li, X.; Sun, J.; Hao, L. RSC Adv. 2020, 10, 2016–2026. doi:10.1039/c9ra10194b |
| 153. | Jin, L.; Zhang, X.-L.; Guo, R.-L.; Wang, M.-Y.; Gao, Y.-R.; Wang, Y.-Q. Org. Lett. 2021, 23, 1921–1927. doi:10.1021/acs.orglett.1c00365 |
| 44. | Petrone, D. A.; Ye, J.; Lautens, M. Chem. Rev. 2016, 116, 8003–8104. doi:10.1021/acs.chemrev.6b00089 |
| 45. | Li, X.; Shi, X.; Li, X.; Shi, D. Beilstein J. Org. Chem. 2019, 15, 2213–2270. doi:10.3762/bjoc.15.218 |
| 46. | Mazeh, S.; Lapuh, M. I.; Besset, T. Chimia 2020, 74, 871. doi:10.2533/chimia.2020.871 |
| 47. | Egami, H. Chem. Pharm. Bull. 2020, 68, 491–511. doi:10.1248/cpb.c19-00856 |
| 48. | Ruyet, L.; Besset, T. Beilstein J. Org. Chem. 2020, 16, 1051–1065. doi:10.3762/bjoc.16.92 |
| 49. | Sindhe, H.; Chaudhary, B.; Chowdhury, N.; Kamble, A.; Kumar, V.; Lad, A.; Sharma, S. Org. Chem. Front. 2022, 9, 1742–1775. doi:10.1039/d1qo01544c |
| 50. | Liu, H.; Gu, Z.; Jiang, X. Adv. Synth. Catal. 2013, 355, 617–626. doi:10.1002/adsc.201200764 |
| 49. | Sindhe, H.; Chaudhary, B.; Chowdhury, N.; Kamble, A.; Kumar, V.; Lad, A.; Sharma, S. Org. Chem. Front. 2022, 9, 1742–1775. doi:10.1039/d1qo01544c |
| 154. | Tamargo, J.; Le Heuzey, J.-Y.; Mabo, P. Eur. J. Clin. Pharmacol. 2015, 71, 549–567. doi:10.1007/s00228-015-1832-0 |
| 155. | Paolini, E.; Stronati, G.; Guerra, F.; Capucci, A. Pharmacol. Res. 2019, 148, 104443. doi:10.1016/j.phrs.2019.104443 |
| 100. | Wang, F.; Zhao, L.; You, J.; Wang, M.-X. Org. Chem. Front. 2016, 3, 880–886. doi:10.1039/c6qo00161k |
| 100. | Wang, F.; Zhao, L.; You, J.; Wang, M.-X. Org. Chem. Front. 2016, 3, 880–886. doi:10.1039/c6qo00161k |
| 164. | Rajesh, N.; Sundararaju, B. Asian J. Org. Chem. 2018, 7, 1368–1371. doi:10.1002/ajoc.201800286 |
| 165. | Zhang, L.-B.; Hao, X.-Q.; Zhang, S.-K.; Liu, Z.-J.; Zheng, X.-X.; Gong, J.-F.; Niu, J.-L.; Song, M.-P. Angew. Chem., Int. Ed. 2015, 54, 272–275. doi:10.1002/anie.201409751 |
| 166. | Zhang, T.; Zhu, H.; Yang, F.; Wu, Y.; Wu, Y. Tetrahedron 2019, 75, 1541–1547. doi:10.1016/j.tet.2019.02.002 |
| 167. | Guo, X.-K.; Zhang, L.-B.; Wei, D.; Niu, J.-L. Chem. Sci. 2015, 6, 7059–7071. doi:10.1039/c5sc01807b |
| 149. | Roane, J.; Daugulis, O. Org. Lett. 2013, 15, 5842–5845. doi:10.1021/ol402904d |
| 163. | Selvakumar, J.; Grandhi, G. S.; Sahoo, H.; Baidya, M. RSC Adv. 2016, 6, 79361–79365. doi:10.1039/c6ra18861c |
| 120. | Xu, J.; Chen, P.; Ye, J.; Liu, G. Acta Chim. Sin. (Chin. Ed.) 2015, 73, 1294–1297. doi:10.6023/a15030211 |
| 121. | Kesavan, A.; Chaitanya, M.; Anbarasan, P. Eur. J. Org. Chem. 2018, 3276–3279. doi:10.1002/ejoc.201800451 |
| 120. | Xu, J.; Chen, P.; Ye, J.; Liu, G. Acta Chim. Sin. (Chin. Ed.) 2015, 73, 1294–1297. doi:10.6023/a15030211 |
| 169. | Sato, T.; Nogi, K.; Yorimitsu, H. ChemCatChem 2020, 12, 3467–3471. doi:10.1002/cctc.202000485 |
| 120. | Xu, J.; Chen, P.; Ye, J.; Liu, G. Acta Chim. Sin. (Chin. Ed.) 2015, 73, 1294–1297. doi:10.6023/a15030211 |
| 169. | Sato, T.; Nogi, K.; Yorimitsu, H. ChemCatChem 2020, 12, 3467–3471. doi:10.1002/cctc.202000485 |
| 119. | Yin, W.; Wang, Z.; Huang, Y. Adv. Synth. Catal. 2014, 356, 2998–3006. doi:10.1002/adsc.201400362 |
| 150. | Yang, L.; Li, S.; Cai, L.; Ding, Y.; Fu, L.; Cai, Z.; Ji, H.; Li, G. Org. Lett. 2017, 19, 2746–2749. doi:10.1021/acs.orglett.7b01103 |
| 119. | Yin, W.; Wang, Z.; Huang, Y. Adv. Synth. Catal. 2014, 356, 2998–3006. doi:10.1002/adsc.201400362 |
| 150. | Yang, L.; Li, S.; Cai, L.; Ding, Y.; Fu, L.; Cai, Z.; Ji, H.; Li, G. Org. Lett. 2017, 19, 2746–2749. doi:10.1021/acs.orglett.7b01103 |
| 118. | Whitfield, S. R.; Sanford, M. S. J. Am. Chem. Soc. 2007, 129, 15142–15143. doi:10.1021/ja077866q |
| 168. | Kumar, S.; Nair, A. M.; Volla, C. M. R. Chem. – Asian J. 2022, 17, e202200801. doi:10.1002/asia.202200801 |
| 149. | Roane, J.; Daugulis, O. Org. Lett. 2013, 15, 5842–5845. doi:10.1021/ol402904d |
© 2023 Monsigny et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.