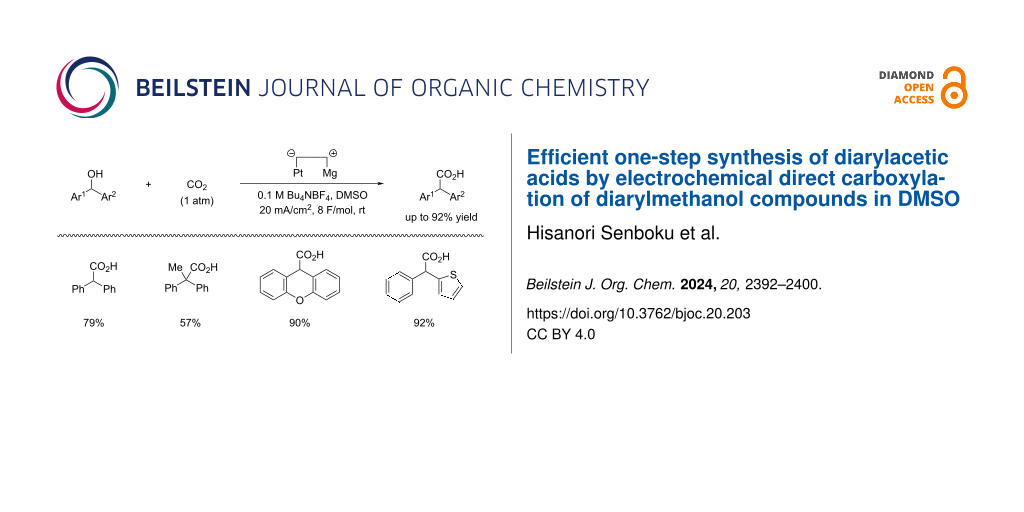
Abstract
An efficient one-step synthesis of diarylacetic acids was successfully performed by electrochemical direct carboxylation of diarylmethanol compounds in DMSO. Constant-current electrolysis of diarylmethanol species in DMSO using a one-compartment cell equipped with a Pt cathode and a Mg anode in the presence of carbon dioxide induced reductive C(sp3)−O bond cleavage at the benzylic position in diarylmethanol compounds and subsequent fixation of carbon dioxide to produce diarylacetic acids in good yield. This protocol provides a novel and simple approach to diarylacetic acids from diarylmethanol species and carbon dioxide without transformation of the hydroxy group into appropriate leaving groups, such as halides and esters including carbonates.
Graphical Abstract
Introduction
Electrochemical reduction of benzyl alcohol derivatives can induce reductive cleavage of a C(sp3)–O bond [1] at the benzylic position to generate the corresponding benzylic anion species. This protocol has been frequently applied to electrochemical carboxylation [2-11], yielding phenylacetic acids. For example, Troupel et al. successfully performed electrochemical reduction of benzyl ethers and several esters such as acetate, trifluoroacetate, benzoate, and dibenzyl carbonate derived from benzyl alcohols, including 1-phenylethanol compounds, in the presence of carbon dioxide to give the corresponding phenylacetic acids [12]. We found that alkyl benzyl carbonates and benzal diacetates (benzylidene diacetates) were also applicable to electrochemical carboxylation with C(sp3)–O bond cleavage at the benzylic position, yielding phenylacetic acids [13] and mandel acetates [14], respectively. Electrolysis of styrene oxide and related 2-phenylcyclic ethers in the presence of carbon dioxide also induced carboxylation at the benzylic position by reductive cleavage of a C(sp3)–O bond to give the corresponding ω-hydroxy-2-phenylalkanoic acids [15,16]. In contrast, little attention has been paid to electrochemical direct carboxylation of benzyl alcohols, although it is a more straightforward and simple protocol toward phenylacetic acids. In 2015, we reported an electrochemical direct carboxylation of benzyl alcohols having an electron-withdrawing group on the phenyl ring [17]. To the best of our knowledge, this is the first report on electrochemical carboxylation of benzyl alcohols. Only benzyl alcohols having an electron-withdrawing group, such as cyano or ester in the ortho- or para-position of the phenyl ring, were efficiently carboxylated by constant-current electrolysis in DMF using an undivided cell equipped with a Pt cathode and a Mg anode in the presence of carbon dioxide. On the other hand, carboxylation scarcely took place in DMF when other benzyl alcohols were used as substrates. Lundberg and co-workers recently reported similar results showing that electrochemical carboxylation of benzyl alcohols in the presence of Bu4NBH4 in DMF using graphite electrodes gave the corresponding carboxylic acids only in poor yield [18]. We recently found that DMSO is a more suitable solvent for electrochemical carboxylation of benzyl alcohols. Even though there was no electron-withdrawing group on the phenyl ring in the benzyl alcohols, electrochemical carboxylation of the benzyl alcohols in DMSO provided the corresponding phenylacetic acids in moderate yield by reductive C(sp3)–O bond cleavage followed by fixation of carbon dioxide at the benzylic position [19]. Notably, when diphenylmethanol (1a) was used as substrate for electrochemical carboxylation in DMSO, the carboxylation efficiently proceeded to give diphenylacetic acid (2a) in high yield. In this paper, we focus on electrochemical carboxylation of diarylmethanol compounds 1 in DMSO and report the efficient one-pot synthesis of diarylacetic acids 2 using this protocol (Scheme 1, bottom). Although photochemical synthesis of diarylacetic acids 2 from diarylmethanol species 1 and carbon dioxide has been reported (Scheme 1, top) [20], to the best of our knowledge, this is the first electrochemical and the second efficient example of diarylacetic acid 2 synthesis from diarylmethanol compounds 1 and carbon dioxide in one step.
Scheme 1: Synthesis of diarylacetic acids 2 from diarylmethanol compounds 1 and carbon dioxide.
Scheme 1: Synthesis of diarylacetic acids 2 from diarylmethanol compounds 1 and carbon dioxide.
Results and Discussion
Although we have previously obtained diphenylacetic acid (2a) in 81% yield by electrochemical carboxylation of diphenylmethanol (1a) [19], screening of reaction conditions for the substrate 1a was carried out. Constant-current electrolysis of 1a using an undivided cell in the presence of carbon dioxide at room temperature was conducted. The effects of the current density (Table 1, entries 1–3), solvent (Table 1, entries 2, 4, and 5), electrodes (Table 1, entries 2, 6, and 7), and electricity (Table 1, entries 2 and 8–10) were investigated. A current density between 10 and 30 mA/cm2 had little effect on the yield of 2a (Table 1, entries 1–3). Carboxylation of 1a also took place in DMF instead of DMSO as solvent. However, the efficiency and yield were lower than those in DMSO (Table 1, entry 4). Acetonitrile seemed to be unsuitable for the reaction (Table 1, entry 5). Glassy carbon (GC) was usable as cathode material to give 2a in 59% yield, slightly lower than when using Pt as cathode, probably due to the high hydrogen overpotential (Table 1, entry 6). In contrast, zinc was not effective as an anode material in the carboxylation, probably due to competitive electrochemical reduction of zinc ions generated by electrochemical oxidation of the zinc anode. The deposition of a black precipitate was observed visually at the cathode (Table 1, entry 7). After tuning of the electricity, electrochemical carboxylation of 1a under the reaction conditions shown in Table 1, entry 8 gave 2a in 79% isolated yield. In all cases, diphenylmethane, probably produced by protonation of the generated benzyl anion species, was detected as a byproduct in less than 10% yield, determined through 1H NMR analysis, except for Table 1, entries 3 (13%) and 5 (25%). It should also be noted that electrolysis was carried out at room temperature, but the temperature of the reaction mixture increased to 40–50 °C at the end of the electrolysis in every case due to heat generation by electric resistance.
Table 1: Screening of reaction conditions in the electrochemical carboxylation of 1a.
|
|
|||||||
| entry | cathode | anode | solvent | current density, mA/cm2 | electricity, F/mol | conversion, %a | yield, %b |
|---|---|---|---|---|---|---|---|
| 1 | Pt | Mg | DMSO | 10 | 6 | 83 | 69 |
| 2 | Pt | Mg | DMSO | 20 | 6 | 84 | 69 |
| 3 | Pt | Mg | DMSO | 30 | 6 | 76 | 61 |
| 4 | Pt | Mg | DMF | 20 | 6 | 48 | 39 |
| 5 | Pt | Mg | CH3CN | 20 | 6 | 42 | 14 |
| 6 | GC | Mg | DMSO | 20 | 6 | 71 | 59 |
| 7 | Pt | Zn | DMSO | 20 | 6 | 38 | 28 |
| 8 | Pt | Mg | DMSO | 20 | 8 | 92 | 79 |
| 9 | Pt | Mg | DMSO | 20 | 10 | 94 | 80 |
| 10 | Pt | Mg | DMSO | 20 | 12 | 98 | 80 |
aDetermined by 1H NMR spectroscopy using 1,4-dinitrobenzene as internal standard. bIsolated yield.
With these results in hand, the substrate scope was investigated. First, the applicability of halogen-containing diphenylmethanol compounds 1b and 1c to the electrochemical carboxylation under the reaction conditions shown in Table 1, entry 8 was investigated, and the results are summarized in Scheme 2. Electrochemical carboxylation of bis(4-chlorophenyl)methanol (1b) unfortunately proceeded along with dehalogenation of the phenyl ring to give 2a in 31% yield. The absence of mono- and dichlorocarboxylic acids such as 2b in the product and the existence of dechlorinated diphenylmethanol (1a) in the recovered starting material were confirmed by 1H NMR analysis. These results suggested that 2a was produced from 1a, which was generated in situ by electroreductive dechlorination of 1b. In other words, electroreductive dechlorination of 1b, producing 1a, took place preferentially over electroreductive C(sp3)–O bond cleavage of 1b. It should also be noted that no aromatic carboxylic acids were detected by 1H NMR analysis. In contrast, the electrochemical carboxylation of 1c, containing a fluorine atom, gave a mixture of fluorine-containing carboxylic acid 2c and defluorinated carboxylic acid 2a. Fluorine-containing starting material 1c and defluorinated diphenylmethanol (1a) were detected by 1H NMR analysis. At the same time, it is presently unclear whether 2a was produced in a carboxylation–defluorination or defluorination–carboxylation sequence.
Scheme 2: Attempted electrochemical carboxylation of halogen-containing diphenylmethanol compounds 1b and 1c.
Scheme 2: Attempted electrochemical carboxylation of halogen-containing diphenylmethanol compounds 1b and 1c.
Other results of the electrochemical carboxylation are summarized in Scheme 3. Electrochemical carboxylation of diphenylmethanol species 1d and 1e, having a methyl or methoxy group on the phenyl ring, also proceeded to give the corresponding diphenylacetic acids 2d and 2e in 61% and 63% yield, respectively. In the electrocarboxylation of 1f and 1g, having a methyl or methoxy group on both phenyl rings, the yield of 2f and 2g was comparably lower, namely 32% and 21%, respectively, probably due to the electron-donating groups. When diphenylmethanol 1h, having a hydroxy group on the phenyl ring, was used as substrate, selective C–O bond cleavage, followed by fixation of carbon dioxide, occurred at the benzylic C(sp3)–O bond rather than the C(sp2)–O bond on the phenyl ring to give the corresponding diphenylacetic acid 2h in 45% yield. Not only a secondary alcohol but also a tertiary alcohol was applicable to the reaction. When 1,1-diphenylethanol (1i) was subjected to electrochemical carboxylation, electroreductive C(sp3)–O bond cleavage and subsequent carboxylation also took place, similarly to that of secondary alcohols, to give 2,2-diphenylpropanoic acid (2i) in 57% yield. Cyclic alcohols and heteroarylmethanol were also suitable and efficient substrates. Electroreductive C(sp3)–O bond cleavage and subsequent carboxylation proceeded efficiently when dibenzocycloheptenol (1j) was used as substrate to give the corresponding dibenzocycloheptenecarboxylic acid (2j) in 84% yield. Electrocarboxylation of xanthenol (1k) provided xanthene-9-carboxylic acid (2k) in an excellent yield of 90%. Phenyl(thiophen-2-yl)acetic acid (2l) could also be synthesized in an excellent yield of 92% yield by electrochemical carboxylation of phenyl(thiophen-2-yl)methanol (1l).
Scheme 3: Synthesis of diarylacetic acids 2 by electrochemical carboxylation of diarylmethanol species 1.
Scheme 3: Synthesis of diarylacetic acids 2 by electrochemical carboxylation of diarylmethanol species 1.
Although a wide range of substrates was shown to be applicable, a limitation of the reaction also existed. Electrochemical carboxylation of 9H-fluoren-9-ol (1m) failed, and only a complex mixture was obtained, probably due to the electrochemical reduction and/or carboxylation of the biphenyl moiety in fluorenol (1m, top of Scheme 4). Similarly, when triphenylmethanol (1n) was subjected to the electrochemical carboxylation, a small amount of carboxylic acid was obtained as a complex mixture. However, in the 1H NMR spectrum of the organic component after extraction with aqueous base, we observed a singlet at δ 5.55 ppm, which could be assigned to the methine proton in triphenylmethane (3, Scheme 4, bottom). Further, the yield was determined to be 67% by 1H NMR spectroscopy. These results indicated that the triphenylmethyl anion species generated by electroreductive C(sp3)–O bond cleavage acted not as nucleophile toward carbon dioxide but as base, performing proton abstraction from DMSO to produce 3 as main product. It was thought that the increase of steric hindrance caused a reduction in nucleophilicity and an increase in basicity in the generated triphenylmethyl anion species, in comparison to the diphenylmethyl anion species.
Scheme 4: Attempted electrochemical carboxylation of 1m and 1n.
Scheme 4: Attempted electrochemical carboxylation of 1m and 1n.
To elucidate the mechanism of the electroreductive C(sp3)–O bond cleavage, one additional experiment was carried out as shown in Scheme 5. Constant-current electrolysis of 1a in DMSO with 20 mA/cm2 of current density and 6 F/mol of electricity at room temperature under a nitrogen atmosphere instead of carbon dioxide resulted in the recovery of 98% of the starting material 1a. This result indicated that carbon dioxide played an important role not only as a carboxy source but also in the electroreductive C(sp3)–O bond cleavage.
Scheme 5: Electrolysis of 1a under a nitrogen atmosphere.
Scheme 5: Electrolysis of 1a under a nitrogen atmosphere.
From these and our previous results for the electrochemical carboxylation of benzyl carbonates [13], plausible reaction pathways are proposed as seen in Scheme 6. At the cathode, one-electron reduction of the hydroxy group in diarylmethanol 1 generates H2 and the corresponding alkoxide ion A, which captures carbon dioxide to form carbonate ion B. Although it is currently unclear whether this proceeds in a stepwise or concerted manner, two-electron reduction of carbonate ion B generates the corresponding diphenylmethyl anion C, which reacts with carbon dioxide to produce a carbon–carbon bond that results in the formation of diphenyl acetate ion D. Electrochemical reduction of carbon dioxide competitively occurs at the cathode, and an excess amount of electricity should therefore be necessary to obtain acceptable results. At the anode, on the other hand, dissolution of magnesium ions by electrochemical oxidation of magnesium metal occurs, preventing electrochemical oxidation of the product and intermediates at the anode. The produced acetate ion D and a magnesium ion form the salt E, which upon acid treatment during workup gives a diphenylacetic acid 2. The effects of DMSO as solvent are not clear at present, and one reasonable and acceptable aspect might be the solubility of the salt of intermediate B in DMSO solvent. Electrochemical reduction of intermediate B should occur in solution, and this would mean that intermediate B must be dissolved in the solvent used. DMSO is well known as a good solvent for dissolving organic metal salts. In this electrochemical reaction medium, a main counter cation of intermediate B is thought to be the magnesium ion, and the magnesium salt of B must be dissolved in the solvent. Although other magnesium salts, such as magnesium carbonate and magnesium oxalate, are also generated during the electrolysis, the magnesium salt of B would be dissolved sufficiently in DMSO for electrochemical reduction at the cathode.
Conclusion
Efficient one-pot synthesis of diarylacetic acids 2 was accomplished by electrochemical direct carboxylation of diarylmethanol species 1 in DMSO. 2,2-Diphenylpropanoic acid (2i) and phenyl(thiophen-2-yl)acetic acid (2l) could also be synthesized from 1,1-diphenylethanol (1i) and phenyl(thiophen-2-yl)methanol (1l), respectively, in one step by this electrochemical method. Notably, synthesis of xanthene-9-carboxylic acid (2k) and dibenzocycloheptene-5-carbocylic acid 2j was conducted with excellent yield. Direct substitution of a hydroxy by a carboxy group using carbon dioxide as carboxy source is generally difficult under neutral and mild conditions without the use of metal catalysts and/or ligands. This protocol represents a novel method for synthesizing diarylacetic acids 2 from the corresponding alcohols 1 in one step by using an electrochemical method. Instead of DMF and acetonitrile, which have frequently been used for electrochemical carboxylation, DMSO was found to be effective for electrochemical carboxylation of diarylmethanol compounds 1 in order to provide diarylacetic acids 2. The present electrochemical synthesis is promising as a novel, efficient, facile, and green organic method.
Experimental
General information
1H (400 MHz) and 13C (100 MHz) NMR spectra were recorded in CDCl3 or DMSO-d6 with a JEOL JNM-ECS400 FT NMR spectrometer. The chemical shifts δ are given in ppm with tetramethylsilane (δ 0 ppm) or DMSO (δ 2.50 ppm) for 1H and CDCl3 (δ 77.0 ppm) or DMSO-d6 (δ 39.5 ppm) for 13C as internal references. J values are in Hz. Peak multiplicities are given as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad. Reagents and solvents, including anhydrous DMSO, are commercially available and were used as received without further purification. Electrochemical reactions were carried out using a constant current power supply (model 5944), Metronix Corp., Tokyo.
Diphenylmethanol (1a) [21], bis(4-chlorophenyl)methanol (1b) [22], (2-fluorophenyl)phenylmethanol (1c) [23], (4-methylphenyl)phenylmethanol (1d) [21], (4-methoxyphenyl)phenylmethanol (1e) [21], bis(4-methylphenyl)methanol (1f) [24], bis(4-methoxyphenyl)methanol (1g) [25], (4-hydroxyphenyl)phenylmethanol (1h) [26], 10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5-ol (1j) [27], 9H-xanthen-9-ol (1k) [28], and phenyl(thiophen-2-yl)methanol (1l) [21] are known compounds and were prepared from the corresponding commercially available ketones by reaction with NaBH4 in EtOH or EtOH/THF 1:1. 1,1-Diphenylethanol (1i) is also a known compound and was prepared according to the reported procedure [29]. 9H-Fluoren-9-ol (1m) and triphenylmethanol (1n) are commercially available.
General procedure for electrochemical carboxylation of 1
A test-tube-like (≈25 mm ⌀) undivided cell equipped with a Pt plate cathode (2 × 2 cm2), a Mg rod anode (6 mm ⌀, 2 cm), and a Teflon® tube (1 mm ⌀) for supplying carbon dioxide were used for the electrolysis. A solution of a diarylmethanol 1 (1 mmol) in anhydrous DMSO (10 mL) containing 0.1 M Bu4NBF4 (329 mg, 1 mmol) was set in the cell under a nitrogen atmosphere. Carbon dioxide was bubbled through the solution at room temperature for 10 min, and then the solution was electrolyzed with a constant current (20 mA/cm2) under atmospheric pressure of bubbling carbon dioxide at room temperature. The temperature of the reaction mixture increased to 40–50 °C at the end of the electrolysis in every case due to generation of heat by electric resistance during the electrolysis. After 8 F/mol of electricity had been supplied, 1 M hydrochloric acid (100 mL) was added to the electrolyzed solution, and then the mixture was extracted with ethyl acetate (30 mL × 5). The combined organic layer was washed with aqueous saturated NaHCO3 (40 mL × 3), and the resulting aqueous solution was acidified with 3 M hydrochloric acid and then extracted with ethyl acetate (30 mL × 5). The combined ethyl acetate solution was washed with H2O (100 mL × 3) and dried over MgSO4. Evaporation of the solvent gave a diarylacetic acid 2. The organic layer, extracted by aqueous saturated NaHCO3, was washed with H2O (100 mL × 3) and dried over MgSO4. Evaporation of the solvent gave a residue that was analyzed by 1H NMR spectroscopy, during which 1,4-dinitrobenzene was used as an internal standard for quantification of the substances.
The products diphenylacetic acid (2a) [30,31], (4-methylphenyl)phenylacetic acid (2d) [32], (4-methoxyphenyl)phenylacetic acid (2e) [32], bis(4-methylphenyl)acetic acid (2f) [30,32], bis(4-methoxyphenyl)acetic acid (2g) [30,33], (4-hydroxyphenyl)phenylacetic acid (2h) [34], 2,2-diphenylpropaoic acid (2i) [35], 10,11-dihydro-5H-dibenzo[a,d]cycloheptene-5-carboxylic acid (2j) [30], 9H-xanthene-9-carboxylic acid (2k) [36], and phenyl(thiophen-2-yl)acetic acid (2l) [30] are known compounds, and their spectral data were good agreement with previously reported values.
Spectral data of the products 2
Diphenylacetic acid (2a): 1H NMR (400 MHz, CDCl3, δ) 5.05 (s, 1H), 7.25–7.34 (m, 10H); 13C NMR (100 MHz, CDCl3, δ) 56.9, 127.5, 128.7 (× 2), 137.8, 178.1.
(4-Methylphenyl)phenylacetic acid (2d): 1H NMR (400 MHz, CDCl3, δ) 2.32 (s, 3H), 5.01 (s, 1H), 7.14 (d, J = 8.2 Hz, 2H), 7.21 (d, J = 8.2 Hz, 2H), 7.25–7.29 (m, 1H), 7.31–7.32 (m, 4H); 13C NMR (100 MHz, CDCl3, δ) 21.0, 56.6, 127.4, 128.5, 128.59, 128.61, 129.3, 134.9, 137.2, 138.0, 178.9.
(4-Methoxyphenyl)phenylacetic acid (2e): 1H NMR (400 MHz, CDCl3, δ) 3.78 (s, 3H), 4.99 (s, 1H), 6.86 (d, J = 8.7 Hz, 2H), 7.24 (d, J = 8.7 Hz, 2H), 7.23–7.32 (m, 5H); 13C NMR (100 MHz, CDCl3, δ) 55.2, 56.1, 114.0, 127.4, 128.5, 128.6, 129.7, 129.9, 138.3, 159.0, 179.2.
Bis(4-methylphenyl)acetic acid (2f): 1H NMR (400 MHz, CDCl3, δ) 2.32 (s, 6H), 4.97 (s, 1H), 7.13 (d, J = 8.2 Hz, 4H), 7.20 (d, J = 8.2 Hz, 4H); 13C NMR (100 MHz, CDCl3, δ) 21.0, 56.2, 128.5, 129.3, 135.1, 137.1, 178.9.
Bis(4-methoxyphenyl)acetic acid (2g): 1H NMR (400 MHz, CDCl3, δ) 3.78 (s, 6H), 4.95 (s, 1H), 6.86 (d, J = 8.7 Hz, 4H), 7.23 (d, J = 8.7 Hz, 4H); 13C NMR (100 MHz, CDCl3, δ) 55.2 (OMe and CHCO2H), 114.0, 129.6, 130.3, 158.9, 179.2.
(4-Hydroxyphenyl)phenylacetic acid (2h): 1H NMR (400 MHz, DMSO-d6, δ) 4.91 (s, 1H), 6.70 (d, J = 8.7 Hz, 2H), 7.10 (d, J = 8.7 Hz, 2H), 7.20–7.36 (m, 5H), 9.35 (s, 1H); 13C NMR (100 MHz, DMSO-d6, δ) 56.0, 115.8, 127.2, 128.8, 128.9, 130.0, 130.2, 140.1, 156.8, 174.3.
2,2-Diphenylpropanoic acid (2i): 1H NMR (400 MHz, CDCl3, δ) 1.94 (s, 3H), 7.26–7.34 (m, 10H); 13C NMR (100 MHz, CDCl3, δ) 26.8, 56.4, 127.0, 128.06, 128.12, 143.7, 181.3.
10,11-Dihydro-5H-dibenzo[a,d]cycloheptene-5-carboxylic acid (2j): 1H NMR (400 MHz, CDCl3, δ) 2.85–2.93 (m, 2H), 3.31–3.39 (m, 2H), 4.81 (s, 1H), 7.14–7.24 (m, 8H); 13C NMR (100 MHz, CDCl3, δ) 32.4, 58.7, 126.3, 128.1, 130.4, 131.3, 135.4, 139.6, 178.2.
9H-Xanthene-9-carboxylic acid (2k): 1H NMR (400 MHz, CDCl3, δ) 4.97 (s, 1H), 7.06–7.10 (m, 2H), 7.12–7.15 (m, 2H), 7.28–7.32 (m, 4H); 13C NMR (100 MHz, CDCl3, δ) 44.9, 117.0, 117.6, 123.4, 129.1, 129.3, 151.3, 177.1.
Phenyl(thiophen-2-yl)acetic acid (2l): 1H NMR (400 MHz, CDCl3, δ) 5.24 (s, 1H), 6.95–6.97 (m, 1H), 7.01–7.02 (m, 1H), 7.24–7.25 (m, 1H), 7.29–7.37 (m, 3H), 7.39–7.42 (m, 2H); 13C NMR (100 MHz, CDCl3, δ) 52.1, 125.5, 126.6, 126.7, 128.0, 128.3, 128.8, 137.6, 140.0, 177.8.
Supporting Information
| Supporting Information File 1: NMR spectra. | ||
| Format: PDF | Size: 3.0 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information to this article.
References
-
Villo, P.; Shatskiy, A.; Kärkäs, M. D.; Lundberg, H. Angew. Chem., Int. Ed. 2023, 62, e202211952. doi:10.1002/anie.202211952
Return to citation in text: [1] -
Senboku, H. Curr. Org. Chem. 2024, 28, 76–88. doi:10.2174/1385272827666230915162055
Return to citation in text: [1] -
Mao, J.; Wang, Y.; Zhang, B.; Lou, Y.; Pan, C.; Zhu, Y.; Zhang, Y. Green Carbon 2024, 2, 45–56. doi:10.1016/j.greenca.2024.02.001
Return to citation in text: [1] -
Zhang, K.; Liu, X.-F.; Ren, W.-M.; Lu, X.-B.; Zhang, W.-Z. Chem. – Eur. J. 2023, 29, e202204073. doi:10.1002/chem.202204073
Return to citation in text: [1] -
Wang, J.; Wei, Z.-F.; Luo, Y.-X.; Lu, C.-H.; Song, R.-J. SynOpen 2024, 8, 116–124. doi:10.1055/s-0043-1763748
Return to citation in text: [1] -
Wang, Q.; Wang, Y.; Liu, M.; Chu, G.; Qiu, Y. Chin. J. Chem. 2024, 42, 2249–2266. doi:10.1002/cjoc.202400008
Return to citation in text: [1] -
Yao, H.; Wang, M.-Y.; Yue, C.; Feng, B.; Ji, W.; Qian, C.; Wang, S.; Zhang, S.; Ma, X. Trans. Tianjin Univ. 2023, 29, 254–274. doi:10.1007/s12209-023-00361-2
Return to citation in text: [1] -
Pradhan, S.; Das, S. Synlett 2023, 34, 1327–1342. doi:10.1055/a-2012-5317
Return to citation in text: [1] -
Mena, S.; Peral, J.; Guirado, G. Curr. Opin. Electrochem. 2023, 42, 101392. doi:10.1016/j.coelec.2023.101392
Return to citation in text: [1] -
Senboku, H. Chem. Rec. 2021, 21, 2354–2374. doi:10.1002/tcr.202100081
Return to citation in text: [1] -
Senboku, H.; Katayama, A. Curr. Opin. Green Sustainable Chem. 2017, 3, 50–54. doi:10.1016/j.cogsc.2016.10.003
Return to citation in text: [1] -
Gal, J.; Folest, J. C.; Troupel, M.; Moingeon, M. O.; Chaussard, J. New J. Chem. 1995, 19, 401–407.
Return to citation in text: [1] -
Ohkoshi, M.; Michinishi, J.-y.; Hara, S.; Senboku, H. Tetrahedron 2010, 66, 7732–7737. doi:10.1016/j.tet.2010.07.067
Return to citation in text: [1] [2] -
Senboku, H.; Sakai, K.; Fukui, A.; Sato, Y.; Yamauchi, Y. ChemElectroChem 2019, 6, 4158–4164. doi:10.1002/celc.201900200
Return to citation in text: [1] -
Zhang, K.; Ren, B.-H.; Liu, X.-F.; Wang, L.-L.; Zhang, M.; Ren, W.-M.; Lu, X.-B.; Zhang, W.-Z. Angew. Chem., Int. Ed. 2022, 61, e202207660. doi:10.1002/anie.202207660
Return to citation in text: [1] -
Wang, Y.; Tang, S.; Yang, G.; Wang, S.; Ma, D.; Qiu, Y. Angew. Chem., Int. Ed. 2022, 61, e202207746. doi:10.1002/anie.202207746
Return to citation in text: [1] -
Senboku, H.; Yoneda, K.; Hara, S. Tetrahedron Lett. 2015, 56, 6772–6776. doi:10.1016/j.tetlet.2015.10.068
Return to citation in text: [1] -
Villo, P.; Lill, M.; Alsaman, Z.; Kronberg, A. S.; Chu, V.; Ahumada, G.; Agarwala, H.; Ahlquist, M.; Lundberg, H. ChemElectroChem 2023, 10, e202300420. doi:10.1002/celc.202300420
Return to citation in text: [1] -
Hayama, M.; Senboku, H. Electrochemistry 2023, 91, 112011. doi:10.5796/electrochemistry.23-67082
Return to citation in text: [1] [2] -
Li, W.-D.; Wu, Y.; Li, S.-J.; Jiang, Y.-Q.; Li, Y.-L.; Lan, Y.; Xia, J.-B. J. Am. Chem. Soc. 2022, 144, 8551–8559. doi:10.1021/jacs.1c12463
Return to citation in text: [1] -
Gaykar, R. N.; Bhunia, A.; Biju, A. T. J. Org. Chem. 2018, 83, 11333–11340. doi:10.1021/acs.joc.8b01549
Return to citation in text: [1] [2] [3] [4] -
Kamijo, S.; Tao, K.; Takao, G.; Tonoda, H.; Murafuji, T. Org. Lett. 2015, 17, 3326–3329. doi:10.1021/acs.orglett.5b01550
Return to citation in text: [1] -
Jakhar, V. K.; Barman, M. K.; Nembenna, S. Org. Lett. 2016, 18, 4710–4713. doi:10.1021/acs.orglett.6b02310
Return to citation in text: [1] -
Han, H. J.; Kim, H. T.; Kim, J.; Jaladi, A. K.; An, D. K. Tetrahedron 2023, 142, 133500. doi:10.1016/j.tet.2023.133500
Return to citation in text: [1] -
Yadav, S.; Kuram, M. R. Eur. J. Org. Chem. 2023, 26, e20220134. doi:10.1002/ejoc.202201344
Return to citation in text: [1] -
Li, C.-J.; Meng, Y. J. Am. Chem. Soc. 2000, 122, 9538–9539. doi:10.1021/ja001699b
Return to citation in text: [1] -
Varjosaari, S. E.; Skrypai, V.; Suating, P.; Hurley, J. J. M.; Gilbert, T. M.; Adler, M. J. Eur. J. Org. Chem. 2017, 229–232. doi:10.1002/ejoc.201601256
Return to citation in text: [1] -
Wei, Z.; Li, H.; Wang, Y.; Liu, Q. Angew. Chem., Int. Ed. 2023, 62, e202301042. doi:10.1002/anie.202301042
Return to citation in text: [1] -
Lei, C.; Yip, Y. J.; Zhou, J. S. J. Am. Chem. Soc. 2017, 139, 6086–6089. doi:10.1021/jacs.7b02742
Return to citation in text: [1] -
Yuan, P.-F.; Yang, Z.; Zhang, S.-S.; Zhu, C.-M.; Yang, X.-L.; Meng, Q.-Y. Angew. Chem., Int. Ed. 2024, 63, e202313030. doi:10.1002/anie.202405333
Return to citation in text: [1] [2] [3] [4] [5] -
Navale, B. S.; Laha, D.; Banerjee, S.; Vanka, K.; Bhat, R. G. J. Org. Chem. 2022, 87, 13583–13597. doi:10.1021/acs.joc.2c01185
Return to citation in text: [1] -
Liao, L.-L.; Cao, G.-M.; Ye, J.-H.; Sun, G.-Q.; Zhou, W.-J.; Gui, Y.-Y.; Yan, S.-S.; Shen, G.; Yu, D.-G. J. Am. Chem. Soc. 2018, 140, 17338–17342. doi:10.1021/jacs.8b08792
Return to citation in text: [1] [2] [3] -
Moore, D. L.; Denton, A. E.; Kohinke, R. M.; Craig, B. R.; Brenzovich, W. E., Jr. Synth. Commun. 2016, 46, 604–612. doi:10.1080/00397911.2016.1158269
Return to citation in text: [1] -
Müller, C.; Gleixner, J.; Tahk, M.-J.; Kopanchuk, S.; Laasfeld, T.; Weinhart, M.; Schollmeyer, D.; Betschart, M. U.; Lüdeke, S.; Koch, P.; Rinken, A.; Keller, M. J. Med. Chem. 2022, 65, 4832–4853. doi:10.1021/acs.jmedchem.1c02033
Return to citation in text: [1] -
Gaydou, M.; Moragas, T.; Juliá-Hernández, F.; Martin, R. J. Am. Chem. Soc. 2017, 139, 12161–12164. doi:10.1021/jacs.7b07637
Return to citation in text: [1] -
Yedage, S. L.; Bhanage, B. M. J. Org. Chem. 2017, 82, 5769–5781. doi:10.1021/acs.joc.7b00570
Return to citation in text: [1]
| 30. | Yuan, P.-F.; Yang, Z.; Zhang, S.-S.; Zhu, C.-M.; Yang, X.-L.; Meng, Q.-Y. Angew. Chem., Int. Ed. 2024, 63, e202313030. doi:10.1002/anie.202405333 |
| 32. | Liao, L.-L.; Cao, G.-M.; Ye, J.-H.; Sun, G.-Q.; Zhou, W.-J.; Gui, Y.-Y.; Yan, S.-S.; Shen, G.; Yu, D.-G. J. Am. Chem. Soc. 2018, 140, 17338–17342. doi:10.1021/jacs.8b08792 |
| 30. | Yuan, P.-F.; Yang, Z.; Zhang, S.-S.; Zhu, C.-M.; Yang, X.-L.; Meng, Q.-Y. Angew. Chem., Int. Ed. 2024, 63, e202313030. doi:10.1002/anie.202405333 |
| 33. | Moore, D. L.; Denton, A. E.; Kohinke, R. M.; Craig, B. R.; Brenzovich, W. E., Jr. Synth. Commun. 2016, 46, 604–612. doi:10.1080/00397911.2016.1158269 |
| 34. | Müller, C.; Gleixner, J.; Tahk, M.-J.; Kopanchuk, S.; Laasfeld, T.; Weinhart, M.; Schollmeyer, D.; Betschart, M. U.; Lüdeke, S.; Koch, P.; Rinken, A.; Keller, M. J. Med. Chem. 2022, 65, 4832–4853. doi:10.1021/acs.jmedchem.1c02033 |
| 1. | Villo, P.; Shatskiy, A.; Kärkäs, M. D.; Lundberg, H. Angew. Chem., Int. Ed. 2023, 62, e202211952. doi:10.1002/anie.202211952 |
| 14. | Senboku, H.; Sakai, K.; Fukui, A.; Sato, Y.; Yamauchi, Y. ChemElectroChem 2019, 6, 4158–4164. doi:10.1002/celc.201900200 |
| 23. | Jakhar, V. K.; Barman, M. K.; Nembenna, S. Org. Lett. 2016, 18, 4710–4713. doi:10.1021/acs.orglett.6b02310 |
| 13. | Ohkoshi, M.; Michinishi, J.-y.; Hara, S.; Senboku, H. Tetrahedron 2010, 66, 7732–7737. doi:10.1016/j.tet.2010.07.067 |
| 21. | Gaykar, R. N.; Bhunia, A.; Biju, A. T. J. Org. Chem. 2018, 83, 11333–11340. doi:10.1021/acs.joc.8b01549 |
| 12. | Gal, J.; Folest, J. C.; Troupel, M.; Moingeon, M. O.; Chaussard, J. New J. Chem. 1995, 19, 401–407. |
| 21. | Gaykar, R. N.; Bhunia, A.; Biju, A. T. J. Org. Chem. 2018, 83, 11333–11340. doi:10.1021/acs.joc.8b01549 |
| 2. | Senboku, H. Curr. Org. Chem. 2024, 28, 76–88. doi:10.2174/1385272827666230915162055 |
| 3. | Mao, J.; Wang, Y.; Zhang, B.; Lou, Y.; Pan, C.; Zhu, Y.; Zhang, Y. Green Carbon 2024, 2, 45–56. doi:10.1016/j.greenca.2024.02.001 |
| 4. | Zhang, K.; Liu, X.-F.; Ren, W.-M.; Lu, X.-B.; Zhang, W.-Z. Chem. – Eur. J. 2023, 29, e202204073. doi:10.1002/chem.202204073 |
| 5. | Wang, J.; Wei, Z.-F.; Luo, Y.-X.; Lu, C.-H.; Song, R.-J. SynOpen 2024, 8, 116–124. doi:10.1055/s-0043-1763748 |
| 6. | Wang, Q.; Wang, Y.; Liu, M.; Chu, G.; Qiu, Y. Chin. J. Chem. 2024, 42, 2249–2266. doi:10.1002/cjoc.202400008 |
| 7. | Yao, H.; Wang, M.-Y.; Yue, C.; Feng, B.; Ji, W.; Qian, C.; Wang, S.; Zhang, S.; Ma, X. Trans. Tianjin Univ. 2023, 29, 254–274. doi:10.1007/s12209-023-00361-2 |
| 8. | Pradhan, S.; Das, S. Synlett 2023, 34, 1327–1342. doi:10.1055/a-2012-5317 |
| 9. | Mena, S.; Peral, J.; Guirado, G. Curr. Opin. Electrochem. 2023, 42, 101392. doi:10.1016/j.coelec.2023.101392 |
| 10. | Senboku, H. Chem. Rec. 2021, 21, 2354–2374. doi:10.1002/tcr.202100081 |
| 11. | Senboku, H.; Katayama, A. Curr. Opin. Green Sustainable Chem. 2017, 3, 50–54. doi:10.1016/j.cogsc.2016.10.003 |
| 22. | Kamijo, S.; Tao, K.; Takao, G.; Tonoda, H.; Murafuji, T. Org. Lett. 2015, 17, 3326–3329. doi:10.1021/acs.orglett.5b01550 |
| 19. | Hayama, M.; Senboku, H. Electrochemistry 2023, 91, 112011. doi:10.5796/electrochemistry.23-67082 |
| 19. | Hayama, M.; Senboku, H. Electrochemistry 2023, 91, 112011. doi:10.5796/electrochemistry.23-67082 |
| 36. | Yedage, S. L.; Bhanage, B. M. J. Org. Chem. 2017, 82, 5769–5781. doi:10.1021/acs.joc.7b00570 |
| 18. | Villo, P.; Lill, M.; Alsaman, Z.; Kronberg, A. S.; Chu, V.; Ahumada, G.; Agarwala, H.; Ahlquist, M.; Lundberg, H. ChemElectroChem 2023, 10, e202300420. doi:10.1002/celc.202300420 |
| 13. | Ohkoshi, M.; Michinishi, J.-y.; Hara, S.; Senboku, H. Tetrahedron 2010, 66, 7732–7737. doi:10.1016/j.tet.2010.07.067 |
| 30. | Yuan, P.-F.; Yang, Z.; Zhang, S.-S.; Zhu, C.-M.; Yang, X.-L.; Meng, Q.-Y. Angew. Chem., Int. Ed. 2024, 63, e202313030. doi:10.1002/anie.202405333 |
| 17. | Senboku, H.; Yoneda, K.; Hara, S. Tetrahedron Lett. 2015, 56, 6772–6776. doi:10.1016/j.tetlet.2015.10.068 |
| 35. | Gaydou, M.; Moragas, T.; Juliá-Hernández, F.; Martin, R. J. Am. Chem. Soc. 2017, 139, 12161–12164. doi:10.1021/jacs.7b07637 |
| 15. | Zhang, K.; Ren, B.-H.; Liu, X.-F.; Wang, L.-L.; Zhang, M.; Ren, W.-M.; Lu, X.-B.; Zhang, W.-Z. Angew. Chem., Int. Ed. 2022, 61, e202207660. doi:10.1002/anie.202207660 |
| 16. | Wang, Y.; Tang, S.; Yang, G.; Wang, S.; Ma, D.; Qiu, Y. Angew. Chem., Int. Ed. 2022, 61, e202207746. doi:10.1002/anie.202207746 |
| 20. | Li, W.-D.; Wu, Y.; Li, S.-J.; Jiang, Y.-Q.; Li, Y.-L.; Lan, Y.; Xia, J.-B. J. Am. Chem. Soc. 2022, 144, 8551–8559. doi:10.1021/jacs.1c12463 |
| 30. | Yuan, P.-F.; Yang, Z.; Zhang, S.-S.; Zhu, C.-M.; Yang, X.-L.; Meng, Q.-Y. Angew. Chem., Int. Ed. 2024, 63, e202313030. doi:10.1002/anie.202405333 |
| 25. | Yadav, S.; Kuram, M. R. Eur. J. Org. Chem. 2023, 26, e20220134. doi:10.1002/ejoc.202201344 |
| 21. | Gaykar, R. N.; Bhunia, A.; Biju, A. T. J. Org. Chem. 2018, 83, 11333–11340. doi:10.1021/acs.joc.8b01549 |
| 24. | Han, H. J.; Kim, H. T.; Kim, J.; Jaladi, A. K.; An, D. K. Tetrahedron 2023, 142, 133500. doi:10.1016/j.tet.2023.133500 |
| 32. | Liao, L.-L.; Cao, G.-M.; Ye, J.-H.; Sun, G.-Q.; Zhou, W.-J.; Gui, Y.-Y.; Yan, S.-S.; Shen, G.; Yu, D.-G. J. Am. Chem. Soc. 2018, 140, 17338–17342. doi:10.1021/jacs.8b08792 |
| 32. | Liao, L.-L.; Cao, G.-M.; Ye, J.-H.; Sun, G.-Q.; Zhou, W.-J.; Gui, Y.-Y.; Yan, S.-S.; Shen, G.; Yu, D.-G. J. Am. Chem. Soc. 2018, 140, 17338–17342. doi:10.1021/jacs.8b08792 |
| 29. | Lei, C.; Yip, Y. J.; Zhou, J. S. J. Am. Chem. Soc. 2017, 139, 6086–6089. doi:10.1021/jacs.7b02742 |
| 30. | Yuan, P.-F.; Yang, Z.; Zhang, S.-S.; Zhu, C.-M.; Yang, X.-L.; Meng, Q.-Y. Angew. Chem., Int. Ed. 2024, 63, e202313030. doi:10.1002/anie.202405333 |
| 31. | Navale, B. S.; Laha, D.; Banerjee, S.; Vanka, K.; Bhat, R. G. J. Org. Chem. 2022, 87, 13583–13597. doi:10.1021/acs.joc.2c01185 |
| 28. | Wei, Z.; Li, H.; Wang, Y.; Liu, Q. Angew. Chem., Int. Ed. 2023, 62, e202301042. doi:10.1002/anie.202301042 |
| 21. | Gaykar, R. N.; Bhunia, A.; Biju, A. T. J. Org. Chem. 2018, 83, 11333–11340. doi:10.1021/acs.joc.8b01549 |
| 26. | Li, C.-J.; Meng, Y. J. Am. Chem. Soc. 2000, 122, 9538–9539. doi:10.1021/ja001699b |
| 27. | Varjosaari, S. E.; Skrypai, V.; Suating, P.; Hurley, J. J. M.; Gilbert, T. M.; Adler, M. J. Eur. J. Org. Chem. 2017, 229–232. doi:10.1002/ejoc.201601256 |
© 2024 Senboku and Hayama; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.