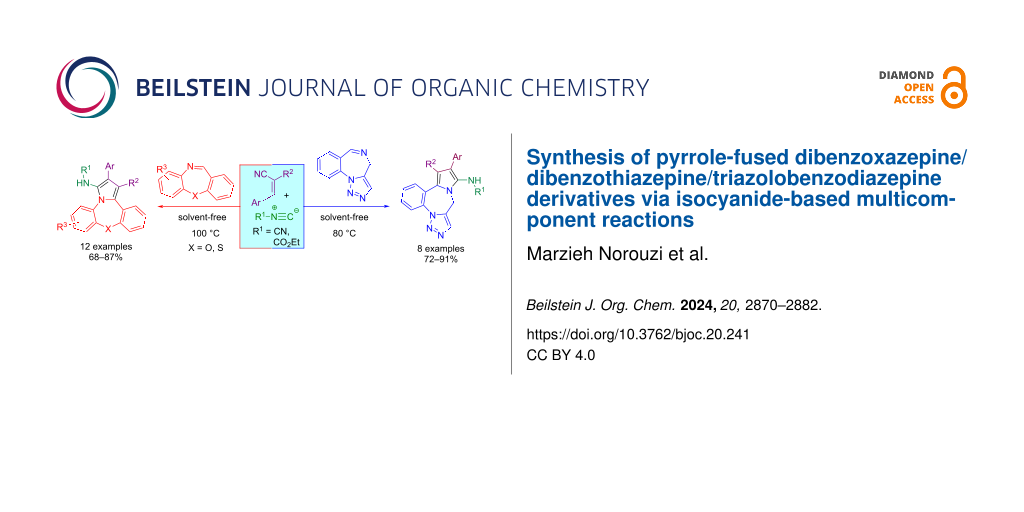
Abstract
An efficient and facile synthesis of pyrrole-fused dibenzoxazepine/dibenzothiazepine/triazolobenzodiazepine derivatives was developed through the isocyanide-based multicomponent reaction of isocyanides, gem-diactivated olefins, and cyclic imines such as dibenzoxazepine, dibenzothiazepine, and triazolobenzodiazepine under solvent- and catalyst-free conditions. Purposefully, this approach produced various bioactive scaffolds using environmentally friendly, mild, and simple conditions. Due to their bioactive moieties, these compounds with exclusive fluorescence properties may attract great attention in biomedical applications, clinical diagnostics, and conjugate materials.
Graphical Abstract
Introduction
Pyrroles and their derivatives are important N-heterocyclic compounds with antibiotic, antiviral, and anticancer properties that are found in many drugs and natural products [1-6]. Pyrroles' biological properties manifest when they are fused to other heterocycles [7-12]. Among them, seven-membered heterocycles of the benzodiazepine, benzoxazepine, and benzothiazepine derivatives are especially important. These consitute the central core of many natural and biological compounds and commercial drugs, including diazepam, clonazepam, lorazepam, telenzepine, chlordiazepoxide, loxapine, and amoxapine [13-21]. Pyrrole-fused benzodiazepines, benzoxazepines, or benzothiazepines exhibit unique biological and pharmacological properties [22,23]. For example, midazolam is a hypnotic-sedative drug with anxiolytic, muscle relaxant, and anticonvulsant properties [24], flumazenil is made to induce general anesthesia for diagnostic and therapeutic procedures [25] or PBOX-6 is a drug for the treatment of depression [26]. Benzothiazepines are known as antidepressants and are molecules with interesting electronic properties (Figure 1) [27]. Accordingly, the synthesis of new derivatives of pyrrole-fused benzodiazepine/benzoxazepine/benzothiazepine is very important.
Figure 1: Representation of distinguished structures of benzodiazepine/benzoxazepine/benzothiazepine with pharmaceutical to electronic applications.
Figure 1: Representation of distinguished structures of benzodiazepine/benzoxazepine/benzothiazepine with pha...
Due to the wide applications of pyrrole-fused heterocycles, very diverse approaches have been developed for their synthesis [28-32]. In recent years, multicomponent reactions (MCRs) have emerged as one of the most efficient and powerful methods to achieve this goal [7,33]. Among them, isocyanide-based multicomponent reactions (I-MCRs) are one of the well-known strategies in this field due to their operational simplicity, one-pot, convergent properties and atom economy, high efficiency, and high levels of chemical selectivity [34-36]. In addition to the application of isocyanides in a variety of MCRs, one of the unique reactions involves the formation of zwitterions from isocyanides upon reaction with acetylene and active olefin compounds such as alkyl acetylenedicarboxylates and gem-diactivated olefins. Due to having nucleophilic and electrophilic sites simultaneously in their structure, these zwitterions are able to participate in various cyclization processes, especially for the synthesis of pyrroles [37-40]. For example, Li et al. developed a one-pot four-component reaction (4-CR) of malononitrile, aldehydes, and isocyanides with 1,10-phenanthroline as cyclic imine under solvent-free conditions for the synthesis of pyrrole-fused phenanthroline. This reaction proceeds via in situ formation of zwitterion I through reaction of the aldehyde and malononitrile followed by 1,3-dipolar cycloaddition (Scheme 1a) [41]. Chen and co-workers reported a one-pot three-component reaction (3-CR) of sulfamate‐derived cyclic imine, isocyanide, and acetylenedicarboxylate. In this reaction too, the pyrrole-fused sulfamate is synthesized through intermediacy of the in situ-formed zwitterion II and [1 + 2 + 2] annulation reaction (Scheme 1b) [42,43]. Another I-MCR for forming pyrroles is the in situ formation reaction of the zwitterion III, known as Huisgens 1,4-dipole. The latter is formed by the reaction of imines with acetylenedicarboxylates and can be trapped by an isocyanide through a [4 + 1] cyclization reaction to synthesize pyrroles. In our recent studies, we prepared pyrrole-fused dibenzoxazepines via an Ugi reaction. Here, the reaction of benzoxazepine imine and acetylenedicarboxylate leads to the Huisgens 1,4-dipole zwitterion in situ, which is trapped by an isocyanide through the cyclization process (Scheme 1c) [44,45].
Scheme 1: Methods for the construction of pyrrole-fused heterocycles through I-MCR reactions.
Scheme 1: Methods for the construction of pyrrole-fused heterocycles through I-MCR reactions.
Here, we report an efficient and facile approach for the synthesis of pyrrole-fused dibenzoxazepine, dibenzothiazepine, and triazolobenzodiazepine derivatives via I-MCRs of gem-diactivated olefins, isocyanides, and cyclic imines (dibenzoxazepines, benzothiazepine, and triazolobenzodiazepine) under solvent- and catalyst-free conditions (Scheme 1d).
Results and Discussion
Synthesis
Dibenzoxazepine as imine component, cyclohexyl isocyanide, and the gem-diactivated olefin (2-benzylidenemalononitrile) were selected as the starting materials to screen the reaction conditions (Scheme 2, Table 1). First, we investigated the reaction in dichloromethane at room temperature and at 40 °C (Table 1, entries 1 and 2) and we found that the reaction progressed slightly at 40 °C. This promising result prompet us to examine the reaction in multiple anhydrous solvents such as CH3CN, toluene, EtOH, THF, EtOAc, and DMF at different temperatures (Table 1, entries 3–9). The result obtained from the study of solvents showed that pyrrole-fused dibenzoxazepine 4a was obtained with a yield of 56% in ethanol as solvent at a temperature of 78 °C (Table 1, entry 4). To achieve a higher yield of product 4a, the reaction was also attempted under solvent-free conditions at different temperatures (Table 1, entries 10–13). Interestingly, the highest yield of 70% of the desired product was achieved by conducting the reaction at 100 °C without using any solvent (Table 1, entry 12).
Scheme 2: The model reaction of dibenzoxazepine, gem-diactivated olefin (2-benzylidenemalononitrile), and cyclohexyl isocyanide.
Scheme 2: The model reaction of dibenzoxazepine, gem-diactivated olefin (2-benzylidenemalononitrile), and cyc...
Table 1: Optimization of the reaction conditions.a
| Entry | Solvent | Temperature (°C) | Time (h) | Yieldb (%) |
| 1 | CH2Cl2 | 25 | 24 | 0 |
| 2 | CH2Cl2 | 40 | 24 | 23 |
| 3 | CH3CN | 82 | 24 | 36 |
| 4 | EtOH | 78 | 24 | 56 |
| 5 | toluene | 110 | 24 | 33 |
| 6 | THF | 66 | 24 | 41 |
| 7 | H2O | 100 | 24 | trace |
| 8 | EtOAc | 77 | 24 | 22 |
| 9 | DMF | 140 | 24 | 38 |
| 10 | solvent-free | 25 | 24 | 0 |
| 11 | solvent-free | 80 | 2 | 61 |
| 12 | solvent-free | 100 | 2 | 70 |
| 13 | solvent-free | 120 | 2 | 70 |
aReaction conditions: 1a (0.55 mmol), 2a (0.55 mmol), and 3a (0.50 mmol) were stirred in 2 mL of solvent in an oil bath at different temperatures and times. bIsolated yields.
After having identified the optimal conditions, we next studied the scope of this reaction with substituted benzoxazepines, gem-diactivated olefins, and isocyanide derivatives (Scheme 3). As illustrated in Scheme 3, both electron-donating (-Me, -OMe) and electron-withdrawing (-NO2, Cl, and Br) groups were well tolerated under the optimal reaction conditions giving the products 4 in yields ranging from 68% to 87% (Scheme 3, 4a–l). In this reaction, the presence of electron-withdrawing substituents in the aromatic rings of the gem-diactivated olefins led to slightly better yields of the products 4 when compared to the substrates having electron-donating substituents (Scheme 3). The cause of this phenomenon is probably related to the electron-widthdawing effect of these substitution groups in olefin, which affects the nucleophilic attack of the isocyanides. When a carboxylate substituent was present instead of the carbonitrile in the gem-diactivated olefins, the desired products were also obtained in good yields (Scheme 3, 4k and 4l). On the other hand, the presence of electron-donating and electron-withdrawing substitutions in the dibenzoxazepine core were also investigated and it was observed, that electron-withdrawing substituent led to an increase and the electron-donating substituents led to a decrease in the reaction efficiency (Scheme 3, 4a–k). In addition, benzothiazepine was used in this protocol and the corresponding pyrrole-fused benzothiazepine was obtained with a yield of 73% (Scheme 3, 4l). Also, various isocyanides were suitable for this reaction. By replacing cyclohexyl isocyanide with tert-butyl- and isopropyl isocyanide, the corresponding products were obtained with similar yields.
Scheme 3: Substrate scope. Conditions: Reactions were carried out using 1 (0.55 mmol), 2 (0.55 mmol), and 3 (0.50 mmol) under solvent-free conditions, stirring in an oil bath at 100 °C for 2 h (monitored by TLC).
Scheme 3: Substrate scope. Conditions: Reactions were carried out using 1 (0.55 mmol), 2 (0.55 mmol), and 3 (...
To investigate the reactivity of other cyclic imines in this protocol, we performed the reaction of triazolobenzodiazepine with gem-diactivated olefins and isocyanides (Scheme 4). As expected, under almost the same conditions as described in Scheme 3 (only at 80 °C), a new type of heterocyclic compounds, pyrrole-fused triazolobenzodiazepines, was obtained in high yield. As summarized in Scheme 4, a variety of gem-diactivated olefins with electron-donating (-Me, -OMe), electron-withdrawing (-NO2), and halogen (-Cl and -Br) substitutions on the aromatic ring were well tolerated under the reaction conditions. Pyrrole-fused triazolobenzodiazepines 6 were obtained in yields ranging from 72% to 91% (Scheme 4, 6a–h). Similar to the reaction of benzoxazepine, the presence of an electron-withdrawing group leads to an increase in the yield of pyrrole-fused triazolobenzodiazepines whereas electron-donating groups led to a decrease in its yield. Also, the substitution of naphthyl used in gem-diactivated olefins increased the efficiency of the desired product compared to the substitution of phenyl (Scheme 4, 6c). Furthermore, n-butyl isocyanide was used to increase the variety of products and the n-butyl-substituted products 6f–h were obtained with 72–78% yield .
Scheme 4: Substrate scope..Conditions: reactions were carried out using 1 (0.55 mmol), 2 (0.55 mmol), and 5 (0.50 mmol) under solvent-free conditions, stirring in an oil bath at 80 °C for 2 h (monitored by TLC).
Scheme 4: Substrate scope..Conditions: reactions were carried out using 1 (0.55 mmol), 2 (0.55 mmol), and 5 (...
All the products were characterized by 1H NMR, 13C NMR, and infrared spectroscopy, and mass spectrometry. Taking 4h as an example for the analysis of its structure, in its 1H NMR spectrum, one singlet signal appears at δ = 0.67 (9H) corresponding to the three methyl groups in the tert-butyl substituent. A singlet at δ = 2.39 (3H) is assigned to the protons of the CH3-group on the phenyl. The signal at δ = 3.42 is the NH group. All the protons of the aromatic rings are located from δ = 7.10 to 7.99. In its 13C NMR spectrum, all of the carbon signals appear at δ = 158.3, 152.5, 134.7, 134.2, 133.9, 133.2, 130.8, 130.4, 129.2, 129.1, 129.0 128.9, 128.4, 127.4, 125.8, 122.6, 121.1, 120.6, 117.1 (CAr), 91.1 (CN), 56.8, 29.4, 20.9 (CAliphatic), respectively. In its mass spectrum, the calculated value matched the found value m/z 419 calculated for [M]+ C28H25N3O. In the IR spectrum, the absorption peak index in 2213 is related to the CN group. For final confirmation, the derivative 4h was studied by X-ray diffraction analysis, and the crystal structure is illustrated in Figure 2 (detailed information can be found in the Supporting Information File 1).
![[1860-5397-20-241-2]](/bjoc/content/figures/1860-5397-20-241-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: The crystal structure of 4h (CCDC 2365305).
Figure 2: The crystal structure of 4h (CCDC 2365305).
The 1H NMR spectrum of product 6f obtained through the I-MCR was investigated and some unexpected chemical shifts were observed at room temperature (Figure 3A) [46]. Therefore, dynamic NMR measurements were performed for compound 6f at various temperatures (25, 35, 45, 55, 65, 75, and 85 °C). As illustrated in Figure 3, all peaks in the spectrum correspond to the structure of 6f. Spectrum A recoreded at 25 °C has two broad singulet signals at δ = 4.65 and δ = 5.84 ppm corresponding to hydrogen I and hydrogen II. Remarkably, at higher temperatures (85 °C), the rapid inversion of the seven-membered ring results in it being observed as a single structure on the 1H NMR time scale (see Figure 3, spectrum F) [19,47,48].
Figure 3: The DNMR (dynamic nuclear magnetic resonance) spectra of compound 6f (DMSO-d6, 300 MHz) at 25–85 °C; spectrum A: 25 °C, spectrum B: 35 °C, spectrum C: 45 °C, spectrum D: 55 °C, spectrum E: 65 °C, spectrum F: 75 °C and spectrum E: 85 °C.
Figure 3: The DNMR (dynamic nuclear magnetic resonance) spectra of compound 6f (DMSO-d6, 300 MHz) at 25–85 °C...
Furthermore, 13C NMR analysis, mass spectrum, and IR are consistent with the structure (for details see Supporting Information File 1). Finally, a single crystal X-ray analysis of compound 6a was performed, confirming the structure (Figure 4).
![[1860-5397-20-241-4]](/bjoc/content/figures/1860-5397-20-241-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: The crystal structure of 6a (CCDC2365306).
Figure 4: The crystal structure of 6a (CCDC2365306).
The proposed mechanism for an isocyanide-based multicomponent domino reaction for synthesizing pyrrole-fused dibenzoxazepine is illustrated in Scheme 5. The reaction is initiated by the nucleophilic attack of the isocyanide 2 on the gem-diactivated olefin 1 to give the zwitterion intermediate 7. The reaction proceeds with the nucleophilic attack of the zwitterion intermediate 8 on the cyclic imine 3 until intermediate 9 is formed. Then, with the cyclization process intermediate 10 is obtained. Finally, pyrrole-fused benzoxazepine is synthesized by successive processes involving the loss of HCN and the tautomeric enamine imine formation.
Scheme 5: A suggested mechanism for compounds 4.
Scheme 5: A suggested mechanism for compounds 4.
The successful synthesis of pyrrole-fused benzoxazepine/triazolobenzodiazepine derivatives via a 3-CR prompted us to investigate the synthesis of these compounds as a 4-CR. Fortunately, the pyrrole-fused benzoxazepine/triazolobenzodiazepines 4a and 6a could also be obtianed through a one-pot 4-CR from benzaldehyde, malononitrile, cyclohexyl isocyanide, and benzoxazepine/triazolobenzodiazepine imine (Scheme 6). However, the yield of the products based on the four-component method was lower compared to those obtained via the three-component strategy.
Scheme 6: Synthesis of pyrrole-fused dibenzoxazepine/triazolobenzodiazepine through a 4-CR.
Scheme 6: Synthesis of pyrrole-fused dibenzoxazepine/triazolobenzodiazepine through a 4-CR.
After the efficient and straightforward synthesis of the pyrrole-fused dibenzoxazepine/triazolobenzodiazepine derivatives on a submillimolar scale, two reactions were conducted on a gram scale to validate the protocol's efficacy (Scheme 7). Reacting each of the cyclic imines at a 3 mmol scale led to the pyrrole-fused dibenzoxazepine/triazolobenzodiazepine 4a and 6a in 80 and 87% yields, respectively. In general, a significant increase in the yield of the products was observed compared to the submillimole state.
Scheme 7: Gram-scale synthesis of pyrrole-fused dibenzoxazepine/triazolobenzodiazepine 4a and 6a via 3-CRs.
Scheme 7: Gram-scale synthesis of pyrrole-fused dibenzoxazepine/triazolobenzodiazepine 4a and 6a via 3-CRs.
Physical properties
The compounds such as benzoxazepines, benzothiazepines, benzodiazepines, and pyrrole have shown promising applications in optoelectronics, biophotonics, and cell imaging due to their suitable fluorophores [49-51]. The UV–vis absorption and emission spectra of products 4 and 6 were studied in ethanol at 298 K. The absorption range (λabs) was 400–225 nm, and the emission range (λem) was 560–400 nm. These findings are shown in Figure S3 in Supporting Information File 1. An interesting trend for the absorption of the synthesized derivatives 4 and 6 was observed when electron-donating substituents were present in the phenyl ring, which led to an increase in the intensity of the absorption and emission wavelengths (Supporting Information File 1, Figure S3). As is evident in numerous reports, quantum yield is one of the most important photophysical parameters for describing luminescent molecules and materials since high quantum efficiency is important for a wide range of applications, including displays, lasers, bio-imaging, solar cells, and accurate measurement of the quantum yield is therefore important [52-54]. Therefore, the quantum yield was determined using the standard of quinine sulfate for all the products (4a–l and 6a–h). Products 4a and 6c, which had the highest intensity of absorption and emission from derivatives of benzoxazepines and triazolobenzodiazepines, respectively, their quantum yield was calculated and 4a was obtained with 48.35% and 6c with 1.04% (Figure 5A and 5B) [55]. According to the results obtained from the quantum yield, we can claim that these synthesized derivatives pyrrole-fused dibenzoxazepine/dibenzothiazepine/triazolobenzodiazepine derivatives could serve as potential candidates for optoelectronic conjugate materials [56].
![[1860-5397-20-241-5]](/bjoc/content/figures/1860-5397-20-241-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: UV–vis absorption for compounds 4a, 6c and QS (quinine sulfate) (a); emission for 4a, 6c and QS (b); c = 75 ppm in ethanol and T = 298 K.
Figure 5: UV–vis absorption for compounds 4a, 6c and QS (quinine sulfate) (a); emission for 4a, 6c and QS (b)...
Conclusion
In summary, we have designed a simple and novel procedure for synthesizing pyrrole-fused dibenzoxazepine/dibenzothiazepine/triazolobenzodiazepine derivatives in high yields. This one-pot 3-CR includes isocyanides, gem-diactivated olefins, and cyclic imines (dibenzoxazepines, dibenzothiazepine, and triazolobenzodiazepine) under catalyst- and solvent-free conditions. Furthermore, the other advantages of this reaction include the manufacturing premium pharmaceutical scaffolds, a wide range of substrates, short reaction times, and simple operation. According to quantum efficiency calculations, all pyrrole-fused dibenzoxazepine/dibenzothiazepine/triazolobenzodiazepine derivatives with excellent emission could serve as potential candidates for optoelectronic conjugate materials.
Experimental
General information
All commercially available reagents and chemicals were bought from Merck & Co. and utilized without extra purification. The melting points of all synthesized compounds were measured utilizing an Electrothermal 9200 apparatus. 1H and 13C NMR and spectra in CDCl3 and DMSO-d6 solvents were recorded on a Bruker Avance spectrometer at 300.13 MHz and 75.47 MHz, respectively. IR spectra were created on a Thermo Nicolet Nexus 470 FT-IR spectrometer in cm−1. Mass spectra with an HP (Agile Technologies) 5975C Mass Selective Detector were used to confirm the mass of the synthesized products. The PL spectra of the products were obtained using an LS45 spectrometer from PerkinElmer. Elemental analyses were conducted using an Vario El CHN mode system from Elementar GmbH. The PL and the UV–vis spectra were obtained using a spectrofluorometer (LS45, PerkinElmer) and a Specord S 600 (Analytik Jena), respectively.
Preventive education for synthesizing triazolobenzodiazepine 6a–h
Azides are highly reactive, toxic, explosive, and shock-sensitive chemicals that can be used under certain conditions. Special safety procedures must be followed during preparation, storage, handling, and disposal. TMSN3 is an organic azide that is very sensitive to external factors such as light, heat, friction, and pressure and should be stored in amber plastic containers without light and at a temperature below zero degree Celsius. Exposure to azide occurs through skin absorption, inhalation, or ingestion through the respiratory tract. Which leads to skin and eye irritation, blurred vision, dizziness, weakness/fatigue, hypotension, seizures, and respiratory failure. The following instructions are required to work with TMSN3. It is necessary to have a silver shield apron, breathing mask, safety glasses, lab coat, and gloves with high chemical resistance. The reactions should be carried out in a hood with a strong suction and with a protective explosion shield, which in this test should be as low as possible. Since exposure to water and strong acids leads to the formation of hydrazoic acid, which is very toxic, volatile, and explosive, the reaction containers must be completely dry and clean. When consuming TMSN3, it should be done cold (below zero degree Celsius) in a container insulated from light. The reaction must be completely closed under the hood and the lid of the reaction container, and the product purification process must be carried out under the mentioned personal protective equipment. The reaction on a larger scale is carried out under special safety conditions [57-59].
3-(Cyclohexylamino)-2-phenyldibenzo[b,f]pyrrolo[1,2-d][1,4]oxazepine-1-carbonitrile (4a); ethyl 2-(4-chlorophenyl)-3-(cyclohexylamino)dibenzo[b,f]pyrrolo[1,2-d][1,4]thiazepine-1-carboxylate (4l) and 11-(cyclohexylamino)-12-phenyl-9H-benzo[f]pyrrolo[1,2-d][1,2,3]triazolo[1,5-a][1,4]diazepine-13-carbonitrile (6a); typical procedure: Cyclic imines of dibenzo[b,f][1,4]oxazepine 3a–c, dibenzo[b,f][1,4]thiazepine 3d, and 4H-benzo[f][1,2,3]triazolo[1,5-a][1,4]diazepine 5 were prepared according to the procedure in the previous report [44,45] and 0.50 mmol (3a, 98 mg; 3d, 105 mg; 5, 92 mg) of each was added separately along with 2-benzylidenemalononitrile (1a, 0.55 mmol, 85 mg), cyclohexyl isocyanide (2a, 0.55 mmol, 60 mg). The reaction mixture was stirred at temperatures of 100 or 80 °C for 2 h using a magnetic stirrer. After making sure that the reactions were completed (monitored by TLC), the reaction mixture was cooled to ambient temperature. Then, the crude mixture was purified using a silica gel chromatography column and washed with n-hexane and ethyl acetate solvent mixtures (1:3 for 3a and 3d; 2:1 for compounds 5).
Supporting Information
| Supporting Information File 1: General synthetic procedures and characterization and copies of 1H NMR, 13C NMR, FTIR and mass spectra. | ||
| Format: PDF | Size: 8.2 MB | Download |
Data Availability Statement
Data generated and analyzed during this study is available from the corresponding author upon reasonable request.
References
-
Wei, L.; Shen, C.; Hu, Y.-Z.; Tao, H.-Y.; Wang, C.-J. Chem. Commun. 2019, 55, 6672–6684. doi:10.1039/c9cc02371b
Return to citation in text: [1] -
Fang, W.-Y.; Ravindar, L.; Rakesh, K. P.; Manukumar, H. M.; Shantharam, C. S.; Alharbi, N. S.; Qin, H.-L. Eur. J. Med. Chem. 2019, 173, 117–153. doi:10.1016/j.ejmech.2019.03.063
Return to citation in text: [1] -
Lamberth, C.; Jeanmart, S.; Luksch, T.; Plant, A. Science 2013, 341, 742–746. doi:10.1126/science.1237227
Return to citation in text: [1] -
Vitaku, E.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2014, 57, 10257–10274. doi:10.1021/jm501100b
Return to citation in text: [1] -
Ahmad, S.; Alam, O.; Naim, M. J.; Shaquiquzzaman, M.; Alam, M. M.; Iqbal, M. Eur. J. Med. Chem. 2018, 157, 527–561. doi:10.1016/j.ejmech.2018.08.002
Return to citation in text: [1] -
Nazeri, M. T.; Farhid, H.; Javanbakht, S.; Shaabani, A.; Notash, B. Synlett 2020, 31, 965–971. doi:10.1055/s-0039-1690887
Return to citation in text: [1] -
Nazeri, M. T.; Shaabani, A. New J. Chem. 2021, 45, 21967–22011. doi:10.1039/d1nj04514h
Return to citation in text: [1] [2] -
Pramanik, C.; Barik, P.; Ali, S. A.; Nayak, D. S.; Ikbal, M.; Mandal, A.; Jana, R.; Giri, S.; Samanta, S. New J. Chem. 2023, 47, 6476–6527. doi:10.1039/d2nj05797b
Return to citation in text: [1] -
Di Santo, R.; Tafi, A.; Costi, R.; Botta, M.; Artico, M.; Corelli, F.; Forte, M.; Caporuscio, F.; Angiolella, L.; Palamara, A. T. J. Med. Chem. 2005, 48, 5140–5153. doi:10.1021/jm048997u
Return to citation in text: [1] -
Dubovtsev, A. Yu.; Dmitriev, M. V.; Silaichev, P. S.; Antonov, D. I.; Maslivets, A. N. Synthesis 2017, 49, 2223–2230. doi:10.1055/s-0036-1588407
Return to citation in text: [1] -
Dai, L.; Shu, P.; Wang, Z.; Li, Q.; Yu, Q.; Shi, Y.; Rong, L. Synthesis 2017, 49, 637–646. doi:10.1055/s-0036-1588605
Return to citation in text: [1] -
Chen, X.-Y.; Han, Y.; Sun, J.; Yan, C.-G. Beilstein J. Org. Chem. 2024, 20, 1436–1443. doi:10.3762/bjoc.20.126
Return to citation in text: [1] -
Farhid, H.; Khodkari, V.; Nazeri, M. T.; Javanbakht, S.; Shaabani, A. Org. Biomol. Chem. 2021, 19, 3318–3358. doi:10.1039/d0ob02600j
Return to citation in text: [1] -
Mandelli, M.; Tognoni, G.; Garattini, S. Clin. Pharmacokinet. 1978, 3, 72–91. doi:10.2165/00003088-197803010-00005
Return to citation in text: [1] -
Browne, T. R. N. Engl. J. Med. 1978, 299, 812–816. doi:10.1056/nejm197810122991505
Return to citation in text: [1] -
Rutgers, J. G.; Shearer, C. M. Lorazepam. In Analytical profiles of drug substances; Florey, K., Ed.; Academic Press, 1981; Vol. 9, pp 397–426. doi:10.1016/s0099-5428(08)60148-3
Return to citation in text: [1] -
Galvan, M.; Boer, R.; Schudt, C. Eur. J. Pharmacol. 1989, 167, 1–10. doi:10.1016/0014-2999(89)90741-3
Return to citation in text: [1] -
López-Muñoz, F.; Álamo, C.; García-García, P. J. Anxiety Disord. 2011, 25, 554–562. doi:10.1016/j.janxdis.2011.01.002
Return to citation in text: [1] -
Mazumdar, W.; Driver, T. G. Synthesis 2021, 53, 1734–1748. doi:10.1055/s-0040-1705995
Return to citation in text: [1] [2] -
Bhattacharya, D.; Mitra, S.; Chattopadhyay, P. Synthesis 2015, 47, 2294–2298. doi:10.1055/s-0034-1379916
Return to citation in text: [1] -
Gómez-Ayuso, J.; Pertejo, P.; Hermosilla, T.; Carreira-Barral, I.; Quesada, R.; García-Valverde, M. Beilstein J. Org. Chem. 2024, 20, 1758–1766. doi:10.3762/bjoc.20.154
Return to citation in text: [1] -
Avendaño, C.; Menéndez, J. C. Medicinal chemistry of anticancer drugs; Elsevier: Amsterdam, Netherlands, 2015.
Return to citation in text: [1] -
Kaufmann, C. N.; Spira, A. P.; Depp, C. A.; Mojtabai, R. Psychiatr. Serv. 2018, 69, 235–238. doi:10.1176/appi.ps.201700095
Return to citation in text: [1] -
Nordt, S. P.; Clark, R. F. J. Emerg. Med. 1997, 15, 357–365. doi:10.1016/s0736-4679(97)00022-x
Return to citation in text: [1] -
Whitwam, J.; Amrein, R. Acta Anaesthesiol. Scand. 1995, 39 (Suppl. 108), 3–14. doi:10.1111/j.1399-6576.1995.tb04374.x
Return to citation in text: [1] -
Sangshetti, J. N.; Altamash Shakeel Ahmad, A.; Kalam Khan, F. A.; Zaheer, Z. Mini-Rev. Org. Chem. 2015, 12, 345–354. doi:10.2174/1570193x12666150930224315
Return to citation in text: [1] -
Lei, X.; Angeli, G.; Dömling, A.; Neochoritis, C. G. Eur. J. Org. Chem. 2022, e202200220. doi:10.1002/ejoc.202200220
Return to citation in text: [1] -
Lee, T.; Christov, P. P.; Shaw, S.; Tarr, J. C.; Zhao, B.; Veerasamy, N.; Jeon, K. O.; Mills, J. J.; Bian, Z.; Sensintaffar, J. L.; Arnold, A. L.; Fogarty, S. A.; Perry, E.; Ramsey, H. E.; Cook, R. S.; Hollingshead, M.; Davis Millin, M.; Lee, K.-m.; Koss, B.; Budhraja, A.; Opferman, J. T.; Kim, K.; Arteaga, C. L.; Moore, W. J.; Olejniczak, E. T.; Savona, M. R.; Fesik, S. W. J. Med. Chem. 2019, 62, 3971–3988. doi:10.1021/acs.jmedchem.8b01991
Return to citation in text: [1] -
Boyer, S. J.; Burke, J.; Guo, X.; Kirrane, T. M.; Snow, R. J.; Zhang, Y.; Sarko, C.; Soleymanzadeh, L.; Swinamer, A.; Westbrook, J.; DiCapua, F.; Padyana, A.; Cogan, D.; Gao, A.; Xiong, Z.; Madwed, J. B.; Kashem, M.; Kugler, S.; O’Neill, M. M. Bioorg. Med. Chem. Lett. 2012, 22, 733–737. doi:10.1016/j.bmcl.2011.10.030
Return to citation in text: [1] -
De Lucca, G. V.; Otto, M. J. Bioorg. Med. Chem. Lett. 1992, 2, 1639–1644. doi:10.1016/s0960-894x(00)80447-3
Return to citation in text: [1] -
Shaabani, A.; Hezarkhani, Z.; Mofakham, H.; Ng, S. W. Synlett 2013, 24, 1485–1492. doi:10.1055/s-0033-1338953
Return to citation in text: [1] -
Donald, J. R.; Martin, S. F. Org. Lett. 2011, 13, 852–855. doi:10.1021/ol1028404
Return to citation in text: [1] -
Nenajdenko, V. G. Russ. Chem. Rev. 2020, 89, 1274–1336. doi:10.1070/rcr5010
Return to citation in text: [1] -
Zlotin, S. G.; Egorova, K. S.; Ananikov, V. P.; Akulov, A. A.; Varaksin, M. V.; Chupakhin, O. N.; Charushin, V. N.; Bryliakov, K. P.; Averin, A. D.; Beletskaya, I. P.; Dolengovski, E. L.; Budnikova, Y. H.; Sinyashin, O. G.; Gafurov, Z. N.; Kantyukov, A. O.; Yakhvarov, D. G.; Aksenov, A. V.; Elinson, M. N.; Nenajdenko, V. G.; Chibiryaev, A. M.; Nesterov, N. S.; Kozlova, E. A.; Martyanov, O. N.; Balova, I. A.; Sorokoumov, V. N.; Guk, D. A.; Beloglazkina, E. K.; Lemenovskii, D. A.; Chukicheva, I. Y.; Frolova, L. L.; Izmest'ev, E. S.; Dvornikova, I. A.; Popov, A. V.; Kutchin, A. V.; Borisova, D. M.; Kalinina, A. A.; Muzafarov, A. M.; Kuchurov, I. V.; Maximov, A. L.; Zolotukhina, A. V. Russ. Chem. Rev. 2023, 92, RCR5104. doi:10.59761/rcr5104
Return to citation in text: [1] -
Nazeri, M. T.; Nasiriani, T.; Farhid, H.; Javanbakht, S.; Bahri, F.; Shadi, M.; Shaabani, A. ACS Sustainable Chem. Eng. 2022, 10, 8115–8134. doi:10.1021/acssuschemeng.2c01030
Return to citation in text: [1] -
Jiang, M.; Hu, K.; Zhou, Y.; Xiong, Q.; Cao, W.; Feng, X. Org. Lett. 2021, 23, 5261–5265. doi:10.1021/acs.orglett.1c01792
Return to citation in text: [1] -
Nair, V.; Rajesh, C.; Vinod, A. U.; Bindu, S.; Sreekanth, A. R.; Mathen, J. S.; Balagopal, L. Acc. Chem. Res. 2003, 36, 899–907. doi:10.1021/ar020258p
Return to citation in text: [1] -
Li, M.; Qiu, Z.-X.; Wen, L.-R.; Zhou, Z.-M. Tetrahedron 2011, 67, 3638–3648. doi:10.1016/j.tet.2011.03.085
Return to citation in text: [1] -
Mironov, M. A.; Shulepov, I. D.; Kozhikhova, K. V.; Ivantsova, M. N.; Tokareva, M. I. Chem. Heterocycl. Compd. (N. Y., NY, U. S.) 2017, 53, 430–433. doi:10.1007/s10593-017-2071-3
Return to citation in text: [1] -
Müller, T. J. J. Multicomponent Reactions; Science of Synthesis, Vol. 2; Thieme: Stuttgart, Germany, 2014. doi:10.1055/b-003-125831
Return to citation in text: [1] -
Li, M.; Lv, X.-L.; Wen, L.-R.; Hu, Z.-Q. Org. Lett. 2013, 15, 1262–1265. doi:10.1021/ol400191b
Return to citation in text: [1] -
Wang, C.-C.; Ma, Z.-W.; Qu, Y.-L.; Liu, Z.-J.; Chen, X.-P.; Zhou, J.; Chen, Y.-J. Chem. – Asian J. 2020, 15, 560–563. doi:10.1002/asia.201901780
Return to citation in text: [1] -
Nazeri, M. T.; Shaabani, A.; Notash, B. Org. Biomol. Chem. 2021, 19, 3722–3734. doi:10.1039/d0ob02339f
Return to citation in text: [1] -
Nazeri, M. T.; Ahmadi, M.; Ghasemi, M.; Shaabani, A.; Notash, B. Org. Biomol. Chem. 2023, 21, 4095–4108. doi:10.1039/d3ob00250k
Return to citation in text: [1] [2] -
Nazeri, M. T.; Ghasemi, M.; Ahmadi, M.; Shaabani, A.; Notash, B. J. Org. Chem. 2023, 88, 13504–13519. doi:10.1021/acs.joc.3c01013
Return to citation in text: [1] [2] -
Donald, J. R.; Wood, R. R.; Martin, S. F. ACS Comb. Sci. 2012, 14, 135–143. doi:10.1021/co2002087
Return to citation in text: [1] -
Newkome, G. R. Eight-membered and larger rings. Progress in Heterocyclic Chemistry; Pergamon Press, 1996; Vol. 8, pp 320–336. doi:10.1016/s0959-6380(96)80019-5
Return to citation in text: [1] -
Anet, F. A. L. J. Am. Chem. Soc. 1964, 86, 458–460. doi:10.1021/ja01057a034
Return to citation in text: [1] -
Taliani, S.; Simorini, F.; Sergianni, V.; La Motta, C.; Da Settimo, F.; Cosimelli, B.; Abignente, E.; Greco, G.; Novellino, E.; Rossi, L.; Gremigni, V.; Spinetti, F.; Chelli, B.; Martini, C. J. Med. Chem. 2007, 50, 404–407. doi:10.1021/jm061137o
Return to citation in text: [1] -
Chen, D.; Shan, Y.; Li, J.; You, J.; Sun, X.; Qiu, G. Org. Lett. 2019, 21, 4044–4048. doi:10.1021/acs.orglett.9b01220
Return to citation in text: [1] -
Meyer, A. G.; Bissember, A. C.; Hyland, C. J. T.; Williams, C. C.; Szabo, M.; Pearsall, M. A.; Hyland, I. K.; Olivier, W. J. Seven-membered rings. Progress in Heterocyclic Chemistry; Elsevier: Amsterdam, Netherlands, 2020; Vol. 31, pp 597–647. doi:10.1016/b978-0-12-819962-6.00016-6
Return to citation in text: [1] -
Levitus, M. Methods Appl. Fluoresc. 2020, 8, 033001. doi:10.1088/2050-6120/ab7e10
Return to citation in text: [1] -
Rubin, M. B.; Braslavsky, S. E. Photochem. Photobiol. Sci. 2010, 9, 670–674. doi:10.1039/b9pp00182d
Return to citation in text: [1] -
Würth, C.; Grabolle, M.; Pauli, J.; Spieles, M.; Resch-Genger, U. Nat. Protoc. 2013, 8, 1535–1550. doi:10.1038/nprot.2013.087
Return to citation in text: [1] -
Sreejith, S.; Divya, K. P.; Ajayaghosh, A. Chem. Commun. 2008, 2903–2905. doi:10.1039/b802958j
Return to citation in text: [1] -
Ghosh, S.; Mandal, S.; Banerjee, C.; Rao, V. G.; Sarkar, N. J. Phys. Chem. B 2012, 116, 9482–9491. doi:10.1021/jp305095n
Return to citation in text: [1] -
Keicher, T.; Löbbecke, S. Lab-Scale synthesis of azido compounds: safety measures and analysis. In Organic Azides: Syntheses and Applications; Bräse, S.; Banert, K., Eds.; John Wiley & Sons: Chichester, UK, 2009; pp 1–27. doi:10.1002/9780470682517.ch1
Return to citation in text: [1] -
González-Bobes, F.; Kopp, N.; Li, L.; Deerberg, J.; Sharma, P.; Leung, S.; Davies, M.; Bush, J.; Hamm, J.; Hrytsak, M. Org. Process Res. Dev. 2012, 16, 2051–2057. doi:10.1021/op3002646
Return to citation in text: [1] -
Bräse, S.; Gil, C.; Knepper, K.; Zimmermann, V. Angew. Chem., Int. Ed. 2005, 44, 5188–5240. doi:10.1002/anie.200400657
Return to citation in text: [1]
| 52. | Levitus, M. Methods Appl. Fluoresc. 2020, 8, 033001. doi:10.1088/2050-6120/ab7e10 |
| 53. | Rubin, M. B.; Braslavsky, S. E. Photochem. Photobiol. Sci. 2010, 9, 670–674. doi:10.1039/b9pp00182d |
| 54. | Würth, C.; Grabolle, M.; Pauli, J.; Spieles, M.; Resch-Genger, U. Nat. Protoc. 2013, 8, 1535–1550. doi:10.1038/nprot.2013.087 |
| 19. | Mazumdar, W.; Driver, T. G. Synthesis 2021, 53, 1734–1748. doi:10.1055/s-0040-1705995 |
| 47. | Newkome, G. R. Eight-membered and larger rings. Progress in Heterocyclic Chemistry; Pergamon Press, 1996; Vol. 8, pp 320–336. doi:10.1016/s0959-6380(96)80019-5 |
| 48. | Anet, F. A. L. J. Am. Chem. Soc. 1964, 86, 458–460. doi:10.1021/ja01057a034 |
| 49. | Taliani, S.; Simorini, F.; Sergianni, V.; La Motta, C.; Da Settimo, F.; Cosimelli, B.; Abignente, E.; Greco, G.; Novellino, E.; Rossi, L.; Gremigni, V.; Spinetti, F.; Chelli, B.; Martini, C. J. Med. Chem. 2007, 50, 404–407. doi:10.1021/jm061137o |
| 50. | Chen, D.; Shan, Y.; Li, J.; You, J.; Sun, X.; Qiu, G. Org. Lett. 2019, 21, 4044–4048. doi:10.1021/acs.orglett.9b01220 |
| 51. | Meyer, A. G.; Bissember, A. C.; Hyland, C. J. T.; Williams, C. C.; Szabo, M.; Pearsall, M. A.; Hyland, I. K.; Olivier, W. J. Seven-membered rings. Progress in Heterocyclic Chemistry; Elsevier: Amsterdam, Netherlands, 2020; Vol. 31, pp 597–647. doi:10.1016/b978-0-12-819962-6.00016-6 |
| 1. | Wei, L.; Shen, C.; Hu, Y.-Z.; Tao, H.-Y.; Wang, C.-J. Chem. Commun. 2019, 55, 6672–6684. doi:10.1039/c9cc02371b |
| 2. | Fang, W.-Y.; Ravindar, L.; Rakesh, K. P.; Manukumar, H. M.; Shantharam, C. S.; Alharbi, N. S.; Qin, H.-L. Eur. J. Med. Chem. 2019, 173, 117–153. doi:10.1016/j.ejmech.2019.03.063 |
| 3. | Lamberth, C.; Jeanmart, S.; Luksch, T.; Plant, A. Science 2013, 341, 742–746. doi:10.1126/science.1237227 |
| 4. | Vitaku, E.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2014, 57, 10257–10274. doi:10.1021/jm501100b |
| 5. | Ahmad, S.; Alam, O.; Naim, M. J.; Shaquiquzzaman, M.; Alam, M. M.; Iqbal, M. Eur. J. Med. Chem. 2018, 157, 527–561. doi:10.1016/j.ejmech.2018.08.002 |
| 6. | Nazeri, M. T.; Farhid, H.; Javanbakht, S.; Shaabani, A.; Notash, B. Synlett 2020, 31, 965–971. doi:10.1055/s-0039-1690887 |
| 24. | Nordt, S. P.; Clark, R. F. J. Emerg. Med. 1997, 15, 357–365. doi:10.1016/s0736-4679(97)00022-x |
| 44. | Nazeri, M. T.; Ahmadi, M.; Ghasemi, M.; Shaabani, A.; Notash, B. Org. Biomol. Chem. 2023, 21, 4095–4108. doi:10.1039/d3ob00250k |
| 45. | Nazeri, M. T.; Ghasemi, M.; Ahmadi, M.; Shaabani, A.; Notash, B. J. Org. Chem. 2023, 88, 13504–13519. doi:10.1021/acs.joc.3c01013 |
| 22. | Avendaño, C.; Menéndez, J. C. Medicinal chemistry of anticancer drugs; Elsevier: Amsterdam, Netherlands, 2015. |
| 23. | Kaufmann, C. N.; Spira, A. P.; Depp, C. A.; Mojtabai, R. Psychiatr. Serv. 2018, 69, 235–238. doi:10.1176/appi.ps.201700095 |
| 46. | Donald, J. R.; Wood, R. R.; Martin, S. F. ACS Comb. Sci. 2012, 14, 135–143. doi:10.1021/co2002087 |
| 13. | Farhid, H.; Khodkari, V.; Nazeri, M. T.; Javanbakht, S.; Shaabani, A. Org. Biomol. Chem. 2021, 19, 3318–3358. doi:10.1039/d0ob02600j |
| 14. | Mandelli, M.; Tognoni, G.; Garattini, S. Clin. Pharmacokinet. 1978, 3, 72–91. doi:10.2165/00003088-197803010-00005 |
| 15. | Browne, T. R. N. Engl. J. Med. 1978, 299, 812–816. doi:10.1056/nejm197810122991505 |
| 16. | Rutgers, J. G.; Shearer, C. M. Lorazepam. In Analytical profiles of drug substances; Florey, K., Ed.; Academic Press, 1981; Vol. 9, pp 397–426. doi:10.1016/s0099-5428(08)60148-3 |
| 17. | Galvan, M.; Boer, R.; Schudt, C. Eur. J. Pharmacol. 1989, 167, 1–10. doi:10.1016/0014-2999(89)90741-3 |
| 18. | López-Muñoz, F.; Álamo, C.; García-García, P. J. Anxiety Disord. 2011, 25, 554–562. doi:10.1016/j.janxdis.2011.01.002 |
| 19. | Mazumdar, W.; Driver, T. G. Synthesis 2021, 53, 1734–1748. doi:10.1055/s-0040-1705995 |
| 20. | Bhattacharya, D.; Mitra, S.; Chattopadhyay, P. Synthesis 2015, 47, 2294–2298. doi:10.1055/s-0034-1379916 |
| 21. | Gómez-Ayuso, J.; Pertejo, P.; Hermosilla, T.; Carreira-Barral, I.; Quesada, R.; García-Valverde, M. Beilstein J. Org. Chem. 2024, 20, 1758–1766. doi:10.3762/bjoc.20.154 |
| 41. | Li, M.; Lv, X.-L.; Wen, L.-R.; Hu, Z.-Q. Org. Lett. 2013, 15, 1262–1265. doi:10.1021/ol400191b |
| 7. | Nazeri, M. T.; Shaabani, A. New J. Chem. 2021, 45, 21967–22011. doi:10.1039/d1nj04514h |
| 8. | Pramanik, C.; Barik, P.; Ali, S. A.; Nayak, D. S.; Ikbal, M.; Mandal, A.; Jana, R.; Giri, S.; Samanta, S. New J. Chem. 2023, 47, 6476–6527. doi:10.1039/d2nj05797b |
| 9. | Di Santo, R.; Tafi, A.; Costi, R.; Botta, M.; Artico, M.; Corelli, F.; Forte, M.; Caporuscio, F.; Angiolella, L.; Palamara, A. T. J. Med. Chem. 2005, 48, 5140–5153. doi:10.1021/jm048997u |
| 10. | Dubovtsev, A. Yu.; Dmitriev, M. V.; Silaichev, P. S.; Antonov, D. I.; Maslivets, A. N. Synthesis 2017, 49, 2223–2230. doi:10.1055/s-0036-1588407 |
| 11. | Dai, L.; Shu, P.; Wang, Z.; Li, Q.; Yu, Q.; Shi, Y.; Rong, L. Synthesis 2017, 49, 637–646. doi:10.1055/s-0036-1588605 |
| 12. | Chen, X.-Y.; Han, Y.; Sun, J.; Yan, C.-G. Beilstein J. Org. Chem. 2024, 20, 1436–1443. doi:10.3762/bjoc.20.126 |
| 42. | Wang, C.-C.; Ma, Z.-W.; Qu, Y.-L.; Liu, Z.-J.; Chen, X.-P.; Zhou, J.; Chen, Y.-J. Chem. – Asian J. 2020, 15, 560–563. doi:10.1002/asia.201901780 |
| 43. | Nazeri, M. T.; Shaabani, A.; Notash, B. Org. Biomol. Chem. 2021, 19, 3722–3734. doi:10.1039/d0ob02339f |
| 28. | Lee, T.; Christov, P. P.; Shaw, S.; Tarr, J. C.; Zhao, B.; Veerasamy, N.; Jeon, K. O.; Mills, J. J.; Bian, Z.; Sensintaffar, J. L.; Arnold, A. L.; Fogarty, S. A.; Perry, E.; Ramsey, H. E.; Cook, R. S.; Hollingshead, M.; Davis Millin, M.; Lee, K.-m.; Koss, B.; Budhraja, A.; Opferman, J. T.; Kim, K.; Arteaga, C. L.; Moore, W. J.; Olejniczak, E. T.; Savona, M. R.; Fesik, S. W. J. Med. Chem. 2019, 62, 3971–3988. doi:10.1021/acs.jmedchem.8b01991 |
| 29. | Boyer, S. J.; Burke, J.; Guo, X.; Kirrane, T. M.; Snow, R. J.; Zhang, Y.; Sarko, C.; Soleymanzadeh, L.; Swinamer, A.; Westbrook, J.; DiCapua, F.; Padyana, A.; Cogan, D.; Gao, A.; Xiong, Z.; Madwed, J. B.; Kashem, M.; Kugler, S.; O’Neill, M. M. Bioorg. Med. Chem. Lett. 2012, 22, 733–737. doi:10.1016/j.bmcl.2011.10.030 |
| 30. | De Lucca, G. V.; Otto, M. J. Bioorg. Med. Chem. Lett. 1992, 2, 1639–1644. doi:10.1016/s0960-894x(00)80447-3 |
| 31. | Shaabani, A.; Hezarkhani, Z.; Mofakham, H.; Ng, S. W. Synlett 2013, 24, 1485–1492. doi:10.1055/s-0033-1338953 |
| 32. | Donald, J. R.; Martin, S. F. Org. Lett. 2011, 13, 852–855. doi:10.1021/ol1028404 |
| 34. | Zlotin, S. G.; Egorova, K. S.; Ananikov, V. P.; Akulov, A. A.; Varaksin, M. V.; Chupakhin, O. N.; Charushin, V. N.; Bryliakov, K. P.; Averin, A. D.; Beletskaya, I. P.; Dolengovski, E. L.; Budnikova, Y. H.; Sinyashin, O. G.; Gafurov, Z. N.; Kantyukov, A. O.; Yakhvarov, D. G.; Aksenov, A. V.; Elinson, M. N.; Nenajdenko, V. G.; Chibiryaev, A. M.; Nesterov, N. S.; Kozlova, E. A.; Martyanov, O. N.; Balova, I. A.; Sorokoumov, V. N.; Guk, D. A.; Beloglazkina, E. K.; Lemenovskii, D. A.; Chukicheva, I. Y.; Frolova, L. L.; Izmest'ev, E. S.; Dvornikova, I. A.; Popov, A. V.; Kutchin, A. V.; Borisova, D. M.; Kalinina, A. A.; Muzafarov, A. M.; Kuchurov, I. V.; Maximov, A. L.; Zolotukhina, A. V. Russ. Chem. Rev. 2023, 92, RCR5104. doi:10.59761/rcr5104 |
| 35. | Nazeri, M. T.; Nasiriani, T.; Farhid, H.; Javanbakht, S.; Bahri, F.; Shadi, M.; Shaabani, A. ACS Sustainable Chem. Eng. 2022, 10, 8115–8134. doi:10.1021/acssuschemeng.2c01030 |
| 36. | Jiang, M.; Hu, K.; Zhou, Y.; Xiong, Q.; Cao, W.; Feng, X. Org. Lett. 2021, 23, 5261–5265. doi:10.1021/acs.orglett.1c01792 |
| 57. | Keicher, T.; Löbbecke, S. Lab-Scale synthesis of azido compounds: safety measures and analysis. In Organic Azides: Syntheses and Applications; Bräse, S.; Banert, K., Eds.; John Wiley & Sons: Chichester, UK, 2009; pp 1–27. doi:10.1002/9780470682517.ch1 |
| 58. | González-Bobes, F.; Kopp, N.; Li, L.; Deerberg, J.; Sharma, P.; Leung, S.; Davies, M.; Bush, J.; Hamm, J.; Hrytsak, M. Org. Process Res. Dev. 2012, 16, 2051–2057. doi:10.1021/op3002646 |
| 59. | Bräse, S.; Gil, C.; Knepper, K.; Zimmermann, V. Angew. Chem., Int. Ed. 2005, 44, 5188–5240. doi:10.1002/anie.200400657 |
| 27. | Lei, X.; Angeli, G.; Dömling, A.; Neochoritis, C. G. Eur. J. Org. Chem. 2022, e202200220. doi:10.1002/ejoc.202200220 |
| 37. | Nair, V.; Rajesh, C.; Vinod, A. U.; Bindu, S.; Sreekanth, A. R.; Mathen, J. S.; Balagopal, L. Acc. Chem. Res. 2003, 36, 899–907. doi:10.1021/ar020258p |
| 38. | Li, M.; Qiu, Z.-X.; Wen, L.-R.; Zhou, Z.-M. Tetrahedron 2011, 67, 3638–3648. doi:10.1016/j.tet.2011.03.085 |
| 39. | Mironov, M. A.; Shulepov, I. D.; Kozhikhova, K. V.; Ivantsova, M. N.; Tokareva, M. I. Chem. Heterocycl. Compd. (N. Y., NY, U. S.) 2017, 53, 430–433. doi:10.1007/s10593-017-2071-3 |
| 40. | Müller, T. J. J. Multicomponent Reactions; Science of Synthesis, Vol. 2; Thieme: Stuttgart, Germany, 2014. doi:10.1055/b-003-125831 |
| 44. | Nazeri, M. T.; Ahmadi, M.; Ghasemi, M.; Shaabani, A.; Notash, B. Org. Biomol. Chem. 2023, 21, 4095–4108. doi:10.1039/d3ob00250k |
| 45. | Nazeri, M. T.; Ghasemi, M.; Ahmadi, M.; Shaabani, A.; Notash, B. J. Org. Chem. 2023, 88, 13504–13519. doi:10.1021/acs.joc.3c01013 |
| 26. | Sangshetti, J. N.; Altamash Shakeel Ahmad, A.; Kalam Khan, F. A.; Zaheer, Z. Mini-Rev. Org. Chem. 2015, 12, 345–354. doi:10.2174/1570193x12666150930224315 |
| 55. | Sreejith, S.; Divya, K. P.; Ajayaghosh, A. Chem. Commun. 2008, 2903–2905. doi:10.1039/b802958j |
| 25. | Whitwam, J.; Amrein, R. Acta Anaesthesiol. Scand. 1995, 39 (Suppl. 108), 3–14. doi:10.1111/j.1399-6576.1995.tb04374.x |
| 7. | Nazeri, M. T.; Shaabani, A. New J. Chem. 2021, 45, 21967–22011. doi:10.1039/d1nj04514h |
| 33. | Nenajdenko, V. G. Russ. Chem. Rev. 2020, 89, 1274–1336. doi:10.1070/rcr5010 |
| 56. | Ghosh, S.; Mandal, S.; Banerjee, C.; Rao, V. G.; Sarkar, N. J. Phys. Chem. B 2012, 116, 9482–9491. doi:10.1021/jp305095n |
© 2024 Norouzi et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.