Abstract
A one-pot three-component synthesis of substituted meta-hetarylanilines from heterocycle-substituted 1,3-diketones has been developed. The electron-withdrawing power of the heterocyclic substituent (which can be estimated on the basis of calculated Hammett constants) in the 1,3-diketone plays a pivotal role in the studied reaction. The series of meta-hetarylanilines prepared (21–85% isolated yield) demonstrates the synthetic utility of the developed method.
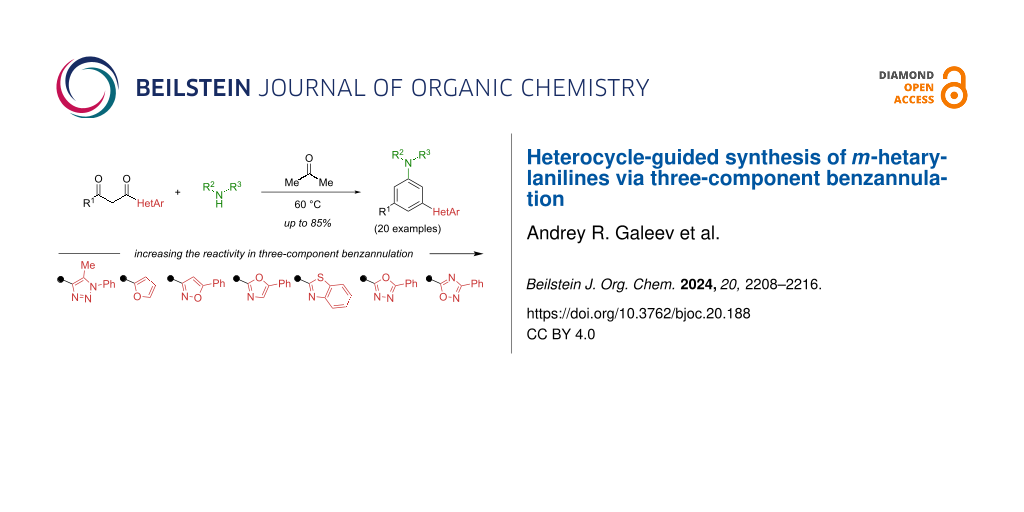
Graphical Abstract
Introduction
The aniline moiety is omnipresent in the synthetic chemistry with applications ranging from building blocks to catalysis [1-4]. Among the possible substitution patterns, meta-substituted anilines hold a special place. These compounds are difficult to access due to the inherent ortho-/para-directional reactivity of the amino group, at the same time they are widely used in medicinal chemistry, resulting in several marketed drugs (Figure 1). On the other hand, 3,5-diarylanilines can be regarded as meta-terphenyls which are of great interest for material and coordination chemistry [5-16]. Moreover, compounds with diverse bioactivities and natural products contain the meta-terphenyl moiety as a key fragment [17-26].
Figure 1: The meta-hetarylaniline motif in bioactive molecules.
Figure 1: The meta-hetarylaniline motif in bioactive molecules.
The aforementioned features have led to an extensive development of novel methods to access meta-substituted anilines which can be divided into two main strategies (Scheme 1A) [27]. The first strategy focuses on the decoration of the aromatic ring mainly via metal-catalyzed C–N or C–C bond formation. Despite recent advances in the area of remote C–H functionalization, this strategy still requires some pre-functionalization of the starting material or the use of directing groups [28-32]. An alternative strategy is based on aromatic ring formation via benzannulative inter- or intramolecular condensation of acyclic precursors [27,32-37].
Scheme 1: Strategies to access meta-substituted anilines.
Scheme 1: Strategies to access meta-substituted anilines.
Within the latter strategy, [3 + 3] condensations are gaining much attention due to the availability of starting materials and the straightforward installation of the aryl(hetaryl) substituent in the meta-position [38-43]. For example, several methods based on the Michael condensation–oxidation sequence starting from α,β-unsaturated ketones have been described (Scheme 1B) [44-50]. Recently, several methods have been developed based on the interrupted Kröhnke reaction (Scheme 1B) [51,52]. The main step of this process is an intermolecular cyclization of the formed 1,5-diketone followed by aromatization.
Previously we have shown that 1,3-diketones bearing an electron-withdrawing group (EWG) adjacent to one of the carbonyls readily react with in situ-generated acetone imines in a (3 + 3) manner to afford meta-substituted anilines (Scheme 1C) [53,54]. Various EWGs (ester, carbamoyl, ketone, trifluoromethyl) have been successfully employed which motivated us to evaluate other possible EWGs.
Results and Discussion
Based on the fact, that many heterocycles are isoelectronic to an ester or a carbamoyl group, we were interested in testing various heterocycles as electron-withdrawing groups for activation of the carbonyl group in 1,3-diketones. In order to compare the electron-withdrawing ability of heterocycles and previously studied EWGs, we tried to utilize Hammett constants. Since only a few numbers of experimentally measured Hammett constants for heterocycles are known [55,56], this approach seems unsuitable at first. However, recently, a web-based tool for the calculation of the substituent descriptors compatible with the Hammett sigma constants was released [57] allowing direct comparison of different substituents.
On the basis of the calculated Hammett constants, we have selected a model series of 1,3-diketones, bearing heterocyclic substituents with a range of electron-withdrawing ability (Figure 2). For the amine model series, we used amines with different nucleophilicity, namely benzylamine (primary alkylamine), morpholine (secondary alkylamine) and aniline (aromatic amine).
Figure 2: The model series of synthesized 1,3-diketones and corresponding calculated Hammett constants of heterocyclic substituents. Previously studied EWGs [53] are shown in the dashed block. The numbers in parentheses are σm and σp calculated [57] constants.
Figure 2: The model series of synthesized 1,3-diketones and corresponding calculated Hammett constants of het...
We first examined the reaction of 1,2,4-oxadiazole-1,3-diketone 1a (σm/σp 0.463/0.575, which is quite close to the constants of the CO2Me group, so a successful reaction was expected, Figure 2) with morpholine under previously found conditions (Scheme 2). To our delight, the meta-1,2,4-oxadiazole aniline 3ab, formed by the sequence of aldol-type reactions, was isolated in 80% yield and its structure was confirmed by NMR and single crystal X-ray analysis (CCDC 2356151). No significant improvement in the yield was observed by varying the reaction conditions. Surprisingly, the reaction of 1,3-diketone 1a, morpholine and acetone without the use of molecular sieves and acid catalysis (conditions A) resulted in 81% yield of meta-substituted aniline 3ab.
Scheme 2: Synthesis of meta-substituted anilines from 1,2,4-oxadiazol-5-yl substituted 1,3-diketone 1a. Conditions A: 1a (0.5 mmol), 2a,b (0.5 mmol) and acetone (2 mL), 60 °C. Conditions B: 1a (0.5 mmol), 2c (0.75 mmol), AcOH (30 mol %), molecular sieves 3 Å (300 mg) and acetone (2 mL), 60 °C.
Scheme 2: Synthesis of meta-substituted anilines from 1,2,4-oxadiazol-5-yl substituted 1,3-diketone 1a. Condi...
Applying the latter conditions to the reaction with benzylamine, the target substituted aniline 3aa was formed in a good 73% yield. However, the reaction with less nucleophilic aniline was sluggish, requiring almost 15 days to achieve full conversion of the 1,3-diketone 1a (TLC control). In this case, performing the reaction in the presence of molecular sieves and 1.5-fold excess of aniline (conditions B) dramatically reduced the reaction time to 1 d, allowing isolation of diarylamine 3ac in 75% yield (Scheme 2).
Next, we started to screen various heterocyclic 1,3-diketones with the model amine series. The reaction of diketone 1b, bearing the less electron-withdrawing 1,3,4-oxadiazole moiety (σm/σp 0.335/0.443), with alkylamines proceeded well (73–74% yields), but required the addition of CHCl3 as co-solvent due to the low solubility of the starting 1,3-diketone 1b. On the other hand, the reaction of 1,3-diketone 1b with aniline resulted in low conversion of 1b even at prolonged reaction times (up to 10 days). The addition of molecular sieves, excess aniline, or acid catalysts did not significantly affect the conversion (Scheme 3).
Scheme 3: Synthesis of meta-substituted anilines from 1,3,4-oxadiazol-substituted 1,3-diketone 1b. Conditions A: 1b (0.5 mmol), 2a,b (0.5 mmol) and acetone/CHCl3 (3 mL, 2:1), 60 °C. Conditions B: 1b (0.5 mmol), 2c (0.75 mmol), AcOH (30 mol %), molecular sieves 3 Å (300 mg) and acetone (2 mL), 60 °C.
Scheme 3: Synthesis of meta-substituted anilines from 1,3,4-oxadiazol-substituted 1,3-diketone 1b. Conditions...
1,3-Diketones with benzothiazole (1c, σm/σp 0.338/0.390) and oxazole (1d, σm/σp 0.267/0.305) substituents reacted with primary and secondary alkylamines, requiring prolonged heating and a 1.5 excess of amine (Scheme 4), to give meta-substituted arylamines in reasonable synthetic yields. In the case of 1,3-diketone 1d, the addition of molecular sieves is necessary in order to reduce the formation of the enamine side-product. Similar to 1,3-diketone 1b, an extremely low conversion of 1c and 1d was observed in the reaction with aniline.
Scheme 4: Synthesis of meta-substituted anilines from benzothiazol-2-yl and oxazol-2-yl-substituted 1,3-diketones. Conditions A: 1c,d (0.5 mmol), 2a,b (0.75 mmol) and acetone/CHCl3 (2 mL, 1:1), 60 °C. Conditions B: 1c,d (0.5 mmol), 2a,c (0.75–2.5 mmol), AcOH (30 mol %), molecular sieves 3 Å (300 mg) and acetone/CHCl3 (2 mL, 1:1), 60 °C.
Scheme 4: Synthesis of meta-substituted anilines from benzothiazol-2-yl and oxazol-2-yl-substituted 1,3-diket...
The isoxazol-3-yl or furan-2-yl substituents have calculated Hammett constants below 0.200 (Figure 2), thus based on the above observations, a slow reaction of 1,3-diketones 1e and 1f with aliphatic amines was expected. Indeed, the reaction of 1,3-diketones 1e or 1f with benzylamine was sluggish, and a number of undefined side-products were formed (LC control). m-Isoxazole arylamine 3ea was isolated in low yield (21%) after heating for 6 days (Scheme 5). In fact, arylamine 3fa, produced from furan-substituted 1,3-diketone 1f and a 5-fold excess of benzylamine, could be prepared in 18% crude yield (after 7 days, see Supporting Information File 1, page S9).
Scheme 5: Synthesis of meta-substituted aniline from isoxazol-3-yl-substituted 1,3-diketone 1e. Conditions B: 1e (0.3 mmol), 2a (0.45 mmol), AcOH (30 mol %), molecular sieves 3 Å (300 mg) and acetone (1 mL), 60 °C.
Scheme 5: Synthesis of meta-substituted aniline from isoxazol-3-yl-substituted 1,3-diketone 1e. Conditions B: ...
Finally, all attempts to perform the reaction with 1,2,3-triazole 1,3-diketone 1g (σm/σp 0.043/0.011) with model amine series failed (7 days reaction time in each case). This result is in good agreement with low Hammett constants of the triazole-substituent of 1,3-diketone 1g, which are close to the calculated Hammett constants of the phenyl group (σm/σp 0.055/0.012).
Quantum-chemical/chemoinformatics calculations were also performed to correlate the observed reactivity with the charges on the carbonyl group of 1,3-diketones. Unfortunately, no reliable correlation was found between the charges and the reactivity of the 1,3-diketones with the present set of heterocyclic substituents or with the EWGs that were previously studied (see Supporting Information File 1).
The presence of electron-donating (3ha) or electron-withdrawing groups (3ia) in the aryl substituent of the 1,3-diketone does not affect the reaction outcome (Figure 3). We have further demonstrated the utility of three-component condensation by introducing additional functional groups into the amine moiety (Figure 3).
Figure 3: Scope of functionalized amines in three-component condensation. Conditions A: 1a,b,h,i (0.2–0.5 mmol), 2e,i–k (0.2–0.75 mmol) and acetone (1–2 mL) or acetone/CHCl3 (3 mL, 2:1), 60 °C. Conditions B: 1a (0.3–0.5 mmol), 2d,f–h (0.45–0.75 mmol), AcOH (30 mol %), molecular sieves 3 Å (300 mg) and acetone (1–2 mL), 60 °C.
Figure 3: Scope of functionalized amines in three-component condensation. Conditions A: 1a,b,h,i (0.2–0.5 mmo...
Substituted arylamines bearing alcohol (3ae), phenol (3ad), alkene (3bi), dimethyl acetal (3bj) functionality can be accessed in good yields. Reaction of 1,3-diketone 1a with a non-amidine type heterocyclic amine, 3-aminopyridine, provided N-hetarylaniline (3af) in moderate yield. Furthermore, it is possible to synthesize sterically hindered anilines such as arylamine 3ah, which was prepared from 2,6-di(isopropyl)aniline in 53% yield. However, in the case of an electron-deficient amine (4-trifluoromethylaniline), the desired meta-heterocycle aniline 3ag was prepared in low yield. Finally, the developed method is suitable for the late-stage arylation of drug-like molecules such as deacetyllinezolid (3ak).
The meta-substituted anilines 3 are formed in a sequence of reactions shown in Scheme 6 [53]. Firstly, the reaction of acetone and amines 2 leads to the formation of acetone imine/enamine (reaction 1, Scheme 6). Nucleophilic addition of an enamine to the most electron-deficient carbonyl group (C1=O, adjacent to the EWG) of the 1,3-diketones 1 gives the acyclic carbinol I (reaction 2, Scheme 6), followed by the intramolecular addition of enamine I to the C3=O to form intermediate II, which dehydrates to cyclic carbinol III. Finally, dehydration of intermediate III yields anilines 3.
Scheme 6: Proposed mechanism for the formation of meta-substituted anilines 3 via three-component benzannulation.
Scheme 6: Proposed mechanism for the formation of meta-substituted anilines 3 via three-component benzannulat...
Conclusion
In summary, a method for the synthesis of substituted meta-hetarylanilines under mild conditions starting from 1,3-diketones with heterocyclic substituents, acetone and various amines has been developed. The success of this three-component reaction is governed by the electron-withdrawing ability of the heterocyclic substituent in the 1,3-diketone, which can be evaluated with computational Hammett constants. As a rule-of-thumb, 1,3-diketones bearing substituents with σm or σp > 0.300 afford meta-anilines from alkylamines in good synthetic yields, and higher σm or σp are required for three-component condensation with less nucleophilic arylamines. The developed one-pot three-component reaction is efficient (yields up to 85%), compatible with many functional groups, and allows to synthesize a series of difficult-to-access meta-substituted anilines of interest for medicinal and material chemistry.
Supporting Information
CCDC 2356152 (1g), 2356151 (3ab), 2356154 (3bb) and 2356153 (3ca) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/structures.
| Supporting Information File 1: Full experimental details, characterization data of new compounds and NMR spectra. | ||
| Format: PDF | Size: 5.3 MB | Download |
| Supporting Information File 2: xyz files with Cartesian atomic coordinates for all model structures and CIF-files for compounds 1g, 3ab, 3bb and 3ca. | ||
| Format: ZIP | Size: 434.6 KB | Download |
Data Availability Statement
The data that supports the findings of this study is available from the corresponding author upon reasonable request.
References
-
Rappoport, Z. The Chemistry of Anilines; John Wiley & Sons: Chichester, UK, 2007.
Return to citation in text: [1] -
Lv, J.; Zhang, Q.; Cai, M.; Han, Y.; Luo, S. Chem. – Asian J. 2018, 13, 740–753. doi:10.1002/asia.201701773
Return to citation in text: [1] -
Wang, C.-J.; Meng, H.-J.; Tang, Y.; Chen, J.; Zhou, L. Org. Lett. 2024, 26, 1489–1494. doi:10.1021/acs.orglett.4c00172
Return to citation in text: [1] -
Sonsona, I. G.; Marqués-López, E.; Gimeno, M. C.; Herrera, R. P. New J. Chem. 2019, 43, 12233–12240. doi:10.1039/c9nj02392e
Return to citation in text: [1] -
Petko, F.; Hola, E.; Jankowska, M.; Gruchała-Hałat, A.; Ortyl, J. Virtual Phys. Prototyping 2023, 18, e2244936. doi:10.1080/17452759.2023.2244936
Return to citation in text: [1] -
Tomal, W.; Szymaszek, P.; Bilut, M.; Popielarz, R.; Świergosz, T.; Ortyl, J. Polym. Chem. 2022, 13, 4650–4665. doi:10.1039/d2py00525e
Return to citation in text: [1] -
Kim, M.-J.; Ahn, M.; Chae, M.; Kim, S.; Kim, D.; Wee, K.-R. RSC Adv. 2021, 11, 34945–34954. doi:10.1039/d1ra06602a
Return to citation in text: [1] -
Wu, C.-A.; Chou, H.-H.; Shih, C.-H.; Wu, F.-I.; Cheng, C.-H.; Huang, H.-L.; Chao, T.-C.; Tseng, M.-R. J. Mater. Chem. 2012, 22, 17792–17799. doi:10.1039/c2jm33376g
Return to citation in text: [1] -
Pavithra, T.; Gnanaoli, K.; Rajkumar, D. B.; Puhazhendhi, A.; Sivalingam, S.; Sampath, N.; Nagarajan, S.; Sridharan, V.; Maheswari, C. U. New J. Chem. 2024, 48, 2175–2182. doi:10.1039/d3nj04721k
Return to citation in text: [1] -
Baykov, S.; Tarasenko, M.; Kotlyarova, V.; Shetnev, A.; Zelenkov, L. E.; Boyarskaya, I. A.; Boyarskiy, V. P. ChemistrySelect 2022, 7, e202201201. doi:10.1002/slct.202201201
Return to citation in text: [1] -
Sasabe, H.; Seino, Y.; Kimura, M.; Kido, J. Chem. Mater. 2012, 24, 1404–1406. doi:10.1021/cm3006748
Return to citation in text: [1] -
Twamley, B.; Haubrich, S. T.; Power, P. P. Element Derivatives of Sterically Encumbering Terphenyl Ligands. In Advances in Organometallic Chemistry; West, R.; Hill, A. F., Eds.; Academic Press, 1999; Vol. 44, pp 1–65. doi:10.1016/s0065-3055(08)60619-x
Return to citation in text: [1] -
Manke, D. R.; Golen, J. A.; Stennett, C. R.; Naeem, M.; Javier-Jimenez, D. R.; Power, P. P. Polyhedron 2022, 222, 115947. doi:10.1016/j.poly.2022.115947
Return to citation in text: [1] -
Clyburne, J. A. C.; McMullen, N. Coord. Chem. Rev. 2000, 210, 73–99. doi:10.1016/s0010-8545(00)00317-9
Return to citation in text: [1] -
Kays, D. L. The Stabilisation of Organometallic Complexes Using M-Terphenyl Ligands. In Organometallic Chemistry; Fairlamb, I. J. S.; Lynam, J. M., Eds.; Royal Society of Chemistry, 2010; Vol. 36, pp 56–76. doi:10.1039/9781847559616-00056
Return to citation in text: [1] -
Rivard, E.; Power, P. P. Inorg. Chem. 2007, 46, 10047–10064. doi:10.1021/ic700813h
Return to citation in text: [1] -
Muszak, D.; Surmiak, E.; Plewka, J.; Magiera-Mularz, K.; Kocik-Krol, J.; Musielak, B.; Sala, D.; Kitel, R.; Stec, M.; Weglarczyk, K.; Siedlar, M.; Dömling, A.; Skalniak, L.; Holak, T. A. J. Med. Chem. 2021, 64, 11614–11636. doi:10.1021/acs.jmedchem.1c00957
Return to citation in text: [1] -
Patrick, D. A.; Ismail, M. A.; Arafa, R. K.; Wenzler, T.; Zhu, X.; Pandharkar, T.; Jones, S. K.; Werbovetz, K. A.; Brun, R.; Boykin, D. W.; Tidwell, R. R. J. Med. Chem. 2013, 56, 5473–5494. doi:10.1021/jm400508e
Return to citation in text: [1] -
Rajakumar, P.; Padmanabhan, R.; Rajesh, N. Bioorg. Med. Chem. Lett. 2012, 22, 3770–3775. doi:10.1016/j.bmcl.2012.04.010
Return to citation in text: [1] -
Heitman, L. H.; Narlawar, R.; de Vries, H.; Willemsen, M. N.; Wolfram, D.; Brussee, J.; IJzerman, A. P. J. Med. Chem. 2009, 52, 2036–2042. doi:10.1021/jm801561h
Return to citation in text: [1] -
Rocchi, D.; González, J. F.; Martín-Cámara, O.; Perrone, M. G.; Miciaccia, M.; Scilimati, A.; Decouty-Pérez, C.; Parada, E.; Egea, J.; Menéndez, J. C. Molecules 2023, 28, 5374. doi:10.3390/molecules28145374
Return to citation in text: [1] -
Bauer, J. D.; Foster, M. S.; Hugdahl, J. D.; Burns, K. L.; May, S. W.; Pollock, S. H.; Cutler, H. G.; Cutler, S. J. Med. Chem. Res. 2007, 16, 119–129. doi:10.1007/s00044-007-9015-x
Return to citation in text: [1] -
Simoni, D.; Giannini, G.; Roberti, M.; Rondanin, R.; Baruchello, R.; Rossi, M.; Grisolia, G.; Invidiata, F. P.; Aiello, S.; Marino, S.; Cavallini, S.; Siniscalchi, A.; Gebbia, N.; Crosta, L.; Grimaudo, S.; Abbadessa, V.; Di Cristina, A.; Tolomeo, M. J. Med. Chem. 2005, 48, 4293–4299. doi:10.1021/jm049080y
Return to citation in text: [1] -
Glombitza, K.-W.; Rauwald, H.-W.; Eckhardt, G. Phytochemistry 1975, 14, 1403–1405. doi:10.1016/s0031-9422(00)98637-0
Return to citation in text: [1] -
Luo, L.; Liu, X.-L.; Li, J.; Mu, R.-H.; Liu, Q.; Yi, L.-T.; Geng, D. Eur. J. Pharmacol. 2015, 762, 357–363. doi:10.1016/j.ejphar.2015.05.036
Return to citation in text: [1] -
Kikuchi, H.; Matsuo, Y.; Katou, Y.; Kubohara, Y.; Oshima, Y. Tetrahedron 2012, 68, 8884–8889. doi:10.1016/j.tet.2012.08.041
Return to citation in text: [1] -
Swami, B.; Yadav, D.; Menon, R. S. Chem. Rec. 2022, 22, e202100249. doi:10.1002/tcr.202100249
Return to citation in text: [1] [2] -
Ruiz-Castillo, P.; Buchwald, S. L. Chem. Rev. 2016, 116, 12564–12649. doi:10.1021/acs.chemrev.6b00512
Return to citation in text: [1] -
Sambiagio, C.; Marsden, S. P.; Blacker, A. J.; McGowan, P. C. Chem. Soc. Rev. 2014, 43, 3525–3550. doi:10.1039/c3cs60289c
Return to citation in text: [1] -
Leitch, J. A.; Frost, C. G. Synthesis 2018, 50, 2693–2706. doi:10.1055/s-0037-1610142
Return to citation in text: [1] -
Mihai, M. T.; Genov, G. R.; Phipps, R. J. Chem. Soc. Rev. 2018, 47, 149–171. doi:10.1039/c7cs00637c
Return to citation in text: [1] -
Jacob, C.; Annibaletto, J.; Maes, B. U. W.; Evano, G. Synthesis 2023, 55, 1799–1823. doi:10.1055/a-2039-7985
Return to citation in text: [1] [2] -
Mies, T.; Ma, T.-K.; Barrett, A. G. M. Russ. Chem. Rev. 2020, 89, 917–965. doi:10.1070/rcr4931
Return to citation in text: [1] -
Zhao, Q.; Peng, C.; Zhan, G.; Han, B. RSC Adv. 2020, 10, 40983–41003. doi:10.1039/d0ra08068c
Return to citation in text: [1] -
Wu, L.; Yu, B.; Li, E.-Q. Adv. Synth. Catal. 2020, 362, 4010–4026. doi:10.1002/adsc.202000608
Return to citation in text: [1] -
Banerjee, A.; Kundu, S.; Bhattacharyya, A.; Sahu, S.; Maji, M. S. Org. Chem. Front. 2021, 8, 2710–2771. doi:10.1039/d1qo00092f
Return to citation in text: [1] -
Poudel, T. N.; Tamargo, R. J. I.; Cai, H.; Lee, Y. R. Asian J. Org. Chem. 2018, 7, 985–1005. doi:10.1002/ajoc.201800080
Return to citation in text: [1] -
Yang, S.; Lu, D.; Huo, H.; Luo, F.; Gong, Y. Org. Lett. 2018, 20, 6943–6947. doi:10.1021/acs.orglett.8b03090
Return to citation in text: [1] -
Goryaeva, M. V.; Kushch, S. O.; Khudina, O. G.; Burgart, Y. V.; Kudyakova, Y. S.; Ezhikova, M. A.; Kodess, M. I.; Slepukhin, P. A.; Sadretdinova, L. S.; Evstigneeva, N. P.; Gerasimova, N. A.; Saloutin, V. I. Org. Biomol. Chem. 2019, 17, 4273–4280. doi:10.1039/c9ob00293f
Return to citation in text: [1] -
Naito, S.; Yokoyama, S.; Asahara, H.; Nishiwaki, N. Tetrahedron Lett. 2017, 58, 4699–4702. doi:10.1016/j.tetlet.2017.11.003
Return to citation in text: [1] -
Volochnyuk, D. M.; Kostyuk, A. N.; Sibgatulin, D. A.; Chernega, A. N.; Pinchuk, A. M.; Tolmachev, A. A. Tetrahedron 2004, 60, 2361–2371. doi:10.1016/j.tet.2004.01.010
Return to citation in text: [1] -
Liu, X.-Y.; Che, C.-M. Angew. Chem., Int. Ed. 2009, 48, 2367–2371. doi:10.1002/anie.200805383
Return to citation in text: [1] -
Davies, I. W.; Marcoux, J.-F.; Kuethe, J. T.; Lankshear, M. D.; Taylor, J. D. O.; Tsou, N.; Dormer, P. G.; Hughes, D. L.; Houk, K. N.; Guner, V. J. Org. Chem. 2004, 69, 1298–1308. doi:10.1021/jo035677u
Return to citation in text: [1] -
Pavithra, T.; Karthiyayini, G.; Nagarajan, S.; Sridharan, V.; Maheswari, C. U. Synlett 2023, 34, 807–814. doi:10.1055/a-1903-5174
Return to citation in text: [1] -
Rocchi, D.; Gómez-Carpintero, J.; González, J. F.; Menéndez, J. C. Molecules 2020, 25, 5565. doi:10.3390/molecules25235565
Return to citation in text: [1] -
Rocchi, D.; González, J. F.; Gómez-Carpintero, J.; González-Ruiz, V.; Martín, M. A.; Sridharan, V.; Menéndez, J. C. ACS Comb. Sci. 2018, 20, 722–731. doi:10.1021/acscombsci.8b00137
Return to citation in text: [1] -
Vachan, B. S.; Ramesh, A.; Karuppasamy, M.; Muthukrishnan, I.; Nagarajan, S.; Menéndez, J. C.; Maheswari, C. U.; Sridharan, V. RSC Adv. 2019, 9, 32946–32953. doi:10.1039/c9ra07108c
Return to citation in text: [1] -
Li, L.; Zhao, M.-N.; Ren, Z.-H.; Li, J.-L.; Guan, Z.-H. Org. Lett. 2012, 14, 3506–3509. doi:10.1021/ol3014733
Return to citation in text: [1] -
Yaragorla, S.; Dada, R. ACS Omega 2017, 2, 4859–4869. doi:10.1021/acsomega.7b00753
Return to citation in text: [1] -
Ma, C.-L.; Yu, X.-L.; Zhu, X.-L.; Hu, Y.-Z.; Dong, X.-W.; Tan, B.; Liu, X.-Y. Adv. Synth. Catal. 2015, 357, 569–575. doi:10.1002/adsc.201400933
Return to citation in text: [1] -
Makarov, A. S.; Bakiev, A. N.; Eshmemeteva, D. A. Org. Chem. Front. 2023, 10, 2760–2765. doi:10.1039/d3qo00583f
Return to citation in text: [1] -
Fang, Z.; Hong, B.; Wu, W.; Weng, Z. Org. Chem. Front. 2023, 10, 3207–3212. doi:10.1039/d3qo00493g
Return to citation in text: [1] -
Galeev, A. R.; Dmitriev, M. V.; Mokrushin, I. G.; Mashevskaya, I. V.; Maslivets, A. N.; Rubin, M. Org. Biomol. Chem. 2019, 17, 10030–10044. doi:10.1039/c9ob02120e
Return to citation in text: [1] [2] [3] -
Galeev, A. R.; Mokrushin, I. G.; Dmitriev, M. V.; Maslivets, A. N. Russ. J. Org. Chem. 2020, 56, 1317–1320. doi:10.1134/s1070428020070295
Return to citation in text: [1] -
Taylor, P. J.; Wait, A. R. J. Chem. Soc., Perkin Trans. 2 1986, 1765–1770. doi:10.1039/p29860001765
Return to citation in text: [1] -
Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165–195. doi:10.1021/cr00002a004
Return to citation in text: [1] -
Ertl, P. Chem.: Methods 2022, 2, e202200041. doi:10.1002/cmtd.202200041
Return to citation in text: [1] [2]
| 1. | Rappoport, Z. The Chemistry of Anilines; John Wiley & Sons: Chichester, UK, 2007. |
| 2. | Lv, J.; Zhang, Q.; Cai, M.; Han, Y.; Luo, S. Chem. – Asian J. 2018, 13, 740–753. doi:10.1002/asia.201701773 |
| 3. | Wang, C.-J.; Meng, H.-J.; Tang, Y.; Chen, J.; Zhou, L. Org. Lett. 2024, 26, 1489–1494. doi:10.1021/acs.orglett.4c00172 |
| 4. | Sonsona, I. G.; Marqués-López, E.; Gimeno, M. C.; Herrera, R. P. New J. Chem. 2019, 43, 12233–12240. doi:10.1039/c9nj02392e |
| 28. | Ruiz-Castillo, P.; Buchwald, S. L. Chem. Rev. 2016, 116, 12564–12649. doi:10.1021/acs.chemrev.6b00512 |
| 29. | Sambiagio, C.; Marsden, S. P.; Blacker, A. J.; McGowan, P. C. Chem. Soc. Rev. 2014, 43, 3525–3550. doi:10.1039/c3cs60289c |
| 30. | Leitch, J. A.; Frost, C. G. Synthesis 2018, 50, 2693–2706. doi:10.1055/s-0037-1610142 |
| 31. | Mihai, M. T.; Genov, G. R.; Phipps, R. J. Chem. Soc. Rev. 2018, 47, 149–171. doi:10.1039/c7cs00637c |
| 32. | Jacob, C.; Annibaletto, J.; Maes, B. U. W.; Evano, G. Synthesis 2023, 55, 1799–1823. doi:10.1055/a-2039-7985 |
| 53. | Galeev, A. R.; Dmitriev, M. V.; Mokrushin, I. G.; Mashevskaya, I. V.; Maslivets, A. N.; Rubin, M. Org. Biomol. Chem. 2019, 17, 10030–10044. doi:10.1039/c9ob02120e |
| 27. | Swami, B.; Yadav, D.; Menon, R. S. Chem. Rec. 2022, 22, e202100249. doi:10.1002/tcr.202100249 |
| 17. | Muszak, D.; Surmiak, E.; Plewka, J.; Magiera-Mularz, K.; Kocik-Krol, J.; Musielak, B.; Sala, D.; Kitel, R.; Stec, M.; Weglarczyk, K.; Siedlar, M.; Dömling, A.; Skalniak, L.; Holak, T. A. J. Med. Chem. 2021, 64, 11614–11636. doi:10.1021/acs.jmedchem.1c00957 |
| 18. | Patrick, D. A.; Ismail, M. A.; Arafa, R. K.; Wenzler, T.; Zhu, X.; Pandharkar, T.; Jones, S. K.; Werbovetz, K. A.; Brun, R.; Boykin, D. W.; Tidwell, R. R. J. Med. Chem. 2013, 56, 5473–5494. doi:10.1021/jm400508e |
| 19. | Rajakumar, P.; Padmanabhan, R.; Rajesh, N. Bioorg. Med. Chem. Lett. 2012, 22, 3770–3775. doi:10.1016/j.bmcl.2012.04.010 |
| 20. | Heitman, L. H.; Narlawar, R.; de Vries, H.; Willemsen, M. N.; Wolfram, D.; Brussee, J.; IJzerman, A. P. J. Med. Chem. 2009, 52, 2036–2042. doi:10.1021/jm801561h |
| 21. | Rocchi, D.; González, J. F.; Martín-Cámara, O.; Perrone, M. G.; Miciaccia, M.; Scilimati, A.; Decouty-Pérez, C.; Parada, E.; Egea, J.; Menéndez, J. C. Molecules 2023, 28, 5374. doi:10.3390/molecules28145374 |
| 22. | Bauer, J. D.; Foster, M. S.; Hugdahl, J. D.; Burns, K. L.; May, S. W.; Pollock, S. H.; Cutler, H. G.; Cutler, S. J. Med. Chem. Res. 2007, 16, 119–129. doi:10.1007/s00044-007-9015-x |
| 23. | Simoni, D.; Giannini, G.; Roberti, M.; Rondanin, R.; Baruchello, R.; Rossi, M.; Grisolia, G.; Invidiata, F. P.; Aiello, S.; Marino, S.; Cavallini, S.; Siniscalchi, A.; Gebbia, N.; Crosta, L.; Grimaudo, S.; Abbadessa, V.; Di Cristina, A.; Tolomeo, M. J. Med. Chem. 2005, 48, 4293–4299. doi:10.1021/jm049080y |
| 24. | Glombitza, K.-W.; Rauwald, H.-W.; Eckhardt, G. Phytochemistry 1975, 14, 1403–1405. doi:10.1016/s0031-9422(00)98637-0 |
| 25. | Luo, L.; Liu, X.-L.; Li, J.; Mu, R.-H.; Liu, Q.; Yi, L.-T.; Geng, D. Eur. J. Pharmacol. 2015, 762, 357–363. doi:10.1016/j.ejphar.2015.05.036 |
| 26. | Kikuchi, H.; Matsuo, Y.; Katou, Y.; Kubohara, Y.; Oshima, Y. Tetrahedron 2012, 68, 8884–8889. doi:10.1016/j.tet.2012.08.041 |
| 53. | Galeev, A. R.; Dmitriev, M. V.; Mokrushin, I. G.; Mashevskaya, I. V.; Maslivets, A. N.; Rubin, M. Org. Biomol. Chem. 2019, 17, 10030–10044. doi:10.1039/c9ob02120e |
| 5. | Petko, F.; Hola, E.; Jankowska, M.; Gruchała-Hałat, A.; Ortyl, J. Virtual Phys. Prototyping 2023, 18, e2244936. doi:10.1080/17452759.2023.2244936 |
| 6. | Tomal, W.; Szymaszek, P.; Bilut, M.; Popielarz, R.; Świergosz, T.; Ortyl, J. Polym. Chem. 2022, 13, 4650–4665. doi:10.1039/d2py00525e |
| 7. | Kim, M.-J.; Ahn, M.; Chae, M.; Kim, S.; Kim, D.; Wee, K.-R. RSC Adv. 2021, 11, 34945–34954. doi:10.1039/d1ra06602a |
| 8. | Wu, C.-A.; Chou, H.-H.; Shih, C.-H.; Wu, F.-I.; Cheng, C.-H.; Huang, H.-L.; Chao, T.-C.; Tseng, M.-R. J. Mater. Chem. 2012, 22, 17792–17799. doi:10.1039/c2jm33376g |
| 9. | Pavithra, T.; Gnanaoli, K.; Rajkumar, D. B.; Puhazhendhi, A.; Sivalingam, S.; Sampath, N.; Nagarajan, S.; Sridharan, V.; Maheswari, C. U. New J. Chem. 2024, 48, 2175–2182. doi:10.1039/d3nj04721k |
| 10. | Baykov, S.; Tarasenko, M.; Kotlyarova, V.; Shetnev, A.; Zelenkov, L. E.; Boyarskaya, I. A.; Boyarskiy, V. P. ChemistrySelect 2022, 7, e202201201. doi:10.1002/slct.202201201 |
| 11. | Sasabe, H.; Seino, Y.; Kimura, M.; Kido, J. Chem. Mater. 2012, 24, 1404–1406. doi:10.1021/cm3006748 |
| 12. | Twamley, B.; Haubrich, S. T.; Power, P. P. Element Derivatives of Sterically Encumbering Terphenyl Ligands. In Advances in Organometallic Chemistry; West, R.; Hill, A. F., Eds.; Academic Press, 1999; Vol. 44, pp 1–65. doi:10.1016/s0065-3055(08)60619-x |
| 13. | Manke, D. R.; Golen, J. A.; Stennett, C. R.; Naeem, M.; Javier-Jimenez, D. R.; Power, P. P. Polyhedron 2022, 222, 115947. doi:10.1016/j.poly.2022.115947 |
| 14. | Clyburne, J. A. C.; McMullen, N. Coord. Chem. Rev. 2000, 210, 73–99. doi:10.1016/s0010-8545(00)00317-9 |
| 15. | Kays, D. L. The Stabilisation of Organometallic Complexes Using M-Terphenyl Ligands. In Organometallic Chemistry; Fairlamb, I. J. S.; Lynam, J. M., Eds.; Royal Society of Chemistry, 2010; Vol. 36, pp 56–76. doi:10.1039/9781847559616-00056 |
| 16. | Rivard, E.; Power, P. P. Inorg. Chem. 2007, 46, 10047–10064. doi:10.1021/ic700813h |
| 51. | Makarov, A. S.; Bakiev, A. N.; Eshmemeteva, D. A. Org. Chem. Front. 2023, 10, 2760–2765. doi:10.1039/d3qo00583f |
| 52. | Fang, Z.; Hong, B.; Wu, W.; Weng, Z. Org. Chem. Front. 2023, 10, 3207–3212. doi:10.1039/d3qo00493g |
| 55. | Taylor, P. J.; Wait, A. R. J. Chem. Soc., Perkin Trans. 2 1986, 1765–1770. doi:10.1039/p29860001765 |
| 56. | Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165–195. doi:10.1021/cr00002a004 |
| 44. | Pavithra, T.; Karthiyayini, G.; Nagarajan, S.; Sridharan, V.; Maheswari, C. U. Synlett 2023, 34, 807–814. doi:10.1055/a-1903-5174 |
| 45. | Rocchi, D.; Gómez-Carpintero, J.; González, J. F.; Menéndez, J. C. Molecules 2020, 25, 5565. doi:10.3390/molecules25235565 |
| 46. | Rocchi, D.; González, J. F.; Gómez-Carpintero, J.; González-Ruiz, V.; Martín, M. A.; Sridharan, V.; Menéndez, J. C. ACS Comb. Sci. 2018, 20, 722–731. doi:10.1021/acscombsci.8b00137 |
| 47. | Vachan, B. S.; Ramesh, A.; Karuppasamy, M.; Muthukrishnan, I.; Nagarajan, S.; Menéndez, J. C.; Maheswari, C. U.; Sridharan, V. RSC Adv. 2019, 9, 32946–32953. doi:10.1039/c9ra07108c |
| 48. | Li, L.; Zhao, M.-N.; Ren, Z.-H.; Li, J.-L.; Guan, Z.-H. Org. Lett. 2012, 14, 3506–3509. doi:10.1021/ol3014733 |
| 49. | Yaragorla, S.; Dada, R. ACS Omega 2017, 2, 4859–4869. doi:10.1021/acsomega.7b00753 |
| 50. | Ma, C.-L.; Yu, X.-L.; Zhu, X.-L.; Hu, Y.-Z.; Dong, X.-W.; Tan, B.; Liu, X.-Y. Adv. Synth. Catal. 2015, 357, 569–575. doi:10.1002/adsc.201400933 |
| 38. | Yang, S.; Lu, D.; Huo, H.; Luo, F.; Gong, Y. Org. Lett. 2018, 20, 6943–6947. doi:10.1021/acs.orglett.8b03090 |
| 39. | Goryaeva, M. V.; Kushch, S. O.; Khudina, O. G.; Burgart, Y. V.; Kudyakova, Y. S.; Ezhikova, M. A.; Kodess, M. I.; Slepukhin, P. A.; Sadretdinova, L. S.; Evstigneeva, N. P.; Gerasimova, N. A.; Saloutin, V. I. Org. Biomol. Chem. 2019, 17, 4273–4280. doi:10.1039/c9ob00293f |
| 40. | Naito, S.; Yokoyama, S.; Asahara, H.; Nishiwaki, N. Tetrahedron Lett. 2017, 58, 4699–4702. doi:10.1016/j.tetlet.2017.11.003 |
| 41. | Volochnyuk, D. M.; Kostyuk, A. N.; Sibgatulin, D. A.; Chernega, A. N.; Pinchuk, A. M.; Tolmachev, A. A. Tetrahedron 2004, 60, 2361–2371. doi:10.1016/j.tet.2004.01.010 |
| 42. | Liu, X.-Y.; Che, C.-M. Angew. Chem., Int. Ed. 2009, 48, 2367–2371. doi:10.1002/anie.200805383 |
| 43. | Davies, I. W.; Marcoux, J.-F.; Kuethe, J. T.; Lankshear, M. D.; Taylor, J. D. O.; Tsou, N.; Dormer, P. G.; Hughes, D. L.; Houk, K. N.; Guner, V. J. Org. Chem. 2004, 69, 1298–1308. doi:10.1021/jo035677u |
| 27. | Swami, B.; Yadav, D.; Menon, R. S. Chem. Rec. 2022, 22, e202100249. doi:10.1002/tcr.202100249 |
| 32. | Jacob, C.; Annibaletto, J.; Maes, B. U. W.; Evano, G. Synthesis 2023, 55, 1799–1823. doi:10.1055/a-2039-7985 |
| 33. | Mies, T.; Ma, T.-K.; Barrett, A. G. M. Russ. Chem. Rev. 2020, 89, 917–965. doi:10.1070/rcr4931 |
| 34. | Zhao, Q.; Peng, C.; Zhan, G.; Han, B. RSC Adv. 2020, 10, 40983–41003. doi:10.1039/d0ra08068c |
| 35. | Wu, L.; Yu, B.; Li, E.-Q. Adv. Synth. Catal. 2020, 362, 4010–4026. doi:10.1002/adsc.202000608 |
| 36. | Banerjee, A.; Kundu, S.; Bhattacharyya, A.; Sahu, S.; Maji, M. S. Org. Chem. Front. 2021, 8, 2710–2771. doi:10.1039/d1qo00092f |
| 37. | Poudel, T. N.; Tamargo, R. J. I.; Cai, H.; Lee, Y. R. Asian J. Org. Chem. 2018, 7, 985–1005. doi:10.1002/ajoc.201800080 |
| 53. | Galeev, A. R.; Dmitriev, M. V.; Mokrushin, I. G.; Mashevskaya, I. V.; Maslivets, A. N.; Rubin, M. Org. Biomol. Chem. 2019, 17, 10030–10044. doi:10.1039/c9ob02120e |
| 54. | Galeev, A. R.; Mokrushin, I. G.; Dmitriev, M. V.; Maslivets, A. N. Russ. J. Org. Chem. 2020, 56, 1317–1320. doi:10.1134/s1070428020070295 |
© 2024 Galeev et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.