Abstract
We report the synthesis of polycyclic uracil derivatives. The method is based on palladium-catalysed Sonogashira–Hagihara and Suzuki–Miyaura cross-coupling reactions followed by Brønsted acid-mediated cycloisomerisation. The developed methodology tolerates various functional groups and leads to moderate up to quantitative yields of the final products. The impact of different functional groups on the optical properties was studied by UV–vis and fluorescence spectroscopy.
Graphical Abstract
Introduction
Nucleobases contain the coded information and give DNA and RNA their typical structure. As a nucleobase, uracil is involved in numerous vital processes and is therefore a promising target and candidate for the development of new drugs against a wide range of diseases [1-4]. As it is not contained in the DNA, it could be used to distinguish between DNA and RNA-based pharmaceutical targets. In previous years, uracil has been successfully used in the development of several drugs that are still important and often used. Examples include 5-fluorouracil and brivudine. 5-Fluoruracil is an important anticancer drug, while brivudine is considered to be one of the most effective antiviral drugs against the HSV-1 virus [4-20]. Consequently, in recent years, new methods have been developed to introduce various functional groups at position 5 or 6 of uracil [19-50]. However, only a few methods are known which allow for an individual introduction of substituents at both positions [37,38,51-61]. In our previous work, we developed a new method which enables both positions to be independently functionalised by Sonogashira- and Suzuki–Miyaura cross-coupling reactions (Figure 1).
Figure 1: Synthesis of uracil-based alkynes and aryl structures [51,62-65].
Figure 1: Synthesis of uracil-based alkynes and aryl structures [51,62-65].
Polycondensed heterocycles containing a uracil moiety have also been studied in recent years. For example, compound A exhibits antitumor and antimicrobial properties (Figure 2) [66,67]. Compounds A and B are used as starting materials for the synthesis of polyaromatic derivatives of other compounds with medicinal or photophysical properties [52-55]. Compounds A are related to the class of natural products known as coumestans, while B resembles flavins. While the medical potential of coumestans is still the subject of current research, interesting photocatalytic properties have already been identified for flavins, making them interesting for photoredox catalysis [68,69]. Inspired by the current interest in the synthesis of novel uracil-derived cyclic compounds and our previous studies, we herein wish to report a new method for the synthesis of a series of novel uracil-based benzo[f]quinazoline-1,3(2H,4H)-diones C [65]. Furthermore, optical properties were analysed by UV–vis and fluorescence spectroscopy.
Figure 2: Structures of uracil derivatives A, B, and C.
Figure 2: Structures of uracil derivatives A, B, and C.
Results and Discussion
Synthesis
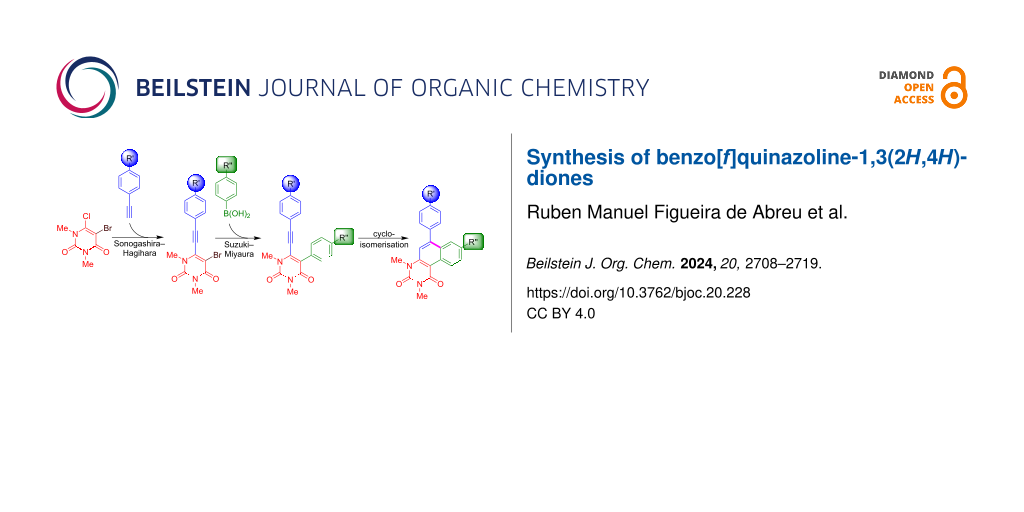
Our strategy for the synthesis of benzo[f]quinazoline-1,3(2H,4H)-diones is based on a four-step sequence and relies on a combination of palladium-catalysed Sonogashira–Hagihara and Suzuki–Miyaura cross-coupling reactions (Scheme 1). The final cyclisation step is accomplished by an acid-mediated cycloisomerisation. The synthesis of starting materials 4 was carried out by our previously reported protocol [65]. While compounds 4a–f are known compounds, the synthesis of derivatives 4g–i has not been previously reported (Scheme 2). Yields were generally lower in case of the presence of electron-withdrawing substituents.
Scheme 1: Strategy for the synthesis of the cyclised product 5. Conditions: i) Br2 (2 equiv), Ac2O (1.5 equiv), AcOH, 25 °C, 1 h [65]; ii) Pd(PPh3)Cl2 (5 mol %), CuI (5 mol %), NEt3 (10 equiv), DMSO, 25 °C, 6 h; iii) Pd(PPh3)4 (10 mol %), NaOH (3 equiv), 1,4-dioxane/water 5:1, 100 °C, 1 h; iv) p-TsOH (20 equiv), toluene, 100 °C, 4 h.
Scheme 1: Strategy for the synthesis of the cyclised product 5. Conditions: i) Br2 (2 equiv), Ac2O (1.5 equiv...
Scheme 2: Synthesis and isolated yields of 1,3-dimethyl-5-aryl-6-[2-(aryl)ethynyl]uracils 4a–i. Reaction conditions: 3 (1 equiv), boronic acid (1.2 equiv), Pd(PPh3)4 (5 mol %), NaOH (3 equiv), 1,4-dioxane/water 5:1, 100 °C, 1 h.
Scheme 2: Synthesis and isolated yields of 1,3-dimethyl-5-aryl-6-[2-(aryl)ethynyl]uracils 4a–i. Reaction cond...
Subsequently, the cyclisation of 4a–i to 5a–i by acid-mediated cycloisomerisation was studied. In our first attempt, we used platinum(II) chloride (PtCl2) as the Lewis acid and 4a as the model starting material. The reaction afforded a mixture of the desired product 5a and the starting material. Separation of both compounds proved to be difficult and, hence, optimisation was carried out as depicted in Table 1. The employment of higher temperatures resulted again in a mixture of 4a and 5a (Table 1, entry 2). Next, p-toluenesulfonic acid (p-TsOH·H2O) was chosen as the Brønsted acid. To our delight, product 5a was obtained in a yield of 59% after 2 hours (Table 1, entry 3). Further investigation showed that the yield could be improved to 99% by increasing the reaction time to 4 hours (Table 1, entry 4), while reduction of the amount of acid resulted in a drop of the yield to 85%.
With the optimised conditions in hand, the scope of the cycloisomerisation was studied and the products 5a, 5d, and 5f–i were isolated in moderate to excellent yields (Scheme 3). The best yield was obtained for 5a with 99%. Several functional groups, such as methyl, N,N-dimethylamino, and trifluoromethyl, are tolerated by the developed procedure. However, a fluorine group was converted to a hydroxy functional group (5h), most likely by nucleophilic aromatic substitution during aqueous workup. Interestingly, electron-donor groups, such as N,N-dimethylamino, proved to be beneficial in terms of yield when they are located at the alkyne-linked aryl group (Scheme 4a). In contrast, the N,N-dimethylanilino group is disadvantageous when located at position 5 of the uracil ring, which might be due to the protonation of the amine under the employed reaction conditions, making the aryl ring less feasible for the SEAr reaction (Scheme 4b). The same effects may explain the cyclisation of 4f to 5f, while 4c is not converted to the respective cyclisation product. Hence, the application of the reaction conditions to starting materials 4b, 4c and 4e resulted in the decomposition of the starting materials and no product could be isolated. The employment of heterocyclic benzothiophene gave a very good yield of 80% for product 5i.
Scheme 3: Scope and isolated yields of the synthesis of 5. Reaction conditions: 4 (1 equiv), p-TsOH·H2O (20 equiv), toluene, 100 °C, 4 h.
Scheme 3: Scope and isolated yields of the synthesis of 5. Reaction conditions: 4 (1 equiv), p-TsOH·H2O (20 e...
Scheme 4: Proposed reaction mechanism of the cyclisation with N,N-dimethylanilino functional groups.
Scheme 4: Proposed reaction mechanism of the cyclisation with N,N-dimethylanilino functional groups.
Optical properties
All synthesized compounds exhibit photoluminescence by excitation with UV light. Hence, we studied the photophysical properties of all obtained derivatives 5 by steady-state absorption and fluorescence spectroscopy. The influence of the substitution pattern on the photophysical properties is displayed in Figure 3. The corresponding photophysical data and quantum yields are described in Table 2.
Figure 3: UV–vis absorption (left) and emission (right, λex = 400 nm) spectra of 5a, 5d, 5f, 5g, 5h, and 5i in dichloromethane (c = 1⋅10−5 M).
Figure 3: UV–vis absorption (left) and emission (right, λex = 400 nm) spectra of 5a, 5d, 5f, 5g, 5h, and 5i i...
Table 2: Photophysical data of 1,3-dimethyl-5-phenyl-6-[2-(phenyl)ethynyl]uracil derivatives 5a, 5d, 5f, 5g, 5h, and 5i in dichloromethane (c = 1·10−5 M) at 20 °C.
| 5a | 5d | 5f | 5g | 5h | 5i | |
|
λ1,abs (nm)
ελ1·104 (M−1 cm−1) |
262
3.1 |
253
3.4 |
258
4.0 |
260
4.1 |
262
3.2 |
258
3.0 |
|
λ2,abs (nm)
ελ2·104 (M−1 cm−1) |
271
2.6 |
262
3.5 |
387
1.1 |
269
2.9 |
274
2.1 |
278
3.6 |
|
λ3,abs (nm)
ελ3·104 (M−1 cm−1) |
327
0.8 |
271
3.4 |
317
0.9 |
339
0.9 |
356
0.6 |
|
|
λ 4,abs (nm)
ελ4·104 (M−1 cm−1) |
347
0.5 |
322
0.7 |
349
0.6 |
353
0.9 |
||
|
λ 5,abs (nm)
ελ5·104 (M−1 cm−1) |
363
0.5 |
374
1.3 |
364
0.6 |
|||
| λem335 (nm) | 408 | 406 | 405 | 408 | ||
| λem355 (nm) | 487 | 512 | ||||
| Φa | 12b | 71c | 51c | 13b | 8b | 3b |
aFluorescence standard: quinine hemisulfate in H2SO4 (0.05 M) (Φ = 0.52) [70,71]; bexcitation at λex = 335 nm; cexcitation at λex = 355 nm.
The analysis of the absorption spectra revealed well-resolved bands for derivatives 5a, 5d, 5f, 5g, and 5i for short wavelengths (250–300 nm). At higher wavelength (300–450 nm) broad absorption bands with a certain fine-structure for compounds 5a, 5g, and 5i and structure-less absorption features for compounds 5d, 5f, and 5h containing electron-donating functional groups are observed. In addition, the presence of strongly electron-donating N,N-dimethylamino groups leads to increased extinction coefficients and bathochromically shifted absorption bands which might be a result of a certain intramolecular charge transfer between the donating N,N-dimethylamino group and the electron-deficient uracil moiety. Similar effects are observed for the emission spectra. The emission maxima of N,N-dimethylamino-group-containing compounds 5d and 5f are bathochromically shifted by ≈80 nm and ≈100 nm, respectively. The other compounds show similar emission maxima at ≈405 nm, hinting to a limited impact of the aryl substituents and their functional groups on the emission properties for these compounds. Moreover, the presence of N,N-dimethylamino groups leads to strongly enhanced quantum yields up to 71%.
The highest quantum yield is observed for 5d (71%), followed by 5f with 51%. However, the presence of a pair of donor and acceptor groups appears to be disadvantageous in terms of quantum yield and the presence of only one donor group is advantageous. Interestingly, the quantum yields of 5a (12%), 5g (13%), 5h (8%), and 5i (3%) are comparatively lower than in case of 5g and 5f. It is reasonable to assume that this large difference is caused by the chosen substitution pattern and can be used for further investigation or modulation of desired properties.
Conclusion
In summary, we have developed the synthesis of novel polycyclic uracil-based compounds. Careful optimisation of the reaction conditions led to the isolation of the desired products in excellent to moderate yields. The developed methodology tolerates various functional groups. The optical properties of as-prepared derivatives were investigated by steady-state absorption and photoluminescence spectroscopy. The photophysical properties are strongly altered by the presence N,N-dimethylaniline functional groups on the scaffold, which leads to strongly, bathochromically shifted absorption and emission spectra with elevated extinction coefficients and quantum yields up to 71%. Further studies will be directed to the synthesis to polycyclic, π-conjugated uracil derivatives.
Experimental
General information
Nuclear magnetic resonance spectra (1H/13C/19F NMR) were recorded on Bruker AVANCE 300 III, 250II, or 500 spectrometers. The analysed chemical shifts δ are referenced to the residual solvent signals of the deuterated solvents CDCl3 (δ = 7.26 ppm/77.16 ppm). Multiplicities due to spin–spin correlation are reported as follows: s = singlet, d = doublet, dd = double doublet, m = multiplet; they are further described by their coupling constants J. Infrared spectra (IR) were measured as attenuated total reflection (ATR) experiments using a Nicolet 380 FT-IR spectrometer. The signals were characterised by their wavenumbers and corresponding absorption as very strong (vs), strong (s), medium (m), weak (w) or very weak (vw). UV–vis spectra were recorded on a Cary 60 UV–vis spectrophotometer and emission spectra were recorded on an Agilent Cary Eclipse fluorescence spectrophotometer. Basic and high-resolution mass spectra (MS/HRMS) were measured on instruments coupled to a preceding gas chromatograph (GC) or liquid chromatograph (LC). Samples were ionised by electron impact ionisation (EI) on an Agilent 6890/5973 or Agilent 7890/5977 GC–MS equipped with an HP-5 capillary column using helium carrier gas or by electron spray ionisation (ESI) on an Agilent 1200/6210 Time-of-Flight (TOF) LC–MS. The solvent, toluene, was purchased as dry solvent and applied without further purification. Other reagents, catalysts, ligands, acids, and bases were used as purchased from commercial suppliers. Column chromatography was performed on Merck Silica gel 60 (particle size 63–200 μm). Solvents for extraction and column chromatography were distilled prior employment.
Synthesis of 4a–i
The synthesis of 4a–f has been previously reported. Novel derivatives 4g–i were prepared according to our previously reported procedure [65].
1,3-Dimethyl-5-phenyl-6-(phenylethynyl)pyrimidine-2,4(1H,3H)-dione (4g). Compound 4g was obtained as a brown solid in 58% yield (58.3 mg, 184 µmol, Rf 0.19 (heptane/ethyl acetate 3:2)); mp 152–154 °C; IR (ATR) ν̃: 1695 (s), 1642 (vs), 1582 (s), 1493 (s), 1440 (s), 1421 (s), 1176 (m), 1079 (m), 756 (s) cm−1; 1H NMR (500 MHz, chloroform-d) δ 7.51–7.48 (m, 2H), 7.45–7.41 (m, 2H), 7.41–7.36 (m, 2H), 7.34–7.29 (m, 2H), 7.24–7.21 (m, 2H), 3.71 (s, 3H), 3.45 (s, 3H); 13C {1H} NMR (126 MHz, chloroform-d) δ 162.1, 151.6, 134.3, 133.2, 131.9, 130.9, 130.5, 128.7, 128.3, 128.0, 120.7, 119.0, 104.2, 81.0, 34.6, 28.7; EIMS (70 eV) m/z (%): 315 (100, M+), 258 (26), 230 (67), 215 (17), 202 (23), 189 (13); HRESIMS-TOF (m/z): [M + H]+ calcd for C20H17N2O2, 317.1290; found, 317.1282.
5-(4-Fluorophenyl)-6-((4-fluorophenyl)ethynyl)-1,3-dimethylpyrimidine-2,4(1H,3H)-dione (4h). Compound 4h was obtained as a brown solid in 43% yield (46.2 mg, 131 µmol, Rf 0.17 (heptane/ethyl acetate 3:2)); mp 187–189 °C; IR (ATR) ν̃: 1708 (s), 1654 (vs), 1574 (s), 1514 (s), 1506 (s), 1446 (s), 1423 (s), 1232 (s), 1158 (s) cm−1; 1H NMR (500 MHz, chloroform-d) δ 7.48–7.44 (m, 2H), 7.26–7.22 (m, 2H), 7.15–7.10 (m, 2H), 7.06–7.02 (m, 2H), 3.70 (s, 3H), 3.44 (s, 3H); 19F NMR (471 MHz, chloroform-d) δ −113.3, −106.3; 13C {1H} NMR (126 MHz, chloroform-d) δ 163.9 (d, J = 253.7 Hz), 162.7 (d, J = 247.8 Hz), 162.0, 151.5, 134.3, 134.1 (d, J = 9.0 Hz), 132.8 (d, J = 8.2 Hz), 129.1 (d, J = 3.3 Hz), 117.9, 116.6 (d, J = 3.6 Hz), 116.4 (d, J = 22.4 Hz), 115.1 (d, J = 21.6 Hz), 103.3, 80.7, 34.7, 28.7; EIMS (70 eV) m/z (%): 352 (96, M+), 294 (28), 266 (67), 251 (14), 238 (30), 160 (100); HRESIMS-TOF(m/z): [M + H]+ calcd for C20H15F2N2O2, 353.1101; found, 353.1100.
5-(Benzo[b]thiophen-3-yl)-1,3-dimethyl-6-(p-tolylethynyl)pyrimidine-2,4(1H,3H)-dione (4i). Compound 4i was obtained as a brownish solid in 54% yield (189 mg, 488 µmol, Rf 0.21 (heptane/ethyl acetate 3:2)); mp 162–164 °C; IR (ATR) ν̃: 1695 (s), 1640 (vs), 1582 (s), 1510 (s), 1446 (s), 1428 (s), 1219 (m), 813 (s) cm−1; 1H NMR (300 MHz, chloroform-d) δ 7.94–7.87 (m, 1H), 7.65–7.59 (m, 1H), 7.58 (s, 1H), 7.39–7.31 (m, 2H), 7.06–7.01 (m, 2H), 6.86–6.80 (m, 2H), 3.73 (s, 3H), 3.48 (s, 3H), 2.30 (s, 3H); 13C {1H} NMR (75 MHz, chloroform-d) δ 161.7, 151.7, 141.2, 139.7, 138.3, 135.9, 131.9, 129.4, 128.5, 128.2, 124.3, 124.2, 123.4, 122.8, 117.2, 112.6, 106.2, 80.4, 34.7, 28.7, 21; EIMS (70 eV) m/z (%): 386 (58, M+), 371 (100), 300 (68), 286 (51), 271 (18), 268 (26); HRESIMS-TOF (m/z): [M + H]+ calcd for C23H19N2O2S, 387.1167; found, 387.1171.
General procedure for the preparation of 5
A mixture of the corresponding starting material 4 (0.145 mmol) and p-TsOH·H2O (20 equiv; 2.94 mmol; 559 mg) was dissolved in dry toluene (2 mL) and stirred for 4 hours under argon atmosphere at 100 °C. The reaction was monitored by TLC until completion. The reaction was neutralised with a saturated NaHCO3 solution and diluted with water (40 mL). The phases were separated, and the aqueous layer was extracted with dichloromethane (3 × 30 mL). The combined organic layers were dried over Na2SO4, concentrated under reduced pressure, and purified by column chromatography (heptane/ethyl acetate).
2,4,8-Trimethyl-6-(p-tolyl)benzo[f]quinazoline-1,3(2H,4H)-dione (5a). According to general procedure, compound 5a was obtained as a brown solid in 99% yield (49.3 mg, 143 µmol, Rf 0.29 (heptane/ethyl acetate 3:2)); mp 185–187 °C; IR (ATR) ν̃: 1685 (s), 1640 (vs), 1508 (s), 1421 (s), 1306 (m), 1121 (m), 1022 (m), 820 (s), 748 (s) cm−1; 1H NMR (500 MHz, chloroform-d) δ 9.82–9.78 (m, 1H), 7.76 (d, J = 8.5 Hz, 1H), 7.39–7.34 (m, 4H), 7.30 (dd, J = 8.6, 1.8 Hz, 1H), 7.27 (s, 1H), 3.72 (s, 3H), 3.59 (s, 3H), 2.60 (d, J = 0.8 Hz, 3H), 2.48 (s, 3H); 13C {1H} NMR (126 MHz, chloroform-d) δ 162.7, 151.5, 149.0, 141.4, 140.0, 138.5, 136.9, 132.6, 129.7, 129.3, 127.7, 126.8, 126.7, 125.5, 113.5, 106.4, 31.8, 28.8, 22.6, 21.4; EIMS (70 eV) m/z (%): 344 (M+, 100), 258 (9), 232 (9), 215 (10), 202 (8); HREIMS (m/z): [M]+ calcd for C22H20N2O2, 344.15173; found, 344.15193.
6-(4-(N,N-Dimethylamino)phenyl)-2,4,8-trimethylbenzo[f]quinazoline-1,3(2H,4H)-dione (5d). According to general procedure, compound 5d was obtained as a brown solid in 92% yield (70 mg, 187 µmol, Rf 0.24 (heptane/ethyl acetate 3:2)); mp: 207–209 °C; IR (ATR) ν̃: 1697 (vs), 1642 (vs), 1512 (s), 1423 (s), 1201 (s), 1129 (s), 1036 (m), 820 (vs), 745 (s) cm−1; 1H NMR (300 MHz, chloroform-d) δ 9.81–9.74 (m, 1H), 7.90 (d, J = 8.6 Hz, 1H), 7.44–7.33 (m, 2H), 7.30 (dd, J = 8.5, 1.7 Hz, 1H), 7.24 (s, 1H), 6.92–6.80 (m, 2H), 3.70 (s, 3H), 3.57 (s, 3H), 3.06 (s, 6H), 2.59 (s, 3H); 13C {1H} NMR (75 MHz, chloroform-d) δ 162.7, 151.5, 150.6, 149.3, 141.5, 139.7, 132.7, 130.8, 127.4, 127.3, 127.0, 126.8, 125.5, 113.2, 112.1, 105.7, 40.6, 31.8, 28.8, 22.5; EIMS (70 eV) m/z (%): 373 (M+, 100), 246 (22), 281 (7), 207 (15), 202 (8); HRESIMS-TOF (m/z): [M + H]+ calcd for C23H24N3O2, 374.1868; found, 374.1859.
6-(4-(N,N-Dimethylamino)phenyl)-2,4-dimethyl-8-(trifluoromethyl)benzo[f]quinazoline-1,3-(2H,4H)-dione (5f). According to general procedure, compound 5f was obtained as a brown solid in 70% yield (45.8 mg, 107 µmol, Rf 0.21 (heptane/ethyl acetate 3:2)); mp 259–261 °C; IR (ATR) ν̃: 1691 (s), 1596 (s), 1473 (s), 1314 (vs), 1147 (s), 1116 (vs), 1075 (vs), 818 (vs), 754 (m) cm−1; 1H NMR (300 MHz, chloroform-d) δ 10.10 (d, J = 9.2 Hz, 1H), 8.32 (s, 1H), 7.86 (dd, J = 9.2, 2.1 Hz, 1H), 7.44–7.35 (m, 3H), 6.92–6.84 (m, 2H), 3.75 (s, 3H), 3.59 (s, 3H), 3.09 (s, 6H); 19F NMR (282 MHz, chloroform-d) δ −62.2; 13C {1H} NMR (75 MHz, chloroform-d) δ 162.3, 151.4, 150.9, 150.2, 142.6, 134.4, 130.8, 127.7, 127.3, 127.1 (q, J = 32.6 Hz), 126.0, 124.9 (q, J = 2.8 Hz), 124.7 (q, J = 4.7 Hz), 124.4 (q, J = 272.1 Hz), 115.3, 112.3, 106.0, 40.5, 31.9, 28.9; EIMS (70 eV) m/z (%): 427 (M+, 100), 369 (5), 270 (4); HRESIMS-TOF (m/z): [M + H]+ calcd for C23H21F3N3O2, 428.1586; found, 428.1577.
2,4-Dimethyl-6-phenylbenzo[f]quinazoline-1,3(2H,4H)-dione (5g). According to general procedure, compound 5g was obtained as a brown solid in 44% yield (22.2 mg, 70.2 µmol, Rf 0.26 (heptane/ethyl acetate 3:2)); mp 193–195 °C; IR (ATR) ν̃: 1693 (s), 1572 (s), 1423 (s), 1341 (s), 1125 (m), 1030 (m), 853 (m), 776 (vs), 766 (s) cm−1; 1H NMR (500 MHz, chloroform-d) δ 10.0–9.9 (m, 1H), 7.8 (dd, J = 8.4, 1.4 Hz, 1H), 7.7 (ddd, J = 8.6, 6.8, 1.5 Hz, 1H), 7.6–7.5 (m, 3H), 7.5–7.4 (m, 3H), 7.4 (s, 1H), 3.7 (s, 3H), 3.6 (s, 3H); 13C {1H} NMR (126 MHz, chloroform-d) δ 162.5, 151.4, 149.0, 141.2, 139.7, 132.3, 129.8, 129.7, 128.7, 128.6, 128.4, 126.9, 126.2, 125.7, 114.6, 107.0, 31.8, 28.9 (signals of two carbons are absent, which may relate to signal overlap); EIMS (70 eV) m/z (%): 316 (M+, 100), 259 (16), 230 (16), 202 (9), 189 (5); HREIMS (m/z): [M]+ calcd for C20H16N2O2, 316.12063; found, 316.12044.
6-(4-Fluorophenyl)-8-hydroxy-2,4-dimethylbenzo[f]quinazoline-1,3(2H,4H)-dione (5h). According to general procedure, compound 5h was obtained as a brown solid in 45% yield (21.7 mg, 61.9 µmol, Rf 0.13 (heptane/ethyl acetate 3:2)); mp 317–319 °C; IR (ATR) ν̃: 1607 (vs), 1506 (s), 1423 (s), 1355 (s), 1230 (vs), 1224 (vs), 1090 (m), 832 (s), 748 (s) cm−1; 1H NMR (300 MHz, chloroform-d) δ 10.2 (s, 1H), 9.3 (d, J = 2.5 Hz, 1H), 7.6–7.5 (m, 3H), 7.4–7.4 (m, 2H), 7.3 (s, 1H), 7.1 (dd, J = 9.1, 2.6 Hz, 1H), 3.6 (s, 3H), 3.4 (s, 3H); 19F NMR (282 MHz, chloroform-d) δ −113.9; 13C {1H} NMR (75 MHz, chloroform-d) δ 162.6 (d, J = 245.3 Hz), 162.3, 159.1, 151.0, 146.9, 142.2, 136.1 (d, J = 3.3 Hz), 134.1, 132.2 (d, J = 8.3 Hz), 128.5, 122.2, 117.7, 115.9 (d, J = 21.4 Hz), 112.6, 108.8, 105.0, 31.9, 28.8; EIMS (70 eV) m/z (%): 350 (M+, 100), 295 (14), 267 (16), 164 (13), 238 (23), 160 (21); HREIMS (m/z): [M]+ calcd for C20H15FN2O3, 350.1062; found, 350.10584.
2,4-Dimethyl-6-(p-tolyl)benzo[4,5]thieno[3,2-f]quinazoline-1,3(2H,4H)-dione (5i). According to general procedure, compound 5i was obtained as a brown solid in 80% yield (60.1 mg, 155 µmol, Rf 0.22 (heptane/ethyl acetate 3:2)); mp 255–257 °C; IR (ATR) ν̃: 1648 (vs), 1483 (s), 1419 (s), 1333 (m), 1158 (m), 1030 (m), 914 (m), 820 (s), 743 (vs) cm−1; 1H NMR (300 MHz, chloroform-d) δ 7.89 (d, J = 7.9 Hz, 1H), 7.39–7.34 (m, 5H), 7.15–7.08 (m, 2H), 7.05 (s, 1H), 3.64 (s, 3H), 3.56 (s, 3H), 2.53 (s, 3H); 13C {1H} NMR (75 MHz, chloroform-d) δ 161.8, 151.2, 145.7, 141.7, 141.7, 139.2, 138.7, 137.3, 133.8, 129.7, 129.5, 128.6, 126.1, 124.2, 124.1, 122.6, 113.0, 108.3, 31.5, 28.7, 21.6; EIMS (70 eV) m/z (%): 386 (M+, 100), 300 (13), 258 (14); HRESIMS-TOF (m/z): [M + H]+ calcd for C23H19N2O2S, 387.1167; found, 387.1169.
Supporting Information
| Supporting Information File 1: Copies of NMR spectra. | ||
| Format: PDF | Size: 3.7 MB | Download |
Acknowledgements
We are grateful for the technical and analytical support of the University of Rostock (Germany) and Leibniz Institute for Catalysis (Germany).
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information to this article.
References
-
Myers, R. L. The 100 Most Important Chemical Compounds, 1st ed.; Greenwood Publishing Group: London, UK, 2007. doi:10.5040/9798400605284
Return to citation in text: [1] -
Casy, A. F.; D’Arcy, P. F.; Scott, E. M.; Kuemmerle, H. P.; Oswald, I.; Schier, O.; Marxer, A.; Sternbach, L. H.; Swallow, D. L.; Venulet, J. Progress in Drug Research; Birkhäuser Verlag: Basel, Switzerland, 2013; Vol. 22. doi:10.1007/978-3-0348-7102-0
Return to citation in text: [1] -
Silverman, R. B.; Holladay, M. W. The Organic Chemistry of Drug Design and Drug Action, 3rd ed.; Academic Press: San Diego, CA, USA, 2015. doi:10.1016/c2009-0-64537-2
Return to citation in text: [1] -
Ramesh, D.; Vijayakumar, B. G.; Kannan, T. Eur. J. Med. Chem. 2020, 207, 112801. doi:10.1016/j.ejmech.2020.112801
Return to citation in text: [1] [2] -
Hitchings, G. H.; Falco, E. A.; Sherwood, M. B. Science 1945, 102, 251–252. doi:10.1126/science.102.2645.251
Return to citation in text: [1] -
Prusoff, W. H.; Holmes, W. L.; Welch, A. D. Cancer Res. 1953, 13, 221–225.
Return to citation in text: [1] -
Fischl, M. A.; Richman, D. D.; Grieco, M. H.; Gottlieb, M. S.; Volberding, P. A.; Laskin, O. L.; Leedom, J. M.; Groopman, J. E.; Mildvan, D.; Schooley, R. T.; Jackson, G. G.; Durack, D. T.; King, D.; The AZT Collaborative Working Group. N. Engl. J. Med. 1987, 317, 185–191. doi:10.1056/nejm198707233170401
Return to citation in text: [1] -
Horwitz, J. P.; Chua, J.; Noel, M. J. Org. Chem. 1964, 29, 2076–2078. doi:10.1021/jo01030a546
Return to citation in text: [1] -
Bergmann, W.; Burke, D. C. J. Org. Chem. 1956, 21, 226–228. doi:10.1021/jo01108a020
Return to citation in text: [1] -
Bergmann, W.; Feeney, R. J. J. Am. Chem. Soc. 1950, 72, 2809–2810. doi:10.1021/ja01162a543
Return to citation in text: [1] -
Prusoff, W. H. Biochim. Biophys. Acta 1959, 32, 295–296. doi:10.1016/0006-3002(59)90597-9
Return to citation in text: [1] -
Geisman, A. N.; Valuev-Elliston, V. T.; Ozerov, A. A.; Khandazhinskaya, A. L.; Chizhov, A. O.; Kochetkov, S. N.; Pannecouque, C.; Naesens, L.; Seley-Radtke, K. L.; Novikov, M. S. Bioorg. Med. Chem. 2016, 24, 2476–2485. doi:10.1016/j.bmc.2016.04.010
Return to citation in text: [1] -
Tănase, C. I.; Drăghici, C.; Cojocaru, A.; Galochkina, A. V.; Orshanskaya, J. R.; Zarubaev, V. V.; Shova, S.; Enache, C.; Maganu, M. Bioorg. Med. Chem. 2015, 23, 6346–6354. doi:10.1016/j.bmc.2015.08.033
Return to citation in text: [1] -
Sneader, W. Drug Discovery: A History, 1st ed.; John Wiley & Sons: Chichester, UK, 2005. doi:10.1002/0470015535
Return to citation in text: [1] -
de Clercq, E. Acta Pharm. Sin. B 2012, 2, 535–548. doi:10.1016/j.apsb.2012.10.001
Return to citation in text: [1] -
De Clercq, E. Adv. Virus Res. 2009, 73, 1–53. doi:10.1016/s0065-3527(09)73001-5
Return to citation in text: [1] -
Sidwell, R. W.; Allen, L. B.; Huffman, J. H.; Witkowski, J. T.; Cook, P. D.; Tolman, R. L.; Revankar, G. R.; Simon, L. N.; Robins, R. K. The Potential of Nucleosides as Antiviral Agents. In Chemotherapy; Williams, J. D.; Geddes, A. M., Eds.; Springer: New York, NY, USA, 1976; Vol. 6, pp 279–294. doi:10.1007/978-1-4684-3129-2_41
Return to citation in text: [1] -
Ghorani-Azam, A.; Balali-Mood, M. Clinical Pharmacology and Toxicology of Mustard Compounds. In Basic and Clinical Toxicology of Mustard Compounds; Balali-Mood, M.; Abdollahi, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Vol. 1, pp 63–99. doi:10.1007/978-3-319-23874-6_4
Return to citation in text: [1] -
De Clercq, E.; Desgranges, C.; Herdewijn, P.; Sim, I. S.; Jones, A. S.; McLean, M. J.; Walker, R. T. J. Med. Chem. 1986, 29, 213–217. doi:10.1021/jm00152a008
Return to citation in text: [1] [2] -
De Clercq, E.; Descamps, J.; De Somer, P.; Barr, P. J.; Jones, A. S.; Walker, R. T. Proc. Natl. Acad. Sci. U. S. A. 1979, 76, 2947–2951. doi:10.1073/pnas.76.6.2947
Return to citation in text: [1] [2] -
Roh, K. R.; Kim, J. Y.; Kim, Y. H. Tetrahedron Lett. 1999, 40, 1903–1906. doi:10.1016/s0040-4039(98)02491-5
Return to citation in text: [1] -
Lin, T. S.; Guo, J. Y.; Schinazi, R. F.; Chu, C. K.; Xiang, J. N.; Prusoff, W. H. J. Med. Chem. 1988, 31, 336–340. doi:10.1021/jm00397a011
Return to citation in text: [1] -
Rahim, S. G.; Trivedi, N.; Bogunovic-Batchelor, M. V.; Hardy, G. W.; Mills, G.; Selway, J. W. T.; Snowden, W.; Littler, E.; Coe, P. L.; Basnak, I.; Whale, R. F.; Walker, R. T. J. Med. Chem. 1996, 39, 789–795. doi:10.1021/jm950029r
Return to citation in text: [1] -
Perman, J.; Sharma, R. A.; Bobek, M. Tetrahedron Lett. 1976, 17, 2427–2430. doi:10.1016/0040-4039(76)90010-1
Return to citation in text: [1] -
Farina, V.; Hauck, S. I. Synlett 1991, 157–159. doi:10.1055/s-1991-20661
Return to citation in text: [1] -
Hudson, R. H. E.; Li, G.; Tse, J. Tetrahedron Lett. 2002, 43, 1381–1386. doi:10.1016/s0040-4039(02)00024-2
Return to citation in text: [1] -
Hirota, K.; Kitade, Y.; Isobe, Y.; Maki, Y. Heterocycles 1987, 26, 355–358. doi:10.3987/r-1987-02-0355
Return to citation in text: [1] -
Stevenson, T. M.; Crouse, B. A.; Thieu, T. V.; Gebreysus, C.; Finkelstein, B. L.; Sethuraman, M. R.; Dubas-Cordery, C. M.; Piotrowski, D. L. J. Heterocycl. Chem. 2005, 42, 427–435. doi:10.1002/jhet.5570420310
Return to citation in text: [1] -
Platonova, Y. B.; Volov, A. N.; Tomilova, L. G. Bioorg. Med. Chem. Lett. 2020, 30, 127351. doi:10.1016/j.bmcl.2020.127351
Return to citation in text: [1] -
Sharma, R. A.; Bobek, M. J. Org. Chem. 1975, 40, 2377–2379. doi:10.1021/jo00904a025
Return to citation in text: [1] -
Shih, Y.-C.; Chien, T.-C. Tetrahedron 2011, 67, 3915–3923. doi:10.1016/j.tet.2011.03.051
Return to citation in text: [1] -
Fang, W.-P.; Cheng, Y.-T.; Cheng, Y.-R.; Cherng, Y.-J. Tetrahedron 2005, 61, 3107–3113. doi:10.1016/j.tet.2005.01.085
Return to citation in text: [1] -
Cho, Y.-M.; Johnson, F. Tetrahedron Lett. 1994, 35, 1149–1152. doi:10.1016/0040-4039(94)88009-3
Return to citation in text: [1] -
Tanaka, H.; Hayakawa, H.; Shibata, S.; Haraguchi, K.; Miyasaka, T.; Hirota, K. Nucleosides Nucleotides 1992, 11, 319–328. doi:10.1080/07328319208021706
Return to citation in text: [1] -
Palmisano, G.; Santagostino, M. Tetrahedron 1993, 49, 2533–2542. doi:10.1016/s0040-4020(01)86332-8
Return to citation in text: [1] -
Nencka, R.; Sinnaeve, D.; Karalic, I.; Martins, J. C.; Van Calenbergh, S. Org. Biomol. Chem. 2010, 8, 5234–5246. doi:10.1039/c0ob00061b
Return to citation in text: [1] -
Hudson, R. H. E.; Moszynski, J. M. Synlett 2006, 2997–3000. doi:10.1055/s-2006-948176
Return to citation in text: [1] [2] -
Kraljević, T. G.; Bistrović, A.; Dedić, M.; Pavelić, S. K.; Sedić, M.; Raić-Malić, S. Tetrahedron Lett. 2012, 53, 5144–5147. doi:10.1016/j.tetlet.2012.07.068
Return to citation in text: [1] [2] -
Janeba, Z.; Balzarini, J.; Andrei, G.; Snoeck, R.; De Clercq, E.; Robins, M. J. Can. J. Chem. 2006, 84, 580–586. doi:10.1139/v06-041
Return to citation in text: [1] -
Park, G.; Ettles, C.; Charles, M.; Hudson, R. H. E. J. Photochem. Photobiol., A 2023, 441, 114653. doi:10.1016/j.jphotochem.2023.114653
Return to citation in text: [1] -
Teppang, K. L.; Lee, R. W.; Burns, D. D.; Turner, M. B.; Lokensgard, M. E.; Cooksy, A. L.; Purse, B. W. Chem. – Eur. J. 2019, 25, 1249–1259. doi:10.1002/chem.201803653
Return to citation in text: [1] -
Heidari, A.; Ghorbani-Choghamarani, A.; Hajjami, M.; Hudson, R. H. E. Molecules 2020, 25, 1995. doi:10.3390/molecules25081995
Return to citation in text: [1] -
Freeman, N. S.; Moore, C. E.; Wilhelmsson, L. M.; Tor, Y. J. Org. Chem. 2016, 81, 4530–4539. doi:10.1021/acs.joc.6b00310
Return to citation in text: [1] -
Suchý, M.; Hudson, R. H. E. J. Org. Chem. 2014, 79, 3336–3347. doi:10.1021/jo402873e
Return to citation in text: [1] -
Kaczmarek, R.; Twardy, D. J.; Olson, T. L.; Korczyński, D.; Andrei, G.; Snoeck, R.; Dolot, R.; Wheeler, K. A.; Dembinski, R. Eur. J. Med. Chem. 2021, 209, 112884. doi:10.1016/j.ejmech.2020.112884
Return to citation in text: [1] -
Bollu, A.; Sharma, N. K. ChemBioChem 2019, 20, 1467–1475. doi:10.1002/cbic.201800822
Return to citation in text: [1] -
Meher, S.; Gade, C. R.; Sharma, N. K. ChemBioChem 2023, 24, e202200732. doi:10.1002/cbic.202200732
Return to citation in text: [1] -
Mikami, A.; Mori, S.; Osawa, T.; Obika, S. Chem. – Eur. J. 2023, 29, e202301928. doi:10.1002/chem.202301928
Return to citation in text: [1] -
Romero-Pérez, S.; López-Martín, I.; Martos-Maldonado, M. C.; Somoza, Á.; González-Rodríguez, D. Org. Lett. 2020, 22, 41–45. doi:10.1021/acs.orglett.9b03801
Return to citation in text: [1] -
Zeng, Y.; Wang, Z.; Zeng, L.; Xiong, H. Anal. Chem. (Washington, DC, U. S.) 2023, 95, 18880–18888. doi:10.1021/acs.analchem.3c04415
Return to citation in text: [1] -
Robins, M. J.; Barr, P. J. Tetrahedron Lett. 1981, 22, 421–424. doi:10.1016/0040-4039(81)80115-3
Return to citation in text: [1] [2] -
Youssefyeh, R. D.; Weisz, M. Tetrahedron Lett. 1973, 14, 4317–4318. doi:10.1016/s0040-4039(01)87209-9
Return to citation in text: [1] [2] -
Senda, S.; Hirota, K.; Takahashi, M. J. Chem. Soc., Perkin Trans. 1 1975, 503–507. doi:10.1039/p19750000503
Return to citation in text: [1] [2] -
Dudkin, S.; Iaroshenko, V. O.; Sosnovskikh, V. Y.; Tolmachev, A. A.; Villinger, A.; Langer, P. Org. Biomol. Chem. 2013, 11, 5351–5361. doi:10.1039/c3ob26837c
Return to citation in text: [1] [2] -
Nishigaki, S.; Sato, J.; Shimizu, K.; Furukawa, K.; Senga, K.; Yoneda, F. Chem. Pharm. Bull. 1980, 28, 142–149. doi:10.1248/cpb.28.142
Return to citation in text: [1] [2] -
Majumdar, K. C.; Sinha, B.; Maji, P. K.; Chattopadhyay, S. K. Tetrahedron 2009, 65, 2751–2756. doi:10.1016/j.tet.2009.01.107
Return to citation in text: [1] -
Sun, G.; Fecko, C. J.; Nicewonger, R. B.; Webb, W. W.; Begley, T. P. Org. Lett. 2006, 8, 681–683. doi:10.1021/ol052876m
Return to citation in text: [1] -
Van Tinh, D.; Fischer, M.; Stadlbauer, W. J. Heterocycl. Chem. 1996, 33, 905–910. doi:10.1002/jhet.5570330358
Return to citation in text: [1] -
Sako, M.; Ichioka, T.; Totani, R.; Hirota, K. J. Org. Chem. 1995, 60, 8115–8116. doi:10.1021/jo00129a068
Return to citation in text: [1] -
Srivastav, N. C.; Rai, D.; Tse, C.; Agrawal, B.; Kunimoto, D. Y.; Kumar, R. J. Med. Chem. 2010, 53, 6180–6187. doi:10.1021/jm100568q
Return to citation in text: [1] -
Petricci, E.; Radi, M.; Corelli, F.; Botta, M. Tetrahedron Lett. 2003, 44, 9181–9184. doi:10.1016/j.tetlet.2003.10.028
Return to citation in text: [1] -
Satoh, K.; Tanaka, H.; Andoh, A.; Miyasaka, T. Nucleosides, Nucleotides Nucleic Acids 1986, 5, 461–469. doi:10.1080/07328318608068688
Return to citation in text: [1] -
Tanaka, H.; Haraguchi, K.; Koizumi, Y.; Fukui, M.; Miyasaka, T. Can. J. Chem. 1986, 64, 1560–1563. doi:10.1139/v86-257
Return to citation in text: [1] -
Wheeler, H. L.; Bristol, H. S. Am. Chem. J. 1905, 33, 437–447.
Return to citation in text: [1] -
de Abreu, R. M. F.; Brockmann, T.; Villinger, A.; Ehlers, P.; Langer, P. Beilstein J. Org. Chem. 2024, 20, 898–911. doi:10.3762/bjoc.20.80
Return to citation in text: [1] [2] [3] [4] [5] -
Hu, Y.-G.; Wang, Y.; Du, S.-M.; Chen, X.-B.; Ding, M.-W. Bioorg. Med. Chem. Lett. 2010, 20, 6188–6190. doi:10.1016/j.bmcl.2010.08.122
Return to citation in text: [1] -
Hossain, M. I.; Bhuiyan, M. M. H. J. Sci. Res. (Rajshahi, Bangladesh) 2009, 1, 317–325. doi:10.3329/jsr.v1i2.2299
Return to citation in text: [1] -
König, B.; Kümmel, S.; Svobodová, E.; Cibulka, R. Phys. Sci. Rev. 2018, 3, 20170168. doi:10.1515/psr-2017-0168
Return to citation in text: [1] -
Tu, Y.; Yang, Y.; Li, Y.; He, C. Pharmacol. Res. 2021, 169, 105615. doi:10.1016/j.phrs.2021.105615
Return to citation in text: [1] -
Brouwer, A. M. Pure Appl. Chem. 2011, 83, 2213–2228. doi:10.1351/pac-rep-10-09-31
Return to citation in text: [1] -
Meech, S. R.; Phillips, D. J. Photochem. 1983, 23, 193–217. doi:10.1016/0047-2670(83)80061-6
Return to citation in text: [1]
| 1. | Myers, R. L. The 100 Most Important Chemical Compounds, 1st ed.; Greenwood Publishing Group: London, UK, 2007. doi:10.5040/9798400605284 |
| 2. | Casy, A. F.; D’Arcy, P. F.; Scott, E. M.; Kuemmerle, H. P.; Oswald, I.; Schier, O.; Marxer, A.; Sternbach, L. H.; Swallow, D. L.; Venulet, J. Progress in Drug Research; Birkhäuser Verlag: Basel, Switzerland, 2013; Vol. 22. doi:10.1007/978-3-0348-7102-0 |
| 3. | Silverman, R. B.; Holladay, M. W. The Organic Chemistry of Drug Design and Drug Action, 3rd ed.; Academic Press: San Diego, CA, USA, 2015. doi:10.1016/c2009-0-64537-2 |
| 4. | Ramesh, D.; Vijayakumar, B. G.; Kannan, T. Eur. J. Med. Chem. 2020, 207, 112801. doi:10.1016/j.ejmech.2020.112801 |
| 51. | Robins, M. J.; Barr, P. J. Tetrahedron Lett. 1981, 22, 421–424. doi:10.1016/0040-4039(81)80115-3 |
| 62. | Satoh, K.; Tanaka, H.; Andoh, A.; Miyasaka, T. Nucleosides, Nucleotides Nucleic Acids 1986, 5, 461–469. doi:10.1080/07328318608068688 |
| 63. | Tanaka, H.; Haraguchi, K.; Koizumi, Y.; Fukui, M.; Miyasaka, T. Can. J. Chem. 1986, 64, 1560–1563. doi:10.1139/v86-257 |
| 64. | Wheeler, H. L.; Bristol, H. S. Am. Chem. J. 1905, 33, 437–447. |
| 65. | de Abreu, R. M. F.; Brockmann, T.; Villinger, A.; Ehlers, P.; Langer, P. Beilstein J. Org. Chem. 2024, 20, 898–911. doi:10.3762/bjoc.20.80 |
| 37. | Hudson, R. H. E.; Moszynski, J. M. Synlett 2006, 2997–3000. doi:10.1055/s-2006-948176 |
| 38. | Kraljević, T. G.; Bistrović, A.; Dedić, M.; Pavelić, S. K.; Sedić, M.; Raić-Malić, S. Tetrahedron Lett. 2012, 53, 5144–5147. doi:10.1016/j.tetlet.2012.07.068 |
| 51. | Robins, M. J.; Barr, P. J. Tetrahedron Lett. 1981, 22, 421–424. doi:10.1016/0040-4039(81)80115-3 |
| 52. | Youssefyeh, R. D.; Weisz, M. Tetrahedron Lett. 1973, 14, 4317–4318. doi:10.1016/s0040-4039(01)87209-9 |
| 53. | Senda, S.; Hirota, K.; Takahashi, M. J. Chem. Soc., Perkin Trans. 1 1975, 503–507. doi:10.1039/p19750000503 |
| 54. | Dudkin, S.; Iaroshenko, V. O.; Sosnovskikh, V. Y.; Tolmachev, A. A.; Villinger, A.; Langer, P. Org. Biomol. Chem. 2013, 11, 5351–5361. doi:10.1039/c3ob26837c |
| 55. | Nishigaki, S.; Sato, J.; Shimizu, K.; Furukawa, K.; Senga, K.; Yoneda, F. Chem. Pharm. Bull. 1980, 28, 142–149. doi:10.1248/cpb.28.142 |
| 56. | Majumdar, K. C.; Sinha, B.; Maji, P. K.; Chattopadhyay, S. K. Tetrahedron 2009, 65, 2751–2756. doi:10.1016/j.tet.2009.01.107 |
| 57. | Sun, G.; Fecko, C. J.; Nicewonger, R. B.; Webb, W. W.; Begley, T. P. Org. Lett. 2006, 8, 681–683. doi:10.1021/ol052876m |
| 58. | Van Tinh, D.; Fischer, M.; Stadlbauer, W. J. Heterocycl. Chem. 1996, 33, 905–910. doi:10.1002/jhet.5570330358 |
| 59. | Sako, M.; Ichioka, T.; Totani, R.; Hirota, K. J. Org. Chem. 1995, 60, 8115–8116. doi:10.1021/jo00129a068 |
| 60. | Srivastav, N. C.; Rai, D.; Tse, C.; Agrawal, B.; Kunimoto, D. Y.; Kumar, R. J. Med. Chem. 2010, 53, 6180–6187. doi:10.1021/jm100568q |
| 61. | Petricci, E.; Radi, M.; Corelli, F.; Botta, M. Tetrahedron Lett. 2003, 44, 9181–9184. doi:10.1016/j.tetlet.2003.10.028 |
| 19. | De Clercq, E.; Desgranges, C.; Herdewijn, P.; Sim, I. S.; Jones, A. S.; McLean, M. J.; Walker, R. T. J. Med. Chem. 1986, 29, 213–217. doi:10.1021/jm00152a008 |
| 20. | De Clercq, E.; Descamps, J.; De Somer, P.; Barr, P. J.; Jones, A. S.; Walker, R. T. Proc. Natl. Acad. Sci. U. S. A. 1979, 76, 2947–2951. doi:10.1073/pnas.76.6.2947 |
| 21. | Roh, K. R.; Kim, J. Y.; Kim, Y. H. Tetrahedron Lett. 1999, 40, 1903–1906. doi:10.1016/s0040-4039(98)02491-5 |
| 22. | Lin, T. S.; Guo, J. Y.; Schinazi, R. F.; Chu, C. K.; Xiang, J. N.; Prusoff, W. H. J. Med. Chem. 1988, 31, 336–340. doi:10.1021/jm00397a011 |
| 23. | Rahim, S. G.; Trivedi, N.; Bogunovic-Batchelor, M. V.; Hardy, G. W.; Mills, G.; Selway, J. W. T.; Snowden, W.; Littler, E.; Coe, P. L.; Basnak, I.; Whale, R. F.; Walker, R. T. J. Med. Chem. 1996, 39, 789–795. doi:10.1021/jm950029r |
| 24. | Perman, J.; Sharma, R. A.; Bobek, M. Tetrahedron Lett. 1976, 17, 2427–2430. doi:10.1016/0040-4039(76)90010-1 |
| 25. | Farina, V.; Hauck, S. I. Synlett 1991, 157–159. doi:10.1055/s-1991-20661 |
| 26. | Hudson, R. H. E.; Li, G.; Tse, J. Tetrahedron Lett. 2002, 43, 1381–1386. doi:10.1016/s0040-4039(02)00024-2 |
| 27. | Hirota, K.; Kitade, Y.; Isobe, Y.; Maki, Y. Heterocycles 1987, 26, 355–358. doi:10.3987/r-1987-02-0355 |
| 28. | Stevenson, T. M.; Crouse, B. A.; Thieu, T. V.; Gebreysus, C.; Finkelstein, B. L.; Sethuraman, M. R.; Dubas-Cordery, C. M.; Piotrowski, D. L. J. Heterocycl. Chem. 2005, 42, 427–435. doi:10.1002/jhet.5570420310 |
| 29. | Platonova, Y. B.; Volov, A. N.; Tomilova, L. G. Bioorg. Med. Chem. Lett. 2020, 30, 127351. doi:10.1016/j.bmcl.2020.127351 |
| 30. | Sharma, R. A.; Bobek, M. J. Org. Chem. 1975, 40, 2377–2379. doi:10.1021/jo00904a025 |
| 31. | Shih, Y.-C.; Chien, T.-C. Tetrahedron 2011, 67, 3915–3923. doi:10.1016/j.tet.2011.03.051 |
| 32. | Fang, W.-P.; Cheng, Y.-T.; Cheng, Y.-R.; Cherng, Y.-J. Tetrahedron 2005, 61, 3107–3113. doi:10.1016/j.tet.2005.01.085 |
| 33. | Cho, Y.-M.; Johnson, F. Tetrahedron Lett. 1994, 35, 1149–1152. doi:10.1016/0040-4039(94)88009-3 |
| 34. | Tanaka, H.; Hayakawa, H.; Shibata, S.; Haraguchi, K.; Miyasaka, T.; Hirota, K. Nucleosides Nucleotides 1992, 11, 319–328. doi:10.1080/07328319208021706 |
| 35. | Palmisano, G.; Santagostino, M. Tetrahedron 1993, 49, 2533–2542. doi:10.1016/s0040-4020(01)86332-8 |
| 36. | Nencka, R.; Sinnaeve, D.; Karalic, I.; Martins, J. C.; Van Calenbergh, S. Org. Biomol. Chem. 2010, 8, 5234–5246. doi:10.1039/c0ob00061b |
| 37. | Hudson, R. H. E.; Moszynski, J. M. Synlett 2006, 2997–3000. doi:10.1055/s-2006-948176 |
| 38. | Kraljević, T. G.; Bistrović, A.; Dedić, M.; Pavelić, S. K.; Sedić, M.; Raić-Malić, S. Tetrahedron Lett. 2012, 53, 5144–5147. doi:10.1016/j.tetlet.2012.07.068 |
| 39. | Janeba, Z.; Balzarini, J.; Andrei, G.; Snoeck, R.; De Clercq, E.; Robins, M. J. Can. J. Chem. 2006, 84, 580–586. doi:10.1139/v06-041 |
| 40. | Park, G.; Ettles, C.; Charles, M.; Hudson, R. H. E. J. Photochem. Photobiol., A 2023, 441, 114653. doi:10.1016/j.jphotochem.2023.114653 |
| 41. | Teppang, K. L.; Lee, R. W.; Burns, D. D.; Turner, M. B.; Lokensgard, M. E.; Cooksy, A. L.; Purse, B. W. Chem. – Eur. J. 2019, 25, 1249–1259. doi:10.1002/chem.201803653 |
| 42. | Heidari, A.; Ghorbani-Choghamarani, A.; Hajjami, M.; Hudson, R. H. E. Molecules 2020, 25, 1995. doi:10.3390/molecules25081995 |
| 43. | Freeman, N. S.; Moore, C. E.; Wilhelmsson, L. M.; Tor, Y. J. Org. Chem. 2016, 81, 4530–4539. doi:10.1021/acs.joc.6b00310 |
| 44. | Suchý, M.; Hudson, R. H. E. J. Org. Chem. 2014, 79, 3336–3347. doi:10.1021/jo402873e |
| 45. | Kaczmarek, R.; Twardy, D. J.; Olson, T. L.; Korczyński, D.; Andrei, G.; Snoeck, R.; Dolot, R.; Wheeler, K. A.; Dembinski, R. Eur. J. Med. Chem. 2021, 209, 112884. doi:10.1016/j.ejmech.2020.112884 |
| 46. | Bollu, A.; Sharma, N. K. ChemBioChem 2019, 20, 1467–1475. doi:10.1002/cbic.201800822 |
| 47. | Meher, S.; Gade, C. R.; Sharma, N. K. ChemBioChem 2023, 24, e202200732. doi:10.1002/cbic.202200732 |
| 48. | Mikami, A.; Mori, S.; Osawa, T.; Obika, S. Chem. – Eur. J. 2023, 29, e202301928. doi:10.1002/chem.202301928 |
| 49. | Romero-Pérez, S.; López-Martín, I.; Martos-Maldonado, M. C.; Somoza, Á.; González-Rodríguez, D. Org. Lett. 2020, 22, 41–45. doi:10.1021/acs.orglett.9b03801 |
| 50. | Zeng, Y.; Wang, Z.; Zeng, L.; Xiong, H. Anal. Chem. (Washington, DC, U. S.) 2023, 95, 18880–18888. doi:10.1021/acs.analchem.3c04415 |
| 65. | de Abreu, R. M. F.; Brockmann, T.; Villinger, A.; Ehlers, P.; Langer, P. Beilstein J. Org. Chem. 2024, 20, 898–911. doi:10.3762/bjoc.20.80 |
| 4. | Ramesh, D.; Vijayakumar, B. G.; Kannan, T. Eur. J. Med. Chem. 2020, 207, 112801. doi:10.1016/j.ejmech.2020.112801 |
| 5. | Hitchings, G. H.; Falco, E. A.; Sherwood, M. B. Science 1945, 102, 251–252. doi:10.1126/science.102.2645.251 |
| 6. | Prusoff, W. H.; Holmes, W. L.; Welch, A. D. Cancer Res. 1953, 13, 221–225. |
| 7. | Fischl, M. A.; Richman, D. D.; Grieco, M. H.; Gottlieb, M. S.; Volberding, P. A.; Laskin, O. L.; Leedom, J. M.; Groopman, J. E.; Mildvan, D.; Schooley, R. T.; Jackson, G. G.; Durack, D. T.; King, D.; The AZT Collaborative Working Group. N. Engl. J. Med. 1987, 317, 185–191. doi:10.1056/nejm198707233170401 |
| 8. | Horwitz, J. P.; Chua, J.; Noel, M. J. Org. Chem. 1964, 29, 2076–2078. doi:10.1021/jo01030a546 |
| 9. | Bergmann, W.; Burke, D. C. J. Org. Chem. 1956, 21, 226–228. doi:10.1021/jo01108a020 |
| 10. | Bergmann, W.; Feeney, R. J. J. Am. Chem. Soc. 1950, 72, 2809–2810. doi:10.1021/ja01162a543 |
| 11. | Prusoff, W. H. Biochim. Biophys. Acta 1959, 32, 295–296. doi:10.1016/0006-3002(59)90597-9 |
| 12. | Geisman, A. N.; Valuev-Elliston, V. T.; Ozerov, A. A.; Khandazhinskaya, A. L.; Chizhov, A. O.; Kochetkov, S. N.; Pannecouque, C.; Naesens, L.; Seley-Radtke, K. L.; Novikov, M. S. Bioorg. Med. Chem. 2016, 24, 2476–2485. doi:10.1016/j.bmc.2016.04.010 |
| 13. | Tănase, C. I.; Drăghici, C.; Cojocaru, A.; Galochkina, A. V.; Orshanskaya, J. R.; Zarubaev, V. V.; Shova, S.; Enache, C.; Maganu, M. Bioorg. Med. Chem. 2015, 23, 6346–6354. doi:10.1016/j.bmc.2015.08.033 |
| 14. | Sneader, W. Drug Discovery: A History, 1st ed.; John Wiley & Sons: Chichester, UK, 2005. doi:10.1002/0470015535 |
| 15. | de Clercq, E. Acta Pharm. Sin. B 2012, 2, 535–548. doi:10.1016/j.apsb.2012.10.001 |
| 16. | De Clercq, E. Adv. Virus Res. 2009, 73, 1–53. doi:10.1016/s0065-3527(09)73001-5 |
| 17. | Sidwell, R. W.; Allen, L. B.; Huffman, J. H.; Witkowski, J. T.; Cook, P. D.; Tolman, R. L.; Revankar, G. R.; Simon, L. N.; Robins, R. K. The Potential of Nucleosides as Antiviral Agents. In Chemotherapy; Williams, J. D.; Geddes, A. M., Eds.; Springer: New York, NY, USA, 1976; Vol. 6, pp 279–294. doi:10.1007/978-1-4684-3129-2_41 |
| 18. | Ghorani-Azam, A.; Balali-Mood, M. Clinical Pharmacology and Toxicology of Mustard Compounds. In Basic and Clinical Toxicology of Mustard Compounds; Balali-Mood, M.; Abdollahi, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Vol. 1, pp 63–99. doi:10.1007/978-3-319-23874-6_4 |
| 19. | De Clercq, E.; Desgranges, C.; Herdewijn, P.; Sim, I. S.; Jones, A. S.; McLean, M. J.; Walker, R. T. J. Med. Chem. 1986, 29, 213–217. doi:10.1021/jm00152a008 |
| 20. | De Clercq, E.; Descamps, J.; De Somer, P.; Barr, P. J.; Jones, A. S.; Walker, R. T. Proc. Natl. Acad. Sci. U. S. A. 1979, 76, 2947–2951. doi:10.1073/pnas.76.6.2947 |
| 65. | de Abreu, R. M. F.; Brockmann, T.; Villinger, A.; Ehlers, P.; Langer, P. Beilstein J. Org. Chem. 2024, 20, 898–911. doi:10.3762/bjoc.20.80 |
| 65. | de Abreu, R. M. F.; Brockmann, T.; Villinger, A.; Ehlers, P.; Langer, P. Beilstein J. Org. Chem. 2024, 20, 898–911. doi:10.3762/bjoc.20.80 |
| 68. | König, B.; Kümmel, S.; Svobodová, E.; Cibulka, R. Phys. Sci. Rev. 2018, 3, 20170168. doi:10.1515/psr-2017-0168 |
| 69. | Tu, Y.; Yang, Y.; Li, Y.; He, C. Pharmacol. Res. 2021, 169, 105615. doi:10.1016/j.phrs.2021.105615 |
| 70. | Brouwer, A. M. Pure Appl. Chem. 2011, 83, 2213–2228. doi:10.1351/pac-rep-10-09-31 |
| 71. | Meech, S. R.; Phillips, D. J. Photochem. 1983, 23, 193–217. doi:10.1016/0047-2670(83)80061-6 |
| 52. | Youssefyeh, R. D.; Weisz, M. Tetrahedron Lett. 1973, 14, 4317–4318. doi:10.1016/s0040-4039(01)87209-9 |
| 53. | Senda, S.; Hirota, K.; Takahashi, M. J. Chem. Soc., Perkin Trans. 1 1975, 503–507. doi:10.1039/p19750000503 |
| 54. | Dudkin, S.; Iaroshenko, V. O.; Sosnovskikh, V. Y.; Tolmachev, A. A.; Villinger, A.; Langer, P. Org. Biomol. Chem. 2013, 11, 5351–5361. doi:10.1039/c3ob26837c |
| 55. | Nishigaki, S.; Sato, J.; Shimizu, K.; Furukawa, K.; Senga, K.; Yoneda, F. Chem. Pharm. Bull. 1980, 28, 142–149. doi:10.1248/cpb.28.142 |
| 66. | Hu, Y.-G.; Wang, Y.; Du, S.-M.; Chen, X.-B.; Ding, M.-W. Bioorg. Med. Chem. Lett. 2010, 20, 6188–6190. doi:10.1016/j.bmcl.2010.08.122 |
| 67. | Hossain, M. I.; Bhuiyan, M. M. H. J. Sci. Res. (Rajshahi, Bangladesh) 2009, 1, 317–325. doi:10.3329/jsr.v1i2.2299 |
| 65. | de Abreu, R. M. F.; Brockmann, T.; Villinger, A.; Ehlers, P.; Langer, P. Beilstein J. Org. Chem. 2024, 20, 898–911. doi:10.3762/bjoc.20.80 |
© 2024 de Abreu et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.