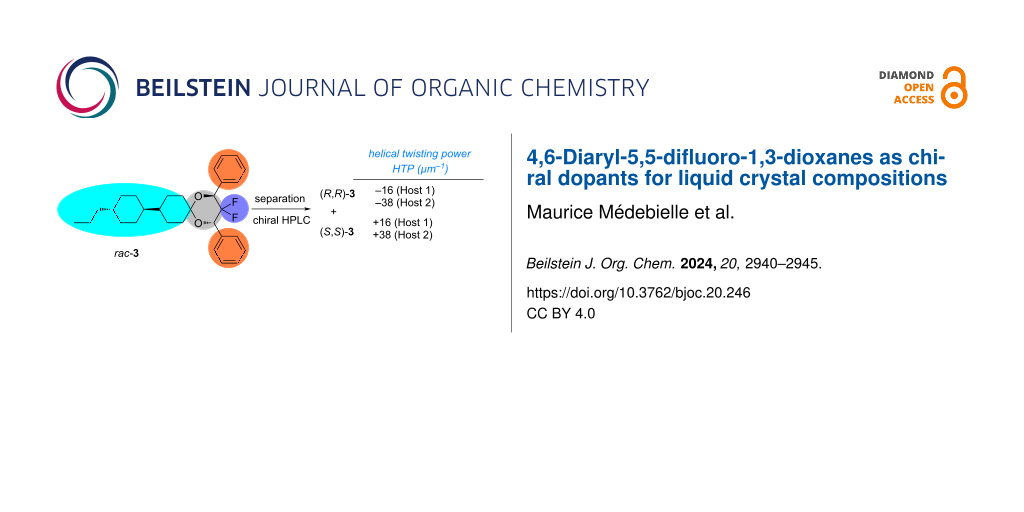
Abstract
Two racemic anti-4,6-diphenyl-5,5-difluoro-1,3-dioxanes were prepared and the corresponding enantiomers were evaluated as potential new chiral dopants for liquid-crystal compositions.
Graphical Abstract
Introduction
Liquid crystals for use in liquid crystal displays (LCDs) have become one of the most prominent application areas of fluoroorganic chemistry [1-3]. In particular, cholesteric, i.e., chiral nematic, liquid crystals (LCs) are attractive for many display applications due to their chiroptical characteristics as well as the selective reflection of light giving rise to Bragg interference colors [4]. Cholesteric LCs can be obtained either from neat chiral mesogens, or through the addition of a chiral dopant to an achiral nematic liquid crystal [5,6]. The ability of the dopant to induce chirality in the nematic phase is defined as the helical twisting power [HTP; β = (pc)−1; with p the helical pitch and c the molar concentration]. The most common type of liquid crystal displays (LCD) is based on the so-called twisted nematic (TN) mode and requires only a quite small HTP (typically around 10–15 µm−1) with a low dopant concentration (around 0.1%) [1,2,7]. However, there are other display modes, such as super-twisted nematic (STN) LCDs which need a higher HTP or increased dopant concentrations [8,9]. TN and STN displays are still based on liquid crystals in the nematic or cholesteric mesophase. Even higher concentrations of chiral dopants with extremely high HTP tend to induce a Blue Phase, which is a cubic mesophase composed of double twist cylinders [10,11]. A prototype of a polymer-stabilized Blue Phase LCD with ultra-fast switching times has been presented in 2008 by Samsung [12]. Another class of materials including high HTP chiral dopants are cholesteric films prepared by the polymerization of reactive mesogens (RMs). They are used, e.g., for improving the viewing angle-dependency of the image quality of LCD panels or as polarizing reflectors [13,14]. For such applications, new dopants with very high HTP are in constant demand. There have been many reported chiral dopants with relatively high HTPs, including 4,5-diaryl-1,3-dioxolanes [15], TADDOLs [16,17], axially chiral alleno-acetylenes [18] and strained axially chiral 1,1’-binaphthyl derivatives [19-22] to cite a few (Figure 1 for selected examples).
Figure 1: Selected examples of chiral dopants with high HTPs in their nematic host LC mixture.
Figure 1: Selected examples of chiral dopants with high HTPs in their nematic host LC mixture.
As a general observation, the more elongated the liquid crystal-like shape of a molecule appears, the more efficient is the induction of chirality in a nematic host. Additionally, the location of the chiral substructure within the dopant molecule seems to play a role. A more ‘central’ location within in the mesogenic core structure corresponds to a higher HTP of the resulting chiral compound. Molecular structures like those depicted in Figure 1 suggest that low conformational flexibility in combination with π-stacking interactions between the aryl moieties of the dopant and those of the LC mixture might be the key parameters for a high HTP. Significant efforts have been made exploring a wide range of chiral, twisted molecules such as binaphthyls [23-25], biphenyls [26,27], TADDOLs [16,17] and 1,2-diphenylethane-1,2-diol [28] to reveal possible relationships between the molecular structure of chiral dopants and their HTP value. However, quantitative structure–property relationships still remain elusive and are not well understood [29].
Recently we have become interested in the preparation of racemic [30] anti- and highly enantioenriched 2,2-difluoro-1,3-diols [30-32] through an acylative double catalytic kinetic resolution (DoCKR) process [33]. While the 1,3-diol motif is found in some natural products [34] with some fluorinated analogues [35,36], this motif is rarely found in LCs [37-39].
Based on these observations and literature data, we embarked in the synthesis and evaluation of enantiomerically pure acetals derived from anti-2,2-difluoro-1,3-diols as potential chiral dopants. Our long-term goal would be to provide a structure–property relationship of this class of molecules by i) modification of the aryl and ii) and/or acetal moieties (Scheme 1) and to elucidate the possible role of fluorine on their physical properties, with comparison to their non-fluorinated analogs [38]. It is anticipated that the introduction of a CF2 could eventually stabilize the conformation of the six-membered ring via stereolectronic interactions of the CF σ* orbitals with the neighboring CH σ orbital [40]. The 180° FCCH dihedral would rigidify the six-membered ring.
Scheme 1: Structure–property relationship of 4,6-diheteroaryl-5,5-difluoro-1,3-dioxanes as potential chiral dopants.
Scheme 1: Structure–property relationship of 4,6-diheteroaryl-5,5-difluoro-1,3-dioxanes as potential chiral d...
We present here preliminary results with the diastereoselective synthesis of two 4,6-diphenyl-5,5-difluoro-1,3-dioxanes (rac-3 and rac-4) and their separation into their corresponding enantiomers ((R,R)-3 and (S,S)-3; (R,R)-4 and (S,S)-4). rac-3 and rac-4 are obtained from readily available racemic 2,2-difluoro-1,3-diphenyl-1,3-propanediol (rac-2). These enantiomers were then evaluated as chiral dopants using two commercially available liquid crystal host mixtures (Host 1 and Host 2 from Merck KGaA) (Scheme 2).
Scheme 2: The syntheses of chiral 4,6-diphenyl-5,5-difluoro-1,3-dioxanes 3 and 4 as dopants for cholesteric liquid crystals.
Scheme 2: The syntheses of chiral 4,6-diphenyl-5,5-difluoro-1,3-dioxanes 3 and 4 as dopants for cholesteric l...
Results and Discussion
Racemic anti-2,2-difluoro-1,3-diol rac-2 was easily prepared through a single step by an aldol-Tishchenko reaction [30] starting from commercially available difluoromethyl phenyl ketone [41,42] and an excess of benzaldehyde (4 equiv), under basic conditions (Scheme 3). Diol rac-2 was obtained in a 62% yield after recrystallization from CHCl3 with no need for chromatography purification. Ketalization of rac-2 with liquid crystal-like ketone 1 [43] (in toluene) or 2,2-dimethoxypropane 5 (in THF) provided dioxanes rac-3 and rac-4 in 56% and 62% yields, respectively (Scheme 2).
Scheme 3: Synthesis of rac-2 as precursor of rac-3 and rac-4.
Scheme 3: Synthesis of rac-2 as precursor of rac-3 and rac-4.
Samples of rac-3 and rac-4 were separated by preparative HPLC on a chiral phase. Suitable crystals of all enantiomers (R,R)-3/(S,S)-3, (R,R)-4/(S,S)-4 were obtained by slow evaporation from n-heptane solution and structures were determined by X-ray diffraction, which allowed the correlation of the chiroptical properties with the configuration (Figure 2 and Figure 3).
![[1860-5397-20-246-2]](/bjoc/content/figures/1860-5397-20-246-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Configuration of (R,R)-3 determined by X-ray crystallography.
Figure 2: Configuration of (R,R)-3 determined by X-ray crystallography.
![[1860-5397-20-246-3]](/bjoc/content/figures/1860-5397-20-246-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Configuration of (R,R)-4 determined by X-ray crystallography.
Figure 3: Configuration of (R,R)-4 determined by X-ray crystallography.
The HTP of all enantiomers were measured with two achiral nematic host mixtures (Host 1 and Host 2 from Merck) (Table 1). (R,R)-3 and (S,S)-3 with a more liquid crystal-like shape have higher HTP [16 µm−1 (Host 1), 38 µm−1 (Host 2)] than the dimethylacetal analogues (R,R)-4 and (S,S)-4 [8 µm−1 (Host 1), 15 µm−1 (Host 2)]. The difference between the two nematic host mixtures is their polarity: whereas Host 1 is only moderately polar (Δε = 4.0), the mixture Host 2 was developed for Blue Mode LCD application and is extremely polar. As compared to chiral dopants depicted in Figure 1, HTPs are lower and closest analogues for comparison could be the 4,5-diaryl-1,3-dioxolanes [15,28]. However, in particular the HTPs obtained for 3 are encouraging, since the values are still equal or higher than those of many commercially used chiral dopants [7]. Both compounds 3 and 4 have good solubilities in LCs hosts, and since their syntheses require few steps and are easily amenable to structural variation (e.g., of the aromatic moieties), there is a clear path towards even higher HTPs values.
Table 1: Comparison of the physical properties of enantiopures (R,R)-3, (S,S)-3, (R,R)-4 and (S,S)-4.
| No | Phase sequencea | ΔHCIb | TNI,virta,c |
|
HTPe |
| (R,R)-3 | C 148 I | 8500 | −58.3 | −5.20 |
−16 (Host 1)
−38 (Host 2) |
| (S,S)-3 | C 149 I | 8500 | −61.2 | + 6.80 |
+16 (Host 1)
+38 (Host 2) |
| (R,R)-4 | C 142 I | 5100 | – | −0.67 |
−8 (Host 1)
−15 (Host 2) |
| (S,S)-4 | C 142 I | 5600 | – | + 0.60 |
+8 (Host 1)
+15 (Host 2) |
aThe phase transition and virtual clearing temperatures TNI,virt are given in °C; bthe melting enthalpies ΔHCI are given in cal mol−1; cthe values are extrapolated from a 5% w/w solution in ZLI-4792; dthe optical rotations are given in °; ethe helical twisting power (HTP) is given in µm−1; C = crystalline, I = isotropic. The "virtual" clearing temperatures TNI were determined by linear extrapolation from a 5% w/w solution in the Merck mixture ZLI-4792 (TNI = 92.8 °C, Δε = 5.3, Δn = 0.0964). The extrapolated values are corrected empirically for differences in the order parameter. For the pure substances the phase transitions were identified by optical microscopy, and the corresponding temperatures by differential scanning calorimetry (DSC).
Conclusion
In general, the C2-symmetric substructure in compounds (R,R)-/(S,S)-3 and (R,R)-/(S,S)-4 is quite efficient in inducing a chiral pitch into the nematic host mixtures Host 1 and Host 2. The extension of the chiral substructure (4 →3) by the liquid crystal building block 1 into a more rod-like shape is further boosting the HTP by roughly a factor of 2. Moreover, the HTP of both compounds 3 and 4 depends strongly on the LC host mixture: for the more polar mixture Host 2 it is twice as high as for less polar Host 1.
Supporting Information
| Supporting Information File 1: Experimental procedures, analytical data and copies of NMR spectra. | ||
| Format: PDF | Size: 1.8 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Kirsch, P.; Bremer, M. Angew. Chem., Int. Ed. 2000, 39, 4216–4235. doi:10.1002/1521-3773(20001201)39:23<4216::aid-anie4216>3.0.co;2-k
Return to citation in text: [1] [2] -
Bremer, M.; Kirsch, P.; Klasen‐Memmer, M.; Tarumi, K. Angew. Chem., Int. Ed. 2013, 52, 8880–8896. doi:10.1002/anie.201300903
Return to citation in text: [1] [2] -
Kirsch, P. J. Fluorine Chem. 2015, 177, 29–36. doi:10.1016/j.jfluchem.2015.01.007
Return to citation in text: [1] -
Kitzerow, H. S.; Bahr, C., Eds. Chirality in Liquid Crystals; Springer: New York, NY, USA, 2001. doi:10.1007/b97374
Return to citation in text: [1] -
Eelkema, R.; Feringa, B. L. Org. Biomol. Chem. 2006, 4, 3729–3745. doi:10.1039/b608749c
Return to citation in text: [1] -
Katsonis, N.; Lacaze, E.; Ferrarini, A. J. Mater. Chem. 2012, 22, 7088–7097. doi:10.1039/c2jm15962g
Return to citation in text: [1] -
Pauluth, D.; Wächtler, A. E. F. Synthesis and Application of Chiral Liquid Crystals. In Chirality in Industry II; Collins, A. N.; Sheldrake, G. N.; Crosby, J., Eds.; John Wiley & Sons: New York, NY, USA, 1997; pp 263–286.
Return to citation in text: [1] [2] -
Scheffer, T. J.; Nehring, J. Appl. Phys. Lett. 1984, 45, 1021–1023. doi:10.1063/1.95048
Return to citation in text: [1] -
Scheffer, T. J.; Nehring, J. J. Appl. Phys. 1985, 58, 3022–3031. doi:10.1063/1.335851
Return to citation in text: [1] -
Kikuchi, H.; Yokota, M.; Hisakado, Y.; Yang, H.; Kajiyama, T. Nat. Mater. 2002, 1, 64–68. doi:10.1038/nmat712
Return to citation in text: [1] -
Wittek, M.; Tanaka, N.; Bremer, M.; Wilkes, D.; Pauluth, D.; Tarumi, K. Novel materials for polymer-stabilized blue phase. In Proc. SPIE 8279, Emerging Liquid Crystal Technologies VII, San Francisco, CA, USA, Feb 10, 2012; 82790W. doi:10.1117/12.908138
Return to citation in text: [1] -
https://phys.org/news/2008-05-samsung-worlds-blue-phase-technology.html (accessed June 21, 2024).
Return to citation in text: [1] -
Allen, J. D. W.; Adlem, K.; Heeney, M. J. Mater. Chem. C 2021, 9, 17419–17426. doi:10.1039/d1tc05068k
Return to citation in text: [1] -
Thiem, H.; Strohriegl, P.; Shkunov, M.; McCulloch, I. Macromol. Chem. Phys. 2005, 206, 2153–2159. doi:10.1002/macp.200500272
Return to citation in text: [1] -
Rosini, C.; Spada, G. P.; Proni, G.; Masiero, S.; Scamuzzi, S. J. Am. Chem. Soc. 1997, 119, 506–512. doi:10.1021/ja962445m
Return to citation in text: [1] [2] -
Kuball, H.-G.; Weiß, B.; Beck, A. K.; Seebach, D. Helv. Chim. Acta 1997, 80, 2507–2514. doi:10.1002/hlca.19970800818
Return to citation in text: [1] [2] -
Seebach, D.; Beck, A. K.; Heckel, A. Angew. Chem., Int. Ed. 2001, 40, 92–138. doi:10.1002/1521-3773(20010105)40:1<92::aid-anie92>3.0.co;2-k
Return to citation in text: [1] [2] -
Wezenberg, S. J.; Ferroni, F.; Pieraccini, S.; Schweizer, W. B.; Ferrarini, A.; Spada, G. P.; Diederich, F. RSC Adv. 2013, 3, 22845–22848. doi:10.1039/c3ra44883e
Return to citation in text: [1] -
Kakisaka, K.; Higuchi, H.; Okumura, Y.; Kikuchi, H. J. Mater. Chem. C 2014, 2, 6467–6470. doi:10.1039/c4tc01049c
Return to citation in text: [1] -
Goh, M.; Park, J.; Han, Y.; Ahn, S.; Akagi, K. J. Mater. Chem. 2012, 22, 25011–25018. doi:10.1039/c2jm35282f
Return to citation in text: [1] -
Taugerbeck, A.; Kirsch, P.; Pauluth, D.; Krause, J.; Suermann, J.; Heckmeier, M. Chiral binaphthol derivatives. WO Patent WO2002034739A1, May 2, 2002.
Return to citation in text: [1] -
Kühn, C.; Bremer, M.; Schreiner, P. R. Liq. Cryst. 2019, 46, 1763–1768. doi:10.1080/02678292.2019.1599455
Return to citation in text: [1] -
Gottarelli, G.; Hibert, M.; Samori, B.; Solladié, G.; Spada, G. P.; Zimmermann, R. J. Am. Chem. Soc. 1983, 105, 7318–7321. doi:10.1021/ja00363a019
Return to citation in text: [1] -
Deuβen, H.-J.; Shibaev, P. V.; Vinokur, R.; Bjørnholm, T.; Schaumburg, K.; Bechgaard, K.; Shibaev, V. P. Liq. Cryst. 1996, 21, 327–340. doi:10.1080/02678299608032841
Return to citation in text: [1] -
Proni, G.; Spada, G. P.; Lustenberger, P.; Welti, R.; Diederich, F. J. Org. Chem. 2000, 65, 5522–5527. doi:10.1021/jo0001683
Return to citation in text: [1] -
Williams, V. E.; Lemieux, R. P. Chem. Commun. 1996, 2259–2260. doi:10.1039/cc9960002259
Return to citation in text: [1] -
di Matteo, A.; Todd, S. M.; Gottarelli, G.; Solladié, G.; Williams, V. E.; Lemieux, R. P.; Ferrarini, A.; Spada, G. P. J. Am. Chem. Soc. 2001, 123, 7842–7851. doi:10.1021/ja010406r
Return to citation in text: [1] -
Superchi, S.; Donnoli, M. I.; Proni, G.; Spada, G. P.; Rosini, C. J. Org. Chem. 1999, 64, 4762–4767. doi:10.1021/jo990038y
Return to citation in text: [1] [2] -
Pieraccini, S.; Ferrarini, A.; Spada, G. P. Chirality 2008, 20, 749–759. doi:10.1002/chir.20482
Return to citation in text: [1] -
Xu, W.; Médebielle, M.; Bellance, M.-H.; Dolbier, W. R., Jr. Adv. Synth. Catal. 2010, 352, 2787–2790. doi:10.1002/adsc.201000548
Return to citation in text: [1] [2] [3] -
Shao, N.; Rodriguez, J.; Quintard, A. Org. Lett. 2020, 22, 7197–7201. doi:10.1021/acs.orglett.0c02536
Return to citation in text: [1] -
Prakash, G. K. S.; Hu, J.; Mathew, T.; Olah, G. A. Angew. Chem., Int. Ed. 2003, 42, 5216–5219. doi:10.1002/anie.200352172
Return to citation in text: [1] -
Desrues, T.; Merad, J.; Andrei, D.; Pons, J.-M.; Parrain, J.-L.; Médebielle, M.; Quintard, A.; Bressy, C. Angew. Chem., Int. Ed. 2021, 60, 24924–24929. doi:10.1002/anie.202107041
Return to citation in text: [1] -
Herkommer, D.; Schmalzbauer, B.; Menche, D. Nat. Prod. Rep. 2014, 31, 456–467. doi:10.1039/c3np70093c
See for a review on polyketide natural products containing the anti-1,3-diol motif.
Return to citation in text: [1] -
Bentler, P.; Bergander, K.; Daniliuc, C. G.; Mück-Lichtenfeld, C.; Jumde, R. P.; Hirsch, A. K. H.; Gilmour, R. Angew. Chem., Int. Ed. 2019, 58, 10990–10994. doi:10.1002/anie.201905452
See for the development of fluorinated analogs of natural 1,3-diols.
Return to citation in text: [1] -
Sperandio, C.; Quintard, G.; Naubron, J.-V.; Giorgi, M.; Yemloul, M.; Parrain, J.-L.; Rodriguez, J.; Quintard, A. Chem. – Eur. J. 2019, 25, 15098–15105. doi:10.1002/chem.201902983
See for the development of fluorinated analogs of natural 1,3-diols.
Return to citation in text: [1] -
Kusumoto, T.; Sato, K.-I.; Ogino, K.; Hiyama, T.; Takehara, S.; Osawa, M.; Nakamura, K. Liq. Cryst. 1993, 14, 727–732. doi:10.1080/02678299308027750
Return to citation in text: [1] -
Farrand, L. D.; Saxton, P. E.; Patrick, J. Mesogenic compounds, liquid crystal medium and liquid crystal composition. Eur. Pat. Appl. EP1690916A1, March 12, 2008.
Return to citation in text: [1] [2] -
Yang, S.; Wang, B.; Cui, D.; Kerwood, D.; Wilkens, S.; Han, J.; Luk, Y.-Y. J. Phys. Chem. B 2013, 117, 7133–7143. doi:10.1021/jp401382h
Return to citation in text: [1] -
O'Hagan, D. Chem. – Eur. J. 2020, 26, 7981–7997. doi:10.1002/chem.202000178
Return to citation in text: [1] -
Amii, H.; Kobayashi, T.; Hatamoto, Y.; Uneyama, K. Chem. Commun. 1999, 14, 1323–1324. doi:10.1039/a903681d
Return to citation in text: [1] -
Prakash, G. K. S.; Hu, J.; Olah, G. A. J. Fluorine Chem. 2001, 112, 355–360. doi:10.1016/s0022-1139(01)00535-8
Return to citation in text: [1] -
CAS 82832-73-3 for the bicyclohexanone derivative 1 which is a common liquid crystal building block and was obtained from Merck KGaA, Darmstadt.
Return to citation in text: [1]
| 43. | CAS 82832-73-3 for the bicyclohexanone derivative 1 which is a common liquid crystal building block and was obtained from Merck KGaA, Darmstadt. |
| 15. | Rosini, C.; Spada, G. P.; Proni, G.; Masiero, S.; Scamuzzi, S. J. Am. Chem. Soc. 1997, 119, 506–512. doi:10.1021/ja962445m |
| 28. | Superchi, S.; Donnoli, M. I.; Proni, G.; Spada, G. P.; Rosini, C. J. Org. Chem. 1999, 64, 4762–4767. doi:10.1021/jo990038y |
| 7. | Pauluth, D.; Wächtler, A. E. F. Synthesis and Application of Chiral Liquid Crystals. In Chirality in Industry II; Collins, A. N.; Sheldrake, G. N.; Crosby, J., Eds.; John Wiley & Sons: New York, NY, USA, 1997; pp 263–286. |
| 1. | Kirsch, P.; Bremer, M. Angew. Chem., Int. Ed. 2000, 39, 4216–4235. doi:10.1002/1521-3773(20001201)39:23<4216::aid-anie4216>3.0.co;2-k |
| 2. | Bremer, M.; Kirsch, P.; Klasen‐Memmer, M.; Tarumi, K. Angew. Chem., Int. Ed. 2013, 52, 8880–8896. doi:10.1002/anie.201300903 |
| 3. | Kirsch, P. J. Fluorine Chem. 2015, 177, 29–36. doi:10.1016/j.jfluchem.2015.01.007 |
| 8. | Scheffer, T. J.; Nehring, J. Appl. Phys. Lett. 1984, 45, 1021–1023. doi:10.1063/1.95048 |
| 9. | Scheffer, T. J.; Nehring, J. J. Appl. Phys. 1985, 58, 3022–3031. doi:10.1063/1.335851 |
| 16. | Kuball, H.-G.; Weiß, B.; Beck, A. K.; Seebach, D. Helv. Chim. Acta 1997, 80, 2507–2514. doi:10.1002/hlca.19970800818 |
| 17. | Seebach, D.; Beck, A. K.; Heckel, A. Angew. Chem., Int. Ed. 2001, 40, 92–138. doi:10.1002/1521-3773(20010105)40:1<92::aid-anie92>3.0.co;2-k |
| 1. | Kirsch, P.; Bremer, M. Angew. Chem., Int. Ed. 2000, 39, 4216–4235. doi:10.1002/1521-3773(20001201)39:23<4216::aid-anie4216>3.0.co;2-k |
| 2. | Bremer, M.; Kirsch, P.; Klasen‐Memmer, M.; Tarumi, K. Angew. Chem., Int. Ed. 2013, 52, 8880–8896. doi:10.1002/anie.201300903 |
| 7. | Pauluth, D.; Wächtler, A. E. F. Synthesis and Application of Chiral Liquid Crystals. In Chirality in Industry II; Collins, A. N.; Sheldrake, G. N.; Crosby, J., Eds.; John Wiley & Sons: New York, NY, USA, 1997; pp 263–286. |
| 28. | Superchi, S.; Donnoli, M. I.; Proni, G.; Spada, G. P.; Rosini, C. J. Org. Chem. 1999, 64, 4762–4767. doi:10.1021/jo990038y |
| 5. | Eelkema, R.; Feringa, B. L. Org. Biomol. Chem. 2006, 4, 3729–3745. doi:10.1039/b608749c |
| 6. | Katsonis, N.; Lacaze, E.; Ferrarini, A. J. Mater. Chem. 2012, 22, 7088–7097. doi:10.1039/c2jm15962g |
| 23. | Gottarelli, G.; Hibert, M.; Samori, B.; Solladié, G.; Spada, G. P.; Zimmermann, R. J. Am. Chem. Soc. 1983, 105, 7318–7321. doi:10.1021/ja00363a019 |
| 24. | Deuβen, H.-J.; Shibaev, P. V.; Vinokur, R.; Bjørnholm, T.; Schaumburg, K.; Bechgaard, K.; Shibaev, V. P. Liq. Cryst. 1996, 21, 327–340. doi:10.1080/02678299608032841 |
| 25. | Proni, G.; Spada, G. P.; Lustenberger, P.; Welti, R.; Diederich, F. J. Org. Chem. 2000, 65, 5522–5527. doi:10.1021/jo0001683 |
| 4. | Kitzerow, H. S.; Bahr, C., Eds. Chirality in Liquid Crystals; Springer: New York, NY, USA, 2001. doi:10.1007/b97374 |
| 26. | Williams, V. E.; Lemieux, R. P. Chem. Commun. 1996, 2259–2260. doi:10.1039/cc9960002259 |
| 27. | di Matteo, A.; Todd, S. M.; Gottarelli, G.; Solladié, G.; Williams, V. E.; Lemieux, R. P.; Ferrarini, A.; Spada, G. P. J. Am. Chem. Soc. 2001, 123, 7842–7851. doi:10.1021/ja010406r |
| 15. | Rosini, C.; Spada, G. P.; Proni, G.; Masiero, S.; Scamuzzi, S. J. Am. Chem. Soc. 1997, 119, 506–512. doi:10.1021/ja962445m |
| 18. | Wezenberg, S. J.; Ferroni, F.; Pieraccini, S.; Schweizer, W. B.; Ferrarini, A.; Spada, G. P.; Diederich, F. RSC Adv. 2013, 3, 22845–22848. doi:10.1039/c3ra44883e |
| 13. | Allen, J. D. W.; Adlem, K.; Heeney, M. J. Mater. Chem. C 2021, 9, 17419–17426. doi:10.1039/d1tc05068k |
| 14. | Thiem, H.; Strohriegl, P.; Shkunov, M.; McCulloch, I. Macromol. Chem. Phys. 2005, 206, 2153–2159. doi:10.1002/macp.200500272 |
| 19. | Kakisaka, K.; Higuchi, H.; Okumura, Y.; Kikuchi, H. J. Mater. Chem. C 2014, 2, 6467–6470. doi:10.1039/c4tc01049c |
| 20. | Goh, M.; Park, J.; Han, Y.; Ahn, S.; Akagi, K. J. Mater. Chem. 2012, 22, 25011–25018. doi:10.1039/c2jm35282f |
| 21. | Taugerbeck, A.; Kirsch, P.; Pauluth, D.; Krause, J.; Suermann, J.; Heckmeier, M. Chiral binaphthol derivatives. WO Patent WO2002034739A1, May 2, 2002. |
| 22. | Kühn, C.; Bremer, M.; Schreiner, P. R. Liq. Cryst. 2019, 46, 1763–1768. doi:10.1080/02678292.2019.1599455 |
| 12. | https://phys.org/news/2008-05-samsung-worlds-blue-phase-technology.html (accessed June 21, 2024). |
| 10. | Kikuchi, H.; Yokota, M.; Hisakado, Y.; Yang, H.; Kajiyama, T. Nat. Mater. 2002, 1, 64–68. doi:10.1038/nmat712 |
| 11. | Wittek, M.; Tanaka, N.; Bremer, M.; Wilkes, D.; Pauluth, D.; Tarumi, K. Novel materials for polymer-stabilized blue phase. In Proc. SPIE 8279, Emerging Liquid Crystal Technologies VII, San Francisco, CA, USA, Feb 10, 2012; 82790W. doi:10.1117/12.908138 |
| 16. | Kuball, H.-G.; Weiß, B.; Beck, A. K.; Seebach, D. Helv. Chim. Acta 1997, 80, 2507–2514. doi:10.1002/hlca.19970800818 |
| 17. | Seebach, D.; Beck, A. K.; Heckel, A. Angew. Chem., Int. Ed. 2001, 40, 92–138. doi:10.1002/1521-3773(20010105)40:1<92::aid-anie92>3.0.co;2-k |
| 30. | Xu, W.; Médebielle, M.; Bellance, M.-H.; Dolbier, W. R., Jr. Adv. Synth. Catal. 2010, 352, 2787–2790. doi:10.1002/adsc.201000548 |
| 31. | Shao, N.; Rodriguez, J.; Quintard, A. Org. Lett. 2020, 22, 7197–7201. doi:10.1021/acs.orglett.0c02536 |
| 32. | Prakash, G. K. S.; Hu, J.; Mathew, T.; Olah, G. A. Angew. Chem., Int. Ed. 2003, 42, 5216–5219. doi:10.1002/anie.200352172 |
| 29. | Pieraccini, S.; Ferrarini, A.; Spada, G. P. Chirality 2008, 20, 749–759. doi:10.1002/chir.20482 |
| 30. | Xu, W.; Médebielle, M.; Bellance, M.-H.; Dolbier, W. R., Jr. Adv. Synth. Catal. 2010, 352, 2787–2790. doi:10.1002/adsc.201000548 |
| 30. | Xu, W.; Médebielle, M.; Bellance, M.-H.; Dolbier, W. R., Jr. Adv. Synth. Catal. 2010, 352, 2787–2790. doi:10.1002/adsc.201000548 |
| 41. | Amii, H.; Kobayashi, T.; Hatamoto, Y.; Uneyama, K. Chem. Commun. 1999, 14, 1323–1324. doi:10.1039/a903681d |
| 42. | Prakash, G. K. S.; Hu, J.; Olah, G. A. J. Fluorine Chem. 2001, 112, 355–360. doi:10.1016/s0022-1139(01)00535-8 |
| 38. | Farrand, L. D.; Saxton, P. E.; Patrick, J. Mesogenic compounds, liquid crystal medium and liquid crystal composition. Eur. Pat. Appl. EP1690916A1, March 12, 2008. |
| 35. |
Bentler, P.; Bergander, K.; Daniliuc, C. G.; Mück-Lichtenfeld, C.; Jumde, R. P.; Hirsch, A. K. H.; Gilmour, R. Angew. Chem., Int. Ed. 2019, 58, 10990–10994. doi:10.1002/anie.201905452
See for the development of fluorinated analogs of natural 1,3-diols. |
| 36. |
Sperandio, C.; Quintard, G.; Naubron, J.-V.; Giorgi, M.; Yemloul, M.; Parrain, J.-L.; Rodriguez, J.; Quintard, A. Chem. – Eur. J. 2019, 25, 15098–15105. doi:10.1002/chem.201902983
See for the development of fluorinated analogs of natural 1,3-diols. |
| 37. | Kusumoto, T.; Sato, K.-I.; Ogino, K.; Hiyama, T.; Takehara, S.; Osawa, M.; Nakamura, K. Liq. Cryst. 1993, 14, 727–732. doi:10.1080/02678299308027750 |
| 38. | Farrand, L. D.; Saxton, P. E.; Patrick, J. Mesogenic compounds, liquid crystal medium and liquid crystal composition. Eur. Pat. Appl. EP1690916A1, March 12, 2008. |
| 39. | Yang, S.; Wang, B.; Cui, D.; Kerwood, D.; Wilkens, S.; Han, J.; Luk, Y.-Y. J. Phys. Chem. B 2013, 117, 7133–7143. doi:10.1021/jp401382h |
| 33. | Desrues, T.; Merad, J.; Andrei, D.; Pons, J.-M.; Parrain, J.-L.; Médebielle, M.; Quintard, A.; Bressy, C. Angew. Chem., Int. Ed. 2021, 60, 24924–24929. doi:10.1002/anie.202107041 |
| 34. |
Herkommer, D.; Schmalzbauer, B.; Menche, D. Nat. Prod. Rep. 2014, 31, 456–467. doi:10.1039/c3np70093c
See for a review on polyketide natural products containing the anti-1,3-diol motif. |
© 2024 Médebielle et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.