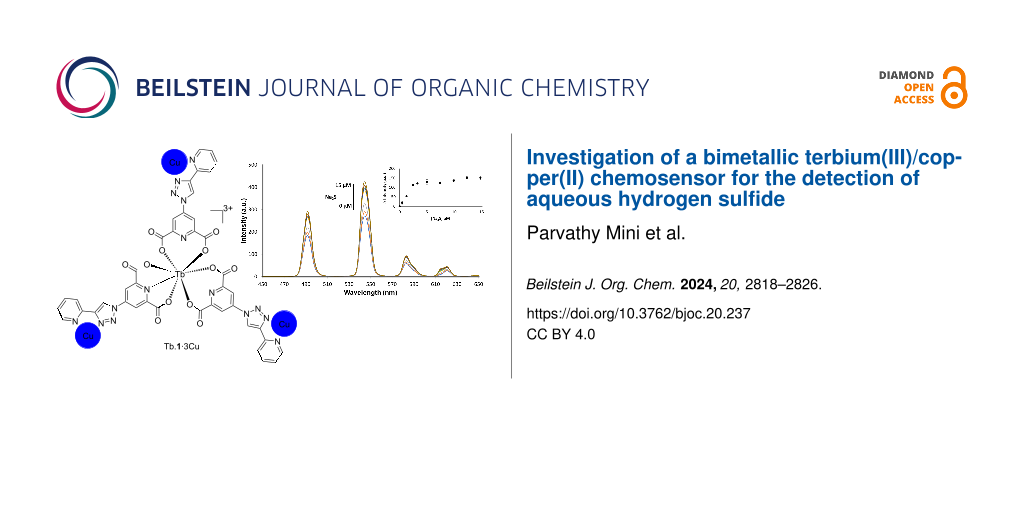
Abstract
The chemosensor properties of a bimetallic terbium(III)/copper(II) complex functionalized with a 4-(2-pyridyl)-1,2,3-triazole ligand for the detection of Cu2+ ions and, aqueous and gaseous hydrogen sulfide was investigated. The 4-(2-pyridyl)-1,2,3-triazole ligand functions both as an antenna chromophore and a receptor for Cu2+ ions; the Cu2+ complex was shown to be a chemosensor for the detection of aqueous hydrogen sulfide. The chemosensor exhibited significant reversibility over multiple cycles, observed with the sequential addition of Na2S followed by Cu2+ ions. The limit of detection for aqueous hydrogen sulfide was 0.63 μM (20 ppb). No luminescent changes of the bimetallic terbium(III)/copper(II) complex were observed in the presence of gaseous hydrogen sulfide, and thus this sensor can only be used for the detection of aqueous hydrogen sulfide.
Graphical Abstract
Introduction
The field of luminescent lanthanide chemosensors is rapidly evolving, driven by the need for more efficient, sensitive, and versatile detection methods for environmentally and biologically relevant analytes. While significant advances have been made, there remain critical challenges and unmet needs that call for innovative approaches. One of the key motivations for this exploration is the increasing complexity and diversity of analytes that require detection in real-world scenarios. Traditional methods, while effective, often fall short in environments where multiple, overlapping signals or low concentrations are present. The emerging strategies discussed in this article aim to overcome these limitations by leveraging novel materials, advanced synthesis techniques, and cutting-edge detection mechanisms for the detection of hydrogen sulfide. Hydrogen sulfide (H2S) is now recognised as a significant gaseous signaling molecule, alongside nitric oxide and carbon monoxide; it belongs to the biologically active group known as "gaseous mediators" or "gasotransmitters" [1]. In mammalian systems, H2S is predominantly biosynthesized at low concentrations through enzymatic conversions of sulfur-containing substrates, and it exerts diverse biological roles across nearly all organ systems. Within the central nervous system, H2S functions as a neuromodulator, influencing pain perception and neuronal potentiation [2]. H2S is implicated in various pathological conditions such as Parkinson's disease, Alzheimer's disease, Down's syndrome, and diabetes [3-5].
H2S naturally occurs in groundwater, originating from the breakdown of organic matter and as a by-product of numerous industrial processes. H2S predominantly exists as HS− in the aqueous state at a pH of 7.4 due to its weak acidic nature and high solubility in water (80 mM at 37 °C) [6]. Elevated levels of H2S in groundwater pose high risks to both human health and aquatic ecosystems [7], compelling rigorous monitoring of water sources. Even though sensors for detecting aqueous H2S are in development [8-14], lack of sensitivity, selectivity, and cost effectiveness remain major challenges.
For a number of years we have explored the properties of lanthanide-based chemosensors (Ln = Eu3+ or Tb3+) due to their significant advantages over fluorescent-based sensors; notable features include large Stokes shifts, extended luminescent lifetimes, and precisely defined emission bands [15]. Typically lasting in the order of milliseconds, their extended luminescent lifetimes enable the implementation of time-gated detection methods, effectively eliminating short-lived background fluorescence. This unique capability enhances the sensitivity and reliability of trivalent lanthanide-based chemosensors, making them invaluable tools for our application in the detection of hydrogen sulfide.
Our previously reported trivalent lanthanide-based chemosensors for the detection of both gaseous and/or aqueous H2S are shown in Figure 1 [12,16,17]. These sensors all function via the copper sequestration mechanism, where upon addition of hydrogen sulfide to the quenched bimetallic species, luminescence modulation occurs. In our quest for highly selective, highly sensitive chemosensors via a facile synthetic route/method, we have explored three chelates for lanthanide ions (DO3A, 2,6-pyridinedicarboxylic acid and DO2A), resulting in complexes with different overall charges. Additionally we have explored two copper(II) binding groups (di(2-picolyl)amine and 4-(2-pyridyl)-1,2,3-triazole). A europium(III)/copper(II) complex [Eu(triazole-DPA)3·3Cu]3+(Figure 1), functionalized with 4-(2-pyridyl)-1,2,3-triazole serving as both an antenna chromophore and a receptor for Cu2+ ions, previously demonstrated theoretical limits of detection (LoD) of 1.1 μM for aqueous hydrogen sulfide and 100 ppb for gaseous hydrogen sulfide [16]. However, due to the limited aqueous solubility and ligand dissociation of this chemosensor, and to the weakly luminescent bis species at usable concentrations, we extended this work to the lanthanide–macrocycle binary complexes [Ln(DO2A)(triazole-DPA)·Cu]+ (Ln = Eu and Tb, Figure 1). We found that both sensors gave good sensitivity for detection of aqueous H2S, however, only the europium variant, [Eu(DO2A)(triazole-DPA)·Cu]+, gave a luminescent increase in the presence of gaseous H2S. Exposure of [Tb(DO2A)(triazole-DPA)·Cu]+ to H2S gas resulted in no modulation of luminescent intensity. With this in mind, it was therefore of interest to investigate the luminescent properties of [Tb(triazole-DPA)3·3Cu]3+, Tb.1·Cu, in the presence of aqueous and gaseous hydrogen sulfide.
Figure 1: Previously reported lanthanide complexes [12,16,17].
Figure 1: Previously reported lanthanide complexes [12,16,17].
Results and Discussion
The Tb.1 complex was synthesized in an analogous fashion to the Eu.1 complex, via a three-step synthesis as reported previously [16] and depicted in Scheme 1. The corresponding terbium(III) species was synthesized by the combination of three equivalents of the ligand L with terbium(III) trifluoromethanesulfonate under basic conditions. High-resolution mass spectrometry (HRMS) analysis and the 1H NMR spectrum were consistent with formation of the Tb.1 complex (Figures S1 and S2 in Supporting Information File 1).
Luminescence characterization of Tb.1
As anticipated, based on the previously reported europium complex ([Eu(triazole-DPA)3]3−, Tb.1 exhibited limited solubility in water, becoming insoluble at concentrations exceeding 100 μM. Therefore, a 1 mM stock solution of Tb.1 was prepared in DMSO, with the final concentration of DMSO in analytical solutions kept at ≤5%. Initial luminescence analysis of a 5 μM solution of Tb.1 exhibited high luminescence (with a quantum yield of 68%) and displayed the characteristic trivalent terbium emission bands with emission peaks at 491 nm, 545 nm, 583 nm, and 621 nm, corresponding to transitions from the 5D4 excited state to the 7F6, 7F5, 7F4, and 7F3 ground states, respectively (Figure 2).
![[1860-5397-20-237-2]](/bjoc/content/figures/1860-5397-20-237-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Absorption (red line), excitation (yellow line, λem = 615 nm), and emission (green line, λex = 250 nm) spectra of 5 µM Tb.1 in 10 mM Tris-HCl buffer, pH 7.4, containing 5% DMSO.
Figure 2: Absorption (red line), excitation (yellow line, λem = 615 nm), and emission (green line, λex = 250 ...
Response of Tb.1 with Cu2+ ions
The addition of excess Cu2+ ions to Tb.1 was expected to result in complete saturation of emission signals. However, upon the titration of Cu2+ ions to 5 μM Tb.1 (10 mM Tris-HCl buffer containing 5% DMSO, pH 7.4), emission signal saturation was not observed until 5 equivalents (25 μM) were added (Figure S3 in Supporting Information File 1). The addition of 5 equivalents of Cu2+ ions led to 70% reduction in the luminescence signal, though complete quenching of luminescence could not be achieved, even with additional Cu2+ ions. However, as greater than 50% of emission quenching had occurred when three equivalents of Cu2+ ions were added, a [Tb(triazole-DPA)3·3Cu]3+ [Tb.1·3Cu]3+ complex was used for the subsequent HS− sensing experiments. We did extend our study to investigate the luminescent quenching of 5 μM Tb.1 upon the addition of Cu2+ ions when 10 mM HEPES buffer containing 5% DMSO, pH 7.4 was used (Figure S4 in Supporting Information File 1), with a similar degree of quenching to that observed in 10 mM Tris-HCl buffer. The incomplete quenching in luminescence was previously also observed for the Eu(III)/Cu(II) complex [16].
Supramolecular.org [18], an Open Access program, was used to determine the binding constant of Cu2+ ions to Tb.1 in both Tris-HCl buffer and HEPES buffer. The host–guest binding modes (1:1, 1:2 or 2:1) were evaluated using the luminescent data (λex = 250 nm, λem = 450–650 nm) from the respective titration experiments. In both cases the 1:1 host–guest binding model gave an acceptable fit with low (co)variance of the fit (Table 1).
Table 1: Binding constant determination for the 1:1 host–guest interaction (Tb.1 + Cu2+ ions), determined using supramolecular.org [18].
| buffer | binding model (host–guest) | K (M−1) |
| 10 mM Tris-HCl | 1:1 | 7.4 × 104 M−1 ± 0.2% |
| 10 mM HEPES | 1:1 | 9.7 × 104 M−1 ± 0.1% |
Aqueous buffer HS− studies of [Tb.1·3Cu]3+
The response of [Tb.1·3Cu]3+ to Na2S (HS− in solution at pH 7.4) in both 10 mM HEPES and 10 mM Tris-HCl was investigated. The addition of HS−(aq) ions to a solution of [Tb.1·3Cu]3+, 10 mM HEPES buffer (pH 7.4) resulted in a sigmoidal growth curve, with a “lag phase” (Figure 3). This was an unexpected result as a linear increase in luminescence regain was anticipated on addition of HS−(aq) ions to the solution. We interpret this “lag phase” as a consequence of Cu2+ ions present in solution, whereby the added HS−(aq) ions are mostly consumed by the unbound Cu2+ ions. This leads to a gradual luminescence increase rather than the anticipated linear response.
![[1860-5397-20-237-3]](/bjoc/content/figures/1860-5397-20-237-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: (a) Changes in the luminescence emission spectrum of 5 µM [Tb.1·3Cu]3+ upon the addition of Na2S (0–15 µM); spectra measured in 10 mM HEPES buffer (pH 7.4) with λex = 250 nm. (b) Change in luminescence intensity detected at 545 nm upon the addition of Na2S (n = 3).
Figure 3: (a) Changes in the luminescence emission spectrum of 5 µM [Tb.1·3Cu]3+ upon the addition of Na2S (0...
The addition of HS− ions to [Tb.1·3Cu]3+ in Tris-HCl buffer resulted in a linear increase in luminescence over a concentration range of 0–15 μM (R2 = 0.974, Figure 4). The linear increase in luminescence, compared to the “lag-phase” observed in HEPES buffer, can be attributed to the Tris-HCl buffer forming a complex with Cu2+ ions, a phenomenon only weakly observed with HEPES buffer [19,20]. We hypothesize that using Tris-HCl buffer minimizes any formation of Cu(OH)2, facilitating the reaction of HS−(aq) with [Tb.1·3Cu]3+, resulting in a linear rate of reaction. A 10-fold increase in luminescence regain was observed by the displacement of Cu2+ as CuS. Saturation in luminescence regain was observed after the addition of 3.0 equivalents of HS−(aq) ions. The calculated theoretical LoD was 0.63 μM (20 ppb), which is comparable to that observed with the europium(III) analogue (1.1 μM, 36 ppb) [16]). The chemosensor exhibited significant reversibility over multiple cycles involving the addition and subsequent removal of Na2S followed by the precipitation and re-addition of Cu2+ ions (Figure 5).
![[1860-5397-20-237-4]](/bjoc/content/figures/1860-5397-20-237-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: (a) Changes in the luminescence emission spectrum of 5 µM [Tb.1·3Cu]3+ upon the addition of Na2S (0–15 µM); spectra measured in 10 mM Tris-HCl buffer (pH 7.4) with λex = 250 nm. (b) Change in luminescence intensity detected at 545 nm upon the addition of Na2S (n = 3).
Figure 4: (a) Changes in the luminescence emission spectrum of 5 µM [Tb.1·3Cu]3+ upon the addition of Na2S (0...
![[1860-5397-20-237-5]](/bjoc/content/figures/1860-5397-20-237-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Luminescence intensity of 5 μM Tb.1 in 10 mM Tris-HCl buffer, pH 7.4, containing 5% DMSO upon the alternate addition of Cu2+ ions (15 μM) ions and Na2S (15 μM); λex = 250 nm, λem = 545 nm.
Figure 5: Luminescence intensity of 5 μM Tb.1 in 10 mM Tris-HCl buffer, pH 7.4, containing 5% DMSO upon the a...
Comprehensive selectivity studies were conducted with various anions/sulfur compounds (SO42−, SO32−, S2O52−, S2O42−, S2O32−, ClO−, OAc−, NO3−, I−, HCO3−, CO32−, Cl−, lipoic acid, and glutathione, as depicted in Figure 6). It was interesting to note that neither of the sulfur-containing compounds caused a remarkable increase in luminescence, especially lipoic acid and glutathione which contain an –S–S (pKa = 4.7) and –SH (pKa = 9.65) group respectively similar to HS−(aq) (pKa = 6.9). This demonstrates that the sensors are highly selective to HS−(aq) ions and are thus suitable for environmental or biological studies where interfering anions may be present.
![[1860-5397-20-237-6]](/bjoc/content/figures/1860-5397-20-237-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: Changes in luminescent intensity of [Tb.1·3Cu]3+ (5 µM) detected at 545 nm in the presence of various anions/sulfur compounds; spectra measured in 10 mM Tris HCl buffer (pH 7.4) with λex = 250 nm. Green bar: [Tb.1·3Cu]3+ alone (5 µM). Blue bar: [Tb.1·3Cu]3+ (5 µM) and Na2S (15 µM). Grey bars: [Tb.1·3Cu]3+ (5 µM) and anion/sulfur compound (50 µM). Black bars: [Tb.1·3Cu]3+ (5 µM), anion/sulfur compound (50 µM) and Na2S (15 µM), n = 3.
Figure 6: Changes in luminescent intensity of [Tb.1·3Cu]3+ (5 µM) detected at 545 nm in the presence of vario...
Gaseous H2S studies of [Tb.1·3Cu]3+
We aimed to further investigate the luminescence response of Tb3+ analogues upon exposure to hydrogen sulfide gas, building upon our previously reported findings. Our earlier work demonstrated an increase in europium luminescence with a LoD of 100 ppb and 665 ppb for two Eu analogues [Eu(triazole-DPA)3·3Cu]3+ [16] and [Eu(DO2A)DPA·Cu]+ [17], while the [Tb(DO2A)DPA·Cu]+ analogue [17] exhibited no significant change in luminescence upon exposure to gaseous H2S. To assess whether this observed behavior is consistent and potentially attributed to the energy levels of the Tb3+ ion, we conducted the gaseous studies with [Tb.1·3Cu]3+. Upon exposure to H2S gas using the established experimental setup, the [Tb.1·3Cu]3+ complex did not exhibit any discernible increase in luminescence.
As far as we are aware, there are only three reports of lanthanide-based probes for the detection of gaseous hydrogen sulfide. Two are the europium(III) complexes from our group ([Eu(triazole-DPA)3·Cu]3+ and [Eu(DO2A)(triazole-DPA)·Cu]+), which are proposed to function by Cu2+ sequestration. The remaining report is of a terbium(III) complex [Tb(DPA-N3)3]3− (Figure 7), which contains an aryl azide-functionalized ligand. In this system the azide functionality prohibits the energy transfer to the lanthanide ion, effectively quenching luminescence. In the presence of gaseous hydrogen sulfide, the aryl azide is reduced to an aniline functionality and luminescence is restored [11]. Drawing our previous findings and insights from the work of Hou, Wu, and co-workers [21], we postulate that gaseous H2S is interacting with the [Tb.1·3Cu]3+ complex as it did with ([Eu(triazole-DPA)3·Cu]3+, however for the terbium(III) complexes, the electronic state of the ligand, [triazole-DPA]2− is altered, resulting in the energy gap of the ligand and the excited energy level of the Tb3+ ion being smaller. This would facilitate efficient back-energy transfer from the excited 5D4 level of the Tb3+ ion, a non-radiative process and consequently, explaining the absence of an observable change in luminescence for this complex.
Figure 7: Chemical structure and mode of action of [Tb(DPA-N3)3]3− for detection of H2S(g) [11].
Figure 7: Chemical structure and mode of action of [Tb(DPA-N3)3]3− for detection of H2S(g) [11].
Conclusion
In conclusion, the Tb.1 complex shows luminescent quenching in the presence of Cu2+ ions in both Tris-HCl and HEPES buffer. Luminescence is restored upon the addition of HS−(aq) ions to [Tb.1·3Cu]3+, with a linear response observed until 1 equivalent of HS−(aq) ions is added and Tris-HCl is used as the buffer (LoD 0.63 μM). Exposure of [Tb.1·3Cu]3+ to gaseous hydrogen sulfide did not result in an increase in the luminescent emission spectrum, unlike that observed with the europium(III) analogue. This result suggests potential variations in the luminescence response among terbium(III) analogues and highlights the complexity of the interaction between lanthanide complexes and gaseous H2S, and understanding the nuances of this interaction is the focus of our future research studies.
Experimental
Synthetic materials and methods
The Cu2+ ions were sourced from Cu(NO3)2·5H2O (Cat. #1027900250, Sigma-Aldrich). The synthesis of 4-(2-pyridyl)-1,2,3-triazole dipicolinic acid (L) was conducted as previously described [16]. Proton nuclear magnetic resonance (1H NMR) spectra was recorded on a Bruker DRX400 spectrometer operating at 400 MHz. High-resolution mass spectrometry (HRMS) was performed using a Bruker BioApex 47e FTMS with an analytical electrospray source employing NaI for accurate mass calibration (ESI). UV–vis absorption spectra were measured at room temperature utilizing a Varian Cary 1E UV–vis spectrophotometer with a quartz cell of 10 mm path length. Luminescence emission spectra of aqueous solutions were captured at 23 °C using a Varian Cary-Eclipse fluorescence spectrophotometer set to phosphorescence mode, employing a quartz cell with a 10 mm path length and a volume of 400 μL. The delay time was 0.1 ms, the gate time was 1 ms, and both the instrument's excitation and emission slit widths were set at 5 nm unless otherwise specified. Gaseous H2S was generated using an Advanced Calibration Designs (ACD) Cal 2000 calibration gas generator.
Synthesis of Tb.1
To a solution of L (155 mg, 0.5 mmol) in water (6 mL) and 1 M NaOH (4 mL) a solution of Tb(OTf)3 (100 mg, 0.166 mmol) in water (2 mL) was added to obtain a white precipitate instantly. The solution was stirred at room temperature for a day. The precipitate was centrifuged, washed with water, and freeze-dried to obtain a white fluffy precipitate in 65% yield. HRESIMS (DMSO m/z): [Tb.1·2Na+]− calcd. for C42H21TbN15O12Na2, 1132.0542; found, 1132.0583.
Luminescence studies – analysis of aqueous solutions
This part was performed in a manner similar to that outlined in reference [17].
Cu2+-dependent luminescence spectra. A solution of Tb.1 (5 μM) in 10 mM Tris–HCl buffer (pH 7.4, containing <5% DMSO), was incrementally spiked with a standard solution of 1 mM Cu(NO3)2·5H2O(aq). The time-gated luminescence emission spectrum (λex = 250 nm) of the solution was recorded after each addition.
In situ preparation of the [Tb.1·3Cu]3+ sensor. Solutions of 5 μM [Tb.1·3Cu]3+ were prepared by combining a DMSO stock solution of Tb.1 (100 μM) with 1 mM Cu(NO3)2·5H2O(aq). Solutions were then diluted with the appropriate amount of buffer (final concentration 10 mM Tris–HCl or 10 mM HEPES buffer (pH 7.4), solutions contained <5% DMSO). The solutions were incubated at 23 °C for 5 min prior to use.
Na2S-dependent luminescence spectra. A solution of [Tb.1·3Cu]3+ (5 μM), in 10 mM buffer (final concentration 10 mM Tris–HCl or 10 mM HEPES buffer (pH 7.4), solutions contained <5% DMSO), was incrementally spiked with a standard solution of 1 mM Na2S(aq). The time-gated luminescence emission spectrum (λex = 250 nm) of the solution was recorded after each addition.
Competition assay with anions and cations: The time-gated luminescence emission change of a solution of [Tb.1·3Cu]3+ (5 μM), in 10 mM Tris–HCl buffer (pH 7.4, containing <5% DMSO), was examined in the absence and presence of 1.0 to 10.0 mol equiv of various anions. The anions were added as 1 mM aqueous solutions; NaCl, NaI, NaHCO3, Na2CO3, NaClO, NaNO2, NaOAc, Na2SO3, Na2SO4, Na2S2O3, Na2S2O4, Na2S2O5, lipoic acid, and glutathione. The change in luminescence emission spectra (λex = 250 nm) of the solutions was also investigated after the subsequent addition of 1.0 molar equivalent of 1 mM Na2S(aq).
Quantum yields. The quantum yield (φ) was determined using a quinine sulfate standard (φ = 0.55) for Tb·1, in water at pH 7.4 at 23 °C, according to the following equation:
where the subscripts X and ST denote sample and standard, respectively, Grad is the gradient of plotted integrated luminescence intensity vs absorbance, and η is the refractive index of the solvent.
Limit of detection (LoD). Solutions of [Tb.1·3Cu]3+ (5 μM) in 10 mM Tris–HCl buffer (pH 7.4, containing <5% DMSO) were incrementally spiked with a standard solution of 1 mM Na2S(aq), with the time-resolved luminescence emission spectra recorded after each addition (λex = 250 nm). From the measured data, the LoD was calculated according to the following equation; LoD = yB + 3sB. yB is the signal associated with the blank and sB corresponds to the standard deviation of the blank [22,23]. LoD: [Tb.1·3Cu]3+ 0.63 μM, λem = 544 nm.
Supporting Information
| Supporting Information File 1: Copies of HRMS, 1H NMR and fluorescence emission spectra. | ||
| Format: PDF | Size: 337.8 KB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information to this article.
References
-
Huang, Y.-Q.; Jin, H.-F.; Zhang, H.; Tang, C.-S.; Du, J.-B. Interaction among Hydrogen Sulfide and Other Gasotransmitters in Mammalian Physiology and Pathophysiology. In Advances in Hydrogen Sulfide Biology; Zhu, Y.-C., Ed.; Springer: Singapore, 2021; pp 205–236. doi:10.1007/978-981-16-0991-6_9
Return to citation in text: [1] -
Abe, K.; Kimura, H. J. Neurosci. 1996, 16, 1066–1071. doi:10.1523/jneurosci.16-03-01066.1996
Return to citation in text: [1] -
Liu, D.; Hessler, W.; Henary, M. Molecules 2023, 28, 1295. doi:10.3390/molecules28031295
Return to citation in text: [1] -
Cirino, G.; Szabo, C.; Papapetropoulos, A. Physiol. Rev. 2023, 103, 31–276. doi:10.1152/physrev.00028.2021
Return to citation in text: [1] -
Fosnacht, K. G.; Pluth, M. D. Chem. Rev. 2024, 124, 4124–4257. doi:10.1021/acs.chemrev.3c00683
Return to citation in text: [1] -
Zhao, Y.; Biggs, T. D.; Xian, M. Chem. Commun. 2014, 50, 11788–11805. doi:10.1039/c4cc00968a
Return to citation in text: [1] -
Letterman, R. D. Water Quality and Treatment: A Handbook of Community Water Supplies, 5th ed.; McGraw-Hill Professional: New York, NY, USA, 1999.
Return to citation in text: [1] -
Thorson, M. K.; Ung, P.; Leaver, F. M.; Corbin, T. S.; Tuck, K. L.; Graham, B.; Barrios, A. M. Anal. Chim. Acta 2015, 896, 160–165. doi:10.1016/j.aca.2015.09.024
Return to citation in text: [1] -
Tropiano, M.; Faulkner, S. Chem. Commun. 2014, 50, 4696–4698. doi:10.1039/c4cc01095g
Return to citation in text: [1] -
Yao, Y.; Kong, C.; Yin, L.; Jain, A. D.; Ratia, K.; Thatcher, G. R. J.; Moore, T. W.; Driver, T. G.; Miller, L. W. Chem. – Eur. J. 2017, 23, 752–756. doi:10.1002/chem.201604786
Return to citation in text: [1] -
Zhang, R.; Liu, S.; Wang, J.; Han, G.; Yang, L.; Liu, B.; Guan, G.; Zhang, Z. Analyst 2016, 141, 4919–4925. doi:10.1039/c6an00830e
Return to citation in text: [1] [2] [3] -
Aulsebrook, M. L.; Biswas, S.; Leaver, F. M.; Grace, M. R.; Graham, B.; Barrios, A. M.; Tuck, K. L. Chem. Commun. 2017, 53, 4911–4914. doi:10.1039/c7cc01764b
Return to citation in text: [1] [2] [3] -
Yao, Y.; Delgado-Rivera, L.; Samareh Afsari, H.; Yin, L.; Thatcher, G. R. J.; Moore, T. W.; Miller, L. W. Inorg. Chem. 2018, 57, 681–688. doi:10.1021/acs.inorgchem.7b02533
Return to citation in text: [1] -
Liang, Z.; Tsoi, T.-H.; Chan, C.-F.; Dai, L.; Wu, Y.; Du, G.; Zhu, L.; Lee, C.-S.; Wong, W.-T.; Law, G.-L.; Wong, K.-L. Chem. Sci. 2016, 7, 2151–2156. doi:10.1039/c5sc04091d
Return to citation in text: [1] -
Aulsebrook, M. L.; Graham, B.; Grace, M. R.; Tuck, K. L. Coord. Chem. Rev. 2018, 375, 191–220. doi:10.1016/j.ccr.2017.11.018
Return to citation in text: [1] -
Mini, P.; Springer, M. A.; Grace, M. R.; Dennison, G. H.; Tuck, K. L. Chem. Commun. 2020, 56, 5605–5608. doi:10.1039/d0cc00745e
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] [8] -
Mini, P.; Walker, S. E.; Grace, M. R.; Dennison, G. H.; Tuck, K. L. Dalton Trans. 2023, 52, 12235–12243. doi:10.1039/d3dt02150e
Return to citation in text: [1] [2] [3] [4] [5] -
Online tools for supramolecular chemistry research and analysis. http://supramolecular.org (accessed May 7, 2024).
Return to citation in text: [1] [2] -
McPhail, D. B.; Goodman, B. A. Biochem. J. 1984, 221, 559–560. doi:10.1042/bj2210559
Return to citation in text: [1] -
Kotuniak, R.; Sudzik, D. Z.; Ufnalska, I. M.; Bal, W. Inorg. Chem. 2024, 63, 12323–12332. doi:10.1021/acs.inorgchem.4c01797
Return to citation in text: [1] -
Zeng, X.; Hu, J.; Zhang, M.; Wang, F.; Wu, L.; Hou, X. Anal. Chem. (Washington, DC, U. S.) 2020, 92, 2097–2102. doi:10.1021/acs.analchem.9b04598
Return to citation in text: [1] -
Miller, J. C.; Miller, J. N. Statistics for analytical chemistry; Ellis Horwood: Chichester, UK, 1988.
Return to citation in text: [1] -
Skoog, D. A.; Holler, F. J.; Crouch, S. R. Principles of instrumental analysis; Thomson Brooks/Cole: Belmont, CA, USA, 2007.
Return to citation in text: [1]
| 1. | Huang, Y.-Q.; Jin, H.-F.; Zhang, H.; Tang, C.-S.; Du, J.-B. Interaction among Hydrogen Sulfide and Other Gasotransmitters in Mammalian Physiology and Pathophysiology. In Advances in Hydrogen Sulfide Biology; Zhu, Y.-C., Ed.; Springer: Singapore, 2021; pp 205–236. doi:10.1007/978-981-16-0991-6_9 |
| 7. | Letterman, R. D. Water Quality and Treatment: A Handbook of Community Water Supplies, 5th ed.; McGraw-Hill Professional: New York, NY, USA, 1999. |
| 19. | McPhail, D. B.; Goodman, B. A. Biochem. J. 1984, 221, 559–560. doi:10.1042/bj2210559 |
| 20. | Kotuniak, R.; Sudzik, D. Z.; Ufnalska, I. M.; Bal, W. Inorg. Chem. 2024, 63, 12323–12332. doi:10.1021/acs.inorgchem.4c01797 |
| 6. | Zhao, Y.; Biggs, T. D.; Xian, M. Chem. Commun. 2014, 50, 11788–11805. doi:10.1039/c4cc00968a |
| 16. | Mini, P.; Springer, M. A.; Grace, M. R.; Dennison, G. H.; Tuck, K. L. Chem. Commun. 2020, 56, 5605–5608. doi:10.1039/d0cc00745e |
| 3. | Liu, D.; Hessler, W.; Henary, M. Molecules 2023, 28, 1295. doi:10.3390/molecules28031295 |
| 4. | Cirino, G.; Szabo, C.; Papapetropoulos, A. Physiol. Rev. 2023, 103, 31–276. doi:10.1152/physrev.00028.2021 |
| 5. | Fosnacht, K. G.; Pluth, M. D. Chem. Rev. 2024, 124, 4124–4257. doi:10.1021/acs.chemrev.3c00683 |
| 18. | Online tools for supramolecular chemistry research and analysis. http://supramolecular.org (accessed May 7, 2024). |
| 2. | Abe, K.; Kimura, H. J. Neurosci. 1996, 16, 1066–1071. doi:10.1523/jneurosci.16-03-01066.1996 |
| 18. | Online tools for supramolecular chemistry research and analysis. http://supramolecular.org (accessed May 7, 2024). |
| 16. | Mini, P.; Springer, M. A.; Grace, M. R.; Dennison, G. H.; Tuck, K. L. Chem. Commun. 2020, 56, 5605–5608. doi:10.1039/d0cc00745e |
| 16. | Mini, P.; Springer, M. A.; Grace, M. R.; Dennison, G. H.; Tuck, K. L. Chem. Commun. 2020, 56, 5605–5608. doi:10.1039/d0cc00745e |
| 12. | Aulsebrook, M. L.; Biswas, S.; Leaver, F. M.; Grace, M. R.; Graham, B.; Barrios, A. M.; Tuck, K. L. Chem. Commun. 2017, 53, 4911–4914. doi:10.1039/c7cc01764b |
| 16. | Mini, P.; Springer, M. A.; Grace, M. R.; Dennison, G. H.; Tuck, K. L. Chem. Commun. 2020, 56, 5605–5608. doi:10.1039/d0cc00745e |
| 17. | Mini, P.; Walker, S. E.; Grace, M. R.; Dennison, G. H.; Tuck, K. L. Dalton Trans. 2023, 52, 12235–12243. doi:10.1039/d3dt02150e |
| 16. | Mini, P.; Springer, M. A.; Grace, M. R.; Dennison, G. H.; Tuck, K. L. Chem. Commun. 2020, 56, 5605–5608. doi:10.1039/d0cc00745e |
| 15. | Aulsebrook, M. L.; Graham, B.; Grace, M. R.; Tuck, K. L. Coord. Chem. Rev. 2018, 375, 191–220. doi:10.1016/j.ccr.2017.11.018 |
| 8. | Thorson, M. K.; Ung, P.; Leaver, F. M.; Corbin, T. S.; Tuck, K. L.; Graham, B.; Barrios, A. M. Anal. Chim. Acta 2015, 896, 160–165. doi:10.1016/j.aca.2015.09.024 |
| 9. | Tropiano, M.; Faulkner, S. Chem. Commun. 2014, 50, 4696–4698. doi:10.1039/c4cc01095g |
| 10. | Yao, Y.; Kong, C.; Yin, L.; Jain, A. D.; Ratia, K.; Thatcher, G. R. J.; Moore, T. W.; Driver, T. G.; Miller, L. W. Chem. – Eur. J. 2017, 23, 752–756. doi:10.1002/chem.201604786 |
| 11. | Zhang, R.; Liu, S.; Wang, J.; Han, G.; Yang, L.; Liu, B.; Guan, G.; Zhang, Z. Analyst 2016, 141, 4919–4925. doi:10.1039/c6an00830e |
| 12. | Aulsebrook, M. L.; Biswas, S.; Leaver, F. M.; Grace, M. R.; Graham, B.; Barrios, A. M.; Tuck, K. L. Chem. Commun. 2017, 53, 4911–4914. doi:10.1039/c7cc01764b |
| 13. | Yao, Y.; Delgado-Rivera, L.; Samareh Afsari, H.; Yin, L.; Thatcher, G. R. J.; Moore, T. W.; Miller, L. W. Inorg. Chem. 2018, 57, 681–688. doi:10.1021/acs.inorgchem.7b02533 |
| 14. | Liang, Z.; Tsoi, T.-H.; Chan, C.-F.; Dai, L.; Wu, Y.; Du, G.; Zhu, L.; Lee, C.-S.; Wong, W.-T.; Law, G.-L.; Wong, K.-L. Chem. Sci. 2016, 7, 2151–2156. doi:10.1039/c5sc04091d |
| 12. | Aulsebrook, M. L.; Biswas, S.; Leaver, F. M.; Grace, M. R.; Graham, B.; Barrios, A. M.; Tuck, K. L. Chem. Commun. 2017, 53, 4911–4914. doi:10.1039/c7cc01764b |
| 16. | Mini, P.; Springer, M. A.; Grace, M. R.; Dennison, G. H.; Tuck, K. L. Chem. Commun. 2020, 56, 5605–5608. doi:10.1039/d0cc00745e |
| 17. | Mini, P.; Walker, S. E.; Grace, M. R.; Dennison, G. H.; Tuck, K. L. Dalton Trans. 2023, 52, 12235–12243. doi:10.1039/d3dt02150e |
| 17. | Mini, P.; Walker, S. E.; Grace, M. R.; Dennison, G. H.; Tuck, K. L. Dalton Trans. 2023, 52, 12235–12243. doi:10.1039/d3dt02150e |
| 16. | Mini, P.; Springer, M. A.; Grace, M. R.; Dennison, G. H.; Tuck, K. L. Chem. Commun. 2020, 56, 5605–5608. doi:10.1039/d0cc00745e |
| 17. | Mini, P.; Walker, S. E.; Grace, M. R.; Dennison, G. H.; Tuck, K. L. Dalton Trans. 2023, 52, 12235–12243. doi:10.1039/d3dt02150e |
| 17. | Mini, P.; Walker, S. E.; Grace, M. R.; Dennison, G. H.; Tuck, K. L. Dalton Trans. 2023, 52, 12235–12243. doi:10.1039/d3dt02150e |
| 22. | Miller, J. C.; Miller, J. N. Statistics for analytical chemistry; Ellis Horwood: Chichester, UK, 1988. |
| 23. | Skoog, D. A.; Holler, F. J.; Crouch, S. R. Principles of instrumental analysis; Thomson Brooks/Cole: Belmont, CA, USA, 2007. |
| 11. | Zhang, R.; Liu, S.; Wang, J.; Han, G.; Yang, L.; Liu, B.; Guan, G.; Zhang, Z. Analyst 2016, 141, 4919–4925. doi:10.1039/c6an00830e |
| 16. | Mini, P.; Springer, M. A.; Grace, M. R.; Dennison, G. H.; Tuck, K. L. Chem. Commun. 2020, 56, 5605–5608. doi:10.1039/d0cc00745e |
| 11. | Zhang, R.; Liu, S.; Wang, J.; Han, G.; Yang, L.; Liu, B.; Guan, G.; Zhang, Z. Analyst 2016, 141, 4919–4925. doi:10.1039/c6an00830e |
| 21. | Zeng, X.; Hu, J.; Zhang, M.; Wang, F.; Wu, L.; Hou, X. Anal. Chem. (Washington, DC, U. S.) 2020, 92, 2097–2102. doi:10.1021/acs.analchem.9b04598 |
© 2024 Mini et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.