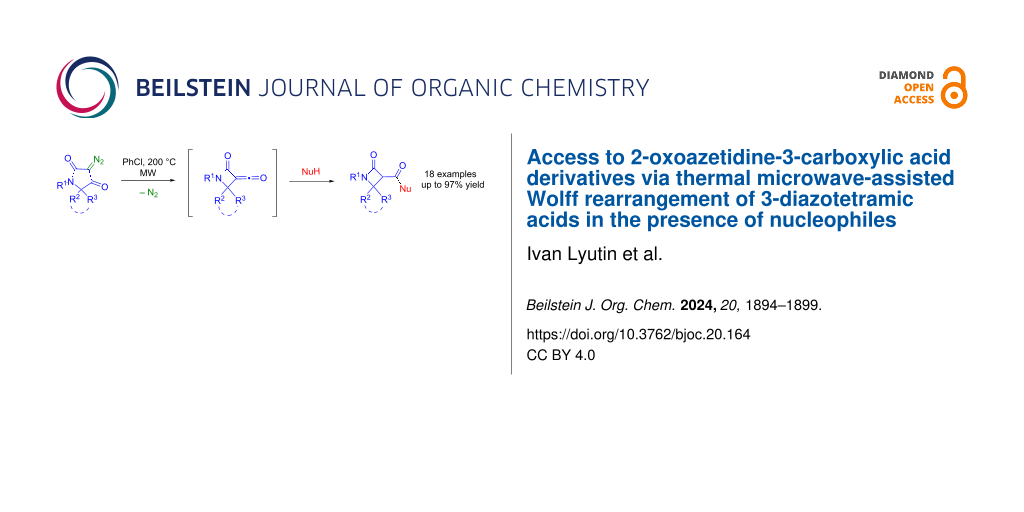
Abstract
In this work, we report an efficient approach to 2-oxoazetidine-3-carboxylic acid derivatives based on a thermally promoted Wolff rearrangement of diazotetramic acids in the presence of nucleophiles. The method allows easy variation of the substituent in the exocyclic acyl group by introducing different N-, O-, and S-nucleophilic reagents into the reaction. The reaction of chiral diazotetramic acids leads exclusively to trans-diastereomeric β-lactams. The use of variously substituted diazotetramic acids, including spirocyclic derivatives, as well as a wide range of nucleophiles provides access to a structural diversity of medically relevant 2-oxoazetidine-3-carboxylic acid amides and esters.
Graphical Abstract
Introduction
The importance of the β-lactam (azetidin-2-one) scaffold to medicinal chemistry and drug design is self-evident. This four-membered heterocycle is a key fragment of many antibiotics [1], including penicillin and its analogues, as well as other pharmacologically important molecules [2]. Therefore, the search for new efficient and versatile methods for the preparation of structurally diverse β-lactam derivatives is of great importance and relevance.
Continuing the investigation of the reactivity and synthetic potential of diazotetramic acids (1), we have recently shown that these diazo reagents can act as precursors of β-lactam ketenes 2 generated by a thermally promoted Wolff rearrangement [3]. The interaction of such ketenes with nucleophiles of different nature could serve as a source of libraries of structurally diverse 2-oxoazetidine-3-carboxylic acid derivatives 3 (Scheme 1).
Scheme 1: Synthetic routes to 2-oxoazetidine-3-carboxylic acid derivatives.
Scheme 1: Synthetic routes to 2-oxoazetidine-3-carboxylic acid derivatives.
The 2-oxoazetidine-3-carboxylic acid derivatives (mainly amides) exhibit various types of biological activity, among which the following can be highlighted: inhibition of β-lactamases [4,5], antitubercular properties [6], antiproliferative and antibacterial activity [7], herbicidal properties [8,9], inhibition of neutral amino acid transporter (SLC6A19) [10]. Hence, developing new synthetic methods to create structurally diverse 2-oxoazetidine-3-carboxylic acid derivatives is a highly valuable endeavour that could have a positive impact on future drug discovery.
Most synthetic approaches to amides and esters of 2-oxoazetidine-3-carboxylic acids reported in the literature are based on the construction of the β-lactam ring (Scheme 1). The main methods include the [2 + 2] cycloaddition of acyl ketenes, generated by various methods, with imines [11-14] and the Wolff rearrangement of γ-amino-α-diazo-β-ketoesters followed by intramolecular cyclization [15,16]. Additionally, the manganese(III)-promoted cyclization of N-alkenyl malonamides [17,18] and the Cu(I)-catalyzed reaction of propiolic acid derivatives with nitrones (Kinugasa reaction) [19-21] should also be mentioned, as well as intramolecular C–H insertion using diazomonomalonamides under the action of various catalysts which is a very efficient method for preparing β-lactam esters [22-25].
At the same time, from the point of view of easy variation of the substituent in the exocyclic acyl group (RX), a method allowing the introduction of this moiety at the last step of the synthesis would be of great demand. We proposed that, besides modifying the 2-oxoazetidine-3-carboxylic acids themselves, such an approach could involve diazotetramic acids, subjected to thermal Wolff rearrangement, with various nucleophiles.
The application of the Wolff rearrangement in organic synthesis as a route to generate ketenes is being actively investigated, involving both acyclic and carbocyclic diazocarbonyl compounds [26]. At the same time, the use of diazoheterocyclic reagents (including diazotetramic acids) in this transformation, with the formation of heterocyclic ring contraction products, is represented in the literature only by isolated examples [27-31]. In addition, photoinitiation is mainly used, while the possibilities of thermolysis remain virtually unexplored.
Herein, we report our findings obtained while investigating a synthetic approach to 2-oxoazetidine-3-carboxylic acid derivatives based on the thermally promoted Wolff rearrangement of diazotetramic acids.
Results and Discussion
Diazotetramic acid derivatives 1 are available in a wide variety using the techniques described previously [32]. The conditions for their thermal decomposition were tested in a previous study [3]. The reaction requires rather severe heating under microwave irradiation (200 °C, chlorobenzene, sealed vial), ensuring complete conversion of the diazo compound in a rather short time.
Initial experiments using p-anisidine as a nucleophile showed that the target β-lactam derivative 3a could be obtained in high yield (83%) after simple chromatographic separation of the reaction mixture (Scheme 2). When the synthesis was carried out using conventional heating in 1,2-dichlorobenzene (200 °C, 1 h), product 3a was obtained in slightly lower yield (75%), so further experiments were carried out using microwave activation. We then introduced various aromatic and aliphatic amines as well as alcohols and mercaptans into the reaction. In order to demonstrate the structural diversity of the compounds obtained, a wide range of diazotetramic acids 1 of different structures was used. It can be observed that the 5-monosubstituted diazo derivatives, and especially those with no substituents in position 5, form the target products in lower, often moderate yields (see products 3i,j,n and 3r,s,t) compared to the 5,5-disubstituted (spirocyclic) analogues. This result may be related to the lower stability of the less-substituted β-lactam derivatives under thermolysis conditions. Additional alkyl substituents sterically shield the ring and prevent an unwanted nucleophilic attack leading to product degradation.
Scheme 2: Scope of diazotetramic acids 1 thermolysis in the presence of various nucleophiles. PMP = p-methoxyphenyl, PCP = p-chlorophenyl, PMB = p-methoxybenzyl, PFB = p-fluorobenzyl; reaction scale – 0.25 mmol; ascaled-up (1.5 mmol) yield.
Scheme 2: Scope of diazotetramic acids 1 thermolysis in the presence of various nucleophiles. PMP = p-methoxy...
In reactions with alcohols and mercaptans, the corresponding esters 3k–n,r and thioesters 3o–q were successfully obtained in moderate to high yields. The synthesis of compound 3k was additionally carried out on a scaled-up experiment (1.5 mmol vs 0.25 mmol), allowing sufficient amounts to be obtained for further modifications (vide infra). However, a marked decrease in the yield (78% vs 96%) was observed upon scaling up.
In the case of products with two stereogenic centers (3r–t), the formation of a single trans-diastereomer was observed. According to literature data, the vicinal coupling constants in the 3,4-disubsituted β-lactam cycle have characteristic values for the two diastereomers, lying in the intervals 5.5–6.0 Hz and 1.5–2.5 Hz for the cis and trans forms, respectively [33,34]. This makes it easy to assign the stereochemistry of the products obtained. Additional confirmation was gained from X-ray analysis data for structure 3t (Scheme 2).
In some cases, we were unable to isolate the target product of the reaction, which was either observed in trace amounts or was not detected in the reaction mixture at all, making it extremely difficult to interpret (Scheme 3). Negative results were observed for dimethyl and tosylhydrazine, N-ethylpiperazine, and β-methoxyethylamine. Attempts to obtain directly 2-oxoazetidine carboxylic acid (or its decarboxylation product) or its trifluoroethyl ester by running the synthesis with water or trifluoroethanol were also unsuccessful. Acylation of the π-excessive double bonds of N-alkylindole and dihydropyran by the in situ-generated ketene, previously described using carbocyclic diazodiketones [35] were also unsuccessful. Of the diazotetramic acids, only the spiro adamantane derivative 1m was not able to form the desired β-lactam. These reactions gave complex mixtures of unidentified products.
Scheme 3: Negative results with several N-, O-, and C-nucleophiles and with diazo reagent 1m.
Scheme 3: Negative results with several N-, O-, and C-nucleophiles and with diazo reagent 1m.
The benzyl esters 3k and 3n were converted into the corresponding acids 4a,b by hydrogenolysis under mild conditions, which proceeded in quantitative yields (Scheme 4). It should be noted that this method of preparing β-lactam acids compares favorably with the alkaline hydrolysis of their methyl and ethyl esters, which does not always give high yields of the target compounds. When stored individually or in solution at room temperature, the acids 4 gradually decompose and undergo decarboxylation and other accompanying processes. The example of acid 4a demonstrates the possibility of easy amidation to form new β-lactam derivatives 3s and 3t (Scheme 4).
Scheme 4: Preparation of acids 4 by hydrogenolysis of benzyl esters and examples of acid 4a amidation.
Scheme 4: Preparation of acids 4 by hydrogenolysis of benzyl esters and examples of acid 4a amidation.
Conclusion
We have developed a straightforward access to 2-oxoazetidine-3-carboxylic acid derivatives based on the thermally promoted Wolff rearrangement of diazotetramic acids in the presence of different nucleophiles. The proposed method allows easy variation of the substituent at the exocyclic carbonyl group by preformed ring contraction and interaction of the intermediate ketene with the selected nucleophile. Various aromatic and aliphatic amines as well as alcohols and thiols can be used as nucleophiles. 5-Monosubstituted diazotetramic acids give exclusively trans-diastereomeric β-lactam products. The use of variously substituted diazotetramic acids, including their spirocyclic derivatives, provides access to a new structural diversity of medically relevant β-lactam derivatives. The possibility of transforming the obtained benzyl esters into 2-oxoazetidine-3-carboxylic acids and their subsequent amidation has been demonstrated.
Supporting Information
Deposition Number CCDC 2323689 (for 3t) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service http://www.ccdc.cam.ac.uk/structures.
| Supporting Information File 1: General experimental information, X-ray crystallographic data, synthetic procedures, analytical data and NMR spectra for the reported compounds. | ||
| Format: PDF | Size: 5.1 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information to this article.
References
-
Lima, L. M.; Silva, B. N. M. d.; Barbosa, G.; Barreiro, E. J. Eur. J. Med. Chem. 2020, 208, 112829. doi:10.1016/j.ejmech.2020.112829
Return to citation in text: [1] -
Fisher, J. F.; Mobashery, S. The β-Lactam (Azetidin-2-one) as a Privileged Ring in Medicinal Chemistry. In Privileged Scaffolds in Medicinal Chemistry: Design, Synthesis, Evaluation; Bräse, S., Ed.; Drug Discovery; The Royal Society of Chemistry: Cambridge, UK, 2015; pp 64–97. doi:10.1039/9781782622246-00064
Return to citation in text: [1] -
Krivovicheva, V.; Lyutin, I.; Kantin, G.; Dar’in, D. J. Org. Chem. 2024, 89, 3585–3589. doi:10.1021/acs.joc.3c02494
Return to citation in text: [1] [2] -
Adediran, S. A.; Lohier, J.-F.; Cabaret, D.; Wakselman, M.; Pratt, R. F. Bioorg. Med. Chem. Lett. 2006, 16, 869–871. doi:10.1016/j.bmcl.2005.11.006
Return to citation in text: [1] -
Punda, P.; Schielmann, M.; Makowiec, S. Lett. Org. Chem. 2017, 14, 337–346. doi:10.2174/1570178614666170321123252
Return to citation in text: [1] -
Banerjee, D. R.; Biswas, R.; Das, A. K.; Basak, A. Eur. J. Med. Chem. 2015, 100, 223–234. doi:10.1016/j.ejmech.2015.06.007
Return to citation in text: [1] -
Filatov, V. E.; Iuzabchuk, D. A.; Tafeenko, V. A.; Grishin, Y. K.; Roznyatovsky, V. A.; Lukianov, D. A.; Fedotova, Y. A.; Sukonnikov, M. A.; Skvortsov, D. A.; Zyk, N. V.; Beloglazkina, E. K. Int. J. Mol. Sci. 2022, 23, 6666. doi:10.3390/ijms23126666
Return to citation in text: [1] -
Zimmermann, G.; Kordes, M.; Seiser, T.; Kramer, G.; Newton, T. W.; Campe, R.; Seitz, T.; Johnen, P. R. Beta-Lactams and their use as herbicides. International Patent WO/2021/209268, Oct 21, 2021.
Return to citation in text: [1] -
Ogawa, Y.; Ishikawa, R.; Sato, T.; Tani, M. Azetidinone derivative and herbicide containing same as active ingredient. Jap. Pat. Appl. JP2021/041945, May 27, 2022.
Return to citation in text: [1] -
Pitzen, J.; de la Higuera, M. M.; Cooper, N.; Sinz, C. J.; Sang Tae, L. P.; Wahlers, J.; Fastman, N.; Tzitzilones, C.; Morgans, J., Jr.; Liu, Y.; Reid, A. N.; Ziebenhaus, C.; Schammel, A. W.; Karmel, C. H.; Mellem, K. Inhibitors of solute carrier family 6A member 19 (SLC6A19) and methods of use thereof. International Pat. Appl. WO/2024/081748A2, April 18, 2024.
Return to citation in text: [1] -
Makowiec, S.; Janikowska, K.; Pawelska, N. Synthesis 2011, 69–72. doi:10.1055/s-0030-1258966
Return to citation in text: [1] -
Zakaszewska, A.; Najda-Mocarska, E.; Makowiec, S. New J. Chem. 2017, 41, 2479–2489. doi:10.1039/c7nj00445a
Return to citation in text: [1] -
Yang, Z.; Li, S.; Zhang, Z.; Xu, J. Org. Biomol. Chem. 2014, 12, 9822–9830. doi:10.1039/c4ob01454e
Return to citation in text: [1] -
Nahmany, M.; Melman, A. J. Org. Chem. 2006, 71, 5804–5806. doi:10.1021/jo0607010
Return to citation in text: [1] -
Gerstenberger, B. S.; Lin, J.; Mimieux, Y. S.; Brown, L. E.; Oliver, A. G.; Konopelski, J. P. Org. Lett. 2008, 10, 369–372. doi:10.1021/ol7025922
Return to citation in text: [1] -
Vaske, Y. S. M.; Mahoney, M. E.; Konopelski, J. P.; Rogow, D. L.; McDonald, W. J. J. Am. Chem. Soc. 2010, 132, 11379–11385. doi:10.1021/ja1050023
Return to citation in text: [1] -
Punda, P.; Ponikiewski, Ł.; Makowiec, S. Helv. Chim. Acta 2013, 96, 2081–2091. doi:10.1002/hlca.201200646
Return to citation in text: [1] -
Attenni, B.; Cerreti, A.; D'Annibale, A.; Resta, S.; Trogolo, C. Tetrahedron 1998, 54, 12029–12038. doi:10.1016/s0040-4020(98)83055-x
Return to citation in text: [1] -
Bilodeau, D. A.; Margison, K. D.; Ahmed, N.; Strmiskova, M.; Sherratt, A. R.; Pezacki, J. P. Chem. Commun. 2020, 56, 1988–1991. doi:10.1039/c9cc09473c
Return to citation in text: [1] -
Zlatopolskiy, B. D.; Krapf, P.; Richarz, R.; Frauendorf, H.; Mottaghy, F. M.; Neumaier, B. Chem. – Eur. J. 2014, 20, 4697–4703. doi:10.1002/chem.201304056
Return to citation in text: [1] -
Qi, Z.; Wang, S. Org. Lett. 2021, 23, 5777–5781. doi:10.1021/acs.orglett.1c01937
Return to citation in text: [1] -
Watanabe, N.; Anada, M.; Hashimoto, S.-i.; Ikegami, S. Synlett 1994, 1031–1033. doi:10.1055/s-1994-23075
Return to citation in text: [1] -
Padwa, A.; Zou, Y. J. Org. Chem. 2015, 80, 1802–1808. doi:10.1021/jo502725d
Return to citation in text: [1] -
Solé, D.; Pérez‐Janer, F.; Bennasar, M.-L.; Fernández, I. Eur. J. Org. Chem. 2018, 4446–4455. doi:10.1002/ejoc.201800666
Return to citation in text: [1] -
Samim Mondal, A. R.; Ghorai, B.; Hari, D. P. Org. Lett. 2023, 25, 4974–4979. doi:10.1021/acs.orglett.3c01549
Return to citation in text: [1] -
Coquerel, Y.; Rodriguez, J. The Wolff Rearrangement: Tactics, Strategies and Recent Applications in Organic Synthesis. In Molecular Rearrangements in Organic Synthesis; Rojas, C. M., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp 59–84. doi:10.1002/9781118939901.ch3
Return to citation in text: [1] -
Lowe, G.; Ridley, D. D. J. Chem. Soc., Perkin Trans. 1 1973, 2024–2029. doi:10.1039/p19730002024
Return to citation in text: [1] -
Stork, G.; Szajewski, R. P. J. Am. Chem. Soc. 1974, 96, 5787–5791. doi:10.1021/ja00825a015
Return to citation in text: [1] -
Schobert, R.; Beneke, J. Synthesis 2013, 45, 773–776. doi:10.1055/s-0032-1316860
Return to citation in text: [1] -
Lawton, G.; Moody, C. J.; Pearson, C. J.; Williams, D. J. J. Chem. Soc., Perkin Trans. 1 1987, 885–897. doi:10.1039/p19870000885
Return to citation in text: [1] -
Moore, H. W.; Arnold, M. J. J. Org. Chem. 1983, 48, 3365–3367. doi:10.1021/jo00167a056
Return to citation in text: [1] -
Dar’in, D.; Kantin, G.; Glushakova, D.; Sharoyko, V.; Krasavin, M. J. Org. Chem. 2024, 89, 7366–7375. doi:10.1021/acs.joc.2c02600
Return to citation in text: [1] -
Ley, S.; Musio, B.; Mariani, F.; Śliwiński, E.; Kabeshov, M.; Odajima, H. Synthesis 2016, 48, 3515–3526. doi:10.1055/s-0035-1562579
Return to citation in text: [1] -
Cordero, F. M.; Salvati, M.; Pisaneschi, F.; Brandi, A. Eur. J. Org. Chem. 2004, 2205–2213. doi:10.1002/ejoc.200300595
Return to citation in text: [1] -
Mohanan, K.; Presset, M.; Mailhol, D.; Coquerel, Y.; Rodriguez, J. Chem. – Eur. J. 2012, 18, 9217–9220. doi:10.1002/chem.201201027
Return to citation in text: [1]
| 35. | Mohanan, K.; Presset, M.; Mailhol, D.; Coquerel, Y.; Rodriguez, J. Chem. – Eur. J. 2012, 18, 9217–9220. doi:10.1002/chem.201201027 |
| 3. | Krivovicheva, V.; Lyutin, I.; Kantin, G.; Dar’in, D. J. Org. Chem. 2024, 89, 3585–3589. doi:10.1021/acs.joc.3c02494 |
| 33. | Ley, S.; Musio, B.; Mariani, F.; Śliwiński, E.; Kabeshov, M.; Odajima, H. Synthesis 2016, 48, 3515–3526. doi:10.1055/s-0035-1562579 |
| 34. | Cordero, F. M.; Salvati, M.; Pisaneschi, F.; Brandi, A. Eur. J. Org. Chem. 2004, 2205–2213. doi:10.1002/ejoc.200300595 |
| 1. | Lima, L. M.; Silva, B. N. M. d.; Barbosa, G.; Barreiro, E. J. Eur. J. Med. Chem. 2020, 208, 112829. doi:10.1016/j.ejmech.2020.112829 |
| 6. | Banerjee, D. R.; Biswas, R.; Das, A. K.; Basak, A. Eur. J. Med. Chem. 2015, 100, 223–234. doi:10.1016/j.ejmech.2015.06.007 |
| 27. | Lowe, G.; Ridley, D. D. J. Chem. Soc., Perkin Trans. 1 1973, 2024–2029. doi:10.1039/p19730002024 |
| 28. | Stork, G.; Szajewski, R. P. J. Am. Chem. Soc. 1974, 96, 5787–5791. doi:10.1021/ja00825a015 |
| 29. | Schobert, R.; Beneke, J. Synthesis 2013, 45, 773–776. doi:10.1055/s-0032-1316860 |
| 30. | Lawton, G.; Moody, C. J.; Pearson, C. J.; Williams, D. J. J. Chem. Soc., Perkin Trans. 1 1987, 885–897. doi:10.1039/p19870000885 |
| 31. | Moore, H. W.; Arnold, M. J. J. Org. Chem. 1983, 48, 3365–3367. doi:10.1021/jo00167a056 |
| 4. | Adediran, S. A.; Lohier, J.-F.; Cabaret, D.; Wakselman, M.; Pratt, R. F. Bioorg. Med. Chem. Lett. 2006, 16, 869–871. doi:10.1016/j.bmcl.2005.11.006 |
| 5. | Punda, P.; Schielmann, M.; Makowiec, S. Lett. Org. Chem. 2017, 14, 337–346. doi:10.2174/1570178614666170321123252 |
| 32. | Dar’in, D.; Kantin, G.; Glushakova, D.; Sharoyko, V.; Krasavin, M. J. Org. Chem. 2024, 89, 7366–7375. doi:10.1021/acs.joc.2c02600 |
| 3. | Krivovicheva, V.; Lyutin, I.; Kantin, G.; Dar’in, D. J. Org. Chem. 2024, 89, 3585–3589. doi:10.1021/acs.joc.3c02494 |
| 22. | Watanabe, N.; Anada, M.; Hashimoto, S.-i.; Ikegami, S. Synlett 1994, 1031–1033. doi:10.1055/s-1994-23075 |
| 23. | Padwa, A.; Zou, Y. J. Org. Chem. 2015, 80, 1802–1808. doi:10.1021/jo502725d |
| 24. | Solé, D.; Pérez‐Janer, F.; Bennasar, M.-L.; Fernández, I. Eur. J. Org. Chem. 2018, 4446–4455. doi:10.1002/ejoc.201800666 |
| 25. | Samim Mondal, A. R.; Ghorai, B.; Hari, D. P. Org. Lett. 2023, 25, 4974–4979. doi:10.1021/acs.orglett.3c01549 |
| 2. | Fisher, J. F.; Mobashery, S. The β-Lactam (Azetidin-2-one) as a Privileged Ring in Medicinal Chemistry. In Privileged Scaffolds in Medicinal Chemistry: Design, Synthesis, Evaluation; Bräse, S., Ed.; Drug Discovery; The Royal Society of Chemistry: Cambridge, UK, 2015; pp 64–97. doi:10.1039/9781782622246-00064 |
| 26. | Coquerel, Y.; Rodriguez, J. The Wolff Rearrangement: Tactics, Strategies and Recent Applications in Organic Synthesis. In Molecular Rearrangements in Organic Synthesis; Rojas, C. M., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp 59–84. doi:10.1002/9781118939901.ch3 |
| 11. | Makowiec, S.; Janikowska, K.; Pawelska, N. Synthesis 2011, 69–72. doi:10.1055/s-0030-1258966 |
| 12. | Zakaszewska, A.; Najda-Mocarska, E.; Makowiec, S. New J. Chem. 2017, 41, 2479–2489. doi:10.1039/c7nj00445a |
| 13. | Yang, Z.; Li, S.; Zhang, Z.; Xu, J. Org. Biomol. Chem. 2014, 12, 9822–9830. doi:10.1039/c4ob01454e |
| 14. | Nahmany, M.; Melman, A. J. Org. Chem. 2006, 71, 5804–5806. doi:10.1021/jo0607010 |
| 17. | Punda, P.; Ponikiewski, Ł.; Makowiec, S. Helv. Chim. Acta 2013, 96, 2081–2091. doi:10.1002/hlca.201200646 |
| 18. | Attenni, B.; Cerreti, A.; D'Annibale, A.; Resta, S.; Trogolo, C. Tetrahedron 1998, 54, 12029–12038. doi:10.1016/s0040-4020(98)83055-x |
| 10. | Pitzen, J.; de la Higuera, M. M.; Cooper, N.; Sinz, C. J.; Sang Tae, L. P.; Wahlers, J.; Fastman, N.; Tzitzilones, C.; Morgans, J., Jr.; Liu, Y.; Reid, A. N.; Ziebenhaus, C.; Schammel, A. W.; Karmel, C. H.; Mellem, K. Inhibitors of solute carrier family 6A member 19 (SLC6A19) and methods of use thereof. International Pat. Appl. WO/2024/081748A2, April 18, 2024. |
| 19. | Bilodeau, D. A.; Margison, K. D.; Ahmed, N.; Strmiskova, M.; Sherratt, A. R.; Pezacki, J. P. Chem. Commun. 2020, 56, 1988–1991. doi:10.1039/c9cc09473c |
| 20. | Zlatopolskiy, B. D.; Krapf, P.; Richarz, R.; Frauendorf, H.; Mottaghy, F. M.; Neumaier, B. Chem. – Eur. J. 2014, 20, 4697–4703. doi:10.1002/chem.201304056 |
| 21. | Qi, Z.; Wang, S. Org. Lett. 2021, 23, 5777–5781. doi:10.1021/acs.orglett.1c01937 |
| 8. | Zimmermann, G.; Kordes, M.; Seiser, T.; Kramer, G.; Newton, T. W.; Campe, R.; Seitz, T.; Johnen, P. R. Beta-Lactams and their use as herbicides. International Patent WO/2021/209268, Oct 21, 2021. |
| 9. | Ogawa, Y.; Ishikawa, R.; Sato, T.; Tani, M. Azetidinone derivative and herbicide containing same as active ingredient. Jap. Pat. Appl. JP2021/041945, May 27, 2022. |
| 7. | Filatov, V. E.; Iuzabchuk, D. A.; Tafeenko, V. A.; Grishin, Y. K.; Roznyatovsky, V. A.; Lukianov, D. A.; Fedotova, Y. A.; Sukonnikov, M. A.; Skvortsov, D. A.; Zyk, N. V.; Beloglazkina, E. K. Int. J. Mol. Sci. 2022, 23, 6666. doi:10.3390/ijms23126666 |
| 15. | Gerstenberger, B. S.; Lin, J.; Mimieux, Y. S.; Brown, L. E.; Oliver, A. G.; Konopelski, J. P. Org. Lett. 2008, 10, 369–372. doi:10.1021/ol7025922 |
| 16. | Vaske, Y. S. M.; Mahoney, M. E.; Konopelski, J. P.; Rogow, D. L.; McDonald, W. J. J. Am. Chem. Soc. 2010, 132, 11379–11385. doi:10.1021/ja1050023 |
© 2024 Lyutin et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.