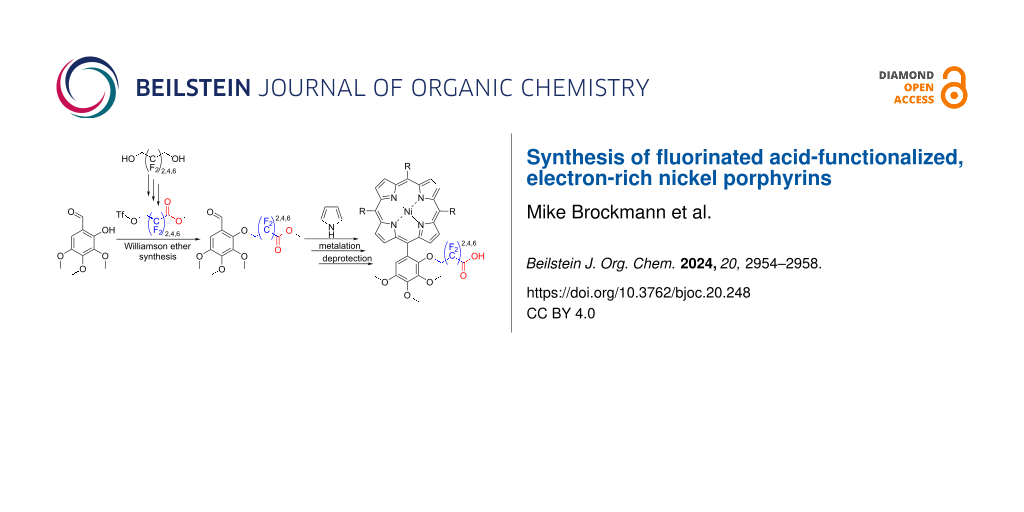
Abstract
In this study, novel fluorinated carboxylic acid esters of the generic structure TfO–CH2–(CF2)n–COOCH3 (n = 2,4,6, Tf = triflate) were synthesized. The triflates were reacted with 2-hydroxy-3,4,5-trimethoxybenzaldehyde via Williamson ether syntheses. The resulting electron-rich compounds were used as aldehydes in the Rothemund reaction with pyrrole to form ester-substituted porphyrins. After metalation with Ni(acac)2 and hydrolysis electron-rich porphyrins were obtained, that are equipped with covalently attached long chain acid substituents. The target compounds have potential applications in catalysis, sensing, and materials science. The fluorinated aliphatic carboxylic acids (TfO–CH2–(CF2)n–COOCH3) with triflate as leaving group in terminal position are easily accessible and versatile building blocks for attaching long chain acids (pKa 0–1) to substrates in Williamson ether-type reactions.
Graphical Abstract
Introduction
Metal porphyrins are prosthetic groups in a number of essential biomolecules, including hemoglobin, chlorophyll, and cytochromes, supporting processes such as oxygen transport, photosynthesis, and electron transfer [1-5]. Beyond their essential biological roles, porphyrins and their derivatives are employed in a number of applications, acting as catalysts in numerous reactions, including oxidation, reduction, and cycloaddition [6-10]. Particularly when electron-rich porphyrins act as reducing agents, e.g. in electrocatalytic hydrogen evolution reactions, a proton source is needed [11]. In this context, trifluoroacetic acid is very frequently chosen as the proton source, because it is a strong acid but just not strong enough to destroy (demetallate) the Ni porphyrin [10]. Covalent attachment of acids facilitates proton transfer and increases the efficiency [12]. Three conditions should be met for the target porphyrins of this study. 1. The acid covalently bound to the porphyrin should have an acid strength similar to trifluoroacetic acid. 2. The length of the tether with which the acid group is bound should be sufficient to serve as a proton source for redox reactions at the metal. 3. The electronic properties of the porphyrin, especially the low oxidation potential, should not be increased. We have chosen four-fold meso-3,4,5-trimethoxyphenyl-substituted Ni porphyrin as the electron-rich system, however, the post-synthetic modification of this porphyrin proved to be difficult. Therefore, we have integrated the acid group into the aldehyde component of the Rothemund reaction to prepare the target porphyrin. In initial tests, we have established that trifluoroacetic acid can be replaced by perfluorinated alkyl carboxylic acids [10]. It was therefore obvious to use a perfluoroalkyl chain as a tether. However, a perfluoroalkyl chain as a substituent on the porphyrin has an electron-withdrawing effect and thus a negative influence on the oxidation potential. We have therefore inserted an O–CH2 group between the phenyl group of the porphyrin and the perfluoroalkyl chain. The oxygen atom, especially in the 2-position, should even improve the electronic properties of the porphyrin.
Results and Discussion
Our synthesis started with the readily available fluorinated symmetric diols HO–CH2–(CF2)n–CH2–OH (n = 2,4,6, see Scheme 1).
Scheme 1: Synthesis of the starting materials 16, 17, and 18 for the subsequent Williamson ether synthesis with 2-hydroxy-3,4,5-trimethoxybenzaldehyde (21). Conditions: a) K2CO3, benzyl bromide, abs. MeCN, N2, reflux, 18 h; b) TEMPO, KBr, NaOCl, NaHCO3, MeCN, rt, 76 h; c) MeOH, H2SO4, reflux, 18 h; d) Pd/C, H2, EtOH, rt, 24 h; e) Tf2O, pyridine, DCM, rt, 18 h.
Scheme 1: Synthesis of the starting materials 16, 17, and 18 for the subsequent Williamson ether synthesis wi...
In order to break the symmetry and to generate the acid function only on one side, benzyl protection was performed. From diols 1, 2, and 3 statistical mixtures of unprotected, mono-, and di-protected products were obtained, from which the isolation of the desired mono-protected products 4 (65%), 5 (50%), and 6 (40%) by chromatography was straightforward. However, the subsequent oxidation of the alcohol with the usual oxidizing agents (Jones reagent, KMnO4, etc.) was not successful. A radical oxidation with TEMPO, potassium bromide (KBr), sodium hypochlorite (NaOCl), and sodium bicarbonate (NaHCO₃) provided acids 7, 8, and 9. A byproduct is obtained during oxidation and it is assumed that this is the molecule oxidized at the benzyl position (see Supporting Information File 1, compounds 35–40). Work-up and isolation proved to be difficult, and therefore, the acids were directly converted into the methyl esters 10 (54%), 11 (52%), and 12 (46%). The benzyl-protecting group was removed hydrogenolytically to give products 13 (85%), 14 (65%), and 15 (99%). The alcohols were then converted to the triflates 16 (28%), 17 (41%), and 18 (63%).
We have chosen 3,4,5-trimethoxybenzaldehyde (19) as the aldehyde component due to its commercial availability. A OH group was introduced to serve as the nucleophile in the Williamson ether synthesis with the triflates 16, 17, and 18 (Scheme 2).
Scheme 2: Synthesis of perfluoroalkyl ester-functionalized aldehydes 22, 23, and 24. Conditions: a) NIS, TFA, Na2CO3, MeCN, reflux, 18 h; b) Cu2O·H2O, 2-pyridinaldoxime, TBAB, CsOH, H2O, N2, rt, 18 h; c) Cs2CO3, DMAc, N2, rt, 3 h.
Scheme 2: Synthesis of perfluoroalkyl ester-functionalized aldehydes 22, 23, and 24. Conditions: a) NIS, TFA,...
Towards this end, 3,4,5-trimethoxybenzaldehyde (19) was iodinated using N-iodosuccinimide (NIS) to give 20 in a yield of 89% [13]. To convert the iodo to an OH group, compound 20 was reacted with Cu2O, 2-pyridinaldoxime and CsOH to give 2-hydroxy-3,4,5-trimethoxybenzaldehyde (21, 65%) [13]. In a subsequent nucleophilic substitution, the fluorinated alkyl chains of 16, 17, and 18 were linked via a Williamson ether synthesis to yield 22 (78%), 23 (44%), and 24 (44%).
Compounds 22, 23, and 24 were used as aldehyde components in the Rothemund-type synthesis of metal-free porphyrins 26 (9%), 27 (18%), and 28 (21%) (see Scheme 3).
Scheme 3: Porphyrin synthesis. a) Rothemund porphyrin synthesis of metal-free porphyrins 26, 27, and 28; b) metalation of porphyrins with Ni(acac)2; c) ester hydrolysis to generate the free acids 32, 33, and 34. Conditions: a) 1) 22/23/24, TFA, abs. DCM, N2, reflux, 30 min, 2) pyrrole, reflux, 2.5 h, 3) DDQ, reflux, 2 h; b) Ni(acac)2, toluene, reflux, 20 h; c) 1) LiOH, MeOH, rt, 1 h, 2) HCl.
Scheme 3: Porphyrin synthesis. a) Rothemund porphyrin synthesis of metal-free porphyrins 26, 27, and 28; b) m...
Metalation was achieved with nickel acetylacetonate to obtain the ester-substituted Ni porphyrins 29 (78%), 30 (97%), and 31 (57%). The latter were treated with LiOH and HCl to give the free acids 32 (94%), 33 (39%), and 34 (45%). The HPLC–ESIMS analysis of 32, 33, and 34 revealed that two major atropisomers of each porphyrin had formed. In 32 both atropisomers exhibit a roughly 1:1 ratio, in 33 we observed a roughly 1:2 ratio, and in 34 almost only one atropisomer was formed (see Figures S102, S106, and S110 in Supporting Information File 1). We attribute this to the increasing sterical hindrance of the increasing chain lengths in compounds 32, 33, and 34, which should favor an alternating sequence of the chains pointing upward and downward.
Conclusion
This study reports the synthesis of perfluoroalkyl carboxylic esters with CH2–OTf groups in the ω-position of the type TfO–CH2–(CF2)n–COOCH3 (n = 2, 4, 6, Tf = triflate). The latter compounds were used in Williamson ether reactions with 2-hydroxy-3,4,5-trimethoxybenzaldehyde (21) to prepare the aldehyde component for a Rothemund-type porphyrin synthesis of acid-functionalized electron-rich porphyrins. The corresponding Ni porphyrins are potential compounds for electrocatalysis and sensor applications. The ω-triflated, perfluoroalkylated carboxylic acids 16, 17, and 18 are easily accessible and versatile building blocks for connecting long chain acids (pKa range between 0 and 1) to substrates in Williamson ether-type reactions.
Supporting Information
| Supporting Information File 1: Experimental procedures, characterization data of all products, and copies of 1H, 13C, and 19F NMR spectra. | ||
| Format: PDF | Size: 5.5 MB | Download |
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Droege, D. G.; Parker, A. L.; Milligan, G. M.; Jenkins, R.; Johnstone, T. C. J. Org. Chem. 2022, 87, 11783–11795. doi:10.1021/acs.joc.2c01538
Return to citation in text: [1] -
Hopp, M.-T.; Schmalohr, B. F.; Kühl, T.; Detzel, M. S.; Wißbrock, A.; Imhof, D. Anal. Chem. (Washington, DC, U. S.) 2020, 92, 9429–9440. doi:10.1021/acs.analchem.0c00415
Return to citation in text: [1] -
Upoma, N. J.; Akter, N.; Ferdousi, F. K.; Sultan, M. Z.; Rahman, S.; Alodhayb, A.; Alibrahim, K. A.; Habib, A. ACS Omega 2024, 9, 22325–22335. doi:10.1021/acsomega.4c01708
Return to citation in text: [1] -
Moore, M. R. An Historical Introduction to Porphyrin and Chlorophyll Synthesis. In Tetrapyrroles: Birth, Life and Death; Warren, M. J.; Smith, A. G., Eds.; Springer Science & Business Media: New York, NY, USA, 2009; pp 1–28. doi:10.1007/978-0-387-78518-9_1
Return to citation in text: [1] -
Munro, A. W.; Givran, H. M.; McLean, K. J.; Cheesman, M. R.; Leys, D. Heme and Hemoproteins. In Tetrapyrroles: Birth, Life and Death; Warren, M.; Smith, A., Eds.; Springer Science & Business Media: New York, NY, USA, 2009; pp 160–183. doi:10.1007/978-0-387-78518-9_10
Return to citation in text: [1] -
Peters, M. K.; Röhricht, F.; Näther, C.; Herges, R. Org. Lett. 2018, 20, 7879–7883. doi:10.1021/acs.orglett.8b03433
Return to citation in text: [1] -
Shaikh, R. R.; Pornpraprom, S.; D’Elia, V. ACS Catal. 2018, 8, 419–450. doi:10.1021/acscatal.7b03580
Return to citation in text: [1] -
Silva, A. M. G.; Tomé, A. C.; Neves, M. G. P. M. S.; Silva, A. M. S.; Cavaleiro, J. A. S. J. Org. Chem. 2005, 70, 2306–2314. doi:10.1021/jo048349i
Return to citation in text: [1] -
Zhou, C.-Y.; Yu, W.-Y.; Che, C.-M. Org. Lett. 2002, 4, 3235–3238. doi:10.1021/ol0201254
Return to citation in text: [1] -
Brockmann, M.; Glotz, G.; von Glasenapp, J.-S.; Unterriker, L.; Neshchadin, D.; Gescheidt, G.; Herges, R. J. Am. Chem. Soc. 2024, 146, 13010–13024. doi:10.1021/jacs.3c14118
Return to citation in text: [1] [2] [3] -
Wu, Z.-Y.; Wang, T.; Meng, Y.-S.; Rao, Y.; Wang, B.-W.; Zheng, J.; Gao, S.; Zhang, J.-L. Chem. Sci. 2017, 8, 5953–5961. doi:10.1039/c7sc02073b
Return to citation in text: [1] -
Castro-Cruz, H. M.; Macías-Ruvalcaba, N. A. Coord. Chem. Rev. 2022, 458, 214430. doi:10.1016/j.ccr.2022.214430
Return to citation in text: [1] -
Gao, W.; Li, Q.; Chen, J.; Wang, Z.; Hua, C. Molecules 2013, 18, 15613–15623. doi:10.3390/molecules181215613
Return to citation in text: [1] [2]
| 1. | Droege, D. G.; Parker, A. L.; Milligan, G. M.; Jenkins, R.; Johnstone, T. C. J. Org. Chem. 2022, 87, 11783–11795. doi:10.1021/acs.joc.2c01538 |
| 2. | Hopp, M.-T.; Schmalohr, B. F.; Kühl, T.; Detzel, M. S.; Wißbrock, A.; Imhof, D. Anal. Chem. (Washington, DC, U. S.) 2020, 92, 9429–9440. doi:10.1021/acs.analchem.0c00415 |
| 3. | Upoma, N. J.; Akter, N.; Ferdousi, F. K.; Sultan, M. Z.; Rahman, S.; Alodhayb, A.; Alibrahim, K. A.; Habib, A. ACS Omega 2024, 9, 22325–22335. doi:10.1021/acsomega.4c01708 |
| 4. | Moore, M. R. An Historical Introduction to Porphyrin and Chlorophyll Synthesis. In Tetrapyrroles: Birth, Life and Death; Warren, M. J.; Smith, A. G., Eds.; Springer Science & Business Media: New York, NY, USA, 2009; pp 1–28. doi:10.1007/978-0-387-78518-9_1 |
| 5. | Munro, A. W.; Givran, H. M.; McLean, K. J.; Cheesman, M. R.; Leys, D. Heme and Hemoproteins. In Tetrapyrroles: Birth, Life and Death; Warren, M.; Smith, A., Eds.; Springer Science & Business Media: New York, NY, USA, 2009; pp 160–183. doi:10.1007/978-0-387-78518-9_10 |
| 12. | Castro-Cruz, H. M.; Macías-Ruvalcaba, N. A. Coord. Chem. Rev. 2022, 458, 214430. doi:10.1016/j.ccr.2022.214430 |
| 10. | Brockmann, M.; Glotz, G.; von Glasenapp, J.-S.; Unterriker, L.; Neshchadin, D.; Gescheidt, G.; Herges, R. J. Am. Chem. Soc. 2024, 146, 13010–13024. doi:10.1021/jacs.3c14118 |
| 11. | Wu, Z.-Y.; Wang, T.; Meng, Y.-S.; Rao, Y.; Wang, B.-W.; Zheng, J.; Gao, S.; Zhang, J.-L. Chem. Sci. 2017, 8, 5953–5961. doi:10.1039/c7sc02073b |
| 6. | Peters, M. K.; Röhricht, F.; Näther, C.; Herges, R. Org. Lett. 2018, 20, 7879–7883. doi:10.1021/acs.orglett.8b03433 |
| 7. | Shaikh, R. R.; Pornpraprom, S.; D’Elia, V. ACS Catal. 2018, 8, 419–450. doi:10.1021/acscatal.7b03580 |
| 8. | Silva, A. M. G.; Tomé, A. C.; Neves, M. G. P. M. S.; Silva, A. M. S.; Cavaleiro, J. A. S. J. Org. Chem. 2005, 70, 2306–2314. doi:10.1021/jo048349i |
| 9. | Zhou, C.-Y.; Yu, W.-Y.; Che, C.-M. Org. Lett. 2002, 4, 3235–3238. doi:10.1021/ol0201254 |
| 10. | Brockmann, M.; Glotz, G.; von Glasenapp, J.-S.; Unterriker, L.; Neshchadin, D.; Gescheidt, G.; Herges, R. J. Am. Chem. Soc. 2024, 146, 13010–13024. doi:10.1021/jacs.3c14118 |
| 13. | Gao, W.; Li, Q.; Chen, J.; Wang, Z.; Hua, C. Molecules 2013, 18, 15613–15623. doi:10.3390/molecules181215613 |
| 13. | Gao, W.; Li, Q.; Chen, J.; Wang, Z.; Hua, C. Molecules 2013, 18, 15613–15623. doi:10.3390/molecules181215613 |
| 10. | Brockmann, M.; Glotz, G.; von Glasenapp, J.-S.; Unterriker, L.; Neshchadin, D.; Gescheidt, G.; Herges, R. J. Am. Chem. Soc. 2024, 146, 13010–13024. doi:10.1021/jacs.3c14118 |
© 2024 Brockmann et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.