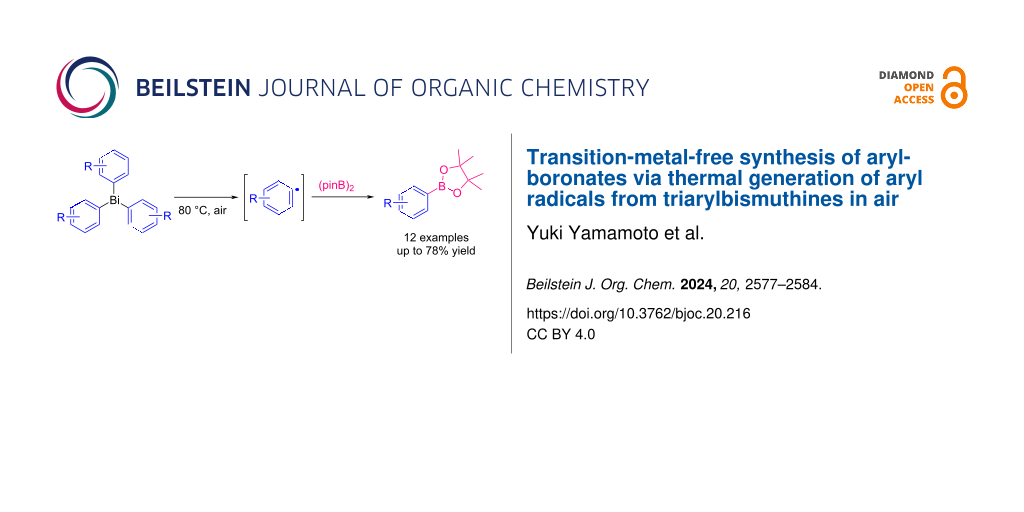
Abstract
A simple and versatile synthesis of arylboronates has been achieved by using triarylbismuthines as aryl radical sources under transition-metal-free and open-air conditions. Conventional methods required photoirradiation or electrolysis to generate aryl radicals from triarylbismuthines. In this study, it was found that simply heating the solution of triarylbismuthines in benzotrifluoride (BTF) in air successfully led to the generation of aryl radicals, and the subsequent reaction with bis(pinacolato)diboron afforded a variety of arylboronates in moderate to good yields.
Graphical Abstract
Introduction
Arylboronates are one of the fundamental aryl compounds in organic synthesis, especially in cross-coupling reactions [1-9], and their applications are widespread, including dye synthesis, pharmaceutical and agrochemical synthesis, and industrial manufacturing [10,11]. In recent years, a variety of transition-metal-catalyzed reactions and photoredox reactions using arylboronates as aryl sources have been energetically investigated for the construction of carbon–carbon or carbon–heteroatom bonds [12-15].
The preparation of arylboronates often requires pre-functionalized substrates with halogen or triflate groups. Recently, transition-metal-catalyzed direct borylation of arenes via C–H bond activation has been reported, although the design of the substrate and ligands is somewhat complicated [16-22]. Since the complete removal of catalyst-derived metal residues from the final products is generally difficult, there is concern about side effects or adverse effects on functional expression when used in pharmaceutical and material synthesis. In addition, many transition metal catalysts are very expensive, unstable, and difficult to handle. For these reasons, the development of new synthetic methods of arylboronates using stable and versatile reagents under transition-metal-free conditions has recently attracted much attention [23-27]. In particular, the use of radical reactions has been considered as one of the effective methods, since diborons can capture the in situ-generated carbon-centered radicals [28-36].
Among the aryl sources in organic synthesis, triarylbismuthines are shelf-stable and easy-to-handle reagents with appropriate reactivities in transition-metal-catalyzed reactions and radical reactions, and their derivatives can be easily synthesized by common Grignard reactions [37-44]. Three activation methods have been reported for their use as aryl radical sources. It has been reported that the homolysis of Ar–Bi bonds could be achieved by photoirradiation in the presence of photocatalysts or UV light irradiation without metal catalysts [45-48]. Similar homolysis by electrolysis has also been reported [49]. These two activation methods required special equipment (i.e., light sources or electronic devices). To achieve thermal homolysis of the Ar–Bi bonds, the reaction conditions were harsh, requiring heating at 260 °C for several days [50]. Thus, only two examples of the use of triarylbismuthines as aryl radical sources have been reported for the synthesis of arylboronates, which proceeds under light irradiation conditions (Scheme 1a) [47,48].
Scheme 1: (a) Conventional methods for the generation of Ar• from Ar3Bi, (b) our previous studies, and (c) this work.
Scheme 1: (a) Conventional methods for the generation of Ar• from Ar3Bi, (b) our previous studies, and (c) th...
Our group has investigated various transition-metal-free methods for the generation of aryl radicals from shelf-stable aryl compounds (Scheme 1b). For example, the heating of arylhydrazine hydrochlorides (ArNHNH2·HCl) in the presence of base under open-air conditions successfully led to the generation of aryl radicals and the subsequent trapping with E–E compounds (E = S, Se, Te, Br, and I) successfully formed new C–E bonds (Scheme 1b) [51-58]. We also demonstrated that the photoirradiation (λ > 300 nm) of the triarylbismuthines in air successfully allowed the generation of the corresponding aryl radicals without photocatalysts, and the trapping with diselenides afforded a variety of diaryl selenides [59]. Based on these backgrounds of our studies and the fundamental property, i.e., the weak bond dissociation energy of the Ph–Bi bond (46 kcal/mol) [60], we hypothesized that aryl radicals generated from triarylbismuthines by our developed methods would be successfully trapped by diboron to form a new C–B bond.
In this study, we report a facile and versatile synthesis of arylboronates using triarylbismuthines as aryl radical sources under transition-metal-free and open-air conditions (Scheme 1c). This method could be carried out without any special apparatus, and the mild conditions led to the wide range of applications.
Results and Discussion
Initially, we used triphenylbismuthine (1a) and bis(pinacolato)diboron (2) as the model substrates to optimize the reaction conditions (Table 1). We first investigated the solubility of 1 and 2 in various solvents. It was found that both 1 and 2 showed excellent solubility towards benzotrifluoride (BTF) [61] (for diboron 2: 637 mg/mL (BTF); 567 mg/mL (AcOEt)). Therefore, BTF is chosen as the solvent.
Table 1: Optimization of the reaction conditions for synthesis of 3a from BiPh3 (1a) and (pinB)2 (2).
|
|
||||||
| Entry | 2 (mmol) | Additive (equiv) | Solvent (mL) | Temp (°C) |
Yield
3a (%)a |
Recovery
1a (%)a |
| 1 | 2.0 | – | BTF (0.4) | 80 | 68 (53) | trace |
| 2 | 0.5 | NaOMe (1.2) | BTF (0.2) | 80 | N. D. | 62 |
| 3 | 3.0 | – | BTF (0.4) | 80 | 62 | trace |
| 4 | 0.5 | – | BTF (0.4) | 80 | 36 | 30 |
| 5b | 2.0 | – | BTF (0.4) | 80 | 58 | trace |
| 6 | 2.0 | – | BTF (0.4) | 60 | 59 | trace |
| 7 | 2.0 | – | BTF (0.4) | 100 | 61 | trace |
| 8 | 2.0 | (PhS)2 (0.01) | BTF (0.4) | 80 | 63 | trace |
| 9 | 2.0 | – | CHCl3 (0.4) | 80 | 36 | 17 |
| 10 | 2.0 | – | CH3CN (0.4) | 80 | 51 | 17 |
| 11 | 2.0 | – | DMF (0.4) | 80 | 38 | 48 |
| 12 | 2.0 | – | DMSO (0.4) | 80 | 18 | 80 |
| 13 | 2.0 | – | EtOH (0.4) | 80 | 45 | 16 |
aYields were determined by 1H NMR spectroscopy based on 1a as three transferable aryl groups (internal standard: 1,3,5-trioxane). Isolated yield was shown in parentheses. bUnder O2 (0.1 MPa).
Surprisingly, heating the mixture of 1 and 2 in BTF (0.4 mL) at 80 °C in air successfully afforded phenylboronic acid pinacol ester 3a in 68% yield (Table 1, entry 1). In the presence of NaOMe as a base, the reaction did not proceed (Table 1, entry 2). Increasing or decreasing the amount of diboron 2 did not improve the yield of 3a (Table 1, entry 2 vs entries 3 and 4). Under atmospheric oxygen, the yield of 3a decreased slightly (Table 1, entry 5). The reaction was investigated at 60 °C and 100 °C, and it was found that the reaction was most efficient at 80 °C (Table 1, entry 2 vs entries 6 and 7). We have previously succeeded in generating a boron radical (pinB•) by photoirradiation of (Bpin)2 and found that the addition of (PhS)2 was effective in generating the boron radical [30]. We therefore investigated this reaction by adding (PhS)2 as a Lewis base, but the yield of 3a was not improved and (PhS)2 was recovered almost quantitatively (Table 1, entry 8). Furthermore, instead of BTF, the reaction was carried out with similarly polar CHCl3, polar and aprotic CH3CN, DMF, DMSO, and protic EtOH, and it was found that BTF was the optimal solvent for the synthesis of 3a (Table 1, entry 2 vs entries 9–13).
Based on the optimized conditions (entry 2 in Table 1), we next investigated the scope and limitations of the transition-metal-free synthesis of arylboronates 3 using functionalized triarylbismuthines 1 (Scheme 2). As shown in Scheme 2, a variety of triarylbismuthines could be used for the transition-metal-free synthesis of arylboronates. For example, the use of triarylbismuthines with o-methyl, m-methyl, p-methyl, p-methoxy, and p-chloro groups successfully afforded the corresponding arylboronates 3a–e and 3g in 62–73% yields, respectively. The low solubility of tri(p-fluorophenyl) and tri(1-naphthyl)bismuthines 1h and 1k in BTF resulted in the low conversion. This system could be applied to the unstable dimethyl acetal-substituted triphenylbismuthine 1f, and 3f was obtained in 51% yield. Interestingly, the use of triarylbismuthines 1i and 1j with strong electron-withdrawing groups such as trifluoromethyl and formyl groups was also tolerable, and the corresponding products 3i and 3j were selectively obtained in moderate yields. Notably, the bulky 2,4,6-trimethylphenyl group of bisumuthine 1l did not inhibit the transformation, and the boronate 3l was obtained in 78% yield. The isolation of arylboronates 3c, 3i, and 3j was somewhat difficult due to strong adsorption or decomposition on silica gel. Since some arylboronates are somewhat unstable, it is desirable to synthesize such compounds and then use them in a one-pot manner for the following reactions without isolation.
Scheme 2: Scope for transition-metal-free synthesis of arylboronates 3 using triaylbismuthines 1 and diboron 2. Yields were determined by 1H NMR spectroscopy based on 1 as three transferable aryl groups (internal standard: 1,3,5-trioxane). Isolated yield was shown in parentheses. aCHCl3 (0.4 mL) was used as the solvent.
Scheme 2: Scope for transition-metal-free synthesis of arylboronates 3 using triaylbismuthines 1 and diboron 2...
To gain insight into the reaction pathways, several control experiments were investigated. When the reaction was carried out in an argon atmosphere using the strict Schlenk technique, the desired product 3a was not obtained at all and 93% of 1a was recovered (Scheme 3).
Scheme 3: Control experiment of the metal-free borylation under an argon atmosphere.
Scheme 3: Control experiment of the metal-free borylation under an argon atmosphere.
Figure 1 shows the comparison of the crude mixture of the reactions under argon atmosphere and in the open-air. In the absence of oxygen, the color of the reaction mixture changed only slightly. In contrast, the reaction in air resulted in the formation of black and a small amount of white insoluble solid (probably metallic bismuth or bismuth oxide) [55], and 3a was successfully obtained with almost complete consumption of 1a. The results clearly indicate that air can play an important role in the thermal activation of triarylbismuthines to generate aryl radicals.
![[1860-5397-20-216-1]](/bjoc/content/figures/1860-5397-20-216-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Comparison of the crude mixture of the reactions under (a) argon atmosphere or (b) open-air.
Figure 1: Comparison of the crude mixture of the reactions under (a) argon atmosphere or (b) open-air.
Furthermore, the yield of 3a was dramatically reduced in the presence of 2,2,6,6-tetramethylpiperidine 1-oxyl free radical (TEMPO) as a radical scavenger, strongly suggesting that a radical pathway is involved in the key step of the arylboronate synthesis (Scheme 4a). In addition, the reaction of triphenylbismuthine 1a (0.07 mmol) and TEMPO (0.4 mmol) under air resulted in the formation of 4 in 10% yield, which was confirmed and characterized by the 1H NMR measurement of the crude reaction mixture (Scheme 4b) [48]. These results clearly showed that the thermal generation of aryl radicals from triarylbismuthines is one of the key factors for the transition-metal-free synthesis of arylboronates, and oxygen (air) would play a role as a radical initiator for the thermal activation of triarylbismuthines.
Scheme 4: Radical-trapping experiments using TEMPO as a radical scavenger.
Scheme 4: Radical-trapping experiments using TEMPO as a radical scavenger.
In this system, an excess amount of diboron 2 was required for the reaction to proceed efficiently. To clarify the transformation of diboron 2 under the reaction conditions, the crude reaction mixture (entry 2 in Table 1) was analyzed by 11B NMR measurement. It was noteworthy that diboron 2 was reactive with air under the reaction conditions, and the decomposition of 2 to form pinB–O–Bpin and pinB–OH was confirmed. The decomposition was also occurred by heating the solution of diboron 2 in BTF in air; however, in the presence of TEMPO, the decomposition of 2 was sightly occurred, and almost all of 2 was recovered (see Supporting Information File 1). Based on the results, diboron 2 could also be activated via the thermal homolysis of the B–B bond in the presence of oxygen (air).
Based on the results of the control experiments and our previous studies, a proposed reaction pathway is shown in Scheme 5. First, thermal activation of triarylbismuthines in air forms aryl radicals together with the bismuth residues (i.e., metal bismuth and bismuth oxide). Alternatively, oxygen in air and/or boron-centered radicals thermally generated from (pinB)2 in air would react with triarylbismuthines to form the aryl radicals. The generated aryl radicals were then captured with (pinB)2 and the corresponding arylboronates were formed. Recombination of simultaneously formed pinB• could regenerate (pinB)2, and some of the pinB• would react with air to form pinB–O–Bpin and pinB–OH (path A). The corresponding arylboronates could also be formed via aryl radical trapping with pinB• generated by heating (pinB)2 in air (path B).
Scheme 5: A proposed reaction pathway for the synthesis of arylboronates.
Scheme 5: A proposed reaction pathway for the synthesis of arylboronates.
Conclusion
In this study, we have developed a novel method for the transition-metal-free synthesis of arylboronates using triarylbismuthines. Most of the previous methods to generate aryl radical species from triarylbismuthines required a special apparatus. In contrast, our method was very simple, and the corresponding aryl radicals were easily accessible by simply heating the solution of triarylbismuthines in air under mild conditions. Therefore, many triarylbismuthines could be used to form a variety of useful arylboronates in moderate to good yields with excellent product selectivity. We hope that this new approach to the generation of aryl radicals from triarylbismuthines will lead to an increased use of organobismuth compounds in synthetic organic chemistry. Further applications of organobismuth compounds as aryl radical precursors are currently under investigation.
Experimental
General comments: Unless otherwise stated, all starting materials were purchased from commercial sources and used without further purification. All solvents were used without distillation. Triarylbismuthines 1 were synthesized according to the previously reported procedures [62]. 1H, 13C{1H}, and 11B NMR spectra were recorded in CDCl3 using a Bruker AVANCE III HD 500 spectrometer at 500, 126, and 160 MHz, respectively. 1H chemical shifts are reported in ppm relative to Me4Si using the solvent residual as the internal standard (δ = 7.26 ppm for chloroform). 13C chemical shifts are reported in ppm relative to Me4Si, referenced to the resonances of CDCl3 (δ = 77.2 ppm). 11B chemical shifts are reported in ppm recorded in CDCl3 using BF3·Et2O (δ = 0.0 ppm) as the internal standard.
General procedure for transition-metal-free synthesis of arylboronates with triarylbismuthines 1 and diboron 2 (Scheme 2): To a 10 mL two-neck flask were added triarylbismuthine 1 (0.07 mmol), bis(pinacolato)diboron 2 (2.0 mmol), and benzotrifluoride (BTF, 0.4 mL). The mixture was heated at 80 °C for 24 h in air. After the reaction was completed, the mixture was filtered through a short Celite pad with AcOEt (20 mL). The filtrate was concentrated under reduced pressure. Finally, the residue was purified by preparative thin-layer chromatography (eluent: AcOEt/hexane) to give the pure product 3. Assuming that three aryl radicals are formed from triarylbismuthine 1, the yield of 3 was determined from the weight of the isolated product based on three times the moles of triarylbismuthine 1. Further details of the experimental procedures and characterization data are provided in Supporting Information File 1.
Supporting Information
| Supporting Information File 1: Investigation of the boron residue in the crude mixture by 11B NMR measurement, characterization data of the compounds, and copies of 1H NMR and 13C{1H} NMR spectra. | ||
| Format: PDF | Size: 1.1 MB | Download |
Acknowledgements
We acknowledged Dr. Tran Dat Phuc and Mr. Soichiro Mita (Osaka Prefecture University) for their initial contribution of this work. We acknowledged Mr. Reiya Kani (University of Yamanashi) for his experimental support. Y.Y. would like to express his deepest gratitude to Prof. Dr. Tetsuo Kuwabara (University of Yamanashi) for his insightful feedback and continuous mentorship that significantly contributed to the success of this work.
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information to this article.
References
-
Ishiyama, T.; Itoh, Y.; Kitano, T.; Miyaura, N. Tetrahedron Lett. 1997, 38, 3447–3450. doi:10.1016/s0040-4039(97)00642-4
Return to citation in text: [1] -
Murata, M.; Watanabe, S.; Masuda, Y. J. Org. Chem. 1997, 62, 6458–6459. doi:10.1021/jo970963p
Return to citation in text: [1] -
Murata, M.; Oyama, T.; Watanabe, S.; Masuda, Y. J. Org. Chem. 2000, 65, 164–168. doi:10.1021/jo991337q
Return to citation in text: [1] -
Ishiyama, T.; Ishida, K.; Miyaura, N. Tetrahedron 2001, 57, 9813–9816. doi:10.1016/s0040-4020(01)00998-x
Return to citation in text: [1] -
Ishiyama, T.; Isou, H.; Kikuchi, T.; Miyaura, N. Chem. Commun. 2010, 46, 159–161. doi:10.1039/b910298a
Return to citation in text: [1] -
Kawamorita, S.; Ohmiya, H.; Iwai, T.; Sawamura, M. Angew. Chem., Int. Ed. 2011, 50, 8363–8366. doi:10.1002/anie.201103224
Return to citation in text: [1] -
Yoshida, T.; Ilies, L.; Nakamura, E. ACS Catal. 2017, 7, 3199–3203. doi:10.1021/acscatal.7b00310
Return to citation in text: [1] -
Alam, S.; Karim, R.; Khan, A.; Pal, A. K.; Maruani, A. Eur. J. Org. Chem. 2021, 6115–6160. doi:10.1002/ejoc.202100817
Return to citation in text: [1] -
Zeng, J.; Naito, M.; Torigoe, T.; Yamanaka, M.; Kuninobu, Y. Org. Lett. 2020, 22, 3485–3489. doi:10.1021/acs.orglett.0c00946
Return to citation in text: [1] -
Miyaura, N.; Yamada, K.; Suzuki, A. Tetrahedron Lett. 1979, 20, 3437–3440. doi:10.1016/s0040-4039(01)95429-2
Return to citation in text: [1] -
Lennox, A. J. J.; Lloyd-Jones, G. C. Chem. Soc. Rev. 2014, 43, 412–443. doi:10.1039/c3cs60197h
Return to citation in text: [1] -
Kakiuchi, F.; Kan, S.; Igi, K.; Chatani, N.; Murai, S. J. Am. Chem. Soc. 2003, 125, 1698–1699. doi:10.1021/ja029273f
Return to citation in text: [1] -
Kakiuchi, F.; Matsuura, Y.; Kan, S.; Chatani, N. J. Am. Chem. Soc. 2005, 127, 5936–5945. doi:10.1021/ja043334n
Return to citation in text: [1] -
Matsuda, N.; Hirano, K.; Satoh, T.; Miura, M. Angew. Chem., Int. Ed. 2012, 51, 3642–3645. doi:10.1002/anie.201108773
Return to citation in text: [1] -
Dastbaravardeh, N.; Schnürch, M.; Mihovilovic, M. D. Org. Lett. 2012, 14, 1930–1933. doi:10.1021/ol300627p
Return to citation in text: [1] -
Kakiuchi, F.; Chatani, N. Adv. Synth. Catal. 2003, 345, 1077–1101. doi:10.1002/adsc.200303094
Return to citation in text: [1] -
Mkhalid, I. A. I.; Barnard, J. H.; Marder, T. B.; Murphy, J. M.; Hartwig, J. F. Chem. Rev. 2010, 110, 890–931. doi:10.1021/cr900206p
Return to citation in text: [1] -
Hartwig, J. F. Acc. Chem. Res. 2012, 45, 864–873. doi:10.1021/ar200206a
Return to citation in text: [1] -
Xu, L.; Wang, G.; Zhang, S.; Wang, H.; Wang, L.; Liu, L.; Jiao, J.; Li, P. Tetrahedron 2017, 73, 7123–7157. doi:10.1016/j.tet.2017.11.005
Return to citation in text: [1] -
Kuleshova, O.; Asako, S.; Ilies, L. ACS Catal. 2021, 11, 5968–5973. doi:10.1021/acscatal.1c01206
Return to citation in text: [1] -
Ramadoss, B.; Jin, Y.; Asako, S.; Ilies, L. Science 2022, 375, 658–663. doi:10.1126/science.abm7599
Return to citation in text: [1] -
Jin, Y.; Ramadoss, B.; Asako, S.; Ilies, L. Nat. Commun. 2024, 15, 2886. doi:10.1038/s41467-024-46893-6
Return to citation in text: [1] -
Légaré, M.-A.; Courtemanche, M.-A.; Rochette, É.; Fontaine, F.-G. Science 2015, 349, 513–516. doi:10.1126/science.aab3591
Return to citation in text: [1] -
Mfuh, A. M.; Doyle, J. D.; Chhetri, B.; Arman, H. D.; Larionov, O. V. J. Am. Chem. Soc. 2016, 138, 2985–2988. doi:10.1021/jacs.6b01376
Return to citation in text: [1] -
Candish, L.; Teders, M.; Glorius, F. J. Am. Chem. Soc. 2017, 139, 7440–7443. doi:10.1021/jacs.7b03127
Return to citation in text: [1] -
Cuenca, A. B.; Shishido, R.; Ito, H.; Fernández, E. Chem. Soc. Rev. 2017, 46, 415–430. doi:10.1039/c6cs00692b
Return to citation in text: [1] -
Mo, F.; Jiang, Y.; Qiu, D.; Zhang, Y.; Wang, J. Angew. Chem., Int. Ed. 2010, 49, 1846–1849. doi:10.1002/anie.200905824
Return to citation in text: [1] -
Yu, J.; Zhang, L.; Yan, G. Adv. Synth. Catal. 2012, 354, 2625–2628. doi:10.1002/adsc.201200416
Return to citation in text: [1] -
Qiu, D.; Jin, L.; Zheng, Z.; Meng, H.; Mo, F.; Wang, X.; Zhang, Y.; Wang, J. J. Org. Chem. 2013, 78, 1923–1933. doi:10.1021/jo3018878
Return to citation in text: [1] -
Yoshimura, A.; Takamachi, Y.; Han, L.-B.; Ogawa, A. Chem. – Eur. J. 2015, 21, 13930–13933. doi:10.1002/chem.201502425
Return to citation in text: [1] [2] -
Yoshimura, A.; Takamachi, Y.; Mihara, K.; Saeki, T.; Kawaguchi, S.-i.; Han, L.-B.; Nomoto, A.; Ogawa, A. Tetrahedron 2016, 72, 7832–7838. doi:10.1016/j.tet.2016.06.040
Return to citation in text: [1] -
Zhang, L.; Jiao, L. J. Am. Chem. Soc. 2017, 139, 607–610. doi:10.1021/jacs.6b11813
Return to citation in text: [1] -
Yan, G.; Huang, D.; Wu, X. Adv. Synth. Catal. 2018, 360, 1040–1053. doi:10.1002/adsc.201701030
Return to citation in text: [1] -
Zhang, L.; Jiao, L. J. Am. Chem. Soc. 2019, 141, 9124–9128. doi:10.1021/jacs.9b00917
Return to citation in text: [1] -
Shiozuka, A.; Sekine, K.; Toki, T.; Kawashima, K.; Mori, T.; Kuninobu, Y. Org. Lett. 2022, 24, 4281–4285. doi:10.1021/acs.orglett.2c01663
Return to citation in text: [1] -
He, T.; Wei, H.; Zhou, Y.; Jiang, L.-y.; Baell, J. B.; Yu, Y.; Huang, F. Org. Chem. Front. 2023, 10, 2918–2926. doi:10.1039/d3qo00458a
Return to citation in text: [1] -
Suzuki, H.; Ikegami, T.; Matano, Y. Synthesis 1997, 249–267. doi:10.1055/s-1997-1194
Return to citation in text: [1] -
Matano, Y.; Miyamatsu, T.; Suzuki, H. Organometallics 1996, 15, 1951–1953. doi:10.1021/om9509709
Return to citation in text: [1] -
Yamago, S.; Kayahara, E.; Kotani, M.; Ray, B.; Kwak, Y.; Goto, A.; Fukuda, T. Angew. Chem., Int. Ed. 2007, 46, 1304–1306. doi:10.1002/anie.200604473
Return to citation in text: [1] -
Kayahara, E.; Yamada, H.; Yamago, S. Chem. – Eur. J. 2011, 17, 5272–5280. doi:10.1002/chem.201100265
Return to citation in text: [1] -
Kobiki, Y.; Kawaguchi, S.-i.; Ogawa, A. Org. Lett. 2015, 17, 3490–3493. doi:10.1021/acs.orglett.5b01566
Return to citation in text: [1] -
Kodama, S.; Yamamoto, Y.; Kobiki, Y.; Matsubara, H.; Tran, C. C.; Kawaguchi, S.-i.; Nomoto, A.; Ogawa, A. Materials 2021, 14, 4271. doi:10.3390/ma14154271
Return to citation in text: [1] -
Birnthaler, D.; Narobe, R.; Lopez-Berguno, E.; Haag, C.; König, B. ACS Catal. 2023, 13, 1125–1132. doi:10.1021/acscatal.2c05631
Return to citation in text: [1] -
Mato, M.; Cornella, J. Angew. Chem., Int. Ed. 2024, 63, e202315046. doi:10.1002/anie.202315046
Return to citation in text: [1] -
Hey, D. H.; Shingleton, D. A.; Williams, G. H. J. Chem. Soc. 1963, 5612–5619. doi:10.1039/jr9630005612
Return to citation in text: [1] -
Yablokov, V. A.; Zelyaev, I. A.; Makarov, E. I.; Lokhov, N. S. Zh. Obshch. Khim. 1987, 57, 2034–2037.
Return to citation in text: [1] -
Nakajima, M.; Nagasawa, S.; Matsumoto, K.; Kuribara, T.; Muranaka, A.; Uchiyama, M.; Nemoto, T. Angew. Chem., Int. Ed. 2020, 59, 6847–6852. doi:10.1002/anie.201915181
Return to citation in text: [1] [2] -
Chiappini, N. D.; Geunes, E. P.; Bodak, E. T.; Knowles, R. R. ACS Catal. 2024, 14, 2664–2670. doi:10.1021/acscatal.3c05598
Return to citation in text: [1] [2] [3] -
Fuchigami, T.; Miyazaki, M. Electrochim. Acta 1997, 42, 1979–1984. doi:10.1016/s0013-4686(97)85470-9
Return to citation in text: [1] -
Razuvaev, G. A.; Petukhov, G. G.; Titov, V. A.; Druzhkov, O. N. Zh. Org. Khim. 1965, 35, 481–485.
Return to citation in text: [1] -
Taniguchi, T.; Imoto, M.; Takeda, M.; Matsumoto, F.; Nakai, T.; Mihara, M.; Mizuno, T.; Nomoto, A.; Ogawa, A. Tetrahedron 2016, 72, 4132–4140. doi:10.1016/j.tet.2016.05.056
Return to citation in text: [1] -
Taniguchi, T.; Naka, T.; Imoto, M.; Takeda, M.; Nakai, T.; Mihara, M.; Mizuno, T.; Nomoto, A.; Ogawa, A. J. Org. Chem. 2017, 82, 6647–6655. doi:10.1021/acs.joc.7b00767
Return to citation in text: [1] -
Taniguchi, T.; Murata, A.; Takeda, M.; Mizuno, T.; Nomoto, A.; Ogawa, A. Eur. J. Org. Chem. 2017, 4928–4934. doi:10.1002/ejoc.201700938
Return to citation in text: [1] -
Dong, C.-p.; Nakamura, K.; Taniguchi, T.; Mita, S.; Kodama, S.; Kawaguchi, S.-i.; Nomoto, A.; Ogawa, A.; Mizuno, T. ACS Omega 2018, 3, 9814–9821. doi:10.1021/acsomega.8b01559
Return to citation in text: [1] -
Yamamoto, Y.; Sato, F.; Kodama, S.; Nomoto, A.; Ogawa, A. Heteroat. Chem. 2018, 29, e21471. doi:10.1002/hc.21471
Return to citation in text: [1] [2] -
Phuc Tran, D.; Nomoto, A.; Mita, S.; Dong, C.-p.; Kodama, S.; Mizuno, T.; Ogawa, A. Tetrahedron Lett. 2020, 61, 151959. doi:10.1016/j.tetlet.2020.151959
Return to citation in text: [1] -
Yamamoto, Y.; Sato, F.; Chen, Q.; Kodama, S.; Nomoto, A.; Ogawa, A. Molecules 2022, 27, 809. doi:10.3390/molecules27030809
Return to citation in text: [1] -
Yamamoto, Y.; Ogawa, A. Molecules 2023, 28, 787. doi:10.3390/molecules28020787
Return to citation in text: [1] -
Kobiki, Y.; Kawaguchi, S.-i.; Ohe, T.; Ogawa, A. Beilstein J. Org. Chem. 2013, 9, 1141–1147. doi:10.3762/bjoc.9.127
Return to citation in text: [1] -
Steele, W. V. J. Chem. Thermodyn. 1979, 11, 187–192. doi:10.1016/0021-9614(79)90170-8
Return to citation in text: [1] -
Ogawa, A.; Curran, D. P. J. Org. Chem. 1997, 62, 450–451. doi:10.1021/jo9620324
Return to citation in text: [1] -
Hébert, M.; Petiot, P.; Benoit, E.; Dansereau, J.; Ahmad, T.; Le Roch, A.; Ottenwaelder, X.; Gagnon, A. J. Org. Chem. 2016, 81, 5401–5416. doi:10.1021/acs.joc.6b00767
Return to citation in text: [1]
| 62. | Hébert, M.; Petiot, P.; Benoit, E.; Dansereau, J.; Ahmad, T.; Le Roch, A.; Ottenwaelder, X.; Gagnon, A. J. Org. Chem. 2016, 81, 5401–5416. doi:10.1021/acs.joc.6b00767 |
| 55. | Yamamoto, Y.; Sato, F.; Kodama, S.; Nomoto, A.; Ogawa, A. Heteroat. Chem. 2018, 29, e21471. doi:10.1002/hc.21471 |
| 48. | Chiappini, N. D.; Geunes, E. P.; Bodak, E. T.; Knowles, R. R. ACS Catal. 2024, 14, 2664–2670. doi:10.1021/acscatal.3c05598 |
| 1. | Ishiyama, T.; Itoh, Y.; Kitano, T.; Miyaura, N. Tetrahedron Lett. 1997, 38, 3447–3450. doi:10.1016/s0040-4039(97)00642-4 |
| 2. | Murata, M.; Watanabe, S.; Masuda, Y. J. Org. Chem. 1997, 62, 6458–6459. doi:10.1021/jo970963p |
| 3. | Murata, M.; Oyama, T.; Watanabe, S.; Masuda, Y. J. Org. Chem. 2000, 65, 164–168. doi:10.1021/jo991337q |
| 4. | Ishiyama, T.; Ishida, K.; Miyaura, N. Tetrahedron 2001, 57, 9813–9816. doi:10.1016/s0040-4020(01)00998-x |
| 5. | Ishiyama, T.; Isou, H.; Kikuchi, T.; Miyaura, N. Chem. Commun. 2010, 46, 159–161. doi:10.1039/b910298a |
| 6. | Kawamorita, S.; Ohmiya, H.; Iwai, T.; Sawamura, M. Angew. Chem., Int. Ed. 2011, 50, 8363–8366. doi:10.1002/anie.201103224 |
| 7. | Yoshida, T.; Ilies, L.; Nakamura, E. ACS Catal. 2017, 7, 3199–3203. doi:10.1021/acscatal.7b00310 |
| 8. | Alam, S.; Karim, R.; Khan, A.; Pal, A. K.; Maruani, A. Eur. J. Org. Chem. 2021, 6115–6160. doi:10.1002/ejoc.202100817 |
| 9. | Zeng, J.; Naito, M.; Torigoe, T.; Yamanaka, M.; Kuninobu, Y. Org. Lett. 2020, 22, 3485–3489. doi:10.1021/acs.orglett.0c00946 |
| 23. | Légaré, M.-A.; Courtemanche, M.-A.; Rochette, É.; Fontaine, F.-G. Science 2015, 349, 513–516. doi:10.1126/science.aab3591 |
| 24. | Mfuh, A. M.; Doyle, J. D.; Chhetri, B.; Arman, H. D.; Larionov, O. V. J. Am. Chem. Soc. 2016, 138, 2985–2988. doi:10.1021/jacs.6b01376 |
| 25. | Candish, L.; Teders, M.; Glorius, F. J. Am. Chem. Soc. 2017, 139, 7440–7443. doi:10.1021/jacs.7b03127 |
| 26. | Cuenca, A. B.; Shishido, R.; Ito, H.; Fernández, E. Chem. Soc. Rev. 2017, 46, 415–430. doi:10.1039/c6cs00692b |
| 27. | Mo, F.; Jiang, Y.; Qiu, D.; Zhang, Y.; Wang, J. Angew. Chem., Int. Ed. 2010, 49, 1846–1849. doi:10.1002/anie.200905824 |
| 61. | Ogawa, A.; Curran, D. P. J. Org. Chem. 1997, 62, 450–451. doi:10.1021/jo9620324 |
| 16. | Kakiuchi, F.; Chatani, N. Adv. Synth. Catal. 2003, 345, 1077–1101. doi:10.1002/adsc.200303094 |
| 17. | Mkhalid, I. A. I.; Barnard, J. H.; Marder, T. B.; Murphy, J. M.; Hartwig, J. F. Chem. Rev. 2010, 110, 890–931. doi:10.1021/cr900206p |
| 18. | Hartwig, J. F. Acc. Chem. Res. 2012, 45, 864–873. doi:10.1021/ar200206a |
| 19. | Xu, L.; Wang, G.; Zhang, S.; Wang, H.; Wang, L.; Liu, L.; Jiao, J.; Li, P. Tetrahedron 2017, 73, 7123–7157. doi:10.1016/j.tet.2017.11.005 |
| 20. | Kuleshova, O.; Asako, S.; Ilies, L. ACS Catal. 2021, 11, 5968–5973. doi:10.1021/acscatal.1c01206 |
| 21. | Ramadoss, B.; Jin, Y.; Asako, S.; Ilies, L. Science 2022, 375, 658–663. doi:10.1126/science.abm7599 |
| 22. | Jin, Y.; Ramadoss, B.; Asako, S.; Ilies, L. Nat. Commun. 2024, 15, 2886. doi:10.1038/s41467-024-46893-6 |
| 30. | Yoshimura, A.; Takamachi, Y.; Han, L.-B.; Ogawa, A. Chem. – Eur. J. 2015, 21, 13930–13933. doi:10.1002/chem.201502425 |
| 12. | Kakiuchi, F.; Kan, S.; Igi, K.; Chatani, N.; Murai, S. J. Am. Chem. Soc. 2003, 125, 1698–1699. doi:10.1021/ja029273f |
| 13. | Kakiuchi, F.; Matsuura, Y.; Kan, S.; Chatani, N. J. Am. Chem. Soc. 2005, 127, 5936–5945. doi:10.1021/ja043334n |
| 14. | Matsuda, N.; Hirano, K.; Satoh, T.; Miura, M. Angew. Chem., Int. Ed. 2012, 51, 3642–3645. doi:10.1002/anie.201108773 |
| 15. | Dastbaravardeh, N.; Schnürch, M.; Mihovilovic, M. D. Org. Lett. 2012, 14, 1930–1933. doi:10.1021/ol300627p |
| 59. | Kobiki, Y.; Kawaguchi, S.-i.; Ohe, T.; Ogawa, A. Beilstein J. Org. Chem. 2013, 9, 1141–1147. doi:10.3762/bjoc.9.127 |
| 10. | Miyaura, N.; Yamada, K.; Suzuki, A. Tetrahedron Lett. 1979, 20, 3437–3440. doi:10.1016/s0040-4039(01)95429-2 |
| 11. | Lennox, A. J. J.; Lloyd-Jones, G. C. Chem. Soc. Rev. 2014, 43, 412–443. doi:10.1039/c3cs60197h |
| 60. | Steele, W. V. J. Chem. Thermodyn. 1979, 11, 187–192. doi:10.1016/0021-9614(79)90170-8 |
| 49. | Fuchigami, T.; Miyazaki, M. Electrochim. Acta 1997, 42, 1979–1984. doi:10.1016/s0013-4686(97)85470-9 |
| 47. | Nakajima, M.; Nagasawa, S.; Matsumoto, K.; Kuribara, T.; Muranaka, A.; Uchiyama, M.; Nemoto, T. Angew. Chem., Int. Ed. 2020, 59, 6847–6852. doi:10.1002/anie.201915181 |
| 48. | Chiappini, N. D.; Geunes, E. P.; Bodak, E. T.; Knowles, R. R. ACS Catal. 2024, 14, 2664–2670. doi:10.1021/acscatal.3c05598 |
| 45. | Hey, D. H.; Shingleton, D. A.; Williams, G. H. J. Chem. Soc. 1963, 5612–5619. doi:10.1039/jr9630005612 |
| 46. | Yablokov, V. A.; Zelyaev, I. A.; Makarov, E. I.; Lokhov, N. S. Zh. Obshch. Khim. 1987, 57, 2034–2037. |
| 47. | Nakajima, M.; Nagasawa, S.; Matsumoto, K.; Kuribara, T.; Muranaka, A.; Uchiyama, M.; Nemoto, T. Angew. Chem., Int. Ed. 2020, 59, 6847–6852. doi:10.1002/anie.201915181 |
| 48. | Chiappini, N. D.; Geunes, E. P.; Bodak, E. T.; Knowles, R. R. ACS Catal. 2024, 14, 2664–2670. doi:10.1021/acscatal.3c05598 |
| 51. | Taniguchi, T.; Imoto, M.; Takeda, M.; Matsumoto, F.; Nakai, T.; Mihara, M.; Mizuno, T.; Nomoto, A.; Ogawa, A. Tetrahedron 2016, 72, 4132–4140. doi:10.1016/j.tet.2016.05.056 |
| 52. | Taniguchi, T.; Naka, T.; Imoto, M.; Takeda, M.; Nakai, T.; Mihara, M.; Mizuno, T.; Nomoto, A.; Ogawa, A. J. Org. Chem. 2017, 82, 6647–6655. doi:10.1021/acs.joc.7b00767 |
| 53. | Taniguchi, T.; Murata, A.; Takeda, M.; Mizuno, T.; Nomoto, A.; Ogawa, A. Eur. J. Org. Chem. 2017, 4928–4934. doi:10.1002/ejoc.201700938 |
| 54. | Dong, C.-p.; Nakamura, K.; Taniguchi, T.; Mita, S.; Kodama, S.; Kawaguchi, S.-i.; Nomoto, A.; Ogawa, A.; Mizuno, T. ACS Omega 2018, 3, 9814–9821. doi:10.1021/acsomega.8b01559 |
| 55. | Yamamoto, Y.; Sato, F.; Kodama, S.; Nomoto, A.; Ogawa, A. Heteroat. Chem. 2018, 29, e21471. doi:10.1002/hc.21471 |
| 56. | Phuc Tran, D.; Nomoto, A.; Mita, S.; Dong, C.-p.; Kodama, S.; Mizuno, T.; Ogawa, A. Tetrahedron Lett. 2020, 61, 151959. doi:10.1016/j.tetlet.2020.151959 |
| 57. | Yamamoto, Y.; Sato, F.; Chen, Q.; Kodama, S.; Nomoto, A.; Ogawa, A. Molecules 2022, 27, 809. doi:10.3390/molecules27030809 |
| 58. | Yamamoto, Y.; Ogawa, A. Molecules 2023, 28, 787. doi:10.3390/molecules28020787 |
| 37. | Suzuki, H.; Ikegami, T.; Matano, Y. Synthesis 1997, 249–267. doi:10.1055/s-1997-1194 |
| 38. | Matano, Y.; Miyamatsu, T.; Suzuki, H. Organometallics 1996, 15, 1951–1953. doi:10.1021/om9509709 |
| 39. | Yamago, S.; Kayahara, E.; Kotani, M.; Ray, B.; Kwak, Y.; Goto, A.; Fukuda, T. Angew. Chem., Int. Ed. 2007, 46, 1304–1306. doi:10.1002/anie.200604473 |
| 40. | Kayahara, E.; Yamada, H.; Yamago, S. Chem. – Eur. J. 2011, 17, 5272–5280. doi:10.1002/chem.201100265 |
| 41. | Kobiki, Y.; Kawaguchi, S.-i.; Ogawa, A. Org. Lett. 2015, 17, 3490–3493. doi:10.1021/acs.orglett.5b01566 |
| 42. | Kodama, S.; Yamamoto, Y.; Kobiki, Y.; Matsubara, H.; Tran, C. C.; Kawaguchi, S.-i.; Nomoto, A.; Ogawa, A. Materials 2021, 14, 4271. doi:10.3390/ma14154271 |
| 43. | Birnthaler, D.; Narobe, R.; Lopez-Berguno, E.; Haag, C.; König, B. ACS Catal. 2023, 13, 1125–1132. doi:10.1021/acscatal.2c05631 |
| 44. | Mato, M.; Cornella, J. Angew. Chem., Int. Ed. 2024, 63, e202315046. doi:10.1002/anie.202315046 |
| 28. | Yu, J.; Zhang, L.; Yan, G. Adv. Synth. Catal. 2012, 354, 2625–2628. doi:10.1002/adsc.201200416 |
| 29. | Qiu, D.; Jin, L.; Zheng, Z.; Meng, H.; Mo, F.; Wang, X.; Zhang, Y.; Wang, J. J. Org. Chem. 2013, 78, 1923–1933. doi:10.1021/jo3018878 |
| 30. | Yoshimura, A.; Takamachi, Y.; Han, L.-B.; Ogawa, A. Chem. – Eur. J. 2015, 21, 13930–13933. doi:10.1002/chem.201502425 |
| 31. | Yoshimura, A.; Takamachi, Y.; Mihara, K.; Saeki, T.; Kawaguchi, S.-i.; Han, L.-B.; Nomoto, A.; Ogawa, A. Tetrahedron 2016, 72, 7832–7838. doi:10.1016/j.tet.2016.06.040 |
| 32. | Zhang, L.; Jiao, L. J. Am. Chem. Soc. 2017, 139, 607–610. doi:10.1021/jacs.6b11813 |
| 33. | Yan, G.; Huang, D.; Wu, X. Adv. Synth. Catal. 2018, 360, 1040–1053. doi:10.1002/adsc.201701030 |
| 34. | Zhang, L.; Jiao, L. J. Am. Chem. Soc. 2019, 141, 9124–9128. doi:10.1021/jacs.9b00917 |
| 35. | Shiozuka, A.; Sekine, K.; Toki, T.; Kawashima, K.; Mori, T.; Kuninobu, Y. Org. Lett. 2022, 24, 4281–4285. doi:10.1021/acs.orglett.2c01663 |
| 36. | He, T.; Wei, H.; Zhou, Y.; Jiang, L.-y.; Baell, J. B.; Yu, Y.; Huang, F. Org. Chem. Front. 2023, 10, 2918–2926. doi:10.1039/d3qo00458a |
| 50. | Razuvaev, G. A.; Petukhov, G. G.; Titov, V. A.; Druzhkov, O. N. Zh. Org. Khim. 1965, 35, 481–485. |
© 2024 Yamamoto et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.